Abstract
The contribution that long-lived bone marrow (BM) plasma cells (PCs) provide to enduring humoral immunity has been underscored by a number of recent studies. However, little is known about the immediate precursors that give rise to long-lived PCs in the BM of immune individuals. We have identified subsets of antigen-experienced B cells within the immune BM that are precursors to PCs. These PC precursors arise in the BM 14 days after immunization and persist for greater than 9 months. Phenotypically distinct subsets of PC precursors give rise to short-lived or long-lived PCs. The differentiation of PC precursors to PCs occurs in the absence of antigen and requires cell division. The functional significance of these newly identified PC precursors in the persistence and quality of the humoral immune response is discussed.
Keywords: B lymphocyte subsets, antibody formation, cell differentiation, cell lineage, immunophenotyping
Introduction
Plasma cells (PCs)* are essential for both the immediate and long-lived antibody response after immunization (1–3). In response to antigen exposure, PCs arise rapidly in the splenic red pulp, allowing for immediate production of protective antibody to acute infections (3–6). These PCs are typically short-lived and can express isotype switched Ig. Although red pulp PCs derived from memory cells could display mutated Ig V regions, the majority of these cells usually have not acquired Ig V region mutations. In contrast, long-lived PCs primarily arise weeks after antigen exposure having accumulated V region mutations due to selection within the germinal center (GC) (3–6). These long-lived PCs ultimately reside in the immune bone marrow (BM) for extended periods and account for over 80% of the antibody present in immune serum (3–7).
Recent studies have underscored the importance of long-lived PC in sustaining humoral immune responses to pathogens and to nominal thymus-dependent (TD) antigens (3–6). It is generally considered that antigen-experienced B cells selected within the GC leave the GC and migrate to the BM as PC precursors or as fully matured PCs (3–6). However, little is known about the identity of the immediate precursors that generate mature, terminally differentiated PCs. Much of what is known about PC intermediaries is derived from studies of multiple myeloma (MM), a human cancer identified by the clonal expansion of PCs in the BM (8). Subsets of PCs within the BM of MM patients have been delineated via expression of a number of cell surface molecules, including CD45, CD38, and CD44, and have been associated with varying proliferative capacities and patient prognosis (9–11). Although a clear identification of a progenitor cell has yet to be made, these studies have revealed a cell lineage representing an intermediary stage before terminal PC differentiation (9, 11).
Whereas little is known about the post-GC B cell lineages that give rise to PCs, there is evidence that cellular intermediaries exist. Advances in our understanding of molecular events regulating PC differentiation are currently being resolved. Analyses of the temporal expression of BLIMP-1 and XBP-1 have identified a distinct maturational pathway of the B cell lineage leading to terminal PC formation and Ig production (12–15). Overexpression of BLIMP-1 stimulates terminal B cell differentiation to PC and deletion of XBP-1 selectively inhibits B cell differentiation before PC formation, but after establishment of GC (12–15). Hence, factors like XBP-1 may function within cells that inhabit an intermediary stage between centrocyte selection and PC formation (13).
We have used B cells from mice expressing a VH transgene that has been inserted into the Ig heavy chain locus (16). The Ig transgenic (Tg) B cells from these mice can be readily used to follow the fate of antigen-activated B cells as they differentiate to terminally committed PCs in vivo (16). Quasi-Monoclonal (QM) Tg B cells bear antigen specificity to 4-hydroxy-3-nitrophenyl acetyl (NP), express the V17.2.25 idiotype, and when transferred into B6-Ly5.2 mice as recipients, are distinctive due to the expression of IgGa allotype immunoglobulin and the Ly5.1 congenic marker (16–18). Previous studies have demonstrated the accurate recapitulation of GC responses by the Tg B cells after immunization with a TD antigen (16, 18, 19). QM Tg B cells within GC express high levels of PNA and GL7 and reduced CD38 expression (16). Data presented in this report demonstrates the ability of these Tg B cells to terminally differentiate to short-lived and long-lived BM PCs after immunization with a TD antigen. Furthermore, we have identified precursors present within immune BM that can differentiate to PC upon transfer into naive recipients. The identity and function of these novel PC precursors is discussed.
Materials and Methods
Antibodies and Reagents.
The following monoclonal antibodies were used during these studies: mouse IgG1 anti–mouse IgMa (clone DS-1), mouse IgG2a anti–mouse IgG1a (clone 10.9), mouse IgM anti–mouse IgG1b (clone B68-2), rat IgG2a anti-mouse B220 (clone RA3-6B2), rat IgG2a anti-mouse CD11a (LFA-1α, clone 2D7), mouse IgG2a anti-mouse CD45.2 (Ly5.1, clone 104), mouse IgG2a anti-mouse CD45.1 (Ly5.2, clone A20), hamster IgG anti-mouse CD80 (B7-1, clone 16-10A1), Rat IgG2a anti-mouse CD86 (B7-2, clone 6L1), rat IgG2a anti-mouse CD126 (IL-6R, clone D7715A7), rat IgG2a anti-mouse CD127 (IL-7R, clone B12-1), rat IgG2a anti-mouse CD138 (Syndecan-1, clone 281-2) (BD PharMingen); rat IgG2b anti-mouse CD49d (VLA4, clone PS/2), rat IgG1 anti-mouse CD44 (clone KM201) (Southern Biotechnology Associates, Inc.); anti-VH17.2.25 cell line (R2348.8), which was provided by T. Imanishi-Kari (University of California San Francisco, San Francisco, CA); and rat IgG2b anti I-A (clone M5/114.15.2). NP-KLH and NP-BSA were purchased from Biosearch Technologies. MultiScreen-HA 96-well plates (Millipore) were used for enzyme-linked immunospot (ELISPOT) assays.
Mice.
QM and B6-Ly5.2 mice were housed within the animal facility at Dartmouth Medical School (Lebanon, NH). Generation of the QM mice has been previously described (20). QM mice were subsequently backbred to the C57BL/6 JH −/− Jk −/− strain for nine generations. Splenocytes from backcrossed mice were used for all adoptive transfers.
Animals, Adoptive Transfer, and Immunization.
After isolation of splenocytes from QM mice, RBC were lysed via incubation with ammonium chloride-Tris buffer (ACT) and then depleted of T cells through anti–Thy-1.2 and complement treatment. The percentage of Tg QM B cells within the remaining population was determined by staining 2 × 105 cells with anti-B220 and anti-idiotype in 5% bovine calf serum in balanced salt solution (BCS/BSS) at 4°C and analyzed via a Becton Dickinson FACScan® or FACSCalibur®. For each primary transfer, 3 × 106 QM B cells were injected via tail vein into B6-Ly5.2 recipient mice. Typically, three mice were used per experimental group. 1 d after transfer, recipient mice were injected intraperitoneally with 100 μg of NP-KLH emulsified in CFA. Secondary transfers were via tail vein injection to B6-Ly5.2 mice as noted. Secondary recipients were not immunized.
