Abstract
Although CD8 T cell–mediated immunosuppression has been a well-known phenomenon during the last three decades, the nature of primary CD8 T suppressor cells and the mechanism underlying their generation remain enigmatic. We demonstrated that naive CD8 T cells primed with allogeneic CD40 ligand–activated plasmacytoid dendritic cells (DC)2 differentiated into CD8 T cells that displayed poor secondary proliferative and cytolytic responses. By contrast, naive CD8 T cells primed with allogeneic CD40 ligand–activated monocyte-derived DCs (DC1) differentiated into CD8 T cells, which proliferated to secondary stimulation and killed allogeneic target cells. Unlike DC1-primed CD8 T cells that produced large amounts of interferon (IFN)-γ upon restimulation, DC2-primed CD8 T cells produced significant amounts of interleukin (IL)-10, low IFN-γ, and no IL-4, IL-5, nor transforming growth factor (TGF)-β. The addition of anti–IL-10–neutralizing monoclonal antibodies during DC2 and CD8 T cell coculture, completely blocked the generation of IL-10–producing anergic CD8 T cells. IL-10–producing CD8 T cells strongly inhibit the allospecific proliferation of naive CD8 T cells to monocytes, and mature and immature DCs. This inhibition was mediated by IL-10, but not by TGF-β. IL-10–producing CD8 T cells could inhibit the bystander proliferation of naive CD8 T cells, provided that they were restimulated nearby to produce IL-10. IL-10–producing CD8 T cells could not inhibit the proliferation of DC1-preactivated effector T cells. This study demonstrates that IL-10–producing CD8 T cells are regulatory T cells, which provides a cellular basis for the phenomenon of CD8 T cell–mediated immunosuppression and suggests a role for plasmacytoid DC2 in immunological tolerance.
Keywords: dendritic cells, T regulatory cells, CD8 T cells, immunosuppression, IL-10
Introduction
The concept of T lymphocyte–mediated immunosuppression was established 30 yr ago, after the observation that immunological tolerance could be induced in naive animals upon the transfer of antigen-primed T lymphocytes (1, 2). The recent identification of regulatory CD4 T cell subsets defined by their surface phenotype (CD45RClow in rat, CD45RBlowCD25+ in mice), their capacity to produce IL-10, or a combination of IL-10, IL-4, and TGF-β, finally provided a cellular basis for this immunological paradigm (3–7). A role for CD8 T cells in the in vivo suppression of self-reactive T cells has also been described (8–10). CD8 T cells may mediate antigen-specific immunosuppression by killing CD4 T helper cells (11, 12) or antigen presenting cells (13), or by noncytolytic pathways that have not been clearly defined. Although a number of noncytolytic CD8 T cell clones with inhibitory activity have been described (14–17), the nature of the primary CD8 T suppressor cells and the mechanism underlying their generation still remain elusive (18–20). Dendritic cells (DC)* were shown to induce Th1 versus Th2 differentiation depending on their maturation stage, the type and duration of activation, and DC subsets (21–24). We have previously shown that mature plasmacytoid DC2 activated by CD40 ligand either for 24 h or 6 d induced Th2 differentiation, whereas mature monocyte-derived DC1 activated by CD40 ligand for 24 h induced Th1 differentiation (25). Here we investigate the role of DC1 and DC2 in activation and differentiation of naive CD8 T cells. We show that in contrast to DC1, which induce the differentiation of naive CD8 T cells into CTL, DC2 induce a population of CD8 T regulatory cells that are anergic, noncytolytic, and capable of inhibiting primary T cell responses through the production of IL-10.
Materials and Methods
Generation of DC1 from Monocytes.
Mononuclear cells were isolated from peripheral blood of healthy donors by Ficoll-Hypaque centrifugation (Amersham Pharmacia Biotech). Cells were then centrifuged on 52% Percoll gradient (Amersham Pharmacia Biotech) for 20 min at 400 g. Residual T, B, and NK cells, as well as erythrocytes were depleted by using mouse anti-CD2 (OKT11), anti-CD3 (OKT3), anti-CD8 (Leu-2a), anti-CD19 (Leu-12), anti-CD20 (Leu-16), anti-CD56 (Leu-19), and anti–glycophorin A (10F7MN) mAbs, and magnetic beads coated with goat anti–mouse IgG (Dynabeads M-450; Dynal). Isolated CD14+ monocytes (purity >90%) were cultured in RPMI 1640 (Biowhittaker) and supplemented with 10% FCS (Biowhittaker), 2 mM l-glutamine, 10 mM Hepes, 1 mM sodium pyruvate, penicillin G, and streptomycin (Life Technologies) in the presence of 100 ng/ml GM-CSF and 200 U/ml of IL-4 (Schering-Plough Research Institute) for 5 d. The resulting immature DC1 were washed and cultured for 24 h with CD40 ligand–transfected L cells (irradiated at 5,500 rad) to obtain mature DC1 (25).
Generation of DC2 from CD4+CD11c−lineage− Blood Precursors.
T, B, and NK cells, monocytes, and erythrocytes were depleted from blood mononuclear cells by using mouse anti-CD3, anti-CD19, anti-CD16 (Immunotech), anti-CD14 (RPA-M1), and anti–glycophorin A mAbs, and magnetic beads coated with goat anti–mouse IgG. The resulting cells were stained with PE-Cy5–conjugated anti-CD4 (Immunotech), PE-conjugated anti-CD11c (Becton Dickinson), and a cocktail of FITC-conjugated anti-CD3, anti-CD14, anti-CD16, and anti-CD20 mAbs (Becton Dickinson). The CD4+CD11c−CD3−CD14−CD16−CD20− cells (DC2 precursors) were isolated by cell sorting (26). These cells (purity >99%) were cultured with 10 ng/ml of IL-3 (R&D Systems) in the presence of irradiated CD40 ligand–transfected L cells for 6 d, or with IL-3 alone for 5 d, followed by a 24-h stimulation with CD40 ligand–transfected L cells (25).
Isolation of Naive CD8 T Lymphocytes from Adult Blood.
Naive CD8 T cells were enriched from peripheral blood mononuclear cells by immunomagnetic depletion using mouse anti-CD4, anti-CD14, anti-CD16, anti-CD19, anti–HLA-DR, and anti-CD45RO mAb, followed by magnetic beads coated with goat anti–mouse IgG. These cells were stained with PE-Cy5–conjugated anti-CD45RA, PE-conjugated anti-CD27 (BD PharMingen), and a cocktail of FITC-conjugated anti-CD4, anti–TCR-γδ, anti-CD14, anti-CD16, and anti-CD20 mAbs (Becton Dickinson). CD27+CD45RA+lineage− were isolated by fluorescence-activated cell sorting and were >98% CD8+ T cells. CD8+ CD27+CD45RA+ have been previously described as naive CD8 T cells (27).
Primary Allogeneic CD8 T Cell Cultures.
4 × 104 freshly isolated CD8+CD27+CD45RA+ T cells were cultured for 6 d with 104 allogeneic DC1 or DC2 in 200 μl of Yssel's medium (Irvine Scientific) containing 10% FCS in 96-well U-bottom culture plates. IL-2 (10 U/ml) was added at day 2 of culture. Naive CD8 T cells were also cultured with plate-bound anti-CD3 mAb SPV-T3b and 1 μg/ml of soluble anti-CD28 mAb L293.1 (Becton Dickinson). In some experiments, recombinant human IL-12 (2 ng/ml; R&D Systems), neutralizing anti–IL-12 mAb AB-219-NA (10 μg/ml; R&D Systems), or neutralizing anti–IL-10 mAb 12G8 (10 μg/ml; DNAX) were added to the cultures.
Proliferation Assays.
Primary and secondary proliferative T cell responses were assessed by stimulating 4 × 104 T cells with 104 CD40 ligand–activated DC1 or DC2 in 96-well round-bottom plates without the addition of IL-2. During the last 12 h of culture, 1 μCi of [3H]thymidine was added to each well and cellular incorporation was determined.
Cytotoxicity Assay.
After 6 d, CD8 T cells, which represented >99% of viable cells in cultures, were tested for the capacity to lyse 51Cr-labeled allogeneic or autologous targets. Target cells included B cells isolated by the positive selection of CD19+ cells (purity >95%) and activated with CD40 ligand–transfected L cells and IL-4 (100 U/ml) for 6–12 d (28). The assay was performed by using 2,500 51Cr-labeled target cells per well and incubated with the indicated effectors at 37°C for 5 h before collecting the supernatants and counting in a gamma counter. We used Triton X to determine the maximum lysis and target cells alone to determine the spontaneous lysis. The percentage of lysis was calculated as follows: (spontaneous cpm − experimental cpm)/spontaneous cpm.
Analysis of Cytokine Production.
