Abstract
Efferent activity in the vagus nerve can prevent endotoxin-induced shock by attenuating tumor necrosis factor (TNF) synthesis. Termed the “cholinergic antiinflammatory pathway,” inhibition of TNF synthesis is dependent on nicotinic α-bungarotoxin-sensitive acetylcholine receptors on macrophages. Vagus nerve firing is also stimulated by CNI-1493, a tetravalent guanylhydrazone molecule that inhibits systemic inflammation. Here, we studied the effects of pharmacological and electrical stimulation of the intact vagus nerve in adult male Lewis rats subjected to endotoxin-induced shock to determine whether intact vagus nerve signaling is required for the antiinflammatory action of CNI-1493. CNI-1493 administered via the intracerebroventricular route was 100,000-fold more effective in suppressing endotoxin-induced TNF release and shock as compared with intravenous dosing. Surgical or chemical vagotomy rendered animals sensitive to TNF release and shock, despite treatment with CNI-1493, indicating that an intact cholinergic antiinflammatory pathway is required for antiinflammatory efficacy in vivo. Electrical stimulation of either the right or left intact vagus nerve conferred significant protection against endotoxin-induced shock, and specifically attenuated serum and myocardial TNF, but not pulmonary TNF synthesis, as compared with sham-operated animals. Together, these results indicate that stimulation of the cholinergic antiinflammatory pathway by either pharmacological or electrical methods can attenuate the systemic inflammatory response to endotoxin-induced shock.
Keywords: vagus nerve, endotoxin, TNF, systemic inflammation, neuroimmunology
Introduction
TNF, high mobility group B (HMGB)1, IL-1, and other inflammatory cytokines released by cells of the innate immune system mediate the systemic response to infection, injury, and endotoxemia. The magnitude and duration of the inflammatory response can influence survival because an unrestrained inflammatory response mediates lethal shock and tissue injury (1, 2). The central nervous system (CNS)* has an important antiinflammatory role in preventing the development of lethal systemic inflammation. For instance, proinflammatory mediators (e.g., LPS, TNF, IL-1) activate afferent neural pathways in the vagus nerve that stimulate release of pituitary adrenocorticotrophic hormone; the resultant increase in serum corticosteroids suppresses cytokine release to prevent excessive inflammation (3, 4). In addition to this sensory function of the vagus nerve in systemic inflammation, we recently described an efferent or motor vagus neural mechanism by which acetylcholine, the principal vagus nerve neurotransmitter, inhibits cytokine release from resident tissue macrophages, termed the “cholinergic antiinflammatory pathway” (5).
Acetylcholine, the principle neurotransmitter of the vagus nerve, binds nicotinic cholinergic receptors on resident macrophages, and this receptor–ligand interaction inhibits the synthesis of TNF at a posttranscriptional level (5). Vagotomy renders animals exquisitely sensitive to endotoxin; vagotomized animals produce significantly more TNF and are significantly more sensitive to endotoxin-induced hypotension (5). Direct electrical stimulation of the efferent vagus nerve confers significant protection against the development of endotoxin-induced shock and prevents the release of TNF in tissue and serum (5). The vagus nerve is distributed throughout the reticuloendothelial system, and since neural signaling is rapid, it now appears that CNS-derived motor output through the cholinergic antiinflammatory pathway is uniquely positioned to modulate the development of systemic inflammation and shock.
CNI-1493, an experimental therapeutic now undergoing testing in Phase II clinical trials for Crohn's disease, is a tetravalent guanylhydrazone inhibitor of macrophage activation that prevents the phosphorylation of p38 mitogen-activated protein kinase (MAPK; references 6–8). During the course of describing the activity of CNI-1493 in cerebral ischemia, we observed that direct application into the cerebral ventricles suppressed cerebral TNF synthesis (9). As further controls in these experiments, the effect of intracerebral CNI-1493 on peripheral endotoxin responses was also studied, and surprisingly, CNI-1493 administered via the intracerebroventricular (i.c.v.) route attenuated the production of systemic TNF. Administration of CNI-1493 directly stimulated neural activity in the cholinergic antiinflammatory pathway (10). Together, these studies suggested the intriguing hypothesis that the TNF-inhibiting activity of CNI-1493 in vivo might require signaling via the cholinergic antiinflammatory pathway. Here we show that administration of CNI-1493 inhibits systemic TNF release and TNF synthesis in tissues during endotoxemia through a mechanism that is dependent upon an intact cholinergic anti-inflammatory pathway.
Materials and Methods
Animals.
Adult male Lewis rats (280–300 g; Charles River Laboratories) were housed at 25°C on a 12 h light/dark cycle. Standard rat chow and water were freely available. All animal experiments were performed in accordance with the National Institutes of Health (NIH) Guidelines under protocols approved by the Institutional Animal Care and Use Committee of North Shore University Hospital and New York University Medical School.
I.C.V. Injections.
Rats were anesthetized with ketamine (10%) in xylazine and placed in a stereotatic head frame (Stoelting Co.). The incisor bar was adjusted until the plane defined by the lambda and bregma was parallel to the base plate. The needle of a Hamilton syringe (25 ml) was stereotactically guided into lateral ventricle (0.8 mm posterior to bregma, 1.5 mm lateral to midline, 3.0 mm below the dura). CNI-1493 was dissolved in sterile endotoxin-free water and administered .2 min. The location of i.c.v. injections was confirmed by histological examination of the brain after the experiment.
Tissue-specific Distribution of CNI-1493.
14C-labeled CNI-1493 (87 μCi/mg; ChemSyn Laboratories) was dissolved in sterile pyrogen-free water and administered via either intravenous (1 mg/kg) or i.c.v. (100 ng/kg) routes. After 1 h, rats underwent transcardial perfusion with 250 ml of NaCl (0.9%) followed by 100 ml of paraformaldehyde (4% in 0.1 M Dulbecco's phosphate-buffered saline, pH 7.3). Radioactivity in tissue samples was counted by a liquid scintillation system (Beckman LS 7800; Beckman Instruments, Inc.), and the content of CNI-1493 calculated by comparison with 14C standards.
Endotoxin Shock.
The left common iliac artery and right common femoral vein were cannulated with a 23-gauge intravenous catheter in anesthetized rats. The arterial catheter was connected to a blood pressure transducer and recorder (Model MP 100 acquisition system; Biopac Systems) for continuous registration of mean arterial blood pressure (MABP) and heart rate (HR). Endotoxin (LPS, Escherichia coli 0111:B4; Sigma-Aldrich) was dissolved in sterile pyrogen-free saline at stock concentrations of 10 mg/ml, and sonicated for 30 min immediately before use for each experiment in the doses indicated. Blood was collected from the right femoral vein 1 h after LPS administration, allowed to clot for 2 h at room temperature, then centrifuged for 20 min at 1,500 g. Serum samples were stored at 20°C before analysis.