Measurement of PC Longevity by Irradiation and BM Reconstitution.
To evaluate the longevity of Tg QM PCs within the BM, we used a whole body irradiation technique previously demonstrated to identify long-lived PCs (3). Naive B cells, memory B cells, and short-lived PCs are susceptible to irradiation whereas long-lived PCs persist after radiation exposure (3). Thus, after adoptive transfer and immunization, primary recipient mice were subjected to whole body irradiation. B6-Ly5.2 recipient mice were irradiated with 600 rad at 7, 14, 28, or 49 d after primary transfer as specified. All irradiated mice were reconstituted with 3 × 107 spleen and 3 × 107 BM cells from a naive B6-Ly5.2 mouse by intravenous tail vein injection. All intravenous reconstitutions were performed within 4 h of radiation treatment. The persistence of long-lived PCs within the BM was measured 3 wk after irradiation by ELISPOT assay.
ELISPOT Assay.
Splenic and BM αNP-specific IgG1 a antibody-secreting cells (ASC) were enumerated by an NP- and allotype-specific ELISPOT assay. After isolation of splenic or BM cells, RBC were lysed via incubation with ACT and T cells depleted through anti–Thy-1.2 and complement treatment. Of the remaining population, 2.4 × 106 cells per well were apportioned to NP-BSA–coated Millipore hemagglutinin 96-well plates and threefold serial dilutions were made before incubation. Plates were incubated for 5 h at 37°C within a CO2 incubator. After the incubation, plates were washed in PBS and 0.5% Tween in PBS and ASC detected by a biotinylated anti–mouse IgG1 a, followed by streptavidin-horseradish peroxidase (Amersham Pharmacia Biotech). ELISPOTS were developed by a chromagen substrate: AEC (Sigma-Aldrich) in DMF (Fisher Scientific) and 0.1 M sodium acetate buffer, pH 4.8–5.0. Immediately before development of ELISPOTS, 3% H2O2 was added to chromagen substrate. ELISPOTS were enumerated via a dual axis light-dissecting microscope.
Flow Cytometry.
After splenic or BM isolation, cells were suspended in ACT for 3 min at 37°C to remove RBC. ACT treatment was not performed with carboxyfluorescein diacetate succinimidyl ester (CFDAse)-labeled cells. After ACT treatment, cells were washed and resuspended in 5% BCS/BSS and stained with the appropriate antibodies as noted. For all studies, nonspecific staining was reduced by the addition of normal rat serum. Incubation with biotinylated antibodies was followed by incubation with streptavidin-PE, peridinine chlorophyll protein, or APC (BD PharMingen). Antibody incubations were for 30 min at 4°C followed by washing in BCS/BSS. Cells labeled with CFDAse (Molecular Probes) were incubated at 37°C for 10 min in RPMI. The final concentration of CFDAse used was 15 μM in RPMI with 10–20 million cells/ml. After incubation with CFDAse, cells were washed in cRPMI. Stained cells were either analyzed immediately or fixed with 1% formaldehyde in 1.25× PBS. Staining was quantified with either a Becton Dickinson FACScan® or FACSCalibur®. A minimum of 500,000 events were collected and fluorescence signals were detected via four-decade logarithmic amplification. Spectral overlap compensation was made with single color-stained samples for each detection channel. For each experiment, data was analyzed using Becton Dickinson CELLQuest™ software.
Magnetic Bead Isolation of Tg B Cells.
QM Tg B cells were isolated from B6-Ly5.2 mice based on expression of Ly5.1. Magnetic bead purifications were performed according to the protocol provided by Miltenyi Biotec. BM cells isolated from recipient B6-Ly5.2 mice were briefly incubated with anti-mouse biotinylated Ly5.1 antibody (BD PharMingen). BM samples were then incubated with streptavidin magnetic beads (Miltenyi Biotec) and cells were positively selected by passage through LS columns attached to magnetic separators. Flow through and eluent populations were collected following Miltenyi Biotec protocol guidelines.
Mitomycin C (MitoC) Treatment.
MitoC (Sigma-Aldrich) was prepared fresh for each experiment. MitoC was resuspended in PBS at a final concentration of 0.5 mg/ml in a light-protected container and filter sterilized. Cells to be treated were resuspended at a concentration of 5 × 107 cells/ml in PBS. MitoC was added to the cell suspension at a final concentration of 50 μg/ml. The cells were then incubated for 20 min at 37°C and protected from light. After the incubation, cells were washed at least three times in cRPMI. The viability of cells posttreatment was analyzed by trypan blue exclusion.
Results
Generation of Tg BM PCs.
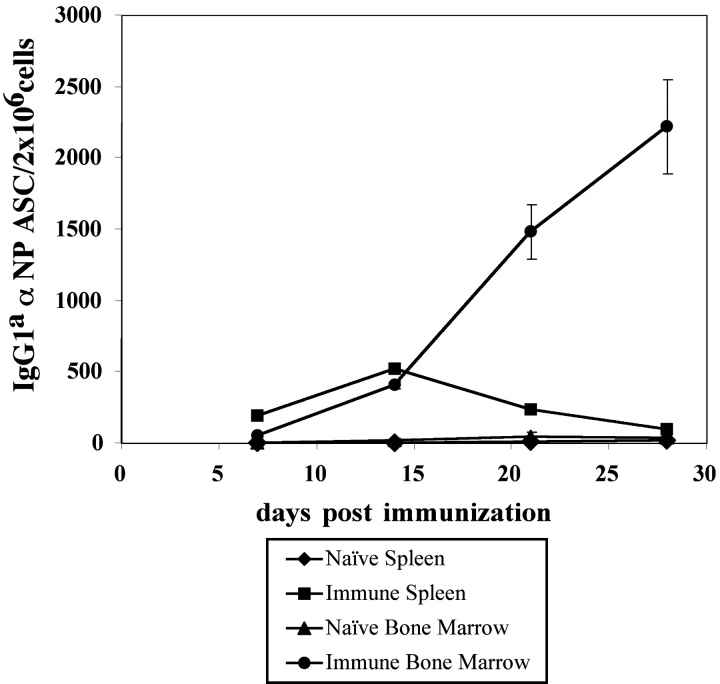
After the adoptive transfer of Tg B cells, the immunization of B6 mice gives rise to a population of Tg GC B cells (PNA+, GL-7+, CD38−) (16), and αNP IgG1a ASC in the spleen and BM (Fig. 1). Through the use of an αNP- and allotype-specific ELISPOT assay, low frequencies of Tg IgG1a ASC were detected early in the spleen (between 7 and 20 d after immunization), whereas much higher frequencies appeared later in the BM after immunization (>20 d) (Fig. 1). For example, at an early stage of the immune response, 7 d after immunization, 26 Tg ASC per 2 × 106 cells were observed in the spleen of immunized mice. At a later stage of the immune response, 21 d after immunization, 470 Tg ASC per 2 × 106 cells were observed in the BM of immunized mice, whereas only 32 Tg ASC were seen within the spleen. In addition to the ELISPOT analysis, Tg B cells expressing cytoplasmic IgGa could be detected in both the spleen and BM (unpublished data).