To test their capacity to secrete cytokines, T cells (106/ml) were restimulated with plate-bound anti-CD3 mAb (10 μg/ml) plus 1 μg/ml of soluble anti-CD28 mAb for 24 h. IFN-γ, TNF-α, IL-4, IL-5, and IL-10 in the culture supernatants were measured by Quantikine ELISA kits (R&D Systems). For flow cytometry analysis of intracellular cytokines, T cells were restimulated with plate-bound anti-CD3 mAb plus soluble anti-CD28 mAb, or with 50 ng/ml PMA plus 500 ng/ml ionomycin. After 2.5 h, 10 μg/ml of Brefeldin A (Epicentre) was added and the cells were cultured for an additional 2.5 h. Harvested cells were fixed with 2% formaldehyde (Sigma-Aldrich) and permeabilized with PBS supplemented with 2% FCS, 0.1% NaN3, and 0.5% saponin (Sigma-Aldrich). The cells were stained with fluorochrome-labeled anti–IFN-γ mAb 4S.B3, anti–IL-10 mAb, and anti–IL-4 mAb (BD PharMingen).
Suppressor Assay.
In direct coculture experiments 4 × 104 naive CD8 T cells were cultured in 96-well round-bottom plates with 4 × 104 allogeneic APC (monocytes, immature DC1, or mature DC1) in the presence of graded doses of DC2-primed CD8 regulatory T (Tr) cells. After 5 d of culture 1 μ/Ci of [3H]thymidine was added to each well and cellular incorporation was determined after 12 h. In some experiments, neutralizing anti–IL-10 mAb (10 μg/ml, 12G8) or anti–TGF-β1, -β2, -β3 mAb (10 μg/ml; catalog number 80-1835-03; Genzyme) that neutralize human TGF-β1 and TGF-β2, was added at the beginning of the assay. Transwell experiments were performed in 24-well plates. 5 × 105 CD8 Tr cells plus 5 × 105 APC were placed in transwell chambers separated from a coculture of 5 × 105 naive CD8 T cells plus 5 × 105 APC by a 0.2-μm permeable membrane (a total culture volume of 600 μl). After 5 d of culture, activated T cells were transferred to 96 wells in triplicates (200 μl/well) and cellular incorporation was determined after a 12-h pulse with [3H]thymidine. To test suppression of secondary responses, 4 × 104 DC1-primed CD8 T cells were restimulated in 96-well round-bottom plates with allogeneic APC in the presence of CD8 Tr cells at ratios of 1:1, 2:1, and 3:1. [3H]thymidine incorporation was determined after 3 d. In addition, culture supernatants were collected for quantitation of IFN-γ.
Results
DC1 and DC2 Induce Strong Primary CD8 T Cell Proliferative Responses.
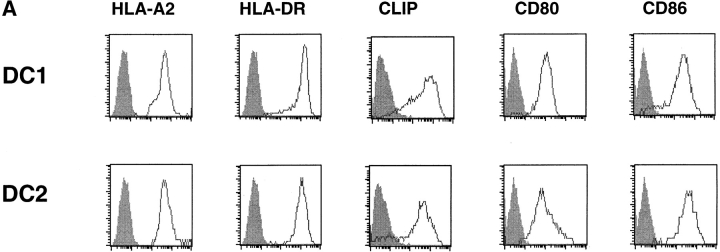
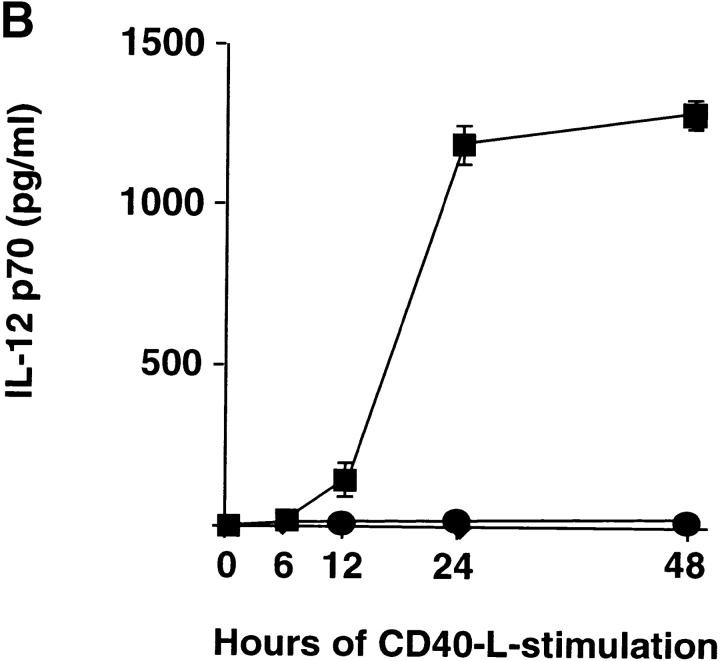
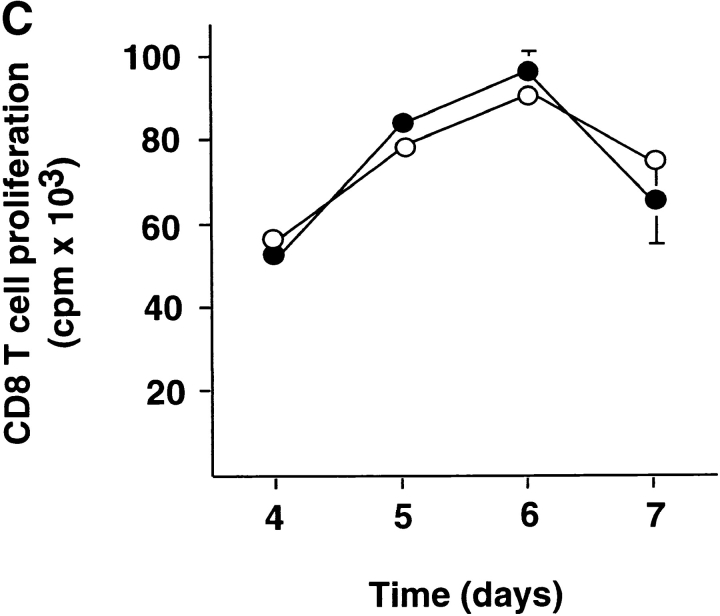
DC1 and DC2 were generated from purified monocytes or plasmacytoid precursors, respectively, and activated with CD40 ligand. DC1 and DC2 expressed similar levels of MHC class I, class II, endogenous class II–associated peptides, and costimulatory molecules CD80 and CD86 (Fig. 1 A). DC1 produced large amounts of IL-12p70 starting as early as 12 h (116 ± 48 pg/ml, n = 2) and reaching a peak at 24 h (1,225 ± 63 pg/ml, n = 2) of CD40 ligand stimulation. In contrast, DC2 maximally produced a mean value of 24.9 pg/ml of IL-12p70 at any time point (6 h, 12 h, 24 h, and 48 h) after CD40 ligand stimulation (Fig. 1 B). This indicates that the inability of mature DC2 to produce large amounts of IL-12 is intrinsic to DC2 and not due to exhaustion after prolonged activation (29, 30). To investigate the ability of DC1 and DC2 to stimulate naive CD8 T cells, we cultured CD45RA+CD27+ naive CD8 T cells with allogeneic DC1 and DC2. Both DC1 and DC2 induced strong proliferation of naive CD8 T cells (Fig. 1 C).
Figure 1.
DC1 and DC2 express similar levels of MHC products and costimulatory molecules, but differ in their ability to produce IL-12. (A) Cell surface expression of HLA-A2, HLA-DR, class II–associated peptides (CLIP), CD80, and CD86 on DC1 and DC2. (B) IL-12p70 in the supernatants of DC1 generated in a 5-d culture with GM-CSF and IL-4 (▪) and DC2 generated in a 5-d culture with IL-3 (•) stimulated at 100,000 cells/ml with CD40 ligand–transfected L cells for 6, 12, 24, and 48 h. Mean value ± SD of two independent experiments are represented. (C) DC1 and DC2 induce strong proliferation of naive CD8 T cells. Naive CD8 T cells were stimulated with allogeneic CD40 ligand–activated DC1 (•) and DC2 (○) and proliferative responses were assessed after 4, 5, 6, or 7 d. Results are expressed as mean cpm ± SD of triplicate wells. Background [3H]thymidine incorporation of CD8 T cells cultured in the absence of DC remained <1,000 cpm at all time points.
