Abstract
By generating four IgG isotype-switch variants of the high affinity 34–3C anti-erythrocyte autoantibody, and comparing them to the IgG variants of the low affinity 4C8 anti-erythrocyte autoantibody that we have previously studied, we evaluated in this study how high affinity binding to erythrocytes influences the pathogenicity of each IgG isotype in relation to the respective contributions of Fcγ receptor (FcγR) and complement. The 34–3C autoantibody opsonizing extensively circulating erythrocytes efficiently activated complement in vivo (IgG2a = IgG2b > IgG3), except for the IgG1 isotype, while the 4C8 IgG autoantibody failed to activate complement. The pathogenicity of the 34–3C autoantibody of IgG2b and IgG3 isotypes was dramatically higher (>200-fold) than that of the corresponding isotypes of the 4C8 antibody. This enhanced activity was highly (IgG2b) or totally (IgG3) dependent on complement. In contrast, erythrocyte-binding affinities only played a minor role in in vivo hemolytic activities of the IgG1 and IgG2a isotypes of 34–3C and 4C8 antibodies, where complement was not or only partially involved, respectively. The remarkably different capacities of four different IgG isotypes of low and high affinity anti-erythrocyte autoantibodies to activate FcγR-bearing effector cells and complement in vivo demonstrate the role of autoantibody affinity maturation and of IgG isotype switching in autoantibody-mediated pathology.
Keywords: autoimmune hemolytic anemia, complement receptor, Fc receptor, IgG isotype, phagocytosis
Introduction
Autoantibodies are the essential factors for particular clinical manifestations associated with a number of autoimmune diseases. The pathogenic potential of autoantibodies is likely to be determined by the combined action of the self-antigen binding properties (specificity and affinity) of the Fab region and the effector functions associated with the Fc region of the different Ig isotypes. Therefore, it is conceivable that a change of Ig isotype may result in a remarkable change of the autoantibody's pathogenic potential, because Ig class switching can alter Fc-dependent effector functions and can be accompanied by concomitant changes in autoantibody affinity. To address this question, we have recently analyzed the pathogenic activity of four different IgG switch variants derived from a murine anti-RBC monoclonal autoantibody, 4C8, originally established from autoimmune-prone NZB mice (1, 2). To our surprise, despite its very poor RBC-binding activity, the IgG2a isotype of the low affinity 4C8 mAb was highly pathogenic and capable of inducing anemia at a dose comparable to that of a high affinity 34–3C IgG2a anti-RBC mAb. This high pathogenicity was apparently due to the capacity of the IgG2a isotype to interact very efficiently with the IgG Fc receptor (FcγR) involved in erythrophagocytosis, consistent with a poor pathogenicity of the three other IgG isotypes (IgG1, IgG2b, and IgG3) of the 4C8 mAb having a limited interaction with FcγR. However, it has not been explored whether high affinity anti-RBC autoantibodies of IgG1, IgG2b, and IgG3 isotypes could become more pathogenic as a result of a markedly increased RBC-binding and thereby enhanced interaction with FcγR and/or activation of complement.
Among the various effector functions mediated by the Ig heavy-chain constant regions, FcγR-mediated erythrophagocytosis has been recognized as the major pathogenic mechanism responsible for autoimmune hemolytic anemia in mice (2–5). Murine phagocytic effector cells express two different classes of phagocytic FcγR, high affinity FcγRI, and low affinity FcγRIII (for a review, see reference 6). These are hetero-oligomeric complexes, in which the respective ligand-binding α-chains are associated with the common γ-chain (FcRγ). FcRγ is required for the assembly of both FcγRI and FcγRIII and for the triggering of their various effector functions, including phagocytosis (7). Our recent analysis by the use of different strains of FcγR-deficient mice revealed that the capacity of each IgG isotype to interact with the low affinity FcγRIII is the critical factor determining the pathogenic potency of individual IgG isotypes of the 4C8 anti-RBC mAb (1, 2), as the high affinity FcγRI apparently plays a relatively limited role, probably because of the competition by circulating monomeric IgG2a.
In contrast, it has been suggested that complement activation plays a minimal role in the development of anemia induced by anti-RBC antibodies (3, 4, 8). However, the interpretation of these results was still tentative, as murine IgG2a, IgG2b, and IgG3 isotypes (but not IgG1) efficiently activate complement (9, 10). The lack of the involvement of complement in anemia induced by the low affinity 4C8 IgG switch variants could be due to a limited opsonization of RBC in mice injected with this mAb (1, 2). Earlier studies in complement-deficient guinea pigs suggested the synergistic cooperation of FcγR and CRs expressed on Kupffer cells in erythrophagocytosis of RBCs sensitized with polyclonal rabbit anti-RBC antibodies (11). Thus, the pathogenicity of high affinity anti-RBC autoantibodies of IgG2b and IgG3 isotypes, which poorly interact with FcγR, could be promoted as a result of complement activation, either by acting synergistically with FcγR or by triggering CR-dependent erythrophagocytosis.
We have generated IgG class-switch variants (IgG1, IgG2b, and IgG3) of the high affinity 34–3C IgG2a anti-RBC mAb derived from NZB mice (3) in order to better define the role of autoantibody binding affinities in relation to Fc-associated effector functions in the development of autoimmune hemolytic anemia. The level of in vivo opsonization of circulating RBCs induced by the high affinity 34–3C mAb was remarkably enhanced, compared with the low affinity 4C8 anti-RBC IgG variants having <1,000 times weaker RBC-binding activity (1). Therefore, by assessing the pathogenic effect of the 34–3C IgG variants in mice deficient in FcγR and/or C3, we should be able to study how high affinity binding to RBCs modulates the pathogenic potential of each IgG isotype, and whether the level of RBC opsonization in vivo could influence the extent of complement activation and FcγR interaction. We show in this study that the pathogenicity of the high affinity 34–3C anti-RBC mAb is markedly enhanced, compared with that of the low affinity 4C8 mAb, but in an IgG isotype-dependent manner. Remarkable and selective enhancement of the pathogenicity of the IgG2b and IgG3 isotypes (>200-fold increases) was associated with a marked activation of complement, as a result of extensive opsonization of circulating RBCs. In contrast, for the IgG1 and IgG2a isotypes, high affinity binding exhibited only a minimal effect on their hemolytic activities in vivo, which were poorly dependent on complement activation.
