Abstract
Localized cutaneous herpes simplex virus type 1 (HSV-1) infection leads to arming and initial expansion of cytotoxic T lymphocytes (CTLs) in the draining popliteal lymph nodes (PLNs) followed by migration and further proliferation in the spleen. To accurately characterize the sequence of events involved in the activation and generation of anti-HSV CTLs, we used T cell receptor (TCR) transgenic mice specific for the immunodominant epitope from HSV glycoprotein B (gB498–505). We describe the detection of the initiation of antigen presentation in the draining lymph nodes by 4–6 h after infection with HSV-1. Analysis of CD69 up-regulation revealed activation of gB-specific CD8+ T cells by 6–8 h after infection. Furthermore, we show that T cell proliferation begins no sooner than 24 h after activation and is marked by the concurrent appearance of CTL activity in the PLNs. These events are not dependent on the presence of virus in the draining lymph nodes, and suggest a requirement for recruitment of professional antigen-presenting cells to the site of T cell activation. Consequently, we have defined the initiation of the CD8+ T cell–mediated response to cutaneous HSV-1 infection, demonstrating that the immune response to localized viral infection depends only on the appearance of cells presenting virus-derived antigen and commences with remarkable swiftness.
Keywords: CD8+ T lymphocyte, HSV, TCR transgenic mice, antigen presentation, CFSE
Introduction
Efficient initiation of the adaptive immune response upon viral infection is essential to combat a rapidly replicating infectious pathogen. The immune system must be able to respond swiftly while maintaining a high degree of specificity toward the foreign organism. Two of the most important factors limiting the rapidity of the immune response soon after infection are the availability of professional APCs presenting viral antigens and their access to specific T lymphocyte precursors in the lymphoid organs draining the site of infection. During localized infection, APCs are thought to acquire viral antigens within infected tissues and migrate to the draining lymphoid tissue where they activate naive T cells (1). While the migration of professional APCs has been carefully examined after contact sensitization (2), it remains unclear how long such migration and antigen transport takes after immunization with an infectious agent.
The adaptive immune response to cutaneous footpad HSV-1 infection peaks in the draining popliteal lymph nodes (PLNs) 5 d after infection (3–5). By this time, there has been a considerable proliferation of CTLs specific for the immunodominant epitope from HSV glycoprotein B (gB498–505) which is followed by migration and further expansion of this population in the spleen. At the peak of the response, as many as 5% of the CD8+ T cells in the PLNs and 10% in the spleen are specific for this determinant (5). Previous work has estimated the frequency of naive virus-specific CTL precursors in the mouse to be in the range of 1 in 105–106 (6). Taken together, this suggests that a massive expansion of the antigen-specific population must occur relatively soon after infection in order to achieve the production of such a large CTL population at the height of the response.
Recently, efforts have been made to quantitate specific antigen-presenting cells (7), and to follow the development of effector functions by CD8+ T cells (8) after infection. Despite this, the kinetics of initial antigen presentation in combination with the activation and generation of a functional CTL population have not yet been fully elucidated. To overcome the limitation of low naive CTL precursor frequencies and effectively analyze the events that occur immediately after cutaneous infection of C57BL/6 mice with HSV-1, we have made use of an MHC class I–restricted TCR transgenic mouse line specific for the gB498–505 determinant (9). Here we describe the rapid activation of the transgenic CD8+ T cells coinciding with the earliest appearance of specific APCs in the draining PLNs within hours of cutaneous infection with HSV-1.
Materials and Methods
Mice, Viruses, and Peptide.
C57BL/6 and the gB-specific, TCR transgenic MHC class I–restricted (gBT-I.1) mice were obtained from the Department of Microbiology and Immunology, University of Melbourne, animal house and kept in specific pathogen-free conditions. The gBT-I.1 transgenic mice express a TCR from a CTL clone (HSV-2.3) that recognizes the HSV-1 gB498–505 determinant complexed with H-2Kb and is described in detail elsewhere (9). The KOS strain of HSV-1 was propagated and titered using VERO cells grown in Minimal Essential Media plus 10% FCS (MEM-10). The mutant KΔ318 and KΔ5C viruses were propagated and titered on D6•VERO cells grown in MEM-10 (10). The gB498–505 peptide with the sequence SSIEFARL was obtained from Auspep.
Monoclonal Antibodies and 5-Carboxyfluorescein Diacetate Succinimidyl Ester Labeling.
Cells were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) as described previously (5). Single cell suspensions prepared from lymph nodes or spleen were stained with anti-CD8α-allophycocyanin (APC; 53–6.7) and anti-CD69-PE (H1.2F3) obtained from BD PharMingen. 2 × 106 cells were stained for 20 min on ice and washed with PBS-azide containing 1% BSA. Dead cells were excluded using propidium iodide and 5–10,000 live CFSE+ events collected on a Becton Dickinson FACSort™.