Figure 1.
The generation of splenic and BM PCs by Tg αNP-specific B cells. All transfers and immunizations were performed as previously described (16). 3 × 106 Tg cells were transferred to B6-Ly5.2 recipients, with three mice per experimental group. 1 d after transfer, recipient mice were injected intraperitoneally with 100 μg of NP-KLH emulsified in CFA. At 7, 14, 21, and 28 d after immunization, BM and spleen cells were isolated from recipient mice. Splenic and BM αNP-specific IgGa ASC were enumerated by an NP- and allotype-specific ELISPOT assay. ELISPOT assays were performed according to standard Millipore protocol using Millipore HA 96-well plates coated with NP-BSA. Data is representative of four independent experiments.
Characterization of Tg B Cells Present in the BM of Immune Recipients.
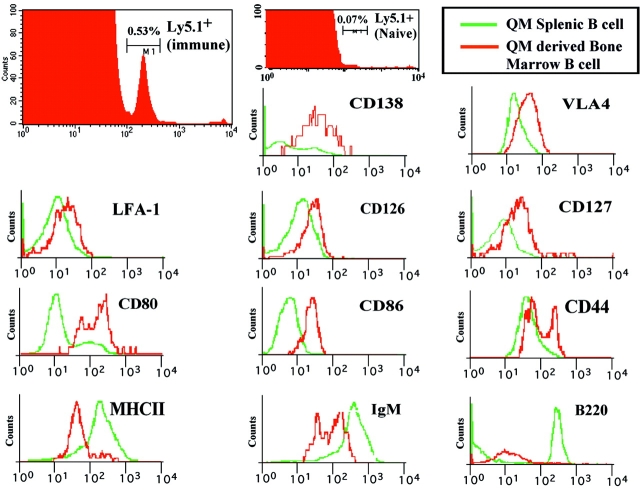
Flow cytofluorimetric analysis of immune BM revealed the presence of a population of Tg B cells as evidenced by the expression of the congenic marker Ly5.1 (Fig. 2). This population encompassed 0.6% (±0.3) of BM cells in over 10 experiments (Fig. 2, and unpublished data). In nonimmune mice, this population represented <0.07% of the total BM cells (Fig. 2). A phenotypic analysis of the Ly5.1+ Tg B cells present in the immune BM revealed characteristics that distinguish these cells from the phenotype of naive Tg splenic B cells. Multiparameter analysis of the Ly5.1+ B cells in the BM revealed that the Tg B cells expressed higher levels of CD80, CD86, CD126, CD127, CD138, VLA4, LFA-1, and CD44 when compared with naive Tg B cells isolated from the spleen (Fig. 2). The Tg B cells in the BM down-regulated surface MHCII, B220, and IgMa expression when compared with naive Tg B cells (Fig. 2). The Ly5.1+ Tg BM B cells, based on the down-regulation of MHCII and B220, and the elevated levels of CD80, CD86, CD138, and CD44, appeared to exhibit a phenotype that is intermediate between splenic B cells and end-stage PCs.
Figure 2.
A novel population of Tg B cells in the BM of immune mice that is distinct from naive B cells and PC. Immunization and transfer of Tg B cells to B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells were isolated from primary recipients for phenotype analysis. As a comparison to the BM cells, Tg splenocytes from naive mice were also isolated for phenotype analysis. Expression levels for a number of cell surface markers were then determined by three-color flow cytometry as previously described (16). All samples were stained with anti-Ly5.1 and anti-CD138 antibodies. Expression patterns for a number of surface markers on Ly5.1+/CD138+ BM cells or Ly5.1+/CD138low splenocytes were then determined via staining with either anti-VLA4, LFA-1, CD126, CD127, CD80, CD86, CD44, MHCII, B220, or IgM antibodies.
Isolation of PC Precursors.
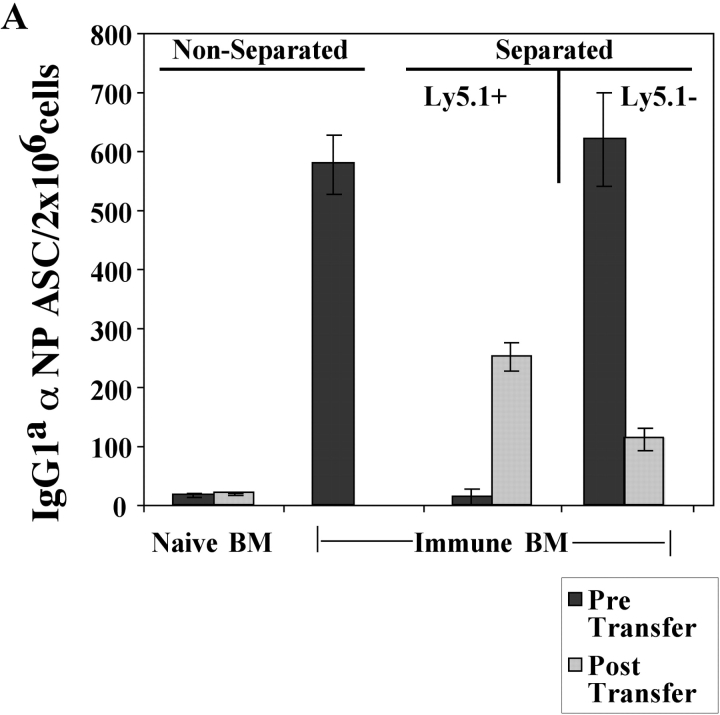
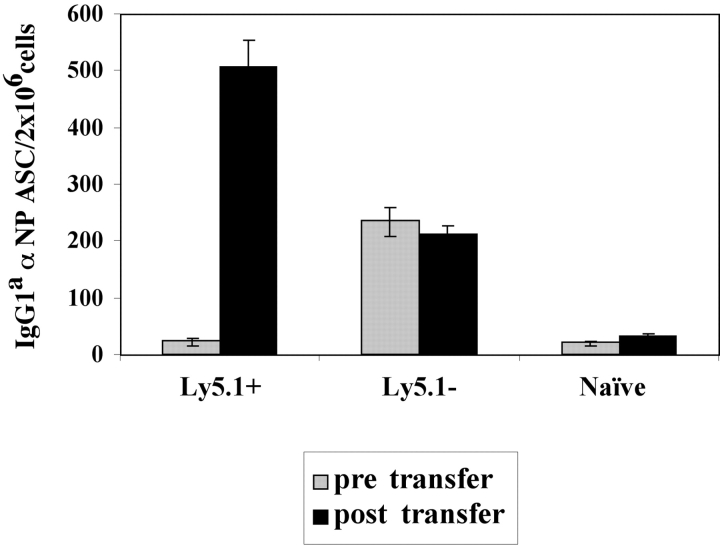
To determine if the Ly5.1+ B cells in the BM of immune mice were PCs, Ig production was examined. Ly5.1+ and Ly5.1− B cells were purified by Ly5.1 magnetic bead selection and analyzed for antibody secretion using an αNP- and allotype-specific ELISPOT assay. All of the Tg IgGa αNP ASC were restricted to the Ly5.1− population with none seen in the purified Ly5.1+ pool (Fig. 3 A). Hence, the Tg PCs lost the expression of the CD45.1 allele (Ly5.1), yet retained their capacity to produce αNP IgG1a (Fig. 3 A).