Materials and Methods
Mice.
BALB/c mice were purchased from Gl. Bomholtgard Ltd. FcRγ-deficient (FcRγ2/−) mice (lacking functional expression of both FcγRI and FcγRIII) with a pure C57BL/6 (B6) background, and their corresponding wild-type (WT)* littermates were developed as described previously (12). C1q- and C3-deficient mice were backcrossed for 10 and 5 generations with B6 mice, respectively (13, 14). FcRγ and C3 double-deficient mice (FcRγ/C3−/−) were obtained from intercross between FcRγ2/− and C3−/− B6 mice. Mice deficient in C3 were identified by the absence of serum C3, as determined by ELISA, and the FcRγ2/− genotype was determined by PCR analysis using a combination of the following sets of primers: WT-specific sense primer (5′-CCAACGCTATGTCCTGATAG-3′), mutant-specific sense primer (5′-TGCTGTCCTGTTTTTGTATGG-3′), and common antisense primer (5′-GCTGCCTTTCGGACCTGGAT-3′). μMT B6 mice deficient in B cells (15) were obtained from B&K Universal.
DNA Constructions.
The VDJH34–3C-Cγ1, -Cγ2b, and -Cγ3 plasmids containing the complete 34–3C IgG heavy-chain gene of the respective IgG subclass were constructed using the following DNA fragments: the rearranged VDJ (variable-diversity-joining) region isolated from cDNA encoding the variable region of the heavy chain of the 34–3C mAb, the promoter region isolated from pSV-Vμ1 (16), the heavy-chain enhancer region isolated from pSVE2-neo (17) and the Cγ1, Cγ2b, or Cγ3 region derived from the respective genomic clones, pEVHCγ1 (17), pIgH22 (18), and pJW7 (19).
mAb.
The hybridoma secreting the 34–3C IgG2a high affinity anti-RBC monoclonal autoantibody was derived from unmanipulated NZB mice (3). The 34–3C IgG1, IgG2b, and IgG3 class-switch variants were obtained by transfecting 34–3C heavy-chain-loss mutant cells by electroporation with the respective VDJH34–3C-Cγ plasmids together with a pSVE2-neo plasmid containing the neomycin-resistant gene, as described previously (1). IgG mAb were purified from culture supernatants by protein G column chromatography. The purity of IgG was >90% as documented by SDS/PAGE. The 34–3C IgG class-switch variants exhibited comparable mouse RBC-binding activity in vitro, as assessed by a flow cytometric analysis (1). The generation of IgG switch variants of the 4C8 low affinity anti-RBC mAb was described previously (1, 2).
Detection of C3 in Sera and of Opsonized RBCs in the Circulating Blood.
The presence or absence of C3 in sera from mice were determined by ELISA. Briefly, IgG goat anti–mouse C3 antibodies (Capell Laboratories) were used for coating microtiter plates, and incubated overnight at 4°C with serum samples at a dilution of 1/1,000. Then, the assay was developed with alkaline phosphatase-labeled goat anti–mouse C3 conjugates. The presence of opsonized RBCs in mice injected with 34–3C or 4C8 anti-RBC mAb was detected by a similar flow cytometric assay, using biotinylated goat anti–mouse C3 or rat anti–mouse k chain mAb (H139.52.1.5), followed by PE-conjugated streptavidin, as described (1). The specificity of the assay for C3 opsonization was confirmed by the absence of staining on circulating RBCs in C3−/− mice.
Experimental Autoimmune Hemolytic Anemia.
Autoimmune hemolytic anemia was induced by a single intraperitoneal injection of purified anti-RBC mAb into two to three mo-old mice. Blood samples were collected into heparinized microhematocrit tubes every 2 d after the injection, and hematocrits (Hts) were directly determined after centrifugation, as described previously (2). The injection of mAb was controlled 24 h later by assessing the level of antibody opsonization of circulating RBCs by a flow cytometric analysis using biotinylated rat anti–mouse k chain mAb. Livers were obtained 8 d after injection of mAb, processed for histological examination, and stained with hematoxylin and eosin. The extent of in vivo RBC destruction by Kupffer cell-mediated phagocytosis was determined by Perls iron staining.
Statistical Analysis.
Statistical analysis was performed with the Wilcoxon two-sample test. Probability values <5% were considered significant.
Results
Efficient Activation of Complement by the High Affinity 34–3C IgG Class-switch Variants, but Not by the Low Affinity 4C8 IgG Variants.
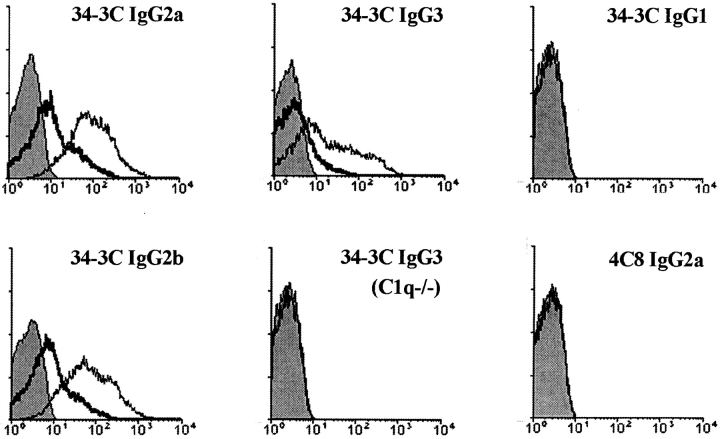
To assess the ability of individual IgG isotypes of the 34–3C anti-RBC mAb to activate complement in vivo, we analyzed by flow cytometry the extent of C3 deposition on circulating RBCs 24 h after a single intraperitoneal injection of 50 or 200 μg of purified mAb into BALB/c mice. The highest and comparable levels of C3 opsonization were observed in mice injected with the IgG2a and IgG2b isotypes; the IgG3 isotype was less efficient (Fig. 1) . As expected, no significant C3 opsonization was observed in mice receiving the IgG1 isotype, which is known not to activate complement efficiently (9, 10). As it has been claimed that murine IgG3 isotype failed to activate the classical pathway of complement (20), the levels of C3 opsonization were evaluated in C1q-deficient B6 mice. When these mice were injected with 200 μg of the 34–3C IgG3 variant, C3-opsonized RBCs were no longer detectable in the blood (Fig. 1), indicating the activation of the classical complement pathway after the binding of the 34–3C IgG3 mAb to RBCs. In contrast, the injection of the low affinity 4C8 IgG variants of any isotype failed to induce a significant C3 opsonization on circulating RBCs (Fig. 1).
