That the mind can influence the body during health and disease is an ancient notion which, in part because of its origins in anecdotal observations, has been an often and sometimes hotly debated concept. Over 20 years ago, Dr. Robert A. Good formulated a concise and elegant description of the problem as viewed from an immunological perspective. To quote Dr. Good:
“Immunologists are often asked whether the state of mind can influence the body's defenses. Can positive attitude, a constructive frame of mind, grief, depression, or anxiety alter ability to resist infections, allergies, autoimmunities, or even cancer? Such questions leave me with a feeling of inadequacy because I know deep down that such influences exist, but I am unable to tell how they work, nor can I in any scientific way prescribe how to harness these influences, predict or control them. Thus they cannot usually be addressed in scientific perspective. In the face of this inadequacy, most immunologists are naturally uneasy and usually plead not to be bothered with such things” (1).
Today we have established that the phenomenon of central nervous system (CNS) regulation of immune function exists and, at least roughly, elucidated the how and why of Dr. Good's “influences.” Perhaps the first experimental evidence that the mind can influence the immune system was the demonstration by Metal'nikov and Chorine in 1926 that an immune response can be conditioned in a classic Pavlovian fashion (2). The essential paradigm is to repeatedly pair administration of an immunoregulatory substance as an unconditioned stimulus with an external stimulation, which is the conditioned stimulus (i.e., in the case of Pavlov, a ringing bell). With sufficient association, the conditioned stimulus alone is able to cause immunoregulation. This finding, in various forms, has stood the test of time with numerous replications beginning most recently in 1975 (for a review, see reference 3). Among many examples, other convincing evidence for the concept is found in the numerous effects of stress on immune function (for a review, see reference 4) and the observation that behavioral characteristics can predict susceptibility to autoimmunity in an animal model of multiple sclerosis (5). Collectively, findings such as these leave little doubt that the mind is capable of influencing the immune system. The findings that stimulation or ablation of various regions of the brain could negatively, as well as positively, alter immune responses strongly suggested that immunoregulatory moieties resided in the CNS (for a review, see reference 6). This being the case, how do they work? Arguably two observations set the stage for unraveling the puzzle. First, it was established that peripheral immune responses could alter the firing rate of neurons in the CNS (7). Thus, information can flow not only from the CNS to the immune system but also in the opposite direction. Further, innervation of immune tissues and organs provides a conduit for such information (for a review, see reference 8). But how? This became clear with the second observation that immune cells can produce neuropeptides such as β-endorphin and other neurotransmitters and neurons can make cytokines such as IL-1 (9). Furthermore, cells of the immune system and the CNS each have receptors for both cytokines as well as neuropeptides and neurotransmitters. Thus, the two systems can communicate in a bidirectional fashion as a result of “speaking the same chemical language” in terms of a common set of signal molecules and their receptors (10, 11). It has long been our contention that among other functions, the immune system serves as a sensory organ (12). A sixth sense, if you will, that completes our ability to be cognizant not only of things we can see, feel, taste, touch, and smell but also those things we cannnot. These would include bacteria, viruses, antigens, tumor cells, etc. Recognition of such “noncognitive stimuli” by the immune system would result in transmission of information to the CNS via the aforementioned shared signal molecules to cause a physiological response that is ultimately beneficial to the host and detrimental to the infectious agent. An example of such communication involving cytokines is the findings that upon peritoneal infection with bacteria or their products the constellation of changes such as fever that are associated with sickness behavior come about as a direct result of immune cell–derived proinflammatory cytokines signaling the CNS via the subdiaphragmatic vagus nerve (references 13 and 14; Fig. 1). An example involving a neuropeptide is found in the demonstration that an antinociceptive system can be activated by inflammation. This apparently results from the production of β-endorphin by immune cells at the site of inflammation. Such β-endorphin in turn acts on local sensory nerve fibers to cause analgesia (15).