Adoptive Transfers, Virus Infections, and Peptide Immunizations.
Lymph node cells obtained from gBT-I.1 mice containing ∼106 CD8+ T cells as determined by flow cytometry were either labeled with CFSE or left untreated and transferred into C57BL/6 mice by intravenous injection. 24 h after transfer, mice were injected in each hind footpad with 4 × 105 PFU HSV-1 KOS, KΔ318, or KΔ5C in PBS, or infected with 106 pfu HSV-1 by flank scarification (11). For peptide injection, mice were immunized intravenously with 1 μg of gB498–505 peptide in PBS. The draining PLN (footpad infection), pooled axillary and inguinal lymph nodes (flank infection), or spleen (intravenous peptide injection) were removed and single cell suspensions produced and counted by trypan blue exclusion before antibody staining for FACS®.
In Vivo CTL Assay.
Splenocyte target cell suspensions from C57BL/6 were evenly split into two populations. One was pulsed with 10−6 M gB498–505 peptide for 45 min at 37°C and then labeled with a high concentration (2.5 μM) of CFSE (CFSEhi population). The other was incubated for 45 min at 37°C without peptide and labeled with a low concentration (0.25 μM) of CFSE (CFSElo population). An equal number of cells from each population (107) were mixed together and adoptively transferred into HSV-1–infected mice which were then killed 4 h later. Cell suspensions were analyzed by FACS® and each population distinguished by their different fluorescent intensities. Percent specific lysis was determined by loss of the peptide-pulsed CFSEhi population compared with control CFSElo population using the formula described previously (5).
LacZ-inducible Hybridomas, APC Assays, and X-gal Assays.
The CTL clone HSV-2.3 was fused with the BWZ.36 cell line containing the NFAT-lacZ construct (12) to produce the T cell hybridoma HSV-2.3.2E2. For detection of APCs, PLNs were removed from mice infected with HSV-1 in the footpad at various times and separately teased into tubes containing 0.5 ml collagenase/DNase solution (1 mg/ml collagenase type II [Worthington Biochemicals] and 0.1% grade II bovine pancreatic DNase I [Boehringer] in Dulbeccos modified eagles medium plus 10% FCS). The suspension was then pipetted for 20 min and 50 μl 0.1 M EDTA added before a further 5 min of pipetting. Twofold serial dilutions of the cells were made in flat-bottom, 96-well plates starting at 106 cells/well. Hybridoma cells (105) were added to each well before overnight culture. Background lacZ expression was determined using hybridoma cells alone or with EL4 cells minus peptide, while positive controls consisted of peptide or peptide-pulsed EL4 cells. X-Gal assays were performed on the cultures to identify the responding hybridomas as described previously (7). Cultures were examined microscopically for the presence of blue cells after 8–12 h incubation at 37°C.
PCR Detection of HSV DNA.
The draining PLNs from HSV-1 footpad infected mice were removed at various times after infection and placed into DNAsol™ (Molecular Research Center) supplemented with proteinase K (50 μg) at room temperature overnight. A footpad from a mouse primed 24 h earlier was also removed and digested as above to act as a positive control. Genomic DNA was isolated as described by the manufacturer's instructions. The HSV-1 DNA was amplified by PCR by using 100 ng of genomic DNA, 50 pmol of primers, 0.2 mM deoxynucleoside triphosphate, and 1.5 U of Taq polymerase (GIBCO BRL). The primers used for PCR amplification were HSV-1a (5′-CCCTGTCTCGCGCGACGGAC-3′) and HSV-1b (5′-TCACCGACCCATACGCGTAA-3′) (13). Amplification involved 35 cycles of 93°C for 30 s, 55°C for 1 min, and 72°C for 1 min followed by one cycle of 93°C 30 s, 55°C for 1 min, and 72°C for 7 min with a DNA Thermal Cycler (PerkinElmer). For each specimen, 100 ng of genomic DNA was evaluated for amplification competence by 25 PCR cycles using insulin primers (5′-CGAGCTCGAGCCTGCCTATCTTTCAGGTC-3′ and 5′-CGGGATCCTAGTTGCAGTAGTTCTCCAG-3′). A 20-μl aliquot of each PCR product was analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide.
Results and Discussion
Rapid Recruitment of gB-specific T Cells into the Dividing Pool and Concurrent Differentiation into Effector Cells.