Figure 1.
Flow cytometric analysis of complement activation in vivo by the 34–3C and 4C8 IgG class-switch variants. Mouse RBCs were obtained 24 h after an intraperitoneal injection of 50 or 200 μg of 34–3C or 4C8 IgG variants into mice, and then stained with biotinylated goat anti–mouse C3 antibodies, followed by PE-conjugated streptavidin. Results obtained with 50 μg (thick lines) and 200 μg (thin lines) of the 34–3C IgG2a, IgG2b, and IgG3 variants in BALB/c mice, 200 μg of the 34–3C IgG3 variant in C1q−/− mice, and 200 μg of the 34–3C IgG1 or 4C8 IgG2a variant in BALB/c mice are shown. Shaded areas indicate the background staining with PE-conjugated streptavidin.
Markedly Enhanced Pathogenicity of IgG2b and IgG3, but not IgG1 and IgG2a Isotypes of the High Affinity 34–3C Anti-RBC mAb, Compared with the Low Affinity 4C8 IgG Variants.
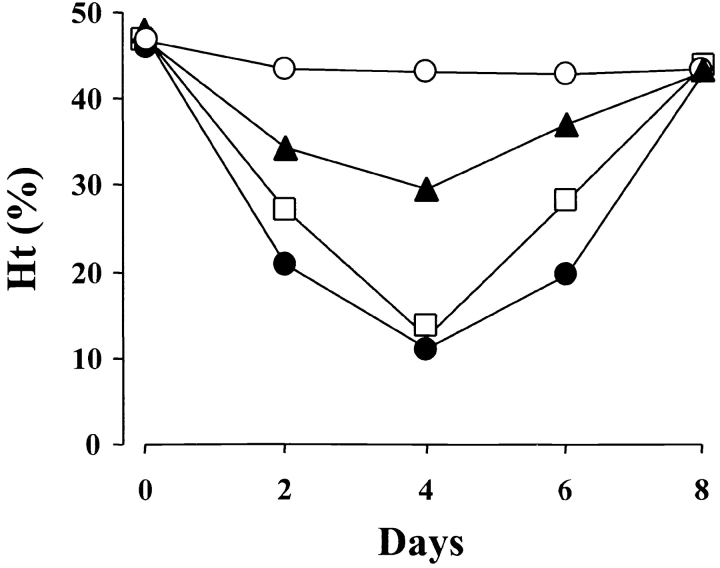
The pathogenic activity of individual IgG isotypes of the 34–3C mAb was analyzed by a single intraperitoneal injection of 200 μg of purified mAb into BALB/c mice. The IgG2a and IgG2b isotypes of the 34–3C mAb induced the most severe form of anemia (a decrease in mean Ht values to 10–12% at day 4), the IgG3 variant a mild anemia (average Ht of 30%), and the IgG1 variant was unable to significantly decrease Ht levels (Fig. 2) . Notably, the extent of opsonization of circulating RBCs 24 h after mAb injection was comparable in mice treated with different IgG variants, as revealed by rat anti–mouse k chain mAb (data not shown).
Figure 2.
Development of anemia induced by the 34–3C IgG class-switch variants in mice. BALB/c mice were injected intraperitoneally with 200 μg of purified 34–3C IgG variants (IgG1, ○; IgG2a, •; IgG2b, □; IgG3, ▴) on day 0. Results are expressed as mean Ht values of five mice.
To compare more quantitatively the pathogenic activity of individual IgG isotypes of the 34–3C mAb, various amounts of mAb were intraperitoneally injected into BALB/c mice and the quantities of mAb required to induce anemia (decreasing Ht values to <40%) were estimated. Mild anemia was induced by the injection of 25 μg of the 34–3C IgG2a and IgG2b mAb, while 100 μg of the IgG3 isotype and 500 μg of the IgG1 isotype were required to provoke a drop of Ht values below 40% (Table I). Thus, the pathogenic potency of the 34–3C IgG2a and IgG2b isotypes was approximately 4- and 20-fold higher than that of the IgG3 and IgG1 isotypes, respectively. Histological examinations showed that the extent of erythrophagocytosis, documented by iron deposits in hepatic Kupffer cells, correlated in all these cases with the level of anemia (data not shown).
Table I.
Estimation of Quantities of the High Affinity 34–3C and Low Affinity 4C8 IgG Class-switch Variants Required for Inducing Mild and Severe Anemia
| 34-3C mAb
|
4C8 mAb
|
|||
|---|---|---|---|---|
| Isotype | Mild anemiaa |
Severe anemiab |
Mild anemiaa |
Severe anemiab |
| IgG1 | 500 μg | NDc | 1 mg | ND |
| IgG2a | 25 μg | 100 μg | 50 μg | 1 mg |
| IgG2b | 25 μg | 100 μg | >5 mg | ND |
| IgG3 | 100 μg | ND | Not pathogenic | |
The quantity of the 34-3C and 4C8 IgG variants required for inducing mild anemia (decreasing Ht values to <40%) in BALB/c mice.
The quantity of the 34-3C and 4C8 IgG variants required for inducing severe anemia (causing a 50% decrease in Ht values) in BALB/c mice.