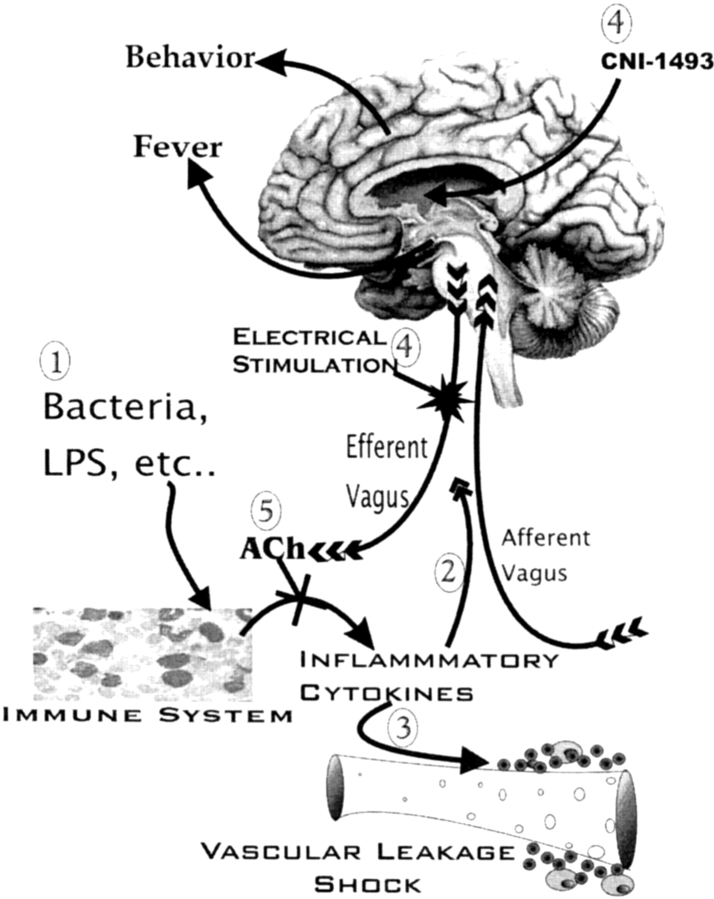
Figure 1.
A scheme for a vagal immune circuit. (1) Bacteria, LPS, or other noncognitive stimuli are recognized by cells of the immune system and cause the production of inflammatory cytokines such as TNF. The inflammatory cytokines: (2) stimulate the afferent vagus nerve causing sickness behavior and fever; (3) and also act to cause hypotension and shock. (4) CNI-1493 or electrical stimulation of the efferent vagus nerve activates a cholinergic antiinflammatory pathway. (5) The resulting ACh inhibits immune cell production of inflammatory cytokines and consequently blocks shock and possibly afferent vagal stimulation.
Contrariwise, the CNS alerts the immune system to environmental changes using the shared neuropeptide, neurotransmitter, and cytokine receptors on immune cells. An example of this is the aforementioned effects of stress to dampen immune function. This apparently occurs via the effects of products of the hypothalamic pituitary adrenal axis on immune cells. An impairment of this axis to appropriately respond to inflammatory stressors has been shown to predispose an animal to autoimmune disease as a result of lack of regulation of the immune system (16). Thus, we have proceeded quite a way in understanding the hows and whys of CNS regulation of immune function.
In this issue, Bernik et al. (17) are among the first to fulfill the final challenge in Dr. Good's narrative by finding a way to harness an influence of the brain over the immune system. In earlier studies, this group, led by Kevin J. Tracey, demonstrated that the neurotransmitter, acetylcholine (ACh), had the ability to inhibit LPS-induced human macrophage production of proinflammatory cytokines (TNF, IL-16, and IL-6), but not an antiinflammatory cytokine (IL-10; reference 18). This effect occurred through an action of the neurotransmitter on nicotinic ACh receptors that were previously known to reside on macrophages and other cells of the immune system (for a review, see reference 19). Parenthetically, mononuclear leukocytes also express choline acetyl-transferase and synthesize ACh (20, 21).
Endotoxin (or LPS) is a product of all gram-negative bacteria, which can cause shock (hypotension) and ultimately, death. This occurs as a result of LPS activation of macrophages to release TNF, which is a principle mediator of acute LPS-induced shock (18). Building on the aforementioned ability of ACh to suppress macrophage TNF production in vitro, the Tracey group tested whether stimulation of efferent vagus nerve activity might inhibit the systemic inflammatory response to endotoxin. The rationale for this being the well known fact that ACh is the principle vagal neurotransmitter and that the vagus nerve is distributed throughout the reticuloendothelial system. Consequently, Lewis rats underwent cervical vagotomy or a sham surgical procedure and efferent vagus nerve activity was stimulated in vagotomized animals. Remarkably, compared with sham-operated controls, electrical stimulation of the efferent vagus nerve very markedly inhibited TNF levels in serum and in the liver and almost totally blocked hypotension caused by a lethal intravenous dose of LPS. Interestingly, vagotomy without stimulation resulted in a significant increase in TNF levels and shortened the time to development of shock as compared with sham-operated controls. This, of course, might be expected if tonic suppression of the aforementioned responses by ACh was removed as a result of vagotomy. These findings were the first to demonstrate a previously unrecognized, parasympathetic antiinflammatory pathway by which the CNS modulates systemic inflammatory responses. The Tracey group termed this the “cholinergic antiinflammatory pathway” (Fig. 1).