Vagotomy and Atropine Blockade.
Anesthetized rats were subjected to bilateral cervical vagotomy or sham surgery as described previously (5). A ventral cervical midline incision was used to expose both vagus trunks, which were ligated with 4–0 silk sutures and divided. In sham-operated animals both vagus nerve trunks were exposed and isolated from surrounding tissue but not transected. Atropine (Sigma-Aldrich) was dissolved in sterile saline and administered intravenously at a dose of 1 mg/kg/h; control animals received a comparable volume of saline.
Electrical Stimulation of Intact Vagus Nerve.
Where indicated, the vagus nerve was exposed and isolated from surrounding tissue with parafilm. Intact vagus nerves were stimulated with constant voltage at either 1V (2 ms, 5 Hz) or 5V (2 ms, 5 Hz) for 10 min intervals before and after LPS injection, for a total of 20 continuous min, using bipolar platinum electrodes connected to the stimulation module. Rats were subjected to either left vagus stimulation, right vagus stimulation, or sham operation. MABP, HR, and respiratory rate were recorded every 5 min, beginning 30 min before, and ending 150 min after LPS injection. Blood was collected from the right femoral vein after 180 min and serum TNF measured. At the completion of monitoring, animals were killed, the heart, liver, and lung rapidly excised, rinsed of blood, homogenized by polytron (Brinkman) in homogenization buffer (PBS containing 0.05% Triton X-100 and a protease inhibitor cocktail, pH 7.2, 4°C), and sonicated for 10 min. Homogenates were centrifuged at 12,000 g for 10 min, and TNF measured using the L929 bioassay. Liver, lung, and heart TNF were normalized to the protein concentration in the sample.
Statistical Analysis.
All data in the figures, tables, and text are expressed as mean ±SEM for at least 4–5 animals per condition. Student's t test was used to compare mean values between groups (P = 0.05).
Results
I.C.V. CNI-1493 Is 100,000-fold More Effective Than Intravenous CNI-1493 at Attenuating Endotoxin-induced Hypotension and Serum TNF.
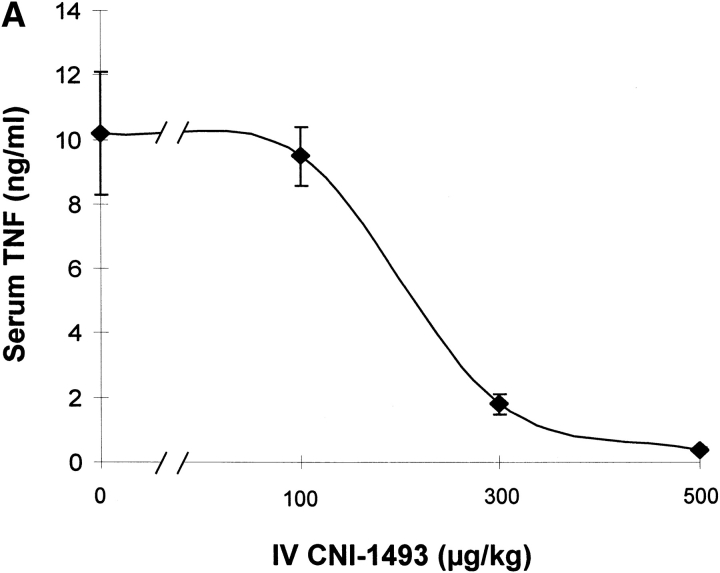
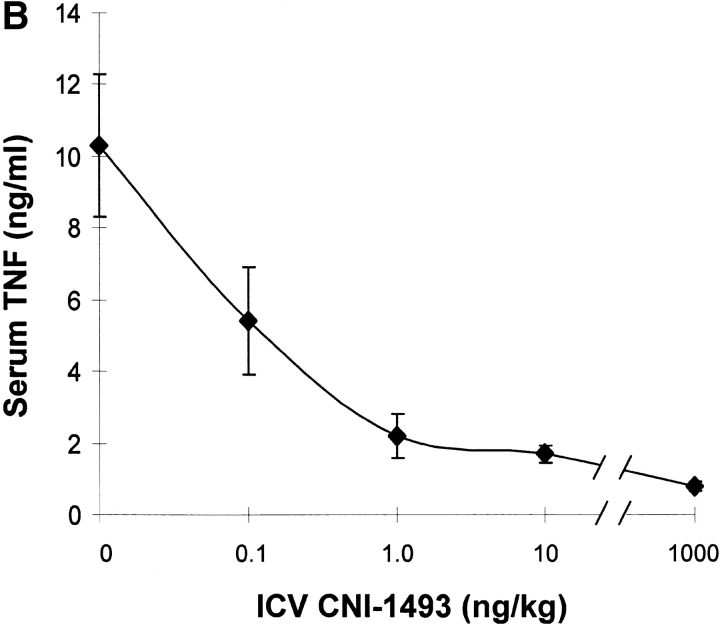
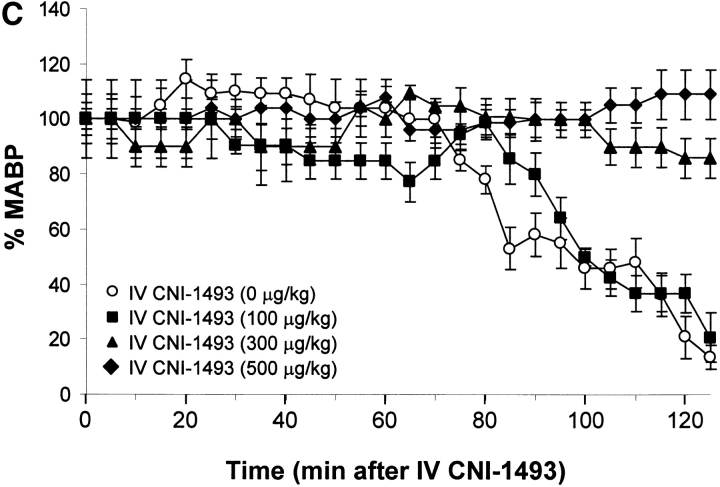
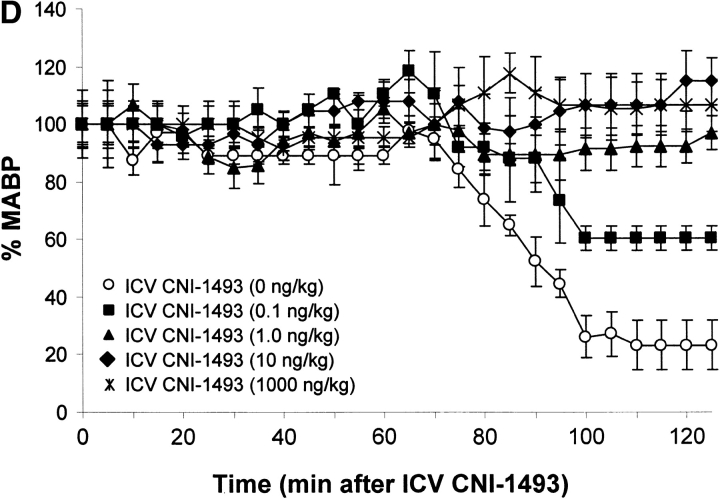
Vehicle-treated endotoxemic rats developed significant hypotension and increased serum TNF levels within 1 h after exposure to a lethal dose of LPS (Fig. 1). Pretreatment with intravenous CNI-1493 significantly and dose–dependently inhibited serum TNF release (vehicle control = 10 ± 2 ng TNF/ml vs. 300 μg CNI-1493/kg, intravenous = 2 ± 0.3 ng TNF/ml; P < 0.05) and prevented the development of LPS-induced hypotension (vehicle control = 14 ± 5% of starting MABP vs. 300 μg CNI-1493/kg, intravenous = 86 ± 7% of starting MABP; P < 0.05) (Fig. 1). The lowest intravenous CNI-1493 dose tested (100 μg/kg) failed to prevent TNF release (vehicle control = 10 ± 2 ng TNF/ml vs. 100 μg CNI-1493/kg, intravenous = 10 ± 1 ng TNF/ml; P > 0.05) or hypotension (vehicle control = 14 ± 5% of starting MABP vs. 100 μg CNI-1493/kg, intravenous = 21 ± 9% of starting MABP; P > 0.05). i.c.v. administration of a 100-fold dilution of this ineffective intravenous dose significantly attenuated serum TNF release (vehicle control = 10 ± 2 ng TNF/ml vs. 1,000 ng CNI-1493/kg, i.c.v. = 1 ± 0.2 ng TNF/ml; P < 0.05) and protected against the development of hypotension (vehicle control = 23 ± 9% of starting MABP vs. 1,000 ng CNI-1493/kg, i.c.v. = 107 ± 9% of starting MABP; P < 0.05) (Fig. 1). Surprisingly, much lower i.c.v. doses of CNI-1493 (10, 1.0, and 0.1 ng CNI-1493/kg) conferred significant protection against