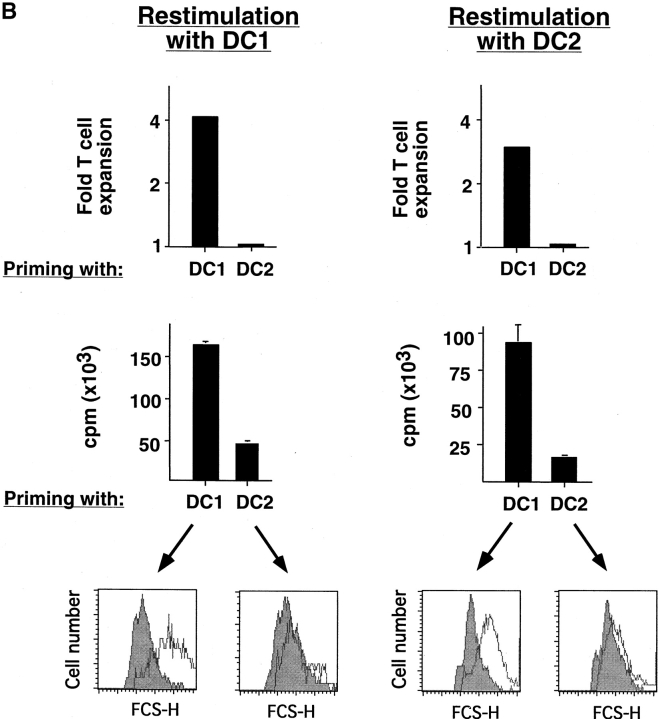
DC2-primed CD8 T Cells Show Impaired Ability to Mount Cytolytic and Secondary Proliferative Responses.
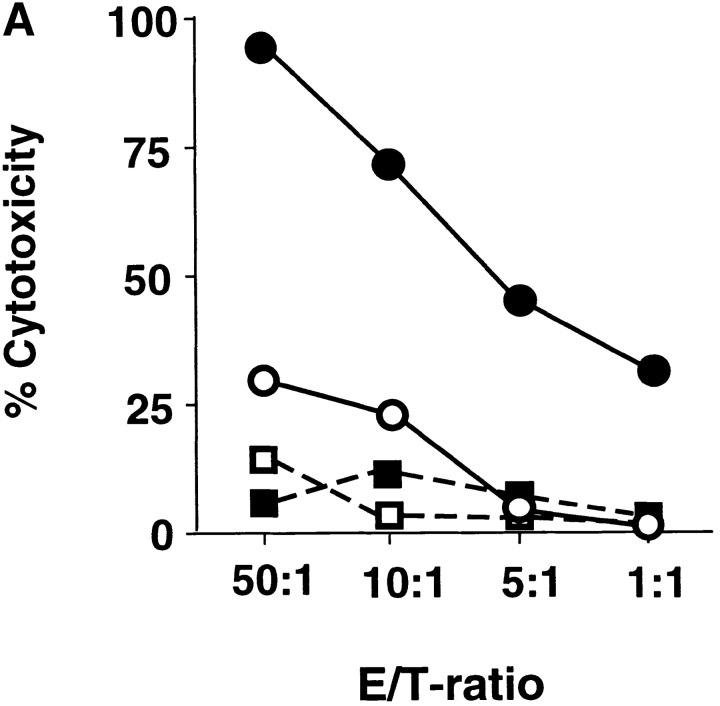
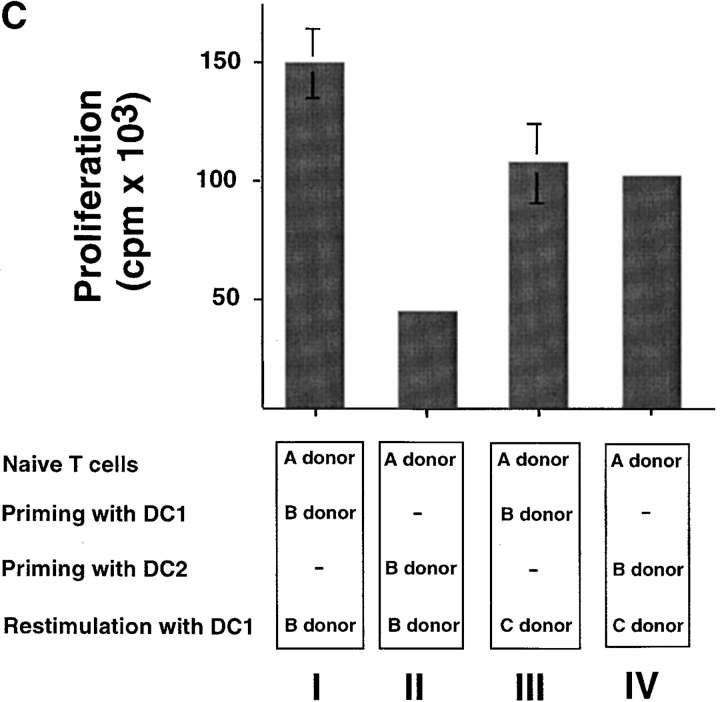
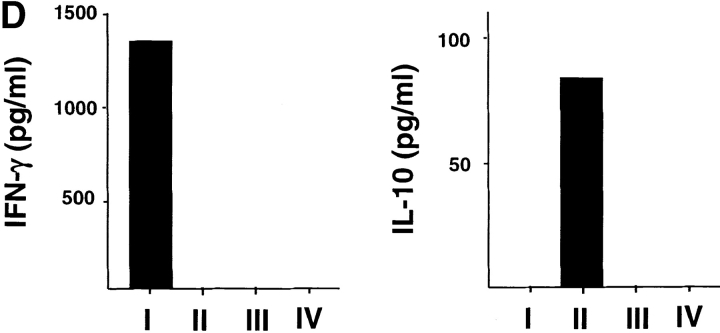
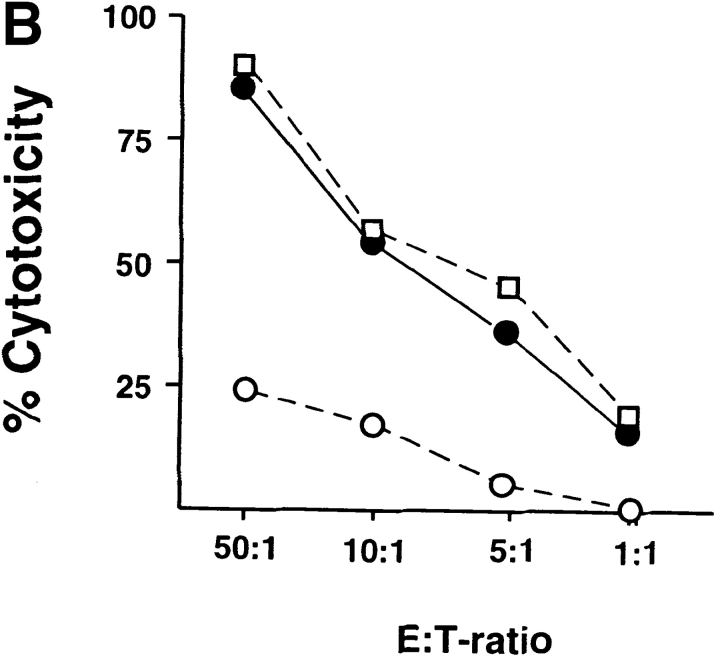
Whereas CD8 T cells primed by DC1 were potent cytotoxic effectors lysing allogeneic targets at low E/T ratios, CD8 T cells primed with DC2 displayed poor alloantigen-specific cytotoxicity (Fig. 2 A), but expressed similar levels of perforin and granzyme B (unpublished data). In addition, CD8 T cells primed with DC1 responded strongly to secondary restimulation with allogeneic DC1 or DC2, whereas CD8 T cells primed with DC2 were hyporesponsive to secondary restimulation with DC1 or DC2, as determined by proliferation and blastogenesis (Fig. 2 B). However, DC2-primed CD8 T cells were able to mount a proliferative response to third-party alloantigens (Fig. 2 C). This is because in contrast to previously described IL-10–producing Tr1 cell clones that display the same allo-specificity (7), DC2-primed CD8 T cells derived from a 6-d primary cell culture not only contain antigen-specific anergic CD8 T cells, but also unprimed naive T cells with other specificities. Therefore, upon restimulation with the original alloantigen, DC2-primed anergic CD8 T cells will not proliferate, whereas unprimed CD8 T cells specific to the third-party alloantigen will undergo primary proliferation. Indeed, in contrast to the secondary responses of DC1- or DC2-primed CD8 T cells to the original alloantigen that respectively produce cytokine IFN-γ or IL-10, their responses to the third-party alloantigen produce neither IFN-γ, nor IL-10 during the 48 h of stimulation, a feature of primary response (Fig. 2 D). These data indicate that DC2 induce alloantigen-specific anergy of CD8 T cells, which display an impaired ability to mount cytolytic and secondary proliferative responses.
Figure 2.
DC2-primed CD8 T cells exhibit poor cytolytic activity and are hyporesponsive to secondary restimulation. (A) CD8 T cells were primed for 6 d with DC1 (•, ▪) and DC2 (○, □), and collected and tested for their ability to lyse allogeneic (•, ○) or autologous (▪, □) B cells. The results represent six independent experiments. (B) CD8 T cells primed with DC1 and DC2 for 6 d were collected and restimulated with DC1 and DC2 derived from the donor used for priming. CD8 T cell expansion (upper panels), proliferation (middle panels), and increase in cell size (lower panel) were determined after 3 d of restimulation. Histograms represent CD8 T cell size before (filled) and after restimulation (open histograms). The results represent three independent experiments. (C) CD8 T cells from donor A were primed for 6 d with DC1 and DC2 from donor B, and then restimulated with DC1 from donor B or donor C. Proliferation was determined after 3 d of restimulation. (D) IFN-γ and IL-10 in the supernatants of CD8 T cells, restimulated as described in C for 2 d.