In this study (17), Bernik et al. have expanded and markedly extended their work on the cholinergic antiinflammatory pathway with an experimental therapeutic. This compound, CNI-1493, is a tetravalent guanylhydrazone molecule that inhibits systemic inflammation. CNI-1493 is currently undergoing testing in Phase II clinical trials for Crohn's disease and in preclinical testing has been shown to be protective in a number of models, including endotoxic shock, acute respiratory distress syndrome, sepsis, pancreatitis, experimental allergic encephalitis, stroke, rheumatoid arthritis, and dextran sulfate colitis (for a review, see reference 22). Prior to the present report, the antiinflammatory effect of CNI-1493 was assumed to be through its known ability to inhibit the phosphorylation of p38 mitogen-activated protein kinase (MAPK), which plays a key role in regulation of proinflammatory cytokine synthesis (22). However, while evaluating the activity of CNI-1493 in cerebral ischemia the authors made an unexpected observation. As expected, the compound when injected intracerebroventricularly inhibited cerebral TNF synthesis. Surprisingly, by an intracerebroventricular route it also inhibited the systemic TNF response to LPS, which was used as a control. CNI-1493 also directly stimulated vagal nerve activity. These observations led this group to a remarkable possibility. Could the systemic antiinflammatory action of CNI-1493 actually be mediated by a central activation of the cholinergic antiinflammatory pathway? This is precisely the conclusion that is reached, and it is based on quite persuasive results. First, CNI-1493 administered intracerebroventricularly was 100,000-fold more effective in suppressing LPS-induced TNF release and shock as compared with intravenous administration. Thus, doses that are ineffective systemically are active when given centrally. Second, surgical or chemical (using the ACh antagonist atropine) vagotomy ablated the effects of CNI-1493 on LPS-induced TNF synthesis and shock whether the compound was given intracerebroventricular or intravenous. Lastly, electrical stimulation of the intact vagus nerve conferred protection against endotoxic shock and TNF release (Fig. 1).
These findings have some truly provocative implications. Perhaps the most profound is the possibility of developing new classes of systemic antiinflammatory agents that act centrally. Prior studies implicating the CNS in mediating certain antiinflammatory actions of α-melanocyte–stimulating hormone (23) and salicylates (24) via a sympathetic neural route suggest that there are pathways in addition to the one herein described that might be exploited. CNI-1493 may well serve as a prototype for the developments of such new drugs. The identification of the CNS receptor(s) for CNI-1493, as well as the central neural fiber tract(s) that mediate its action, will be important first steps in this process. A particularly interesting future question will be whether CNI-1493 inhibition of inflammatory cytokine production will also cause a blockade of cytokine-mediated afferent vagal stimulation. If so, the compound might ameliorate fever and sickness behavior as well as shock (Fig. 1). It will also be important to determine whether vagus nerve stimulation, which is a clinically approved therapy for epilepsy and depression (with minimal morbidity) (25), can alter proinflammatory cytokines in humans. If so, this might represent another modality for activation of the cholinergic antiinflammatory pathway for the treatment of inflammatory diseases. Bringing these ideas to fruition may now provide a means for harnessing the possibly powerful influences of the CNS over the immune system.
Acknowledgments
The author acknowledges Nate Weathington for help with the artwork and Diane Weigent for editorial assistance.
This work is supported by National Institutes of Health grants AI37670, DK55647, and HL68806, and grants from the Muscular Dystrophy Association and the National Multiple Sclerosis Society (RG20667B8/1).
References
- 1.Good, R.A. 1981. Foreword: interactions of the body's major networks. In Psychoneuroimmunology. R. Ader, editor. Academic Press, Inc., Orlando. xvii–xix.
- 2.Metal'nikov, S., and V. Chorine. 1926. Role des reflexes conditionnels dans l'immunite. Annales de l'Institute Pasteur. 40:893–900. [Google Scholar]
- 3.Ader, R., and N. Cohen. 1991. The influence of conditioning on immune responses. Psychoneuroimmunology. R. Ader, D.L. Felten, and N. Cohen, editors. Academic Press, Inc., San Diego. 611–646.
- 4.Keller, S.E., S.J. Schleifer, and M.K. Demetrikopoulos. 1991. Stress-induced changes in immune function in animals: hypothalamo-pituitary-adrenal influences. Psychoneuroimmunology. R. Ader, D.L. Felten, and N. Cohen, editors. Academic Press, Inc., San Diego. 771–787.
- 5.Kavelaars, A., C.J. Heijnen, R. Tennekes, J.E. Bruggink, and J.M. Koolhaas. 1999. Individual behavioral characteristics of wild-type rats predict susceptibility to experimental autoimmune encephalomyelitis. Brain Behav. Immun. 13:279–286. [DOI] [PubMed] [Google Scholar]
- 6.Felten, D.L., N. Cohen, R. Ader, S.Y. Felten, S.L. Carlson, and T.L. Roszman. 1991. Central neural circuits involved in neural-immune interactions. Psychoneuroimmunology. R. Ader, D.L. Felten, and N. Cohen, editors. Academic Press, Inc., San Diego. 3–25.