Figure 3.
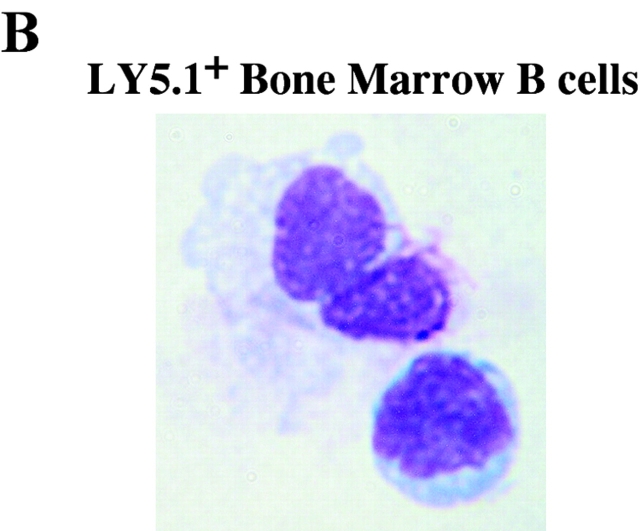
Identification of a Tg PC precursor. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells from primary recipients were isolated, depleted of T cells, and then separated by magnetic bead purification for Ly5.1 expression. (A) After separation, 105 Ly5.1+ or Ly5.1− cells were transferred to each secondary recipient B6-Ly5.2 mouse, with three mice per group. 2 wk after transfer, the total of BM cells were isolated from secondary recipients. The levels of αNP IgGa ASC within BM populations were enumerated by αNP- and allotype-specific ELISPOT assays at the time of isolation and postsecondary transfer for separated and unseparated BM populations. ELISPOT assays were performed as described in Fig. 1. Tg B cells express Ly5.1, whereas endogenous host cells express Ly5.2. Data is representative of over 10 independent experiments. (B) After magnetic bead selection, cytospin preparations of the Ly5.1+ cells were stained with Wright-Giemsa. ×1,000.
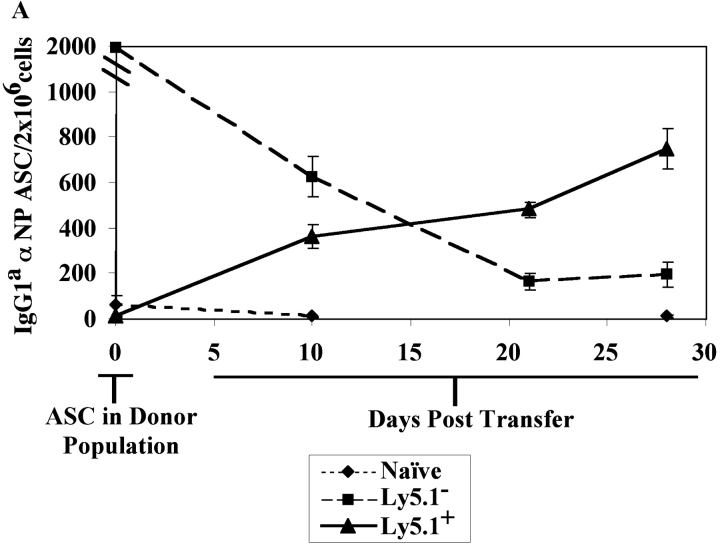
To address whether the Ly5.1+ cells found in the immune BM could give rise to PCs, purified Ly5.1+ B cells (>90% purity in 10 experiments) were transferred into naive recipients and the emergence of Tg PC in recipient mice was evaluated. Ly5.1+ cells, which do not produce antibody upon isolation (Fig. 3 A), give rise to a population of αNP IgG1a ASCs in the BM upon transfer to naive, secondary recipients (Fig. 3 A). Thus, the Ly5.1+ cells appear to be PC precursors as defined by their ability to give rise to PCs after adoptive transfer to naive recipients in the absence of antigen (Fig. 3 A). Moreover, PC precursors give rise to PC after adoptive transfer to recombination activating gene knockout in the absence of antigen and T cell help (unpublished data), distinguishing the differentiation of precursors from that of memory B cells (2). Morphologically, the Ly5.1+ PC precursors are a heterogeneous population containing lymphoblastoid and plasmacytoid cells (Fig. 3 B).
Tg PC Precursors and Short-lived PC in the BM.