DC2 Induce IL-10–producing CD8 T Cells.
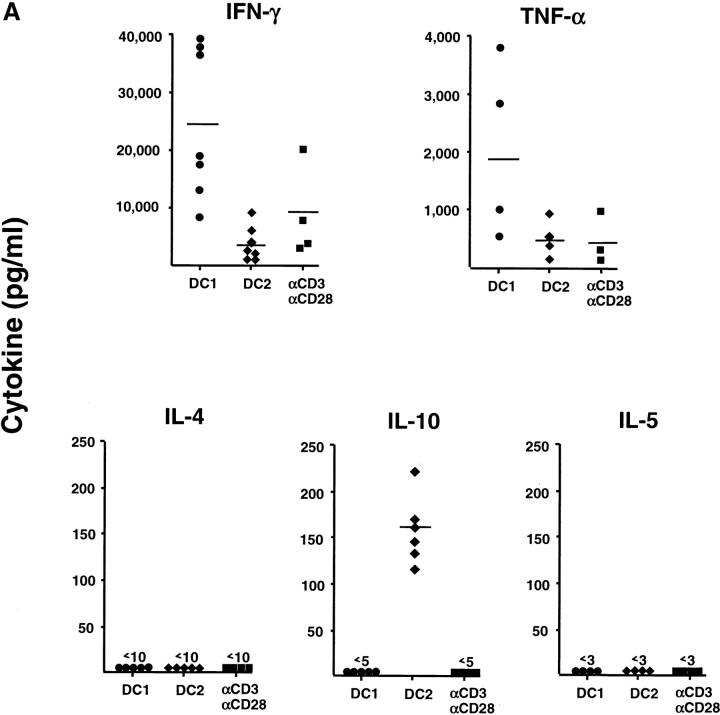
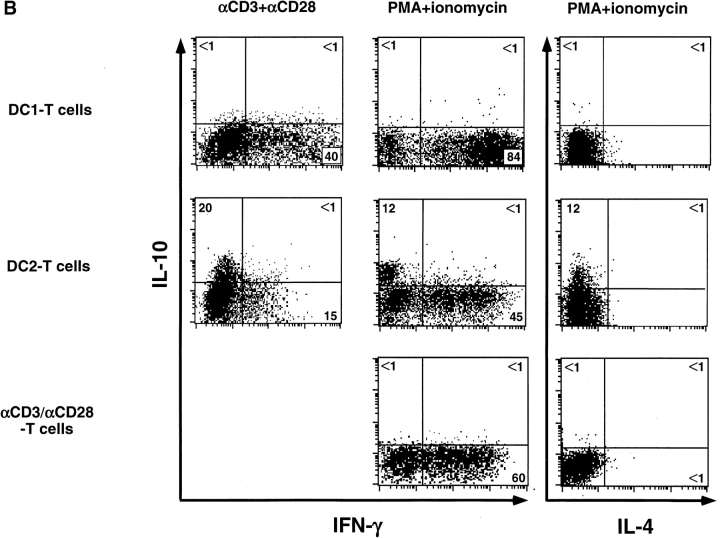
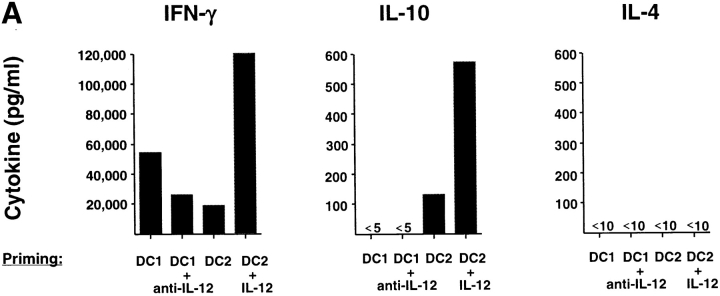
We analyzed the cytokine production by primed CD8 T cells in response to a 24-h restimulation with anti-CD3 plus anti-CD28 (Fig. 3 A). CD8 T cells primed with anti-CD3 and anti-CD28 for 6 d produced moderate levels of IFN-γ (2,500–21,000 pg/ml) and TNF-α (10–1,000 pg/ml), but no IL-4, IL-5, nor IL-10. CD8 T cells primed with DC1 secreted high amounts of IFN-γ (9,000–39,000 pg/ml) and TNF-α (500–3,800 pg/ml), but no detectable IL-4, IL-5, nor IL-10. CD8 T cells primed with DC2 secreted significantly lower amounts of IFN-γ (400–9,500 pg/ml), and neither IL4 nor IL5. Strikingly, DC2-primed CD8 T cells produced significant amounts of IL-10 (120–230 pg/ml). CD8 T cells primed with DC1, DC2, or anti-CD3 plus anti-CD28 did not secrete detectable levels of TGF-β protein. This was confirmed by quantitative PCR analyses showing that no significant levels of TGF-β mRNA were detected in CD8 T cells primed with DC1 or DC2 after restimulation. Immunofluorescence flow cytometry analysis (Fig. 3 B, left) revealed that 40% of DC1-primed CD8 T cells and 15% of DC2-primed CD8 T cells, respectively, differentiated into IFN-γ–producing cells upon anti-CD3 plus anti-CD28 restimulation. DC2-primed CD8 T cells, but not DC1-primed CD8 T cells, differentiated into IL-10–producing cells (20%). In a separate experiment, primed CD8 T cells were reactivated by a stronger stimulus such as PMA plus ionomycin (Fig. 3 B, middle and right). Although PMA plus ionomycin induced higher numbers of IFN-γ–producing CD8 T cells in all priming conditions, it did not stimulate CD8 T cells to produce IL-4. However, DC2-primed CD8 T cells produced IL-10 after restimulation with PMA and ionomycin. Because DC1 produced 40–200 times more IL-12 than DC2 after CD40 ligand activation, we determined whether the generation of IL-10–producing CD8 T cells was due to the absence of IL-12. The addition of neutralizing monoclonal antibody to IL-12 in the DC1–T cell culture significantly reduced the ability of CD8 T cells to produce IFN-γ, but did not promote the generation of IL-10–producing CD8 T cells (Fig. 4 A). The addition of IL-12 to the DC2–T cell culture strongly enhanced the IFN-γ production and, surprisingly, the IL-10 production by CD8 T cells (Fig. 4 A). Therefore, the ability of CD40 ligand–activated DC2 to induce IL-10–producing CD8 T cells was not due to the absence of IL-12.
Figure 3.
DC2-primed CD8 T cells produce IL-10 and low levels of IFN-γ. (A) Secretion of IFN-γ, TNF-α, IL-4, IL-5, and IL-10 by CD8 T cells primed for 6 d with DC1, DC2, or anti-CD3 plus anti-CD28, and restimulated with anti-CD3 plus anti-CD28 for 24 h. Each symbol represent an independent experiment. (B) Two-color staining of intracellular IFN-γ and IL-10, or IL-4 and IL-10, produced by DC1- and DC2-primed CD8 T cells after a 5-h restimulation with anti-CD3 plus anti-CD28 (left), or PMA plus ionomycin (middle and right). 104 cells were analyzed and the percentages of each population are indicated in the plots.
Figure 4.
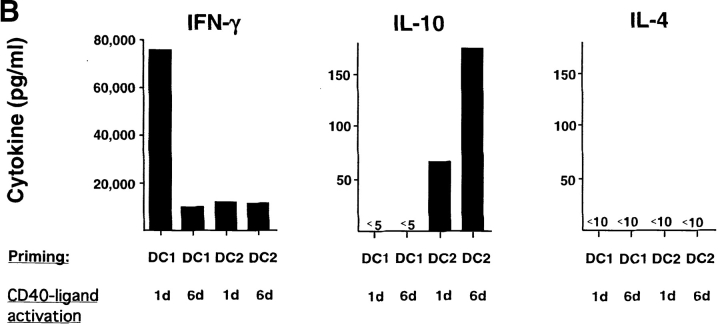
The generation of IL-10–producing CD8 T cells is not due to the absence of IL-12 nor to DC exhaustion caused by prolonged CD40 ligand activation. (A) Cytokine secretion by CD8 T cells primed for 6 d with DC1 plus neutralizing anti–IL-12, DC2, or DC2 plus IL-12. (B) Cytokine production of CD8 T cells primed for 6 d with DC1 and DC2 activated with CD40 ligand for either 1 d (5 d of GM-CSF/IL-4 or IL-3, respectively, plus an additional day of CD40 ligand), or 6 d (6 d of GM-CSF/IL-4 or IL-3, respectively, in the presence of CD40 ligand).