Not done.
When the pathogenic activity of the high affinity 34–3C IgG variants was compared with that of the low affinity 4C8 IgG variants (2), the ability to induce mild anemia was dramatically enhanced (200-fold and even more) in the 34–3C IgG2b and IgG3 isotypes (Table I). In contrast, the minimal amount of the IgG1 and IgG2a isotypes required for the induction of mild anemia was almost comparable between the 34–3C and 4C8 mAb. However, for the induction of a severe form of anemia (causing a 50% decrease in Ht values), 10 times lesser amounts of the 34–3C IgG2a mAb (100 μg) were sufficient, compared with the 4C8 IgG2a mAb (1 mg), indicating that the high affinity 34–3C IgG2a mAb became more pathogenic at higher doses than the low affinity 4C8 IgG2a mAb.
Major Role of FcγR and Secondary Role of Complement in the Development of Anemia Induced by 34–3C IgG2a Anti-RBC mAb.
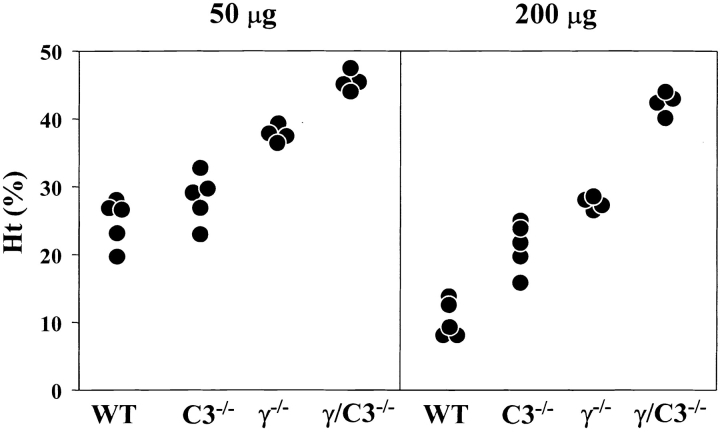
As the 34–3C IgG2a mAb efficiently activated complement in vivo, we reevaluated the respective contributions of FcγR and complement to the anemia induced by two different doses (50 and 200 μg) of the 34–3C IgG2a mAb in B6 mice deficient in FcRγ (i.e. lacking functional expression of both phagocytic FcγRI and FcγRIII) and/or C3. The development of anemia occurring in WT mice injected with 50 μg of the 34–3C IgG2a mAb was almost completely prevented in FcRγ2/− mice (P < 0.01), while no significant protection was observed in C3−/− mice (P > 0.05; Fig. 3 and Table II). Histological analysis still revealed the presence of substantial iron deposits in Kupffer cells in FcRγ2/− mice receiving the 34–3C IgG2a mAb. However, such deposits were no longer detectable in 34–3C IgG2a mAb-injected FcRγ/C3−/− mice. In contrast, the level of protection from anemia provoked by the injection of 200 μg mAb was only moderate in FcRγ2/− mice, while a lower but significant level of protection was observed in C3−/− mice (P < 0.005; Fig. 3 and Table II). Notably, the development of anemia was completely prevented in FcRγ/C3−/− mice injected with this high dose of the 34–3C IgG2a mAb (P < 0.01). These data indicated a minor but still significant contribution of CR-mediated erythrophagocytosis to the development of the severe form of anemia induced by a high dose (200 μg) of the 34–3C IgG2a mAb.
Figure 3.
Development of anemia in WT, C3−/−, FcRγ2/−, or FcRγ/C3−/− mice after the injection of the 34–3C IgG2a mAb. Ht values of individual mice measured 4 d after the intraperitoneal injection of 50 or 200 μg of the mAb are shown.
Table II.
Pathogenic Activities of the 34–3C IgG Class-switch Variants in WT, C3−/−, FcRγ−/−, and FcRγ/C3−/− B6 Mice
| Ht (%)a
|
In vivo erythrophagocytosisb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Isotype | Dose | WT | C3−/− | FcRγ−/− | FcRγ/C3−/− | WT | C3−/− | FcRγ−/− | FcRγ/C3−/− |
| IgG2a | 50 μg | 25 ± 3 | 28 ± 4 | 39 ± 1 | 46 ± 2 | ++ | ++ | + | − |
| 200 μg | 10 ± 3 | 21 ± 4 | 28 ± 1 | 43 ± 2 | +++ | ++/+++ | ++ | − | |
| IgG2b | 50 μg | 28 ± 2 | 38 ± 3 | 44 ± 1 | NDc | +/++ | ± | − | ND |
| 200 μg | 12 ± 3 | 36 ± 3 | 36 ± 1 | 45 ± 2 | +++ | +/++ | +/++ | − | |
| IgG1 | 500 μg | 37 ± 1 | 38 ± 2 | 46 ± 2 | ND | + | + | − | ND |
| IgG3 | 200 μg | 30 ± 3 | 40 ± 1 | 30 ± 3 | ND | + | − | + | ND |
Ht values (mean of 4–5 mice ± 1SD) were determined 4 d after the intraperitoneal injection of purified 34–3C IgG variants (data from Figs. 3, 4, and 5). Ht values before the injection of anti-RBC mAb in WT, C3−/−, FcRγ−/−, and FcRγ/C3−/− mice were in a range from 44–48%.
The extent of erythrophagocytosis by Kupffer cells in the liver is arbitrarily graded, in a blinded fashion, on the basis of the intensity of Perls iron staining on liver sections.
Not done.
Comparable Contributions of FcγR and Complement to the Development of Anemia Induced by 34–3C IgG2b Anti-RBC mAb.