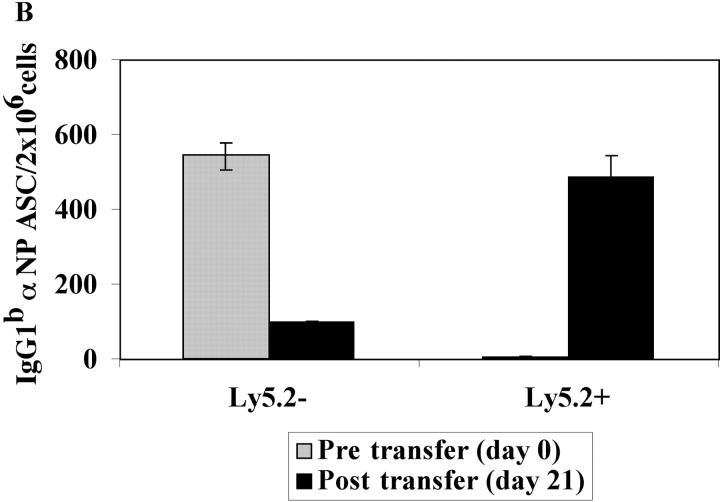
The kinetics of differentiation from precursor to PC and the longevity of PCs in immune mice were determined in vivo. Tg PC precursors (Ly5.1+) and PCs (Ly5.1−) were purified from BM 14 d after immunization. As before, the isolated Ly5.1+ subset did not contain high numbers of αNP IgG1a ASC, unlike the Ly5.1− subset (Fig. 4). Each of these populations was independently adoptively transferred into naive recipients in the absence of antigen. The transfer of the purified Ly5.1+ B cells into naive mice gave rise to increasing numbers of PCs producing αNP IgGa over a 30-d period (Fig. 4 A). In contrast, the transfer of the αNP IgGa-producing PCs demonstrated a progressive decline in the number of ASC that persisted over time (Fig. 4 A). Thus, 2 wk after immunization there is a heterogeneous population of Tg B cells within the immune BM, which contain a population of PC precursors and a population of short-lived PCs (Fig. 4 A). A similar observation was made with endogenous non-Tg PC precursors and PCs (Fig. 4 B).
Figure 4.
Functional capacity of BM PCs and precursors early in the immune response. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM cells were isolated from primary recipients and depleted of T cells. (A) Endogenous and Tg PC precursors and PCs within the BM population were then separated by magnetic bead purification for expression of the Ly5.1 (Tg), or (B) Ly5.2 (endogenous) cell surface marker. 105 Ly5.1+ or Ly5.1− cells were transferred to secondary recipients for measurement of Tg-derived ASC, whereas 3 × 106 Ly5.2+ or Ly5.2− cells were transferred to secondary recipients for measurement of endogenous ASC. Three B6-Ly5.2 mice were used per group. Over a 1-mo period after secondary transfer, αNP IgGa ASC levels within the recipient BM population were enumerated by an αNP- and allotype-specific ELISPOT assay. Endogenous αNP IgGb ASC were enumerated at 3 wk after secondary transfer. Data represent the number of ASC found within the donor populations before adoptive transfer as well as posttransfer. ELISPOT assays were performed as in Fig. 1. Data is representative of at least three independent experiments.
Precursors Generate Long-lived PCs.
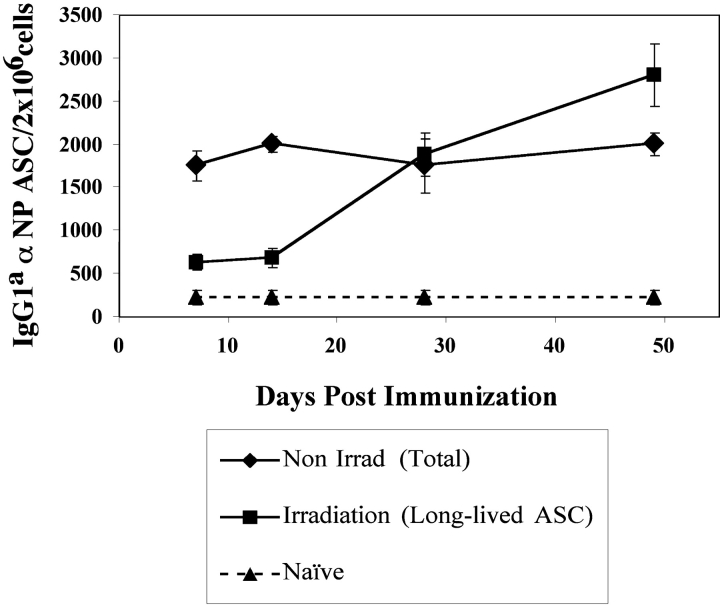
The half-life of Tg PCs at a later time during an immune response to NP was evaluated by the persistence of ASC in the BM of recipient mice after irradiation and reconstitution. Slifka et al. (3) have recently demonstrated that 600 rad of whole body irradiation of mice effectively depletes naive and memory B cells but does not significantly affect the persistence of BM long-lived PC. Therefore, the enduring Tg ASC after irradiation represent long-lived PC (3, 21, 22). At various times after immunization, recipient mice were irradiated and BM was reconstituted to determine the half-life of the ASC in the BM. The levels of αNP IgGa ASC within recipient mice were then assessed 3 wk after irradiation and BM reconstitution. The PCs present in the BM at 7 and 14 d after immunization appeared short-lived since irradiation caused >70% loss in ASC over a 3-wk period (Fig. 5). In contrast, the PCs present in the BM at 28 and 49 d did not decay over this 3-wk period after irradiation (Fig. 5). Hence, the early PCs in the BM are short-lived and the PCs that emerge after 2–3 wk in the BM are long-lived (Fig. 5). Thus, the data obtained with this Tg B cell system substantiates previous observations of PC longevity that have used polyclonal systems in vivo (1, 3).
Figure 5.
Kinetics of appearance of short-lived and long-lived Tg PCs after immunization. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. Primary recipients were irradiated with 600 rads at 7, 14, 28, or 49 d after transfer of Tg B cells and immunization. Irradiation protocols were performed as stated in Materials and Methods and as previously described (3). 3 wk after irradiation, BM cells were isolated from primary recipients. Tg αNP IgGa ASCs within the BM populations were enumerated by αNP- and allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. Data is representative of two independent experiments.
Proliferation of Precursors Is Necessary for Formation of Long-lived BM PC.