Previous studies have shown that after a prolonged activation, DC1 (called exhausted DC) have a reduced ability to prime Th1 responses (29, 30). Here, we determined if the ability of DC to induce CD8 T cells to produce IL-10 was due to DC exhaustion by prolonged CD40 ligand activation. DC1 activated by CD40 ligand for either 1 or 6 d did not prime CD8 T cells to produce IL-10 (Fig. 4 B). In contrast, DC2 activated by CD40 ligand for both 1 or 6 d primed CD8 T cells to produce IL-10 (Fig. 4 B). In accordance with the previous reports, DC1 activated by CD40 ligand for 6 d had a reduced ability to prime CD8 T cells to produce IFN-γ, compared with DC1 activated by CD40 ligand for 1 d (Fig. 4 B). Therefore, the ability of DC to prime CD8 T cells to produce IL-10 is not due to DC exhaustion, but to an intrinsic property of DC2.
Impaired Secondary Responsiveness of DC2-primed CD8 T Cells Is Mediated by IL-10.
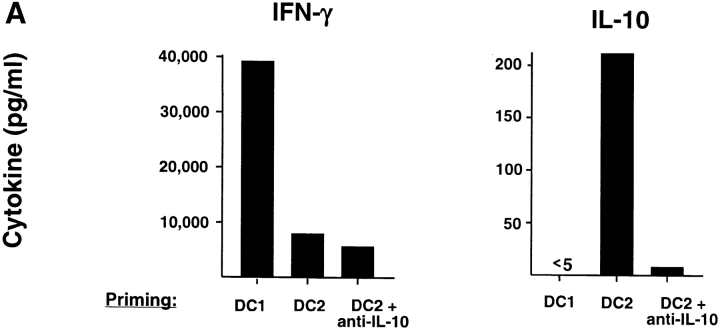
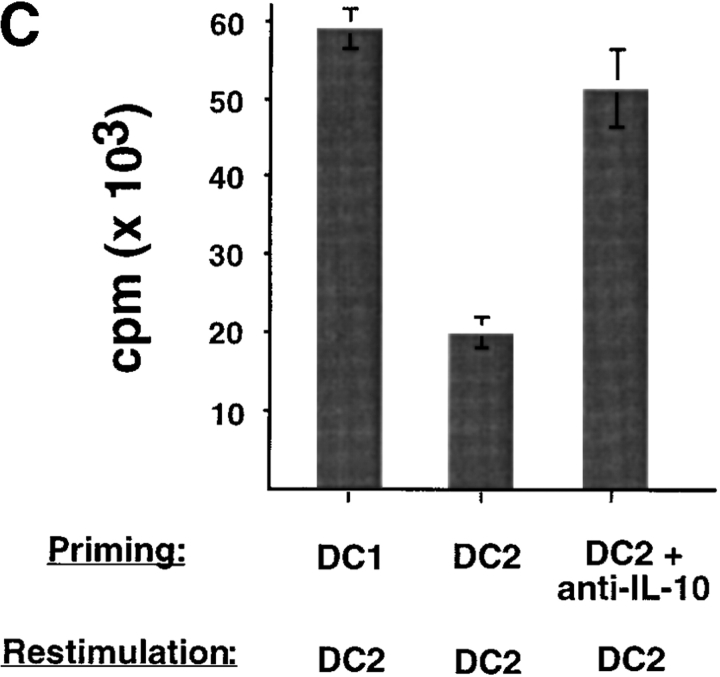
To investigate whether IL-10 is responsible for the impaired secondary responsiveness of DC2-primed CD8 T cells, neutralizing anti–IL-10 mAbs were added into the DC2–CD8 T cell coculture. The addition of anti–IL-10 mAbs resulted in the generation of CD8 T cells that did not produce IL-10 upon restimulation and became strongly cytotoxic and responsive to secondary restimulation (Fig. 5) . DC2 do not produce detectable IL-10 mRNA and protein after CD40 ligand activation (25), which suggests that IL-10 produced by CD8 T cells has an autocrine effect on the differentiation of IL-10–producing CD8 T cells. Furthermore, the data indicate that IL-10–producing CD8 T cells mediated the secondary cytolytic and proliferative hyporesponsiveness of DC2-primed CD8 T cells. Blocking of IL-10 during the secondary restimulation did not enhance the proliferative response of DC2-primed CD8 T cells (unpublished data), indicating that the hyporesponsiveness was induced during the primary cocultures.
Figure 5.
IL-10 is required for the generation of IL-10–producing CD8 T cells and mediates the poor cytolytic activity and secondary hyporesponsiveness of DC2-primed CD8 T cells. (A) Cytokine profile of CD8 T cells primed for 6 d with DC1, DC2, or DC2 plus neutralizing anti–IL-10 (10 μg/ml). (B) CD8 T cells primed with DC1 (•), DC2 (○), or DC2 plus anti–IL-10 (□) were tested for the ability to lyse allogeneic B cells in a 5-h Cr-release assay. Specific lysis of autologous B cells remained below 15% in all conditions (unpublished data). (C) Secondary proliferation of CD8 T cells primed with DC1, DC2, or DC2 plus anti–IL-10 after a 3-d restimulation with DC2. Equivalent results were obtained by restimulation with DC1 (unpublished data). Results are representative of three independent experiments.
IL-10–producing CD8 T Cells Strongly Suppress Naive CD8 T Cell Responses.
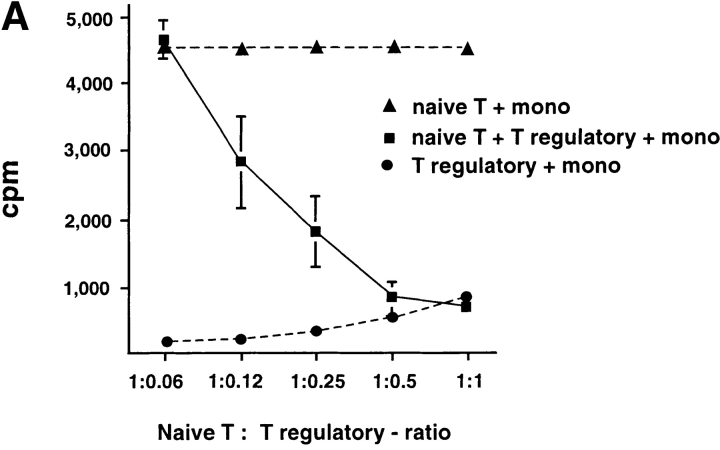
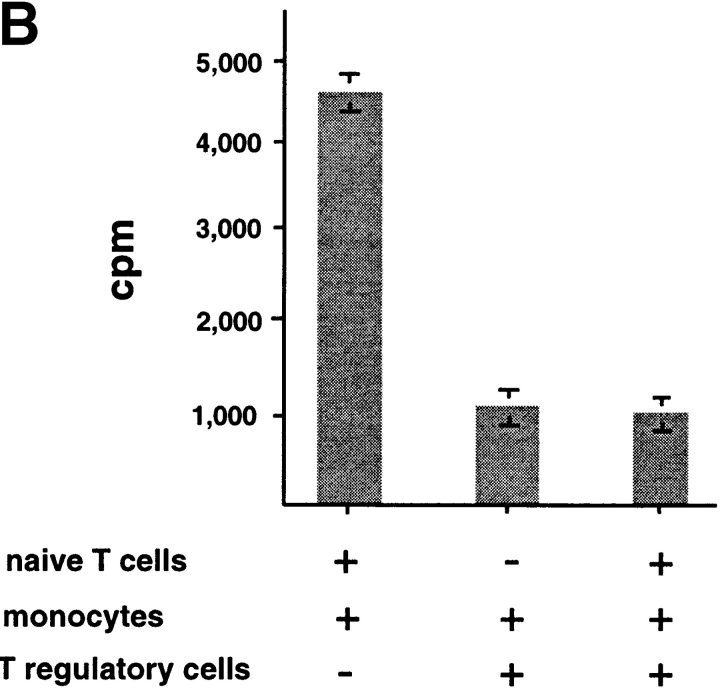
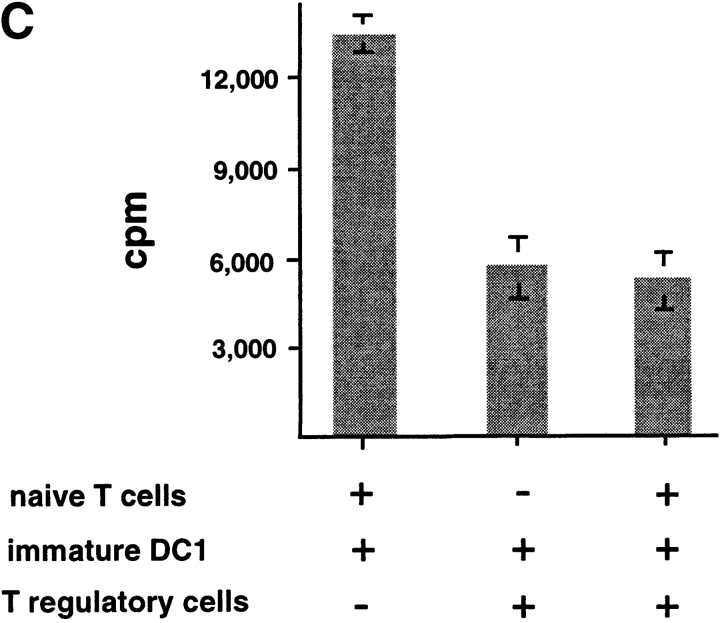
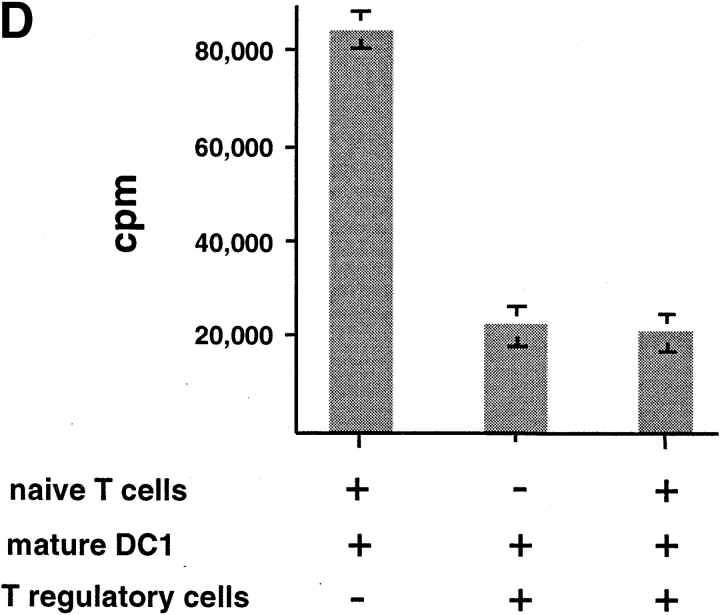
To determine whether IL-10–producing CD8 T cells are immunosuppressive, CD8 T cells cultured with allogeneic DC2 for 6 d were added into a coculture of autologous naive CD8 T cells with APCs from the same donor of priming DC2. DC2-primed CD8 Tr cells strongly inhibited the proliferation of naive CD8 T cells to allogeneic monocytes in a dose-dependent fashion (Fig. 6, A and B) . CD8 Tr cells also inhibited naive CD8 T cell proliferation to immature and mature DC1 (Fig. 6, C and D, respectively).