the development of endotoxin-induced shock (vehicle control = 23 ± 9% of starting MABP vs. 10 ng CNI-1493/kg, i.c.v. = 97 ± 8% of starting MABP, P < 0.05; 1.0 ng CNI-1493/kg, i.c.v. = 115 ± 6% of starting MABP, P < 0.05; and 0.1 ng CNI-1493/kg, i.c.v. = 61 ± 4% of starting MABP, P < 0.05) and inhibited serum TNF (vehicle control = 10 ± 1 ng TNF/ml vs. 10 ng CNI-1493/kg, i.c.v. = 2 ± 0.3 ng TNF/ml, P < 0.05; 1.0 ng CNI-1493/kg, i.c.v. = 2 ± 0.4 ng TNF/ml, P < 0.05; and 0.1 ng CNI-1493/kg, i.c.v. = 5 ± 1 ng TNF/ml, P < 0.05) (Fig. 1). Comparison of dose–response curves constructed from data obtained after CNI-1493 was given via either intravenous or i.c.v. routes revealed that the latter route was at least 100,000 times more effective in preventing TNF release and shock (Fig. 1), suggesting that the CNS participates in the systemic antiinflammatory action of CNI-1493 during endotoxemia.
Figure 1.
CNI-1493 (intravenous or i.c.v.) inhibits endotoxin-induced hypotension and attenuates serum TNF. (A) CNI-1493 was given intravenously in the doses shown; endotoxin (15 mg/kg, intravenously) was administered 60 min later. After 1 h, blood was collected via carotid artery catheter, and serum prepared for TNF assays. (B) CNI-1493 was given i.c.v. in the doses shown; endotoxin (15 mg/kg, intravenously) was administered 60 min later. After 1 h, blood was collected via carotid artery catheter, and serum prepared for TNF assays. (C) Intravenous injection of CNI-1493 60 min before endotoxin exposure prevented endotoxic shock. 1 h after exposure to a lethal dose of LPS (15 mg/kg), vehicle-treated endotoxemic rats developed significant LPS-induced hypotension. Intravenous administration of CNI-1493, in doses of 300 or 500 mg/kg, given 60 min before endotoxin exposure significantly prevented the development of LPS-induced hypotension. (D) i.c.v. injection of CNI-1493 60 min before endotoxin exposure prevented endotoxin-induced shock. 1 h after exposure to a lethal dose of LPS (15 mg/kg), vehicle-treated endotoxemic rats developed significant LPS-induced hypotension. i.c.v. doses of CNI-1493 (1,000, 10, 1.0, and 0.1 ng/kg) given 60 min before endotoxin exposure significantly prevented the development of LPS-induced hypotension.
Intravenous CNI-1493 Accumulates in Brain.
To determine the tissue distribution of CNI-1493 after systemic dosing, we measured radioactivity in rat tissues 1 h after intravenous administration of 14C-labeled CNI-1493 (1 mg/kg). After transcardiac perfusion to minimize nonspecific binding, the highest levels of radioactivity were observed in the spleen, liver, kidney, lungs, and gastrointestinal tract. Lesser accumulation was observed in brain, skin, muscle, and heart (Table I). Although peripheral organs did not accumulate significant radioactivity after i.c.v. injection of 14C-CNI-1493, significant radioactive uptake persisted in brain (Table I). The amount of radioactivity detected in brain after i.c.v. administration of 100 ng/kg of 14C-CNI-1493 was comparable to that observed after intravenous administration of 1 mg/kg of 14C-CNI-1493. Each of these dosing regimens suppressed endotoxin-induced TNF and shock (Fig. 1), so that brain drug levels achieved after intravenous dosing with a higher dose were comparable to those achieved with direct i.c.v. application of a lesser, but pharmacologically active, dose of CNI-1493. The i.c.v. doses that effectively inhibited systemic inflammatory responses were too low to reach detectable levels in peripheral tissues, suggesting that the CNS participates in mediating the systemic antiinflammatory mechanisms of CNI-1493.
Table I.