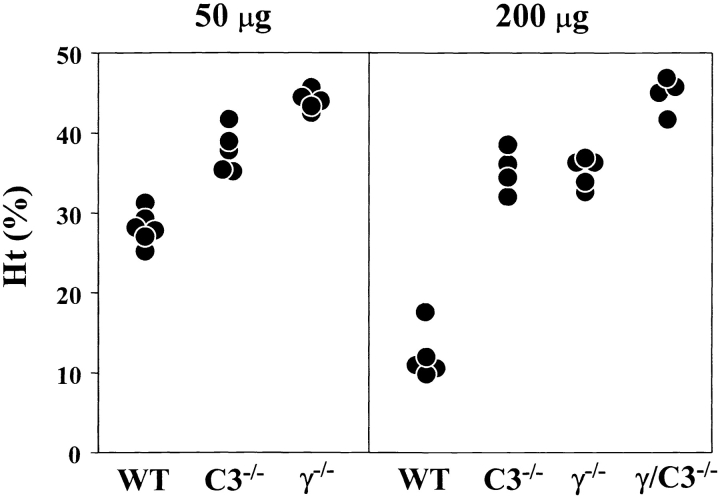
We similarly evaluated the respective roles of FcγR and complement in the development of anemia induced by the 34–3C IgG2b mAb. This antibody is able to activate complement as strongly as the IgG2a isotype, but interacts less efficiently with FcγR (2). The development of anemia occurring in WT B6 mice injected with 50 μg of the 34–3C IgG2b mAb was completely prevented in FcRγ2/− B6 mice (P < 0.005; Fig. 4 and Table II). Histological analysis confirmed the absence of erythrophagocytosis, as documented by the lack of iron deposits in Kupffer cells. However, unlike after injection of the 34–3C IgG2a mAb, the development of anemia was also markedly prevented in C3−/− mice (P < 0.005), which exhibited only a limited extent of erythrophagocytosis. At a highly pathogenic dose (200 μg) of the 34–3C IgG2b mAb, the development of anemia was strongly, though not completely, inhibited in both FcRγ2/− and C3−/− mice (P < 0.005; Fig. 4 and Table II). As in the case of the IgG2a isotype, FcRγ/C3−/− mice were totally resistant to the pathogenic effect of 200 μg of the 34–3C IgG2b mAb (P < 0.01), and did not show any significant iron deposits in Kupffer cells.
Figure 4.
Development of anemia in WT, C3−/−, FcRγ2/−, or FcRγ/C3−/− mice after the injection of the 34–3C IgG2b variant. Ht values of individual mice measured 4 d after the intraperitoneal injection of 50 or 200 μg of the mAb are shown.
Differential Role of FcγR and Complement in the Development of Anemia Induced by 34–3C IgG1 and IgG3 Anti-RBC mAb.
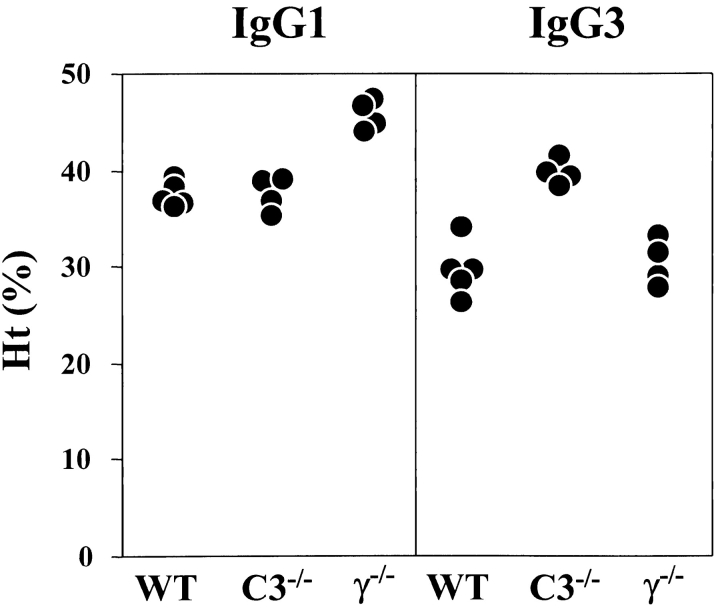
Using high affinity 105–2H IgG1 and low affinity 4C8 IgG1 anti-RBC mAb derived from NZB mice, it has been well established that FcγRIII is the sole FcγR mediating IgG1-dependent phagocytosis in vivo (2, 5, 21). In agreement with these prior observations, FcRγ2/− mice were completely resistant to the pathogenic effect of the 34–3C IgG1 variant (P < 0.01), and failed to show any sign of erythrophagocytosis (Fig. 5 and Table II). Notably, the development of anemia was not prevented in C3−/− mice after the injection of the 34–3C IgG1 isotype.
Figure 5.
Development of anemia in WT, C3−/−, or FcRγ2/− mice after the injection of 34–3C IgG1 or IgG3 variant. Ht values of individual mice measured 4 d after the intraperitoneal injection of 500 μg of 34–3C IgG1 or 200 μg of 34–3C IgG3 mAb are shown.
Unlike the IgG1, IgG2a, and IgG2b isotypes of the 34–3C mAb, FcRγ-deficient mice were not resistant at all to the pathogenic effect of 200 μg of the 34–3C IgG3 variant (Fig. 5 and Table II). In contrast, the development of anemia was completely prevented in C3−/− mice (P < 0.01), in which erythrophagocytosis by Kupffer cells was no longer detectable.
A recent in vitro study has reported that polymeric IgG3 is capable of interacting with FcγRI (22). Thus, an only limited usage of FcγRI in vivo for phagocytosis of 34–3C IgG3-opsonized RBCs could be due to competition with excess amounts of circulating monomeric IgG2a having a high affinity interaction with FcγRI (23). If this were the case, one could expect an enhancement of the pathogenic effect of the 34–3C IgG3 mAb in Ig-deficient μMT B6 mice because of the absence of competition with IgG2a. However, after injection of 50 μg of the 34–3C IgG3 mAb, μMT mice failed to show significant drops in Ht values and increased sign of erythrophagocytosis, compared with WT mice (mean Ht values of four mice 4 d after the injection: μMT mice, 46.9 ± 1.3%; WT mice, 45.5 ± 0.5%).