Figure 6.
DC2-primed CD8 T cells inhibit primary CD8 T cell proliferation induced by various types of APCs, in a dose-dependent manner. (A) Primary CD8 T cell proliferation in response to allogeneic monocytes in the presence of increasing numbers of syngeneic DC2-primed CD8 Tr cells (▪). Primary proliferation of naive CD8 T cells without CD8 Tr (▴) and proliferation of CD8 T regulatory cells (•) are also represented. (B) Proliferation of CD8 T cells stimulated with allogeneic monocytes, (C) immature DC1, or (D) mature DC1 in the presence of equal numbers of CD8 Tr (1:1 ratio). Results are representative of two independent experiments.
CD8 Tr Cells Suppress Naive CD8 T Cell Proliferation through IL-10, but Not TGF-β.
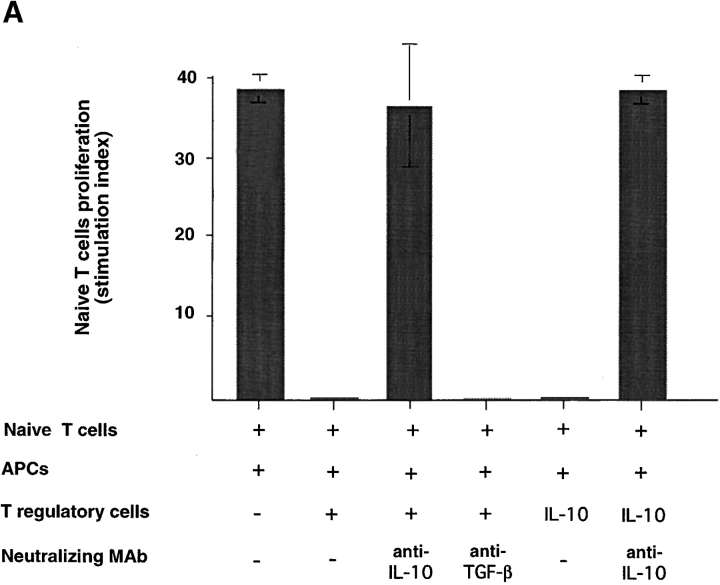
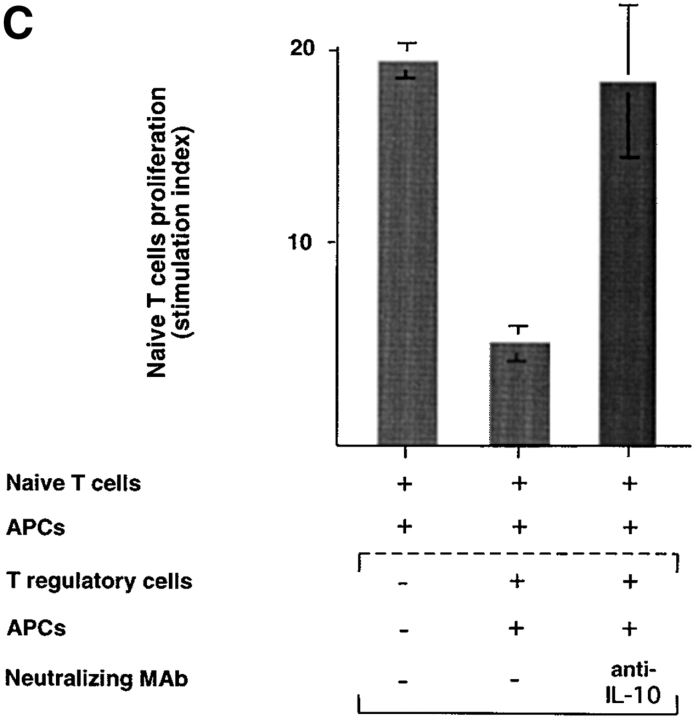
Previous studies demonstrated that CD4 regulatory T cells or CD4 Th3 cells suppress T cell–mediated immune responses, respectively, through IL-10 or TGF-β (4, 7). To further investigate whether IL-10 or TGF-β was involved in the immunosuppression by CD8 Tr cells, neutralizing antibodies to IL-10 or TGF-β were added, respectively, into naive CD8 T cell coculture with allogeneic APCs and syngeneic CD8 Tr cells. Anti–IL-10, but not anti–TGF-β, completely blocked the inhibitory effect of CD8 Tr cells (Fig. 7 A), indicating that the suppression was mediated by IL-10. The antibody against TGF-β used in the neutralization experiments is a monoclonal mouse anti–TGF-β1, -β2, -β3 and recognizes human TGF-β1 and TGF-β2. Its neutralization potential has been previously described (detailed in the manufacturer's instruction). This was further confirmed by the finding that the inhibitory effect of CD8 Tr cells could be mimicked by the addition of low doses of IL-10 in cultures (Fig. 7 A) and the fact that CD8 T cells activated by DCs do not produce detectable levels of TGF-β.
Figure 7.
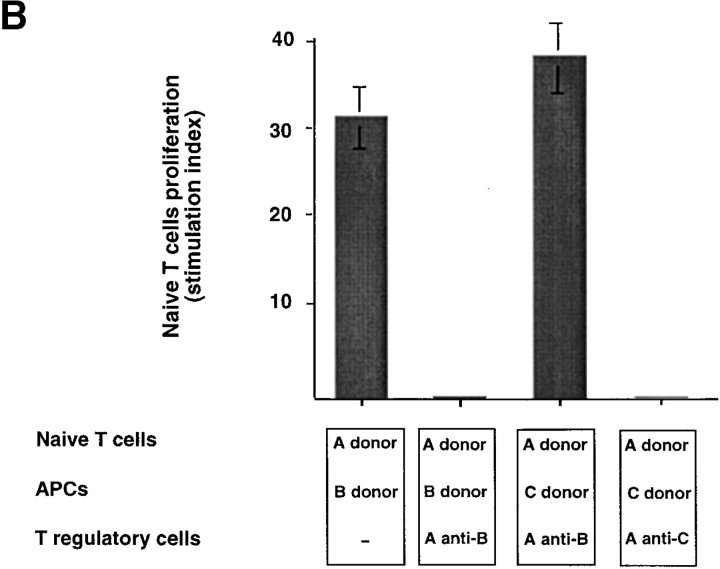
Suppression is mediated by soluble IL-10 and requires Ag-specific stimulation of IL-10+CD8 Tr cells. (A) 5-d primary CD8+T cell proliferation induced by allogeneic monocytes plus equal numbers of CD8 Tr cells in the presence or absence of neutralizing anti–IL-10 or anti–TGF-β. To determine if IL-10 could display a similar inhibitory effect of CD8+ Tr cells, primary CD8+T cell proliferation induced by allogeneic monocytes was performed in the presence of IL-10, or IL-10 plus anti–IL-10. The stimulation index of naive CD8 T cells was calculated by the following formula: cpm [(naive T + T reg + mono) − (T reg + mono)]/cpm (naive T). Results are representative of three independent experiments. (B) Naive CD8 T cells (donor A) were stimulated with allogeneic monocytes from donor B or C in the presence of CD8 Tr cells primed to donor B or C. Results are representative of two independent experiments. (C) CD8 Tr cells stimulated separately with monocytes were added to the primary cultures of naive CD8 T cells plus monocytes in Transwell chambers. The stimulation index of naive CD8 T cells was calculated by the following formula: cpm (naive T + mono)/cpm (naive T).