Distribution of 14C-labeled CNI-1493 in Rat Tissues 60 min After Intravenous (1 mg/kg) and Intracerebroventricular (100 ng/kg) Injections
| Tissue/organ | CNI-1493 after intravenous(1 mg/kg injection) g/mg tissue | CNI-1493 after i.c.v.(100 ng/kg) injection ng/mg tissue |
|---|---|---|
| Blood | 4.5 ± 1.3 | ND |
| Brain | 1.8 ± 0.3 | 1.6 ± 0.6 |
| Heart | 3.3 ± 0.7 | ND |
| Intestine | 12.6 ± 5.1 | ND |
| Kidney | 18.6 ± 1.9 | ND |
| Liver | 19.7 ± 5.4 | ND |
| Lung | 13.0 ± 5.0 | ND |
| Muscle | 2.7 ± 1.1 | ND |
| Skin | 8.5 ± 2.5 | ND |
| Spleen | 21.8 ± 2.1 | ND |
The Protective Effects of CNI-1493 Are Abolished by Surgical or Chemical Vagotomy.
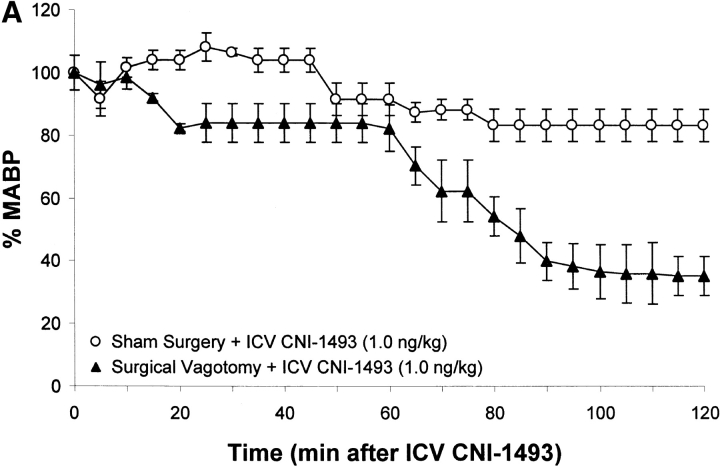
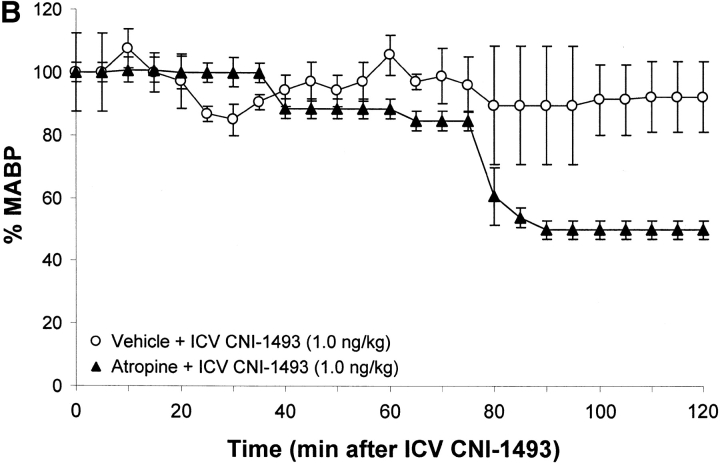
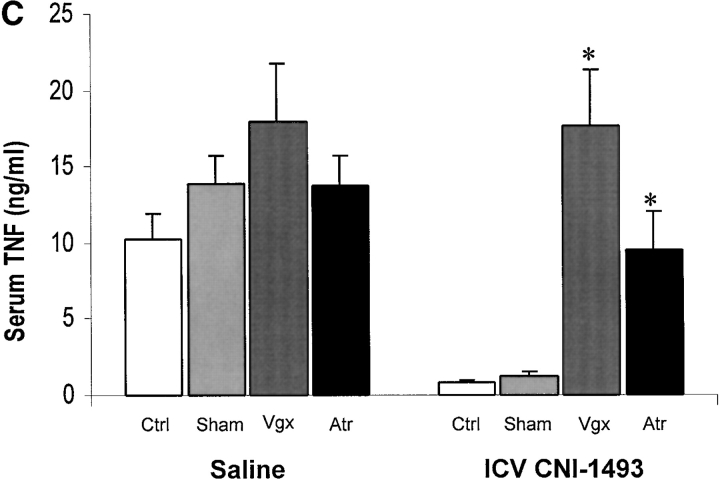
To determine whether an intact cholinergic antiinflammatory pathway is required for inhibition of TNF and protection from endotoxin-induced shock by i.c.v. or intravenous CNI-1493, animals were subjected to either surgical or chemical vagotomy. Surgical vagotomy eliminated the protective effects of i.c.v. CNI-1493 against LPS-induced hypotension (sham surgery plus 1.0 ng CNI-1493/kg, i.c.v. = 83 ± 5% of starting MABP vs. surgical vagotomy plus 1.0 ng CNI-1493/kg, i.c.v. = 35 ± 6% of starting MABP, P < 0.05). Surgical vagotomy also eliminated the protective effect of intravenous CNI-1493 against endotoxin-induced shock (500 μg CNI-1493/kg, intravenous plus sham vagotomy = 89 ± 4 vs. surgical vagotomy plus 500 μg CNI-1493/kg, intravenous = 55 ± 11% of starting MABP; P < 0.05), indicating that the protective effects of CNI-1493, whether administered into the brain or the peripheral circulation, require an intact vagus nerve. Surgical vagotomy also significantly attenuated the protective effects of intracerebral CNI-1493 against LPS-induced TNF release as compared with sham surgery (sham surgery plus 1.0 ng CNI-1493/kg, i.c.v. = 1 ± 0.2 ng TNF/ml vs. vagotomy plus 1.0 ng CNI-1493/kg, i.c.v. = 18 ± 4 ng TNF/ml; P < 0.05) (Fig. 2). Similar observations were made after administration of atropine, an antagonist of cholinergic signaling, because atropine abolished the protective effect of i.c.v. CNI-1493 against LPS-induced hypotension (vehicle plus 1.0 ng CNI-1493/kg, i.c.v. = 93 ± 11% of starting MABP vs. atropine plus 1.0 ng CNI-1493/kg, i.c.v. = 50 ± 3% of starting MABP; P < 0.05). Atropine abolished the protective effect of i.c.v. CNI-1493 against LPS-induced TNF release (vehicle plus 1.0 ng CNI-1493/kg, i.c.v. = 1 ± 0.2 ng TNF/ml vs. 1 mg atropine/kg/h plus 1.0 ng CNI-1493/kg, i.c.v. = 10 ± 3 ng TNF/ml; P < 0.05) (Fig. 2). Considered with the previously described observations that CNI-1493 stimulates increased vagus nerve activity when administered in vivo (10), these results indicate that cholinergic vagus neural signals mediate systemic protection against endotoxin-induced TNF and hypotension.
Figure 2.