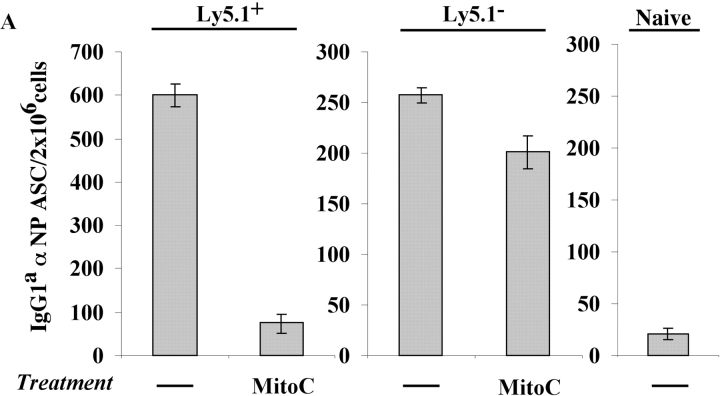
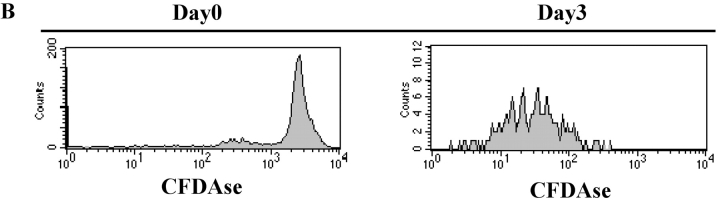
Irradiation not only ablated early PC survival, but also seemed to ablate the ability of PC precursors to generate new PC (Fig. 5). One effect of irradiation would be to inhibit PC precursor proliferation thereby preventing PC formation. Thus, the requirement of PC precursor proliferation for the generation of PCs was addressed. PCs and precursors were isolated, treated with mitoC, and transferred into naive mice. MitoC treatment did not affect PCs as both treated and untreated populations continued to produce antibody at similar levels after secondary transfer (Fig. 6 A). In contrast, mitoC treatment of the PC precursors ablated their capacity to differentiate into PCs upon adoptive transfer in secondary recipients (Fig. 6 A). Furthermore, the ability of PC precursors to proliferate in vivo was visually observed via CFDAse labeling. Precursors were briefly CFDAse labeled before secondary transfer (Fig. 6 B) and cell division was measured as a function of dye dilution. Proliferation of the precursors was visualized in the BM of recipient mice 3 d after transfer as a reduced, heterogeneous CFDAse emission compared with the high, unimodal staining of the input cells (Fig. 6 B).
Figure 6.
Proliferation is required for the differentiation of precursors to PCs early in the immune response. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. 2 wk after immunization, BM was isolated from primary recipients and depleted of T cells. (A) PC precursors and PCs were then separated by magnetic bead purification for Ly5.1 expression and a portion of each fraction was treated with MitoC. MitoC treatment protocol was derived from methods previously described (3). Each population was then transferred separately to B6-Ly5.2 secondary recipients. 105 cells were transferred to each recipient. 2 wk after secondary transfer, BM cells were isolated from secondary recipients. αNP IgGa ASCs within the BM population were then enumerated by αNP- and allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. (B) After Ly5.1 isolation, PC precursors were labeled with CFDAse and transferred (106 per mouse) to B6-Ly5.2 secondary recipients. 3 d after transfer, BM from recipient mice was stained with anti-Ly5.1, and CFDAse expression of the Ly5.1+ cells was determined via FACS® analysis. Data is representative of two independent experiments.
Long-lived PC Precursors.
Given the ability of PC precursors to give rise to long-lived PCs (Figs. 4 and 5), we evaluated their ability to persist in the immune BM over long periods of time. Indeed, PC precursors could be isolated from the BM of mice immunized 9 mo earlier (Fig. 7). Upon transfer of these precursors, PCs were generated in the BM of recipient mice (Fig. 7). Thus, both PC and their precursors are long-lived.
Figure 7.
PC precursors are long-lived. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. BM was isolated from primary recipients 9 mo after immunization and depleted of T cells. PC precursors and PCs were then separated by magnetic bead purification for Ly5.1 expression and transferred separately to B6-Ly5.2 secondary recipients. 6 × 104 PC precursors or PCs were transferred to secondary recipients. 2 mo after secondary transfer, BM cells from secondary recipients were isolated. αNP IgGa ASC levels within the BM were measured by an αNP- and allotype-specific ELISPOT assay both before and postsecondary transfer. ELISPOT assays were performed as described in Fig. 1. Data is representative of two independent experiments.
Subsets of PC Precursors Give Rise to Short-lived or Long-lived PC.
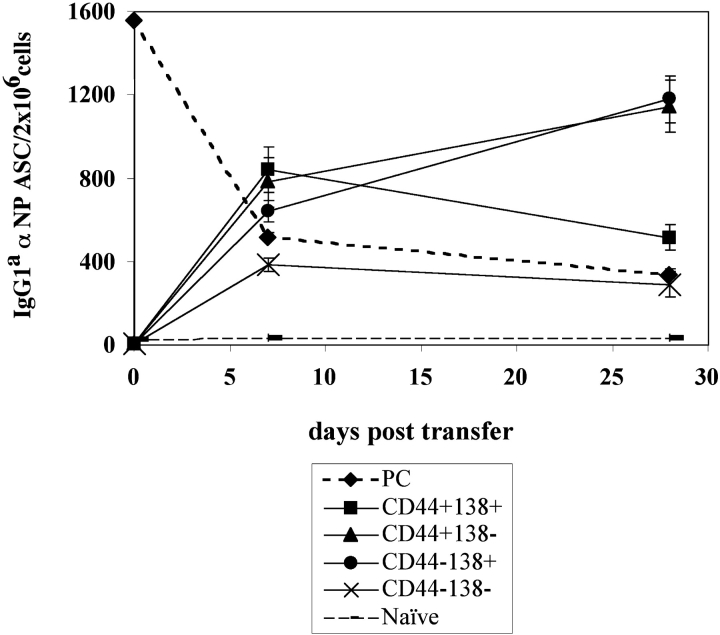
Functional heterogeneity of the Ly5.1+ B cell population in immune BM was evaluated by electronically sorting the precursors based on differences in cell surface antigen expression. Specifically, Ly5.1+ BM B cells were purified and sorted based on their expression of CD44 and CD138 (Fig. 2). Heightened levels of CD138 expression have been correlated with the differentiation of B cells to PC, whereas CD44 expression has been implicated in BM homing and observed on the MM PCs (1, 10, 13). Each of the sorted populations were transferred to naive mice and analyzed for their capacity to give rise to αNP IgG1a-secreting PC. Single-positive CD44+ and single-positive CD138+ cells displayed the greatest capacity to generate long-lived PCs (Fig. 8), whereas the double-positive (CD44+, CD138+) and double-negative (CD44−, CD138−) cells produced primarily short-lived PCs in the BM (Fig. 8). Thus, there appears to be a heterogeneous lineage of PC precursors in the immune BM that give rise to PCs with varying life spans.
Figure 8.
Phenotypic and functional diversity of PC precursor subsets. Immunization and transfer of Tg B cells to primary recipient B6-Ly5.2 mice were performed as described in Fig. 1. PCs and PC precursors were isolated from primary recipient BM 2 wk after immunization via Ly5.1 magnetic bead selection. The precursor fraction was then further separated via FACS® sorting for expression of CD44 and CD138 by fluorescent antibody staining. Each population of sorted precursor cells and PCs were transferred separately to B6-Ly5.2 secondary recipients. 4 × 104 cells were transferred to each recipient. BM cells were isolated from secondary recipients over a 1-mo period after transfer. αNP IgGa ASC were enumerated by an αNP-, allotype-specific ELISPOT assay. ELISPOT assays were performed as described in Fig. 1. Data is representative of four independent experiments.