Discussion
We have generated three IgG class-switch variants bearing identical VH and Vk regions of a high affinity 34–3C IgG2a anti-RBC autoantibody derived from lupus-prone NZB mice, and determined how a high binding affinity to circulating RBCs influenced the pathogenicity of individual IgG isotypes in relation to the respective contributions of FcγR and complement. Strikingly, the pattern of the IgG isotype-dependent pathogenicity of the 34–3C mAb (IgG2a = IgG2b > IgG3 > IgG1) was totally different from that of the low affinity 4C8 mAb (IgG2a > IgG1 > IgG2b > IgG3) (2). The combined analysis in mice deficient in FcRγ or C3 revealed that this difference was primarily due to a marked enhancement of the pathogenic effect of the IgG2b and IgG3 isotypes (>200-fold increases) as a result of complement activation, which not only triggered CR-dependent erythrophagocytosis but also promoted FcγR-mediated erythrophagocytosis through synergistic cooperation with CR. In contrast, a high affinity binding to RBCs barely augmented in vivo hemolytic activities of the IgG1 and IgG2a isotypes, in which complement played no or only a minimal role. Our data thus define the role of autoantibody affinity maturation and IgG isotype switching in relation to the activation of FcγR and complement in vivo in the pathogenesis of autoimmune hemolytic anemia.
The analysis of IgG switch variants of the high affinity 34–3C mAb has clarified the issue concerning the role of complement in the development of autoimmune hemolytic anemia (Table III). As already described previously (2, 4, 5), FcγR-mediated erythrophagocytosis was the major mechanism for the induction of anemia induced by the IgG2a isotype, which most efficiently interacts with both classes of phagocytic FcγR, FcγRI, and FcγRIII (2). C3 deficiency only minimally affected the development of mild anemia at a low dose (50 μg) of this isotype, despite its efficient activation of complement. This indicates that CR-mediated erythrophagocytosis apparently requires an extensive opsonization of C3 fragments on RBCs. On the contrary, a limited opsonization with IgG2a antibodies is sufficient to trigger FcγR-dependent erythrophagocytosis, as observed in mice injected with the low affinity 4C8 IgG2a mAb, which very poorly opsonizes circulating RBCs (1). Notably, CR-dependent erythrophagocytosis significantly contributed to the development of the severe form of anemia induced by a high dose (200 μg) of the 34–3C IgG2a mAb. Thus, differential contribution of complement to the development of anemia induced by low and high doses of the high affinity 34–3C IgG2a mAb would explain why the 34–3C mAb became more pathogenic only at higher doses, but not at lower doses, compared with the low affinity 4C8 mAb, which poorly activated complement in vivo. The latter observation also indicates that relatively high density of IgG is required for efficient binding and activation of C1. Thus, complement activation by the classical pathway could be markedly influenced by antibody affinity, in addition to the density and distribution of epitope, which is responsible for creating an appropriate angle of the Fab arms of IgG required for C1 activation (24, 25).
Table III.
Pathogenic Activities of the 34–3C IgG Class-switch Variants and Respective Contributions of FcγR and Complement to the 34–3C IgG-induced Anemia
| Isotype | Pathogenicity | Effector functions |
|---|---|---|
| IgG2a | +++ | FcγR > complement |
| IgG2b | +++ | FcγR and complement |
| IgG3 | ++ | Complement |
| IgG1 | + | FcγR |
In contrast to the IgG2a isotype, both complement and FcγR contributed almost equally well to the development of anemia induced by the IgG2b isotype (Table III). However, the role of complement is apparently different between anemia induced at low and high doses of this isotype. The development of mild anemia provoked by a low dose (50 μg) was markedly prevented in C3−/− mice, but completely prevented in FcγR-deficient mice. This last observation suggests that FcγR is the sole receptor involved in erythrophagocytosis, but that, because of a very weak affinity of FcγR to the IgG2b isotype (2), moderately opsonized RBCs require an additional involvement of complement to trigger optimally FcγR-dependent phagocytosis. This interpretation is consistent with previous reports suggesting a synergistic cooperation of CR and FcγR for phagocytosis in vivo and in vitro (11, 26, 27). However, this was no longer the case for severe anemia after injection of a high dose (200 μg) of the IgG2b isotype, as anemia developed in mice deficient in either FcγR or C3, but not in those deficient in both FcγR and C3. It is possible that the extensive opsonization resulting from the injection of this high dose could not only overcome the low avidity interaction between FcγR and IgG2b isotype to provoke FcγR-mediated phagocytosis, independently of complement activation, but also trigger CR-mediated erythrophagocytosis. Our data thus indicate a critical role for the autoantibody affinity in the pathogenic activity of the IgG2b isotype by promoting IgG Fc-associated effector functions.
As expected from our previous studies (2, 5), the 34–3C IgG1 isotype triggered erythrophagocytosis only by activating FcγR, but not complement (Table III). It is somehow surprising to see that the pathogenic potential of the high affinity 34–3C IgG1 mAb was poor and almost comparable to that of the low affinity 4C8 IgG1 variant. This is likely to result from the lack of complement activation, which is, in contrast, partly responsible for the markedly enhanced pathogenicity of the IgG2b isotype of the high affinity 34–3C mAb, as discussed above. Another important difference is that the IgG1 isotype interacts only with FcγRIII (2, 5, 21), while the IgG2b isotype is apparently able to interact not only with FcγRIII but also with another phagocytic receptor, FcγRI (2). In this respect, we recently observed that the development of anemia triggered by the 34–3C IgG2b variant was significantly inhibited in FcγRI-deficient mice as well as in FcγRIII-deficient mice (unpublished data). Accordingly, RBCs highly opsonized with 34–3C IgG2b mAb could interact with three different receptors, FcγRI, FcγRIII, and CR, thereby potentiating erythrophagocytosis through synergistic cooperation among these three receptors.