Chemical or surgical vagotomy blocks the protective action of intracerebral CNI-1493. (A) Effects of bilateral cervical vagotomy vs. sham surgery on the action of i.c.v. CNI-1493 (ng/kg) during endotoxemia, expressed as the percentage of starting MABP. CNI-1493 (1.0 ng/kg) was administered i.c.v., followed 60 min later by endotoxin (15 mg/kg, intravenously). Note that surgical vagotomy eliminated the protective effects of 1.0 ng CNI-1493/kg, i.c.v. against LPS-induced hypotension. (B) Effects of intravenous saline treatment (control) vs. atropine blockade on the action of i.c.v. administered CNI-1493 during endotoxemia, expressed as the percentage of starting MABP. CNI-1493 (1.0 ng/kg, i.c.v.) was administered to animals treated with atropine (1 mg/kg/h, intravenously) to block vagus nerve activity. Note that chemical vagotomy abolished the protective effect of i.c.v. CNI-1493 against LPS-induced hypotension. (C) Serum TNF levels after i.c.v. CNI-1493 injection 60 min after LPS infusion. On the left, all animals received an i.c.v. injection of vehicle. On the right, animals received CNI-1493 (1.0 ng/kg, i.c.v.). In both panels, “Ctrl” refers to animals that received atropine vehicle (saline), while “Sham” identifies those animals in which the vagus nerves were exposed, but not surgically divided. Note that chemical vagotomy and surgical vagotomy both significantly attenuated the protective effects of CNI-1493 (1.0 ng/kg, i.c.v.) against LPS-induced TNF release as compared with vehicle.
Intact Vagus Nerve Stimulation Protects Against Endotoxin-induced Hypotension and Endotoxin-induced Shock.
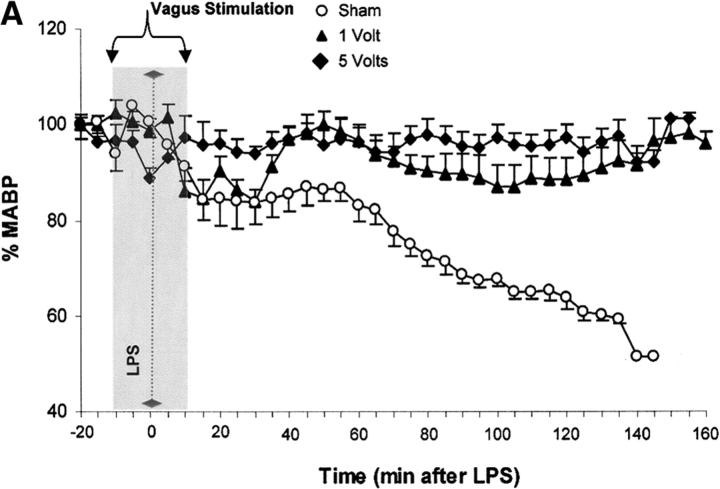
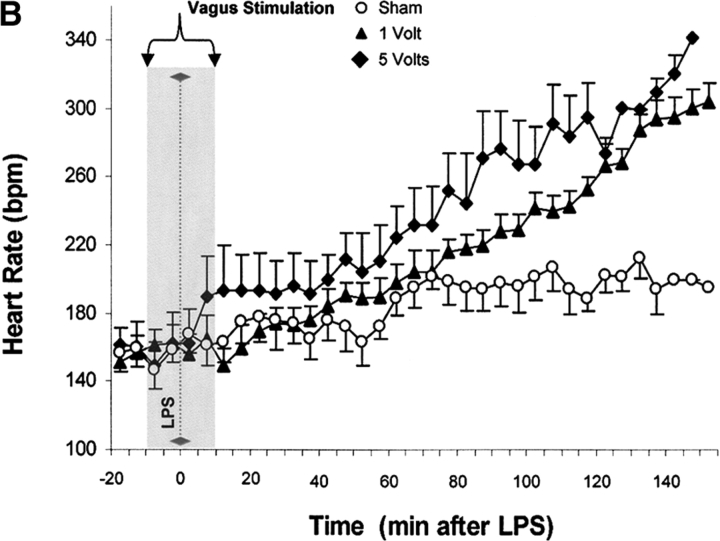
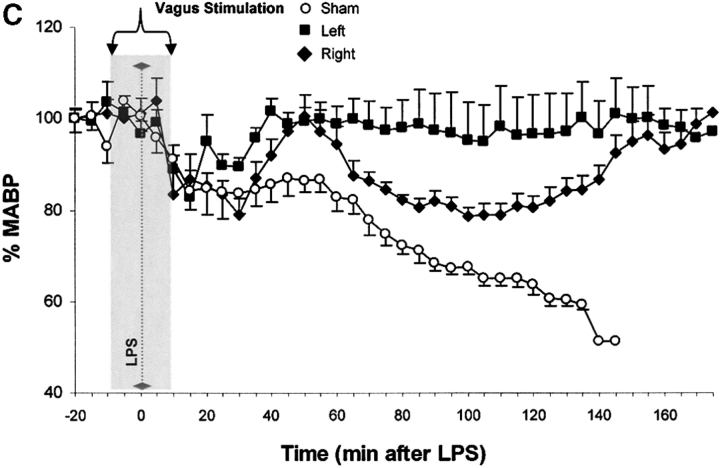
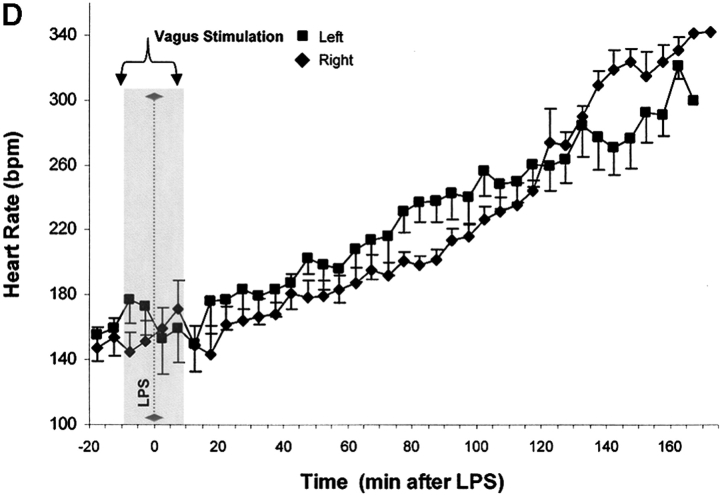
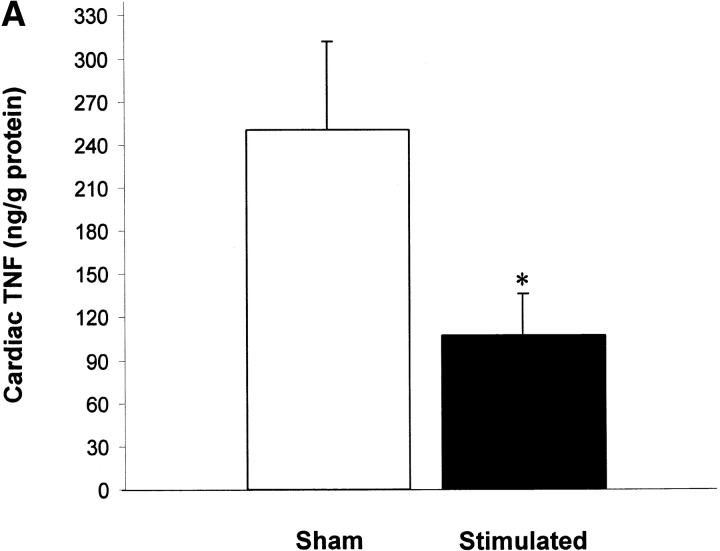
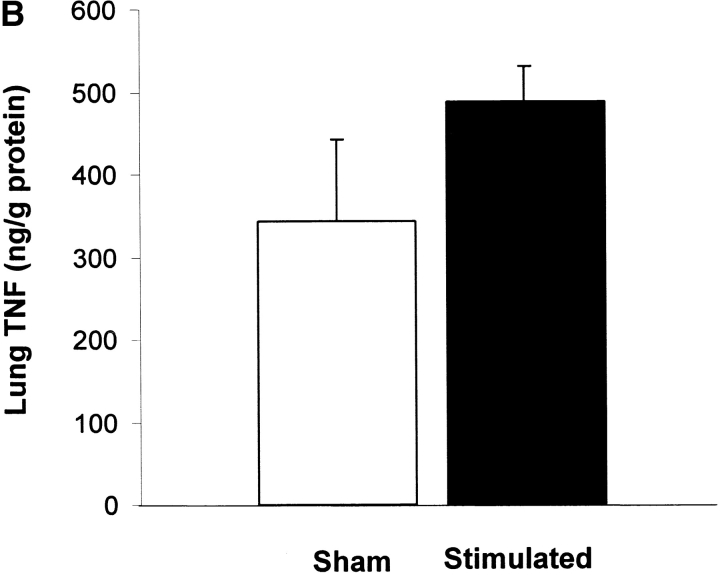
Vagus nerve stimulators are used clinically for the treatment of seizure disorders and depression, but the effects of this treatment applied to intact vagus nerves on the development of systemic inflammatory responses are unknown (11). The above observations with a pharmacological vagus nerve stimulator (CNI-1493) predict that electrical stimulation of the vagus nerve might recapitulate the antiinflammatory action of CNI-1493. We electrically stimulated intact vagus nerves and observed that this procedure significantly prevented the development of hypotension during endotoxemia (Fig. 3). Stimulation with either 1 V or 5 V impulses at 2 ms intervals (5 Hz) prevented the development of significant hypotension (control = 51 ± 1% of starting MABP vs. 1 V stimulation = 96 ± 2% of starting MABP, P < 0.05; and 5 V stimulation = 101 ± 1% of starting MABP, P < 0.05) (Fig. 3). HR did not increase significantly in nonstimulated endotoxemic animals, despite the development of hypotension. Stimulation of the vagus nerve, however, was associated with a significant increase in HR; the increase in HR was voltage stimulus dependent (sham HR = 195 ± 15 bpm vs. 1 V vagus stimulation = 304 ± 12 bpm, P < 0.05, and 5 V vagus stimulation = 342 ± 11 bpm, P < 0.05) (Fig. 3). To assess whether the