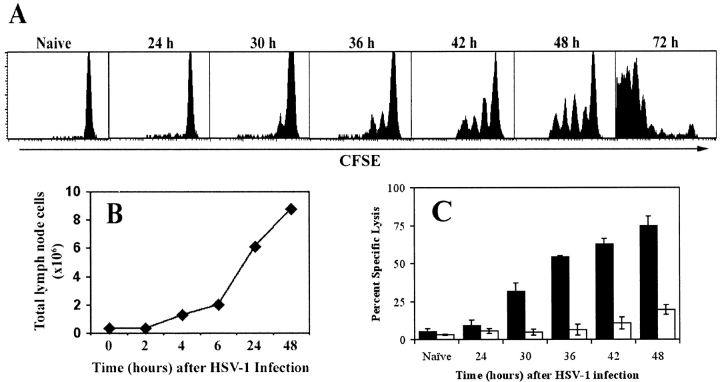
To examine the early events that give rise to the large CTL pool detectable at the peak of the response to HSV-1 infection, gBT-I.1 CD8+ T cells were labeled with CFSE (14) and transferred into normal mice before footpad infection with HSV-1. The first appearance of a dividing cohort of CD8+ T cells appeared in the PLNs at ∼30 h after infection (Fig. 1 A). Progressive analysis indicated that these cells then proceeded to double consistently every 5–6 h thereafter, having undergone four divisions after 48 h and >7 divisions by 72 h. Initially, very few cells were present in the dividing pool, as indicated by the relatively small peak of divided cells at 30 h. However, as the response proceeded the number of cells that were recruited into the dividing pool continued to increase, thereby reducing the size of the undivided population.
Figure 1.
Concurrent in vivo proliferation and CTL activity by gB-specific CD8+ T cells in the PLNs after cutaneous infection with HSV-1. (A) CFSE-labeled lymph node cells from gBT-I.1 mice were transferred into C57BL/6 mice before infection with HSV-1. PLN cells were isolated at various times after infection (24–72 h) and dilution of the CFSE fluorescence analyzed by gating on live CD8+ T cells. (B) Cellularity within the draining lymph nodes over a 48-h period was determined using cell suspensions obtained from the PLNs of mice after foot-pad HSV-1 infection. (C) Mice that had (black bars) or had not (white bars) received 106 gBT-I.1 cells 24 h earlier were infected with HSV-1 in the footpad and left for various times as shown before intravenous transfer of CFSE-labeled syngeneic target cells. gB-peptide–pulsed splenocytes were labeled with a high concentration of CFSE (CFSEhi) while unpulsed control targets were labeled with a low concentration of CFSE (CFSElo). 4 h after target cell transfer, mice were killed and PLN cells analyzed for relative elimination of the CFSEhi versus CFSElo populations. Percent specific lysis was calculated as described in reference 5. Error bars represent SD.
The relatively small pool of dividing cells observed early in the response may reflect either the small number of gB-specific precursor cells present within the PLNs at the time of infection, or a limited level of initial antigen presentation in the PLNs, or both. Due to the limited size of the PLNs, the number of gB-specific CTL precursors present here in a naive mouse would be very small, yet these cells would be stimulated first due to their proximity to the site of infection. A 15–20-fold increase in total cellularity within the PLNs is observed over the first 24 h after infection (Fig. 1 B), most of which is due to an influx of resting lymphocytes (3). The multiple peaks of proliferating cells indicate that the time for recruitment into the dividing pool varies as more naive T cells enter the PLNs, yet once activated all cells divide at a regular rate (15).
While we show that CD8+ T cells first divide at 30 h after infection, significant CTL activity is not detectable in the PLNs in normal mice until 2–3 d after infection with HSV-1 (5), even though CTLs are thought to be armed after one cell division (16). One possible reason for this delay is the low precursor frequency in C57BL/6 mice which prevents detection earlier in the response when cell division begins. To overcome this limitation we used an in vivo CTL assay method (17) in combination with the adoptive transfer of naive gBT-I.1 CD8+ T cells in numbers (106) that would greatly exceed the natural precursor frequency of host gB-specific cells. This enabled the direct detection of significant CTL activity in the PLNs as early as 30 h after infection, which continued to increase, more than doubling over the next 18 h (Fig. 1 C). This data corresponds closely to the increase in proliferating cells over this same period (Fig. 1 A), and provides in vivo evidence suggesting that the first round of division and the development of effector functions by CD8+ T cells occur simultaneously.
De Novo Antigen Synthesis Is Required for Initiation of the gB-specific Response.