In marked contrast to the three other IgG isotypes, the development of anemia provoked by the high affinity 34–3C IgG3 variant was totally dependent on complement activation, and this isotype apparently fails to interact with phagocytic FcγR (Table III). There has been a controversy about the presence of any receptor for murine IgG3 isotype. Diamond and Yelton have proposed the presence of an IgG3-specific phagocytic FcγR expressed on macrophages (28). This has also been supported by a recent study, demonstrating the possible involvement of nonconventional FcγR in the phagocytosis of IgG3-opsonized cryptococci by peritoneal macrophages in vitro (29). In contrast, using bone marrow–derived macrophages from FcγRI-deficient mice, FcγRI has been claimed to be the sole receptor for the IgG3 isotype (22). The present results showing that Kupffer cell–mediated phagocytosis of RBCs highly opsonized with the 34–3C IgG3 mAb is not inhibited in FcRγ-deficient mice, but completely abrogated in C3-deficient mice strongly argue not only against any significant affinity of conventional FcγR to murine IgG3 isotype in vivo, but also against the presence of a novel IgG3-specific FcγR mediating phagocytosis. The absence of interaction of 34–3C IgG3-opsonized RBCs with FcγRI in vivo could be explained by competition with circulating monomeric IgG2a having a high affinity interaction with FcγRI (23). However, the lack of enhanced erythrophagocytosis by Kupffer cells in Ig-deficient μMT mice after injection of the 34–3C IgG3 mAb argues against this hypothesis.
The use of four different anti-RBC IgG switch variants of the high affinity 34–3C and the low affinity 4C8 mAb in this and previous studies (2) has provided a unique opportunity to define the pathogenic potency of individual murine IgG isotypes in relation to autoantibody affinity and to IgG Fc-dependent effector functions in the development of autoimmune hemolytic anemia. Taken together, our studies have demonstrated the importance of the autoantigen-binding affinity for the pathogenicity of certain IgG isotypes (IgG2b and IgG3) by promoting the activation of complement and/or FcγR-bearing effector cells. Studies with an IgG2a anti-platelet monoclonal autoantibody have shown a minimal role of complement in immune elimination of platelets (4, 8, 30); however, more extensive analysis with other IgG isotypes of anti-platelet autoantibodies is awaited to elucidate the potential role of complement in immune thrombocytopenic purpura. Furthermore, it should also be stressed that, in addition to the role of FcγR in activation of inflammatory effector cells (12, 31, 32), complement is apparently required for the full-blown expression of immune complex–mediated inflammatory reactions in several experimental models including Arthus reaction, immune complex alveolitis, and anti-glomerular basement membrane nephritis (33–37). Thus, complement is actively implicated in autoantibody- and immune complex–triggered inflammatory cascades, in addition to its essential role in innate immune responses (14, 38, 39). Given the role of FcγR and complement in phagocytosis, cellular cytotoxicity, and inflammatory reactions, further analysis of autoantibody-triggered cellular and tissue injuries in relation to the respective roles of FcγR and complement would help establish new strategies for the development of therapeutic approaches in autoantibody- and immune complex–mediated inflammatory disorders.
Acknowledgments
We thank Dr. P. Vassalli for his critical reading of the manuscript, and Ms. G. Leyvraz, Ms. G. Lange, Mr. G. Brighouse, and Mr. G. Celetta for their excellent technical assistance.
This work was supported by grants from the Swiss National Foundation for Scientific Research, and La Fondation Dr. Dubois-Ferrière – Dinu Lipatti.
Footnotes
Abbreviations used in this paper: Ht, hematocrit; WT, wild-type.
References
- 1.Fossati-Jimack, L., L. Reininger, Y. Chicheportiche, R. Clynes, J.V. Ravetch, T. Honjo, and S. Izui. 1999. High pathogenic potential of low-affinity autoantibodies in experimental autoimmune hemolytic anemia. J. Exp. Med. 190:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fossati-Jimack, L., A. Ioan-Facsinay, L. Reininger, Y. Chicheportiche, N. Watanabe, T. Saito, F.M.A. Hofhuis, J.E. Gessner, C. Schiller, R.E. Schmidt, et al. 2000. Markedly different pathogenicity of four IgG isotype-switch variants of an anti-erythrocyte autoantibody is based on their respective capacity to interact in vivo with the low-affinity FcγRIII. J. Exp. Med. 191:1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibata, T., T. Berney, L. Reininger, Y. Chicheportiche, S. Ozaki, T. Shirai, and S. Izui. 1990. Monoclonal anti-erythrocyte autoantibodies derived from NZB mice cause autoimmune hemolytic anemia by two distinct pathogenic mechanisms. Int. Immunol. 2:1133–1141. [DOI] [PubMed] [Google Scholar]
- 4.Clynes, R., and J.V. Ravetch. 1995. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 3:21–26. [DOI] [PubMed] [Google Scholar]
- 5.Meyer, D., C. Schiller, J. Westermann, S. Izui, W.L.W. Hazenbos, J.S. Verbeek, R.E. Schmidt, and J.E. Gessner. 1998. FcγRIII (CD16)-deficient mice show IgG isotope-dependent protection to experimental autoimmune hemolytic anemia. Blood. 92:3997–4002. [PubMed] [Google Scholar]
- 6.Ravetch, J.V. 1994. Fc receptors: rubor redux. Cell. 78:553–560. [DOI] [PubMed] [Google Scholar]
- 7.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J.V. Ravetch. 1994. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell. 76:519–529. [DOI] [PubMed] [Google Scholar]
- 8.Sylvestre, D.L., R. Clynes, M. Ma, H. Warren, M.C. Carroll, and J.V. Ravetch. 1996. Immunoglobulin G-mediated inflammatory responses develop normally in complement deficient mice. J. Exp. Med. 184:2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaus, G.G., M.B. Pepys, K. Kitajima, and B.A. Askonas. 1979. Activation of mouse complement by different classes of mouse antibody. Immunology. 38:687–695. [PMC free article] [PubMed] [Google Scholar]