right and left vagus nerves contribute a differential protective effect, we measured blood pressure and HR in animals after separately stimulating either nerve. As demonstrated in Fig. 3, we observed no significant difference between right and left cervical vagus nerve stimulation. Cardiac and pulmonary TNF synthesis has been implicated in the development of endotoxin-induced myocardial depression and acute lung injury (12, 13). To address the specificity of stimulating intact vagus nerves on inhibition of TNF, we measured cardiac and pulmonary TNF during lethal endotoxemia. Vagus nerve stimulation (5 V, 2 ms, 5 Hz) during lethal endotoxemia significantly attenuated cardiac TNF levels (unstimulated = 251 ± 61 ng TNF/g protein vs. stimulated = 107 ± 29 ng TNF/g protein; P < 0.05), but failed to inhibit pulmonary TNF levels (Fig. 4), indicating that cholinergic anti-inflammatory pathway inhibition of TNF in liver (5) and heart is specific.
Figure 3.
Electrical stimulation of intact vagus nerves protects against endotoxic shock. Electrical stimulation at the indicated voltage was applied to the exposed, intact vagus nerve of anethesized rats. Endotoxin (50–60 mg/kg, intravenously) was given 10 min after beginning electrical stimulation; electrical stimulation was continued for an additional 10 min as shown. (A) Blood pressure responses to electrical stimulation of the intact right cervical vagus nerve (1 V and 5 V) in the presence of endotoxemia. Note that stimulation with either 1 V or 5 V at 2 ms intervals (5 Hz) prevented the development of significant hypotension. (B) HR responses to electrical stimulation of the intact right cervical vagus nerve (1 V and 5 V) in the presence of endotoxemia. Right vagus nerve stimulation with either 1 V or 5 V was associated with a significant, voltage stimulus-dependent increase in HR, but HR did not increase significantly in nonstimulated endotoxemic animals, despite the development of hypotension. (C) Blood pressure responses to electrical stimulation of the intact right and left cervical vagus nerves in the presence of endotoxemia. Electrical stimulation (5V) was applied to the either the left or right intact vagus nerves of anesthesized rats where indicated. Note that the difference in MABP between right and left cervical vagus nerve stimulation was not statistically significant. (D) HR responses to right versus left vagus nerve stimulation in the presence of endotoxemia. Electrical stimulation (5V) was applied to the either the left or right intact vagus nerves of anesthesized rats, where indicated.
Figure 4.
Vagus nerve stimulation attenuates cardiac, but not pulmonary TNF. Electrical stimulation (5 V) was applied to the exposed, intact vagus nerve of anethesized rats as described in the legend to Fig. 3. (A) Cardiac homogenate TNF 180 min after LPS administration. Note that vagus nerve stimulation (5 V, 2 ms, 5 Hz) during lethal endotoxemia significantly attenuated cardiac TNF levels. (B) Lung homogenate TNF 180 min after LPS administration. Note that vagus nerve stimulation (5 V, 2 ms, 5 Hz) during lethal endotoxemia failed to inhibit pulmonary TNF levels.