CD8 Tr cells primed by DC2 from donor B only inhibited naive CD8 T cell proliferation induced by APCs from donor B, but not APCs from donor C (Fig. 7 B). These data indicate that immunosuppression requires antigen-specific restimulation of CD8 Tr cells to produce IL-10.
CD8 Tr Cells Can Inhibit Bystander Activation of Naive T Cells through IL-10.
To investigate whether CD8 Tr cells have the ability to inhibit bystander CD8 T cell proliferation, transwell culture experiments were performed in which CD8 Tr cells were separated from primary naive CD8 T cells by permeable membranes (Fig. 7 C). CD8 Tr cells reactivated by the priming APCs in the upper well strongly inhibited the proliferation of naive CD8 T cells in response to a third-party APC in the lower wells. The addition of anti–IL-10 mAbs completely blocked the inhibition effects. These data suggest that CD8 Tr cells have the ability to inhibit bystander activation of naive CD8 T cells if the site of activation of CD8 Tr cells is close enough to deliver secreted IL-10 to the adjacent naive T cells. Because IL-10 is known to inhibit the proliferation of naive CD4 T cells (28), bystander suppression of CD4 T cells may also occur when CD8 Tr cells are simultaneously activated to produce IL-10 by the same or an adjacent APC.
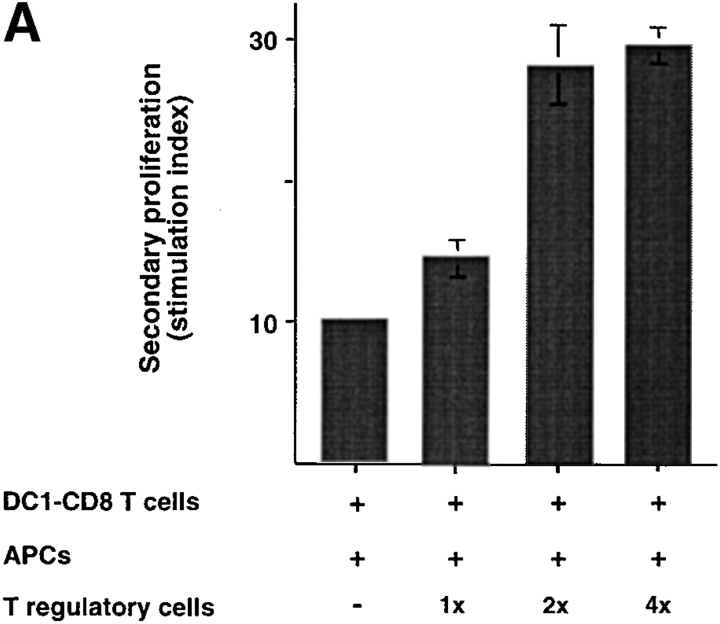
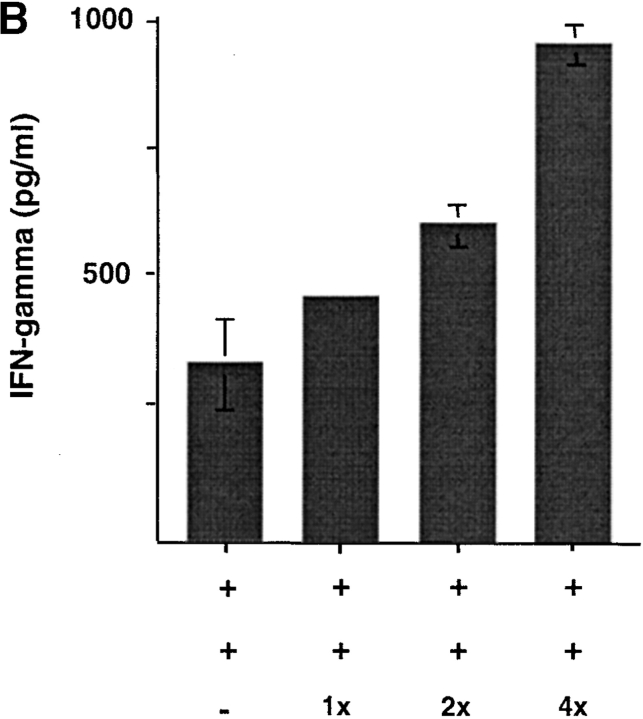
CD8 Tr Cells Cannot Suppress Preactivated CD8 T Cells.
To test whether CD8 Tr cells could inhibit preactivated CD8 T cells, different numbers of DC2-primed CD8 Tr cells were added to a coculture of autologous DC1-preactivated CD8 T cells with APCs. As shown in Fig. 8, A and B , CD8 Tr cells were unable to suppress both the proliferative responses and the IFN-γ secretion of activated DC1-primed CD8 T cells. The increased proliferation and IFN-γ production detected in cultures with increasing numbers of CD8 Tr cells may simply reflect the additive effect of low levels of proliferation and IFN-γ production of CD8 Tr cells.
Figure 8.
CD8 Tr cells do not suppress secondary responses. (A) 3-d secondary proliferation and (B) IFN-γ production by DC1-primed CD8 T cells induced by allogeneic monocytes plus CD8 Tr cells (1:1, 1:2, and 1:4 ratio). The stimulation index of naive CD8 T cells was calculated by the following formula: cpm [(DC1 − T + T reg + mono) − (T reg + mono)]/cpm (DC1 − T).
Discussion
During the last three decades, there have been extensive efforts to understand the cellular and molecular mechanisms underlying CD8 T cell–mediated immunosuppression. Hypothetical suppressor cascades, involving the participation of multiple effector T cell subsets and a variety of secreted mediators, have been proposed (31–33). These models have not been confirmed due to the difficulties in isolating the primary CD8 T suppressor cells, the failure in characterizing the soluble suppressive factors, and the inability to convincingly explain the induction and antigen specificity of CD8 T cell–mediated suppression (19, 20).
In this study, we describe the generation of a population of CD8 Tr cells from naive CD8 T cells during a 6-d culture with mature lymphoid DC2. These primary CD8 Tr cells, which represent 10–20% of primed CD8 T cells, can now be directly visualized by immunofluorescence flow cytometry according to intracellular expression of IL-10. In addition, we have demonstrated that IL-10 represents the soluble inhibitory factor, which is responsible for the poor cytolytic and secondary hypoproliferative responses, as well as for the suppression of primary naive CD8 T cell responses. IL-10–producing CD8 Tr cells are very potent, because one IL-10–producing CD8 Tr cell can actually suppress the proliferation of 10 to 20 naive CD8 T cells. Previous models of CD8 T cell–mediated immunosuppression, based on inducer and effector T suppressor cells with distinct T cell determinants, failed to provide a solid basis for the antigen specificity of suppression (19). Our present study demonstrates that IL-10–producing CD8 Tr cells are directly induced via antigen presentation by DC2 and require antigen-specific restimulation to deliver their immunosuppression through the production of IL-10. Therefore, this study provides direct experimental evidence for the existence of CD8 Tr cells, identifies IL-10 as soluble inhibitory factor, and explains the antigen specificity of CD8 T cell–mediated immunosuppression.
A subset of CD8 T suppressor cells lacking CD28 expression has been reported (16, 34). IL-10–producing CD8 Tr cells differ from CD28−CD8 T cells in several aspects. Whereas IL-10–producing CD8 Tr cells can be directly generated from naive CD8 T cells by one round of stimulation with DC2, CD28−CD8 T suppressor cells appear to represent end-stage CTL differentiation after repetitive stimulation (35). Furthermore, IL-10–producing CD8 Tr cells inhibit primary T cell responses through IL-10, whereas CD28−CD8 T suppressor cells inhibit the ability of APC to induce T cell activation by direct cell contact (34). Therefore, there may be two distinct subsets of CD8 Tr suppressor cells that are responsible for CD8 T cell–mediated immunosuppression at different stages of immune response and by different mechanisms. IL-10–producing CD8 Tr cells share many similarities with the CD4 Tr1 cells (7): (a) they are both anergic; (b) their generation depends on IL-10; and (c) they both suppress primary T cell responses through IL-10, but not through TGF-β. Therefore, this study suggests that IL-10-producing regulatory cells exist both in CD4 and CD8 T cell compartments.