- 10.Neuberger, M.S., and K. Rajewsky. 1981. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 11:1012–1016. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber, A.D., and M.M. Frank. 1972. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J. Clin. Invest. 51:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park, S.Y., S. Ueda, H. Ohno, Y. Hamano, M. Tanaka, T. Shiratori, T. Yamazaki, H. Arase, N. Arase, A. Karasawa, et al. 1998. Resistance of Fc receptor-deficient mice to fatal glomerulonephritis. J. Clin. Invest. 102:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. [DOI] [PubMed] [Google Scholar]
- 14.Wessels, M.R., P. Butko, M. Ma, H.B. Warren, A.L. Lage, and M.C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA. 92:11490–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura, D., J. Roses, R. Kühn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 350:423–427. [DOI] [PubMed] [Google Scholar]
- 16.Neuberger, M.S. 1983. Expression and regulation of immunoglobulin heavy gene transfected into lymphoid cells. EMBO J. 2:1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon, T., and K. Rajewsky. 1988. “Enhancer-constitutive” vectors for the expression of recombinant antibodies. Nucleic Acids Res. 16:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamawaki-Kataoka, Y., T. Kataoka, N. Takahashi, M. Obata, and T. Honjo. 1980. Complete nucleotide sequence of immunoglobulin γ2b chain gene cloned from newborn mouse DNA. Nature. 283:786–789. [DOI] [PubMed] [Google Scholar]
- 19.Wels, J.A., C.J. Word, D. Rimm, G.P. Der-Balan, H.M. Martinez, P.W. Tucker, and F.R. Blattner. 1984. Structural analysis of the murine IgG3 constant region gene. EMBO J. 3:2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grey, H.M., J.W. Hirst, and M. Cohn. 1971. A new mouse immunoglobulin: IgG3. J. Exp. Med. 133:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazenbos, W.L.W., I.A.F.M. Heijnen, D. Meyer, F.M.A. Hofhuis, C.R. de Lavalette, R.E. Schmidt, P.J.A. Capel, J.G.J. Van de Winkel, J.E. Gessner, T.K. van den Berg, and J.S. Verbeek. 1998. Murine IgG1 complexes trigger immune effector functions predominantly via FcγRIII (CD16). J. Immunol. 161:3026–3032. [PubMed] [Google Scholar]
- 22.Gavin, A.L., N. Barnes, H.M. Dijstelbloem, and P.M. Hogarth. 1998. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J. Immunol. 160:20–23. [PubMed] [Google Scholar]
- 23.Unkeless, J.C., and H.N. Eisen. 1975. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J. Exp. Med. 142:1520–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borson, T., and A. Circolo. 1983. Binding and activation of C1 by cell bound IgG: activation depends on cell surface hapten density. Mol. Immunol. 20:433–438. [DOI] [PubMed] [Google Scholar]
- 25.Kratz, H.J., and H. Isliker. 1985. Mouse monoclonal antibodies at the red cell surface. II. Effect of hapten density on complement fixation and activation. Mol. Immunol. 22:229–235. [DOI] [PubMed] [Google Scholar]
- 26.Brown, E.J., J.F. Bohnsack, and H.D. Gresham. 1988. Mechanism of inhibition of immunoglobulin G-mediated phagocytosis by monoclonal antibodies that recognize the Mac-1 antigen. J. Clin. Invest. 81:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, M.J., and E.J. Brown. 1994. CR3 and FcγRIII cooperate in generation of a neutrophil respiratory burst: requirement for FcγRII and tyrosine phosphorylation. J. Cell Biol. 125:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond, B., and D.E. Yelton. 1981. A new Fc receptor on mouse macrophages binding IgG3. J. Exp. Med. 153:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan, R., R. Clynes, J.V. Ravetch, and M.D. Scharff. 1998. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J. Exp. Med. 187:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuelsson, A., T.L. Towers, and J.V. Ravetch. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory receptor. Science. 291:484–486. [DOI] [PubMed] [Google Scholar]
- 31.Sylvestre, D.L., and J.V. Ravetch. 1994. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 265:1095–1098. [DOI] [PubMed] [Google Scholar]
- 32.Clynes, R., J.S. Maizes, R. Guinamard, M. Ono, T. Takai, and J.V. Ravetch. 1999. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 189:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Höpken, U.E., B. Lu, N.P. Gerard, and C. Gerard. 1997. Impaired inflammatory responses in the reverse Arthus reaction through genetic deletion of the C5a receptor. J. Exp. Med. 186:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazenbos, W.L.W., J.E. Gessner, F.M.A. Hofhuis, H. Kuipers, D. Meyer, I.A.F.M. Heijnen, R.E. Schmidt, M. Sandor, P.J.A. Capel, M. Daëron, et al. 1996. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcγRIII (CD16) deficient mice. Immunity. 5:181–188. [DOI] [PubMed] [Google Scholar]
- 35.Baumann, U., J. Köhl, T. Tschernig, K. Schwerter-Strumpf, J.S. Verbeek, R.E. Schmidt, and J.E. Gessner. 2000. A codominant role of FcγRI/III and C5aR in the reverse Arthus reaction. J. Immunol. 164:1065–1070. [DOI] [PubMed] [Google Scholar]
- 36.Bozic, C.R., B. Lu, U.E. Höpken, C. Gerard, and N.P. Gerard. 1996. Neurogenic amplification of immune complex inflammation. Science. 273:1722–1725. [DOI] [PubMed] [Google Scholar]
- 37.Tang, T., A. Rosenkranz, K.J.M. Assmann, M.J. Goodman, J.C. Gutierrez-Ramos, M.C. Carroll, R.S. Cotran, and T.N. Mayadas. 1997. A role of Mac-1(CD11b/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcγ receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J. Exp. Med. 186:1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prodeus, A.P., X. Zhou, M. Maurer, S.J. Galli, and M.C. Carroll. 1997. Impaired mast cell-dependent natural immunity in complement C3 deficient mice. Nature. 390:172–175. [DOI] [PubMed] [Google Scholar]
- 39.Hopken, U.E., B. Lu, N.P. Gerard, and C. Gerard. 1997. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 383:86–89. [DOI] [PubMed] [Google Scholar]