Discussion
CNI-1493 is a tetravalent guanylhydrazone that inhibits phosphorylation of p38 MAPK and suppresses proinflammatory cytokine release from monocytes and macrophages (6–8). Systemic administration of CNI-1493 is effective in the treatment of experimental endotoxemia, sepsis, allergic encephalitis, cerebral ischemia, and arthritis (6, 9, 14–16). Administration of CNI-1493 to cancer patients undergoing IL-2 therapy significantly attenuated systemic TNF (17). The present results now indicate that an intact cholinergic antiinflammatory pathway is required for the antiinflammatory action of CNI-1493 in vivo. Here, i.c.v. CNI-1493 attenuated endotoxin-induced proinflammatory cytokine release and shock at doses that are ineffective via the intravenous route. Moreover, surgical or chemical (atropine) vagotomy completely abolished the antiinflammatory effects of CNI-1493. Thus, an intact cholinergic antiinflammatory pathway is required for the macrophage-deactivating effects of CNI-1493 given via either the intracerebral or intravenous routes. Indeed, it now appears that the primary antiinflammatory mechanism of systemically administered CNI-1493 is centrally mediated.
Previous studies implicated the CNS in preferentially mediating the antiinflammatory action of α-melanocyte–stimulating hormone (α-MSH; reference 18), and the nonsteroidal antiinflammatory drugs lysine acetylsalicylate and sodium salicylate (19). Systemically administered α-MSH inhibits activation of NF-κB (20), a transcription factor that is required for the transcription of TNF (21). Significantly lower doses of centrally administered α-MSH modulate cytokine-induced cutaneous inflammation (19), through a sympathetic neural route that is acetylcholine independent and β2-adrenergic receptor dependent (22, 23). Considered with the present findings, it appears that the CNS is positioned to rapidly and effectively modulate acute innate immune responses by innervation of immune tissues. The identities of the CNS receptor(s) for CNI-1493, and the central neural fiber tract(s) that mediate activation of the cholinergic antiinflammatory pathway are unknown. CNI-1493 is an effective inhibitor of the phosphorylation of p38 MAPK, an intracellular signal transduction mechanism that plays a critical role in the production and activity of many proinflammatory mediators in macrophages (24, 25). p38 MAPK is expressed in the brain, but it is not known whether it participates in the mediation of the systemic antiinflammatory effects of CNI-1493, α-MSH, or nonsteroidal antiinflammatory drugs (26–28).
Administration of CNI-1493 activates vagus nerve firing (10), and electrical stimulation of the distal end of transected vagus nerves recapitulates the central antiinflammatory activity of CNI-1493 (5). Here we show that electrical stimulation of intact vagus nerves also recapitulates the antiinflammatory action of CNI-1493, attenuates LPS-induced systemic TNF release, and TNF synthesis in the heart and prevents the development of endotoxin-induced shock. The protective effects of vagus nerve stimulation were partially dependent on stimulus voltage, but not nerve laterality. No significant differences were observed between left or right vagus stimulation with regard to protection against endotoxin-induced shock, demonstrating that stimulation of either vagus nerve is effective. Electrical stimulation of the vagus nerve was associated with increased heart rates that correlated to the applied voltage. Although it may seem paradoxical that vagus verve stimulation is associated with the development of tachycardia (instead of bradycardia), when the endotoxin-induced changes in heart rate are considered in the context of ongoing hypotension, the observed tachycardia actually reflects normalization of an adaptive stress response in the vagus nerve–stimulated animals.
It is well established that tissue macrophages are the primary source of increased serum TNF levels during endo-toxemia, but recent studies suggest that production of TNF by cardiac myocytes contributes to the development of myocardial depression and shock (13). TNF has been implicated in the development of cardiac failure secondary to septic cardiomyopathy, biventricular dysfunction, and pulmonary edema (29). The vagus nerve innervates the heart through two cervical branches; the right vagus regulates HR primarily through modulation of the sinoatrial node, and the left branch influences conduction primarily through the atrioventricular node. The observation that direct vagus nerve stimulation inhibits cardiac TNF raises the possibility that this strategy should be explored further for regulating cardiac TNF levels in vivo. Vagus nerve stimulation is clinically approved as therapy for epilepsy and depression and is associated with minimal morbidity. Surgically implanted vagus nerve stimulating devices are widely used, but it is not known at present whether stimulation of the intact vagus nerve in humans influences proinflammatory cytokines in liver, heart, or blood, or whether this modality can be used to treat cytokine-mediated inflammatory diseases (e.g., rheumatoid arthritis or inflammatory bowel disease).
Two major considerations are raised by this study. First, it now appears that the central neural mechanism of CNI-1493 represents a previously unrecognized method for suppressing systemic inflammation. Prior to these studies, it was certainly not anticipated that a drug which inhibits p38 MAPK signaling would be 100,000-fold more effective as an inhibitor of TNF in vivo through a distinct, neural-based mechanism. A better understanding of the underlying neural signaling pathways and associated receptors that activate the cholinergic antiinflammatory pathway may yield new insights into the design of agents that act centrally to suppress systemic inflammation. Second, electrical stimulation of the intact vagus nerve inhibits excessive systemic inflammation; this activity is both tissue specific and efficacious. Thus, the cholinergic antiinflammatory pathway is uniquely positioned to effectively counterregulate final common pathways to tissue injury which converge on the activity of proinflammatory cytokines.
Acknowledgments
The authors thank Lyudmila Borovikova for technical contributions.
This work was supported in part by National Institutes of Health grants GM62508 and GM63075.
Footnotes
Abbreviations used in this paper: α-MSH, α-melanocyte–stimulating hormone; CNS, central nervous system; HR, heart rate; i.c.v., intracerebroventricular; MABP, mean arterial blood pressure; MAPK, mitogen-activated protein kinase.