A recent study described IL-10–producing CD8 T cells in vivo in concomitance with a decreased influenza-specific CD8 T cell response after vaccination with immature DC1 pulsed with influenza matrix peptide (36). Although the function of these anti-influenza IL-10–producing CD8 T cells was not characterized, this study suggests that IL-10–producing CD8 Tr cells may indeed exist and operate in vivo. Furthermore, the existence of IL-10–producing CD8 Tr cells in vivo has been suggested in a long-term acceptor of allogeneic kidney transplant (37).
We showed that DC2-primed CD8 Tr cells are unable to suppress secondary responses of activated effector cells such as DC1-primed CD8 T cells. This finding does not exclude the possibility that CD8 Tr cells may suppress secondary responses of quiescent memory T cells, as described in a recent study (36)
Our study further demonstrates the functional differences between CD40 ligand–activated mature DC1 and DC2, which induce strong IFN-γ–producing cytotoxic CD8 T cells and IL-10–producing CD8 Tr cells, respectively. During bone marrow transplantation or solid organ transplantation, it is likely that donor or host DCs are activated by CD40 ligand expressed by allogeneic T cells. CD40 ligand–activated DC1 may induce strong IFN-γ–producing alloantigen-specific Th1 and CTL responses, which will trigger and accelerate GVHD or graft rejection. In contrast, CD40 ligand–activated DC2 may induce Th2 (25) and IL-10–producing CD8 Tr cells, which will inhibit GVHD or graft rejection. This hypothesis is supported by a recent study showing that transplantation of GM-CSF–mobilized blood cells, which contain increased numbers of DC2 precursors, induced less severe GVHD (38). The study suggests that transplanted DC2 precursors may capture host alloantigen, mature into DC2, and present the host alloantigen to tolerize the donor T cell by the induction of Th2 (25) and IL-10–producing CD8 Tr cells. Therefore, DC2 lineage may play a key role in transplantation-associated immunological tolerance.
The generation of primary IL-10–producing CD8 Tr cells provides the direct evidence supporting the immunological paradigms of CD8 T cell–mediated immunosuppression. Further understanding of the molecular mechanism regulating the generation of CD8 Tr cells by human-mature DC2 may have important clinical implications for the development of more effective therapies for autoimmune and transplantation-associated diseases.
Acknowledgments
We thank J. Sedgwick, K. Moore, W. Chen, H. Kanzler, and V. Soumelis for critical reading of the manuscript.
This work was supported in part by an award from the Swiss National Science Foundation to M. Gilliet. The DNAX Research Institute is supported by the Schering-Plough Corporation.
Footnotes
Abbreviations used in this paper: DC, dendritic cell(s); Tr, regulatory T.
References
- 1.Gershon, R.K., and K. Kondo. 1970. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 2.Kilshaw, P.J., L. Brent, and M. Pinto. 1975. Suppressor T cells in mice made unresponsive to skin allografts. Nature. 255:489–491. [DOI] [PubMed] [Google Scholar]
- 3.Fowell, D., and D. Mason. 1993. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J. Exp. Med. 177:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Y., V.K. Kuchroo, J. Inobe, D.A. Hafler, and H.L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 265:1237–1240. [DOI] [PubMed] [Google Scholar]
- 5.Powrie, F., M.W. Leach, S. Mauze, S. Menon, L.B. Caddle, and R.L. Coffman. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1:553–562. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 7.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 8.Lider, O., T. Reshef, E. Beraud, A. Ben-Nun, and I.R. Cohen. 1988. Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science. 239:181–183. [DOI] [PubMed] [Google Scholar]
- 9.Koh, D.R., W.P. Fung-Leung, A. Ho, D. Gray, H. Acha-Orbea, and T.W. Mak. 1992. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 256:1210–1213. [DOI] [PubMed] [Google Scholar]
- 10.Gaur, A., G. Ruberti, R. Haspel, J.P. Mayer, and C.G. Fathman. 1993. Requirement for CD8+ cells in T cell receptor peptide-induced clonal unresponsiveness. Science. 259:91–94. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, H., R. Ware, A. Stall, L. Flaherty, L. Chess, and B. Pernis. 1995. Murine CD8+ T cells that specifically delete autologous CD4+ T cells expressing V beta 8 TCR: a role of the Qa-1 molecule. Immunity. 2:185–194. [DOI] [PubMed] [Google Scholar]
- 12.Lanzavecchia, A., E. Roosnek, T. Gregory, P. Berman, and S. Abrignani. 1988. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 334:530–532. [DOI] [PubMed] [Google Scholar]
- 13.Simpson, E. 1988. Suppression of the immune response by cytotoxic T cells. Nature. 336:426. [DOI] [PubMed] [Google Scholar]
- 14.Salgame, P., R. Modlin, and B.R. Bloom. 1989. On the mechanism of human T cell suppression. Int. Immunol. 1:121–129. [DOI] [PubMed] [Google Scholar]
- 15.Hisatsune, T., A. Enomoto, K. Nishijima, Y. Minai, Y. Asano, T. Tada, and S. Kaminogawa. 1990. CD8+ suppressor T cell clone capable of inhibiting the antigen- and anti-T cell receptor-induced proliferation of Th clones without cytolytic activity. J. Immunol. 145:2421–2426. [PubMed] [Google Scholar]
- 16.Koide, J., and E.G. Engleman. 1990. Differences in surface phenotype and mechanism of action between alloantigen-specific CD8+ cytotoxic and suppressor T cell clones. J. Immunol. 144:32–40. [PubMed] [Google Scholar]
- 17.Hu, F.Y., Y. Asano, K. Sano, T. Inoue, M. Furutani-Seiki, and T. Tada. 1992. Establishment of stable CD8+ suppressor T cell clones and the analysis of their suppressive function. J. Immunol. Methods. 152:123–134. [DOI] [PubMed] [Google Scholar]
- 18.Moller, G. 1988. Do suppressor T cells exist? Scand. J. Immunol. 27:247–250. [DOI] [PubMed] [Google Scholar]
- 19.Bloom, B.R., P. Salgame, and B. Diamond. 1992. Revisiting and revising suppressor T cells. Immunol. Today. 13:131–136. [DOI] [PubMed] [Google Scholar]
- 20.Dorf, M.E., V.K. Kuchroo, and M. Collins. 1992. Suppressor T cells: some answers but more questions. Immunol. Today. 13:241–243. [DOI] [PubMed] [Google Scholar]
- 21.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 22.Moser, M., and K.M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199–205. [DOI] [PubMed] [Google Scholar]
- 23.Kalinski, P., C.M. Hilkens, E.A. Wierenga, and M.L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 20:561–567. [DOI] [PubMed] [Google Scholar]
- 24.Shortman, K. 2000. Burnet oration: dendritic cells: multiple subtypes, multiple origins, multiple functions. Immunol. Cell Biol. 78:161–165. [DOI] [PubMed] [Google Scholar]
- 25.Rissoan, M.C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y.J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 26.Grouard, G., M.C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y.J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamann, D., P.A. Baars, M.H. Rep, B. Hooibrink, S.R. Kerkhof-Garde, M.R. Klein, and R.A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banchereau, J., P. de Paoli, A. Valle, E. Garcia, and F. Rousset. 1991. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 251:70–72. [DOI] [PubMed] [Google Scholar]
- 29.Reis e Sousa, C., G. Yap, O. Schulz, N. Rogers, M. Schito, J. Aliberti, S. Hieny, and A. Sher. 1999. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 11:637–647. [DOI] [PubMed] [Google Scholar]
- 30.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 4:311–316. [DOI] [PubMed] [Google Scholar]
- 31.Groux H., M. Bigler, J.E. de Vries, and M.G. Roncarolo. 1996. IL-10 induces a long-term antigen-specific anergic state in human CD4+ cells. J. Immunol. 184:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorf, M.E., and B. Benacerraf. 1984. Suppressor cells and immunoregulation. Annu. Rev. Immunol. 2:127–157. [DOI] [PubMed] [Google Scholar]
- 33.Green, D.R., P.M. Flood, and R.K. Gershon. 1983. Immunoregulatory T-cell pathways. Annu. Rev. Immunol. 1:439–463. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Z., S. Tugulea, R. Cortesini, S. Lederman, and N. Suciu-Foca. 1999. Inhibition of CD40 signaling pathway in antigen presenting cells by T suppressor cells. Hum. Immunol. 60:568–574. [DOI] [PubMed] [Google Scholar]
- 35.Borthwick, N.J., M. Lowdell, M. Salmon, and A.N. Akbar. 2000. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int. Immunol. 12:1005–1013. [DOI] [PubMed] [Google Scholar]
- 36.Dhodapkar, M.V., R.M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai, J., J.L. Lee, E. Jankowska-Gan, L. De Vito-Haynes, S. Kusaka, J. Schneck, and W. Burlingham. 2000. Minor H-specific CD8+ T cells may play a regulatory role in human allograft acceptors. 29th Annual Autumn Immunology Conference, Chicago. p 38 (Abstr. 93).
- 38.Arpinati, M., C.L. Green, S. Heimfeld, J.E. Heuser, and C. Anasetti. 2000. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 95:2484–2490. [PubMed] [Google Scholar]