Discussion
Among the multiple subsets of mature B cells found within the immune BM, a novel post-GC precursor to BM PCs has been identified. Thus, in addition to the existence of memory B cells and PCs, this newly identified PC precursor contributes to the development of long-lived humoral immunity (23–25). This novel subset within the BM is phenotypically distinct from either splenic B cells or PCs. Unlike mature B cells, this post-GC population in the BM downregulates B220, MHCII, and surface Ig, but up-regulates the expression of LFA-1, VLA4, CD80, CD86, CD126, and CD127. Unlike PCs the precursors express CD45 and do not secrete antibody. Moreover, the precursors are distinct from memory B cells in the BM due to their down-regulation of B220 (1). The precursors also exhibit an increased expression of CD138 compared with naive splenic B cells, although this level is still lower than previously observed on PCs (1). This intermediate level of CD138 might be an indication of the precursor's transitional stage of development. These studies are the first to establish that PC precursors migrate to the BM, can persist in the BM for extended periods of time, and can differentiate into PCs in the absence of antigen. Furthermore, multiple, phenotypically distinct PC precursors appear to exist within the BM; some give rise to short-lived (CD138+, CD44+) PC, and some give rise to long-lived (CD138+, CD44− and CD138−, CD44+) PC. Finally, upon trafficking to the BM the PC precursors proliferate, which is essential for the differentiation and generation of mature PCs.
Similar to previous studies of the humoral response to peptide antigens (1), QM Tg PC formation initiates with a rise in spleen ASC after immunization with NP. The spleen ASCs eventually decrease to naive levels, followed by a sustained increase in BM ASC (Fig. 1) (1). This is in contrast to the sustained ASC levels observed in both the spleen and BM after viral activation of B cells (3). Although the levels of Tg ASC in the spleen are lower than previously observed with either viral or peptide antigen (1, 3), the dissimilarity can be attributed to contrasts between non-Tg polyclonal responses and Tg immune responses. Unlike the non-Tg response to NP that is characterized by a prolonged GC, which peaks ∼2 wk after immunization, QM Tg B cells are synchronized in such a way that the peak of GC formation occurs at day 7, and then wanes soon thereafter (16 and unpublished data). Given the brief QM GC reaction, it is not surprising that the spleen Tg ASC levels would be lower than observed in non-Tg systems.
An unresolved issue regarding PC maturation is whether long-lived BM PC are established through the migration of fully differentiated PCs from secondary lymphoid organs or through alternative means, such as homing of a PC precursor to the BM (1–3, 24–28). Although it has been shown that mature PCs can be guided to the BM by a coordinated pattern of cell surface protein and chemokine receptor expression (12, 29–31), it is not clear if this is a major means for the generation of long-lived PCs in the BM. Based on the data presented we would hypothesize that PC precursors traffic from the spleen and LN and, upon arrival in the BM, proliferate and differentiate to PCs. If one examines the kinetics of PC generation in the BM, there is a rapid rise in the number of PC from 2 to 4 wk after immunization. During this period of time the number of mature PCs being generated in the spleen is declining. Thus, a tenable hypothesis is that the migration of PC precursors and their differentiation upon arrival in the BM, and not the migration of fully matured PCs, is responsible for the burst of PCs in the BM 3–4 wk after immunization.
The studies presented demonstrate that the immune BM harbors complex populations of both progenitors and PCs. Through electronic sorting and transfer, subsets of PC precursors were identified that gave rise to either short-lived or long-lived PCs. Even though these newly identified precursors may be essential to “seed” the BM to give rise to short-lived and long-lived PCs, the question emerges as to why these precursors persist for long periods of time in the face of long-lived PCs. First, it is possible that these precursors replace PCs upon PC attrition. If this were the case, one would envision a homeostatic mechanism controlling the total number of PC. Thus, as the numbers of long-lived PCs decline, or as the levels of immune complexes in the BM decline, the differentiation of precursors to PCs would be triggered. Another possible role of precursors might be that of affinity maturation. A number of studies have observed post-GC affinity maturation (26, 27, 32), and it is possible that affinity selection of long-lived precursors occurs within the BM. Currently, studies are underway to determine if the number of PCs or circulating immune complex regulates the differentiation of the PC precursors to PCs and if PC precursors can contribute to affinity maturation of the humoral immune response.
The identification of a PC precursor should provide the opportunity to discover the factors that govern their trafficking and proliferation. Heightened expression of VLA4, IL-7 receptor, and IL-6 receptor all conform to our current understanding of the gene products that regulate homing, growth, and survival of cells destined to traffic to the BM (29, 30, 33–36). In addition to the importance of the PC precursors to the regulation of long-lived humoral immunity, the identification of a proliferative precursor in MM has also been vigorously sought (8, 11, 37). MM, a highly aggressive tumor involving clonogenic PCs of the BM, is fatal for the majority of patients diagnosed (8, 11, 37). Traditional treatments are not effective in MM (8, 11, 37). This resistance is thought to be maintained by a progenitor cell that survives primary treatment after the majority of malignant PCs have been removed (8, 11, 37). The MM progenitor may then be capable of giving rise to new tumors leading to clinical relapse. The phenotype, proliferative potential, and differentiative capacities of the PC precursors are similar to what has been described for possible MM progenitors (8, 11, 37). Thus, PC precursors are attractive candidates for the normal counterpart of transformed MM progenitors.
Acknowledgments
We thank L. Vogel, B. Durell, and L. Erickson for their support and expert advice; Gary Ward, Kenneth Orndorff, and Alice Givan for their expertise in Flow Cytometry and FACS® sorting; E. Lind, S. Hopkins, and E. Westover for assistance with histology; and J. O'Connor, A. O'Connor, and R. Fleming for support in preparation of the manuscript.
Supported by National Institutes of Health grant AI-26296.
Footnotes
Abbreviations used in this paper: ACT, ammonium chloride-Tris buffer; ASC, antibody-secreting cells; BCS/BSS, bovine calf serum in balanced salt solution; BM, bone marrow; CFDAse, carboxyfluorescein diacetate succinimidyl ester; ELISPOT, enzyme-linked immunospot; GC, germinal center; MitoC, Mitomycin C; MM, multiple myeloma; NP, 4-hydroxy-3-nitrophenyl acetyl; PC, plasma cell; QM, quasi-monoclonal; TD, thymus-dependent; Tg, transgenic.