References
- 1.Tracey, K.J., B. Beutler, S.F. Lowry, J. Merryweather, S. Wolpe, I.W. Milsark, R.J. Hariri, T.J. Fahey, III, A. Zentalla, J.D. Albert, et al. 1986. Shock and tissue injury induced by recombinant human cachectin. Science. 234:470–474. [DOI] [PubMed] [Google Scholar]
- 2.Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, A. Frazier, H. Yang, S. Ivanova, L. Borovikova, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 285:248–251. [DOI] [PubMed] [Google Scholar]
- 3.Watkins, L.R., and S.F. Maier. 1999. Implications of immune-to-brain communication for sickness and pain. Proc. Natl. Acad. Sci. USA. 96:7710–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sternberg, E.M. 1997. Neural-immune interactions in health and disease. J. Clin. Invest. 100:2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovikova, L.V., S. Ivanova, M. Zhang, H. Yang, G.I. Botchkina, L.R. Watkins, H. Wang, N. Abumrad, J.W. Eaton, and K.J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:468–462. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi, M., O. Bloom, B. Sherry, J. Chesney, P. Cohen, P. Ulrich, T. Raabe, M. Bukrinsky, A. Cerami, and K.J. Tracey. 1996. Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone. J. Exp. Med. 83:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, H., M. Zhang, M. Bianchi, B. Sherry, A. Sama, and K.J. Tracey. 1988. Fetuin (α-2-HS-Glycoprotein) opsonizes cationic macrophage-deactivating molecules. Proc. Natl. Acad. Sci. USA. 95:14429–14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracey, K.J. 1998. Suppression of TNF and other proinflammatory cytokines by the tetravalent guanylhydrazone CNI-1493. Prog. Clin. Biol. Res. 397:335–343. [PubMed] [Google Scholar]
- 9.Meistrell, M.E. III, G.I. Botchkina, H. Wang, E. DeSanto, K.M. Cockroft, O. Bloom, J.M. Vishnubhakat, P. Ghezzi, and K.J. Tracey. 1997. TNF is a brain-damaging cytokine in cerebral ischemia. Shock. 8:341–348. [PubMed] [Google Scholar]
- 10.Borovikova, L.V., S. Ivanova, D. Nardi, M. Zhang, H. Yang, M. Ombrellino, and K.J. Tracey. 2000. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci. 85:141–147. [DOI] [PubMed] [Google Scholar]
- 11.De Giorgio, C.M., S.C. Schachter, A. Handforth, M. Salinsky, J. Thompson, B. Uthman, R. Reed, S. Collins, E. Tecoma, G.L. Morris, et al. 2000. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 41:1195–2000. [DOI] [PubMed] [Google Scholar]
- 12.Song, Y., L. Ao, C.D. Raeburn, C.M. Calkins, E. Abraham, A.H. Harken, and X. Meng. 2001. A low level of TNF-α mediates hemorrhage-induced acute lung injury via p55 TNF receptor. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L677–L684. [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt, B., S.B. Kribbs, F.J. Clubb, Jr., L.H. Michael, V.V. Didenko, P.J. Hornsby, Y. Seta, H. Oral, F.G. Spinale, and D.L. Mann. 1998. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 97:1382–1391. [DOI] [PubMed] [Google Scholar]
- 14.Villa, P., C. Meazza, M. Sironi, M. Bianchi, P. Ulrich, G. Botchkina, K.J. Tracey, and P. Ghezzi. 1997. Inhibition of multiple proinflammatory mediators (TNF, IL-6 and NO) abrogate lethality in a murine mode of polymicrobial sepsis. J. Endotoxin Research. 4:197–204. [Google Scholar]
- 15.Martiney, J.A., A.J. Rajan, P.C. Charles, A. Cerami, P.C. Ulrich, S. MacPhail, K.J. Tracey, C.F. Brosnan. 1998. Prevention and treatment of experimental autoimmune encephalomyelitis by CNI-1493, a macrophage deactivating agent. J. Immunol. 160:5588–5595. [PubMed] [Google Scholar]
- 16.Akerlund, K., H. Erlandsson-Harris, K.J. Tracey, H. Wang, T. Fehniger, L. Klareskog, J. Andersson, and U. Andersson. 1999. Anti-inflammatory effects of a new TNFα inhibitor (CNI-1493) in collagen-induced arthritis in rats. J. Clin. Exp. Immunol. 115:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins, M.B., B. Redman, J. Mier, J. Gollob, J. Weber, J. Sosman, B.L. MacPherson, and T. Plasse. 2001. A phase I study of CNI-1493, an inhibitor of cytokine release, in combination with high-dose interleukin-2 in patients with renal cancer and melanoma. Clin. Cancer Res. 7:486–492. [PubMed] [Google Scholar]
- 18.Delgado Hernandez, R., M.T. Demitri, A. Carlin, C. Meazza, P. Villa, P. Ghezzi, J.M. Lipton, A. Catania. 1999. Inhibition of systemic inflammation by central action of the neuropeptide α-melanocyte stimulating hormone. Neuroimmunomodulation. 6:187–92. [DOI] [PubMed] [Google Scholar]
- 19.Catania, A., J. Arnold, A. Macaluso, M.E. Hiltz, and J.M. Lipton. 1991. Inhibition of acute inflammation in the periphery by central action of salicylates. Proc. Natl. Acad. Sci. USA. 88:8544–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manna, S.K., and B.B. Aggarwal. 1998. α-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-κB activation induced by various inflammatory agents. J. Immunol. 161:2873–2880. [PubMed] [Google Scholar]
- 21.Cao, Z., M. Tanaka, C. Regnier, M. Rothe, A. Yamit-hezi, J.D. Woronicz, M.E. Fuentes, M.H. Durnin, S.A. Dalrymple, and D.V. Goeddel. 1999. NF-κB activation by tumor necrosis factor and interleukin-1. Cold Spring Harb. Symp. Quant. Biol. 64:473–483. [DOI] [PubMed] [Google Scholar]
- 22.Ceriani, G., A. Macaluso, A. Catania, and J.M. Lipton. 1994. Central neurogenic antiinflammatory action of α-MSH: modulation of peripheral inflammation induced by cytokines and other mediators of inflammation. Neuroendocrinology. 59:138–143. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso, A., D. McCoy, G. Ceriani, T. Watanabe, J. Biltz, A. Catania, and J.M. Lipton. 1994. Antiinflammatory influences of α-MSH molecules: central neurogenic and peripheral actions. J. Neurosci. 14:2377–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herlaar, E., and Z. Brown. 1999. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today. 5:439–447. [DOI] [PubMed] [Google Scholar]
- 25.Obata, T., G.E. Brown, and M.B. Yaffe. 2000. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit. Care Med. 28:N67–N77. [DOI] [PubMed] [Google Scholar]
- 26.Prasad, A.V., W.H. Pilcher, and S.A. Joseph. 1994. Nuclear factor-κB in rat brain: enhanced DNA-binding activity following convulsant-induced seizures. Neurosci. Lett. 170:145–148. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S.J., K. Drabik, N.J. Van Wagoner, S. Lee, C. Choi, Y. Dong, and E.N. Benveniste. 2000. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. J. Immunol. 165:4658–4666. [DOI] [PubMed] [Google Scholar]
- 28.Gass, P., M. Kiessling, and H. Bading. 1993. Regionally selective stimulation of mitogen activated protein (MAP) kinase tyrosine phosphorylation after generalized seizures in the rat brain. Neurosci. Lett. 162:39–42. [DOI] [PubMed] [Google Scholar]
- 29.Mann, D.L. 2001. Recent insights into the role of tumor necrosis factor in the failing heart. Heart Fail. Rev. 6:71–80. [DOI] [PubMed] [Google Scholar]