- 7.Besedovsky, H.O., E. Sorkin, D. Felix, and H. Haas. 1977. Hypothalamic changes during the immune response. Euro. J. Immunol. 7:323–325. [DOI] [PubMed] [Google Scholar]
- 8.Stevens-Felten, S.Y., and D.L. Bellinger. 1997. Noradrenergic and peptidergic innervation of lymphoid organs. Neuroimmunoendocrinology. J.E. Blalock, editor. Karger AG, Basel, Switzerland. 99–131. [DOI] [PubMed]
- 9.Blalock, J.E. 1989. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol. Rev. 69:1–32. [DOI] [PubMed] [Google Scholar]
- 10.Blalock, J.E. 1994. The syntax of immune-neuroendocrine communication. Immunol. Today. 15:504–511. [DOI] [PubMed] [Google Scholar]
- 11.Blalock, J.E. 1994. The immune system: our sixth sense. The Immunologist. 2:8–15. [Google Scholar]
- 12.Blalock, J.E. 1984. The immune system as a sensory organ. J. Immunol. 132:1067–1070. [PubMed] [Google Scholar]
- 13.Watkins, L.R., and S.F. Maier. 1999. Implications of immune-to-brain communication for sickness and pain. Proc. Natl. Acad. Sci. USA. 96:7710–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laye, S., R.M. Bluthe, S. Kent, C. Combe, C. Medina, P. Parnet, K. Kelley, and R. Dantzer. 1995. Subdiaphragmatic vagotomy blocks induction of IL-1 β mRNA in mice brain in response to peripheral LPS. Am. J. Physiol. 268:R1327–R1331. [DOI] [PubMed] [Google Scholar]
- 15.Machelska, H., and C. Stein. 2000. Pain control by immune-derived opioids. Clin. Exp. Pharmacol. Physiol. 27:533–536. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg, E.M. 1997. Neural-immune interactions in health and disease. J. Clin. Invest. 100:2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernik, T.R., S.G. Friedman, M. Ochani, R. DiRaimo, L. Ulloa, H. Yang, S. Sudan, C.J. Czura, S.M. Ivanova, and K.J. Tracey. 2002. Systemic anti-inflammatory action of CNI-1493 mediated by the cholinergic anti-inflammatory pathway. J. Exp. Med. 195:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borovikova, L.V., S. Ivanova, M. Zhang, H. Yang, G.I. Botchkena, L.R. Watkins, H. Wang, N. Abumrad, J.E. Eaton, and K.J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–462. [DOI] [PubMed] [Google Scholar]
- 19.Sato, K.Z., T. Fujii, Y. Watanabe, S. Yamada, T. Ando, F. Kazuko, and K. Kawashima. 1999. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci. Lett. 266:17–20. [DOI] [PubMed] [Google Scholar]
- 20.Fujii, T., S. Yamada, Y. Watanabe, H. Misawa, S. Tajima, K. Fujimoto, T. Kasahara, and K. Kawashima. 1999. Constitutive expression of mRNA for the same choline acetyltransferase as that in the nervous system, an acetylcholine-synthesizing enzyme, in human leukemic T-cell lines. Neurosci. Lett. 259:71–74. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima, K., T. Fujii, Y. Watanabe, and H. Misawa. 1998. Synthesis of acetylcholine and expression of mRNA for muscarinic receptor subtypes in T-lymphocytes. Life Sci. 62:1701–1705. [DOI] [PubMed] [Google Scholar]
- 22.Hommes, D., B. VandenBlink, T. Plasse, J. Bartelsman, C. Xu, B. Macpherson, G. Tytgat, M. Peppelenbosch, and S. van Deventer. 2002. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology. 122:7–14. [DOI] [PubMed] [Google Scholar]
- 23.Delgado Hernandez, R., M.T. Demitri, A. Carlin, C. Meazza, P. Villa, P. Ghezzi, J.M. Lipton, and A. Catania. 1999. Inhibition of systemic inflammation by central action of the neuropeptide α-melanocyte stimulating hormone. Neuroimmunomodulation. 6:187–192. [DOI] [PubMed] [Google Scholar]
- 24.Catania, A., J. Arnold, A. Macaluso, M.E. Hiltz, and J.M. Lipton. 1991. Inhibition of acute inflammation in the periphery by central action of salicylates. Proc. Natl. Acad. Sci. USA. 88:8544–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeGiorgio, C.M., S.C. Schachter, A. Handforth, M. Salinsky, J. Thompson, B. Uthman, R. Reed, S. Collins, E. Tecoma, G.L. Morris, et al. 2000. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 41:1195–2000. [DOI] [PubMed] [Google Scholar]