References
- 1.Manz, R.A., M. Lohning, G. Cassese, A. Thiel, and A. Radbruch. 1998. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10:1703–1711. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama, M., K.P. Lam, and K. Rajewsky. 2000. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 407:636–642. [DOI] [PubMed] [Google Scholar]
- 3.Slifka, M.K., R. Antia, J.K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity. 8:363–372. [DOI] [PubMed] [Google Scholar]
- 4.McHeyzer-Williams, M.G., and R. Ahmed. 1999. B cell memory and the long-lived plasma cell. Curr. Opin. Immunol. 11:172–179. [DOI] [PubMed] [Google Scholar]
- 5.Slifka, M.K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slifka, M.K., and R. Ahmed. 1996. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 4:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benner, R., W. Hijmans, and J.J. Haaijman. 1981. The bone marrow: the major source of serum immunoglobulins but still a neglected site of antibody production. Clin. Exp. Immunol. 46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Hallek, M., P.L. Bergsagel, and K.C. Anderson. 1998. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 91:3–21. [PMC free article] [PubMed] [Google Scholar]
- 9.Billadeau, D., G. Ahmann, P. Greipp, and B. Van Ness. 1993. The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J. Exp. Med. 178:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster-Kolbe, J., H. Ludwig, G.R. Adolf, and K.H. Heider. 1999. Expression of CD44 isoforms on isolated bone marrow plasma cells and peripheral CD19+ B cells of patients with multiple myeloma and healthy individuals. Leuk. Lymphoma. 34:95–103. [DOI] [PubMed] [Google Scholar]
- 11.Yaccoby, S., and J. Epstein. 1999. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 94:3576–3582. [PubMed] [Google Scholar]
- 12.Hargreaves, D.C., P.L. Hyman, T.T. Lu, V.N. Ngo, A. Bidgol, G. Suzuki, Y.R. Zou, D.R. Littman, and J.G. Cyster. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimold, A.M., N.N. Iwakoshi, J. Manis, P. Vallabhajosyula, E. Szomolanyi-Tsuda, E.M. Gravallese, D. Friend, M.J. Grusby, F. Alt, and L.H. Glimcher. 2001. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 412:300–307. [DOI] [PubMed] [Google Scholar]
- 14.Lin, K.I., Y. Lin, and K. Calame. 2000. Repression of c-myc is necessary but not sufficient for terminal differentiation of B lymphocytes in vitro. Mol. Cell. Biol. 20:8684–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelin-Duclos, C., G. Cattoretti, K.I. Lin, and K. Calame. 2000. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 165:5462–5471. [DOI] [PubMed] [Google Scholar]
- 16.Erickson, L.D., L.A. Vogel, M. Cascalho, J. Wong, M. Wabl, B.G. Durell, and R.J. Noelle. 2000. B cell immunopoiesis: visualizing the impact of CD40 engagement on the course of T cell-independent immune responses in an Ig transgenic system. Eur. J. Immunol. 30:3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cascalho, M., J. Wong, C. Steinberg, and M. Wabl. 1998. Mismatch repair co-opted by hypermutation. Science. 279:1207–1210. [DOI] [PubMed] [Google Scholar]
- 18.Cascalho, M., J. Wong, J. Brown, H.M. Jack, C. Steinberg, and M. Wabl. 2000. A B220(−), CD19(−) population of B cells in the peripheral blood of quasimonoclonal mice. Int. Immunol. 12:29–35. [DOI] [PubMed] [Google Scholar]
- 19.Cascalho, M., J. Wong, C. Steinberg, and M. Wabl. 1998. Mismatch repair co-opted by hypermutation. Science. 279:1207–1210. [DOI] [PubMed] [Google Scholar]
- 20.Cascalho, M., A. Ma, S. Lee, L. Masat, and M. Wabl. 1996. A quasi-monoclonal mouse. Science. 272:1649–1652. [DOI] [PubMed] [Google Scholar]
- 21.Anderson, R.E., and I. Lefkovits. 1980. Effects of irradiation on the in vitro immune response. Exp. Cell Biol. 48:255–278. [PubMed] [Google Scholar]
- 22.Okudaira, H., and K. Ishizaka. 1981. Reaginic antibody formation in the mouse. XI. Participation of long-lived antibody-forming cells in persistent antibody formation. Cell. Immunol. 58:188–201. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. [DOI] [PubMed] [Google Scholar]
- 24.Smith, K.G., A. Light, G.J. Nossal, and D.M. Tarlinton. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16:2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, K.G., A. Light, L.A. O'Reilly, S.M. Ang, A. Strasser, and D. Tarlinton. 2000. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J. Exp. Med. 191:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linton, P.L., D.J. Decker, and N.R. Klinman. 1989. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 59:1049–1059. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi, Y., P.R. Dutta, D.M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3- nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarlinton, D.M., and K.G. Smith. 2000. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol. Today. 21:436–441. [DOI] [PubMed] [Google Scholar]
- 29.Koni, P.A., S.K. Joshi, U.A. Temann, D. Olson, L. Burkly, and R.A. Flavell. 2001. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 193:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuker, C.E., M. Labow, W. Muller, and N. Wagner. 2001. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell–dependent humoral immune response. J. Exp. Med. 193:755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, Q., D. Jones, and T.A. Springer. 1999. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 10:463–471. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Y., G. Huang, J. Wang, H. Molina, D.D. Chaplin, and Y.X. Fu. 2000. Antigen persistence is required for somatic mutation and affinity maturation of immunoglobulin. Eur. J. Immunol. 30:2226–2234. [DOI] [PubMed] [Google Scholar]
- 33.Fujii, R., H. Ishikawa, M.S. Mahmoud, H. Asaoku, and M.M. Kawano. 1999. MPC-1-CD49e-immature myeloma cells include CD45+ subpopulations that can proliferate in response to IL-6 in human myelomas. Br. J. Haematol. 105:131–140. [PubMed] [Google Scholar]
- 34.Kawano, M.M., K. Mihara, N. Huang, T. Tsujimoto, and A. Kuramoto. 1995. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood. 85:487–494. [PubMed] [Google Scholar]
- 35.Rawstron, A.C., J.A. Fenton, J. Ashcroft, A. English, R.A. Jones, S.J. Richards, G. Pratt, R. Owen, F.E. Davies, J.A. Child, et al. 2000. The interleukin-6 receptor alpha-chain (CD126) is expressed by neoplastic but not normal plasma cells. Blood. 96:3880–3886. [PubMed] [Google Scholar]
- 36.Liu, Y.J., and J. Banchereau. 1997. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin. Immunol. 9:235–240. [DOI] [PubMed] [Google Scholar]
- 37.Jego, G., N. Robillard, D. Puthier, M. Amiot, F. Accard, D. Pineau, J.L. Harousseau, R. Bataille, and C. Pellat-Deceunynck. 1999. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood. 94:701–712. [PubMed] [Google Scholar]