Given the very rapid activation of specific T cells in the PLNs, it was possible that the antigen was derived from the injected virions rather than synthesized de novo upon infection. To exclude this, we made use of mutant strains of the HSV-1 KOS virus (KΔ318 and KΔ5C) that express defective forms of the gB protein (10). While both mutants are packaged with a cell line that supplies the wild-type gB protein, they express distinct truncated forms of this protein, with only one strain (KΔ318) containing the gB498–505 epitope. Mice containing CFSE-labeled gBT-I.1 CD8+ T cells were infected cutaneously with the mutant viruses or the wild-type KOS strain and analyzed for proliferating cells. The absence of dividing cells after infection with the KΔ5C strain, but not the KΔ318 mutant, indicates that de novo synthesis of the viral peptides is required in order to stimulate a response by CD8+ T cells (Fig. 2) . This data rules out activation of the T cells via antigen obtained from the injected virions.
Figure 2.
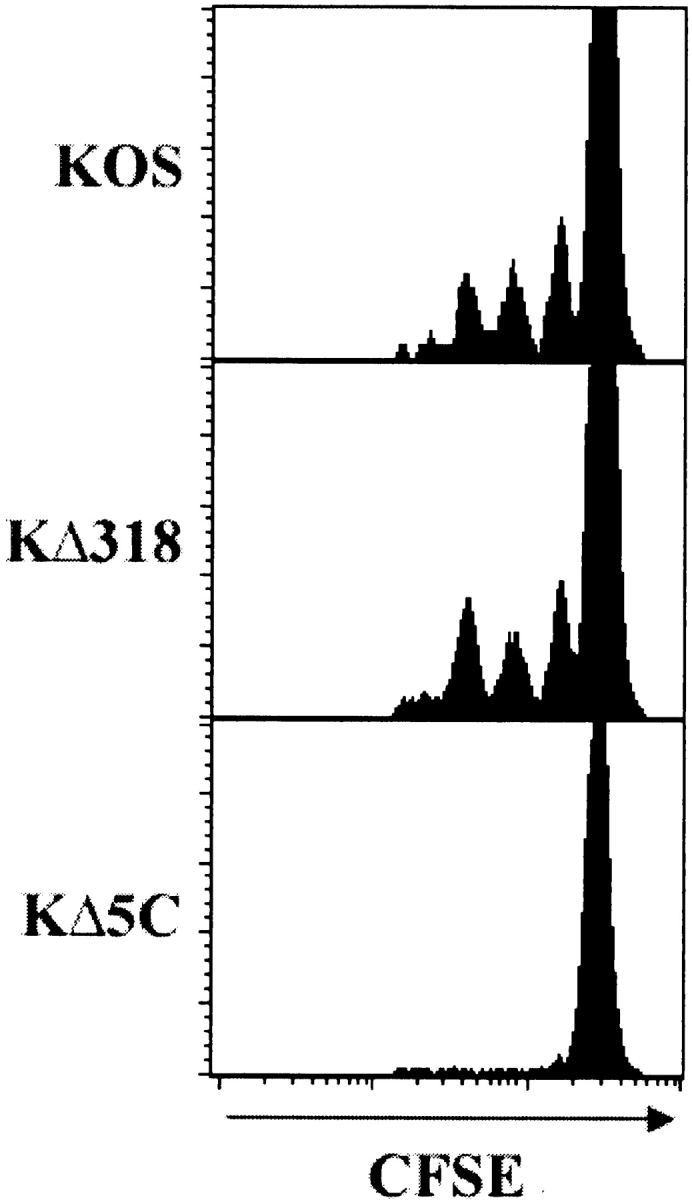
De novo synthesis of viral peptides is required to elicit a gB-specific T cell response. Mice receiving CFSE-labeled gBT-I.1 CD8+ T cells were infected with wild-type HSV-1 KOS or the gB mutant strains KΔ318 or KΔ5C. 42 h after infection, CD8+ T cells from draining PLNs were analyzed for dilution of the CFSE stain caused by cell division. Histograms represent 5–10,000 live events.
There Is a 6-h Lag between Infection and T Cell Activation in the PLNs.
It has been shown that CD8+ T cells require a 24-h lag between TCR stimulation and the first division, possibly to recruit the various cellular machinery required for division (16). To determine whether this is the case for gBT-I T cells, mice were immunized intravenously with 1 μg gB498–505 peptide after the transfer of CFSE-labeled transgenic T cells. The use of peptide should provide immediate TCR engagement. As shown in Fig. 3 , proliferation was first observed in the spleen 24 h after immunization. This data agrees with the results of Ohen et al. using LCMV transgenic T cells and peptide stimulation (16). More importantly, given the observation that cutaneous infection results in appearance of the first cell division within PLNs at 30 h, we calculate that the HSV-1–specific T cells are most likely activated 6 h after virus inoculation.
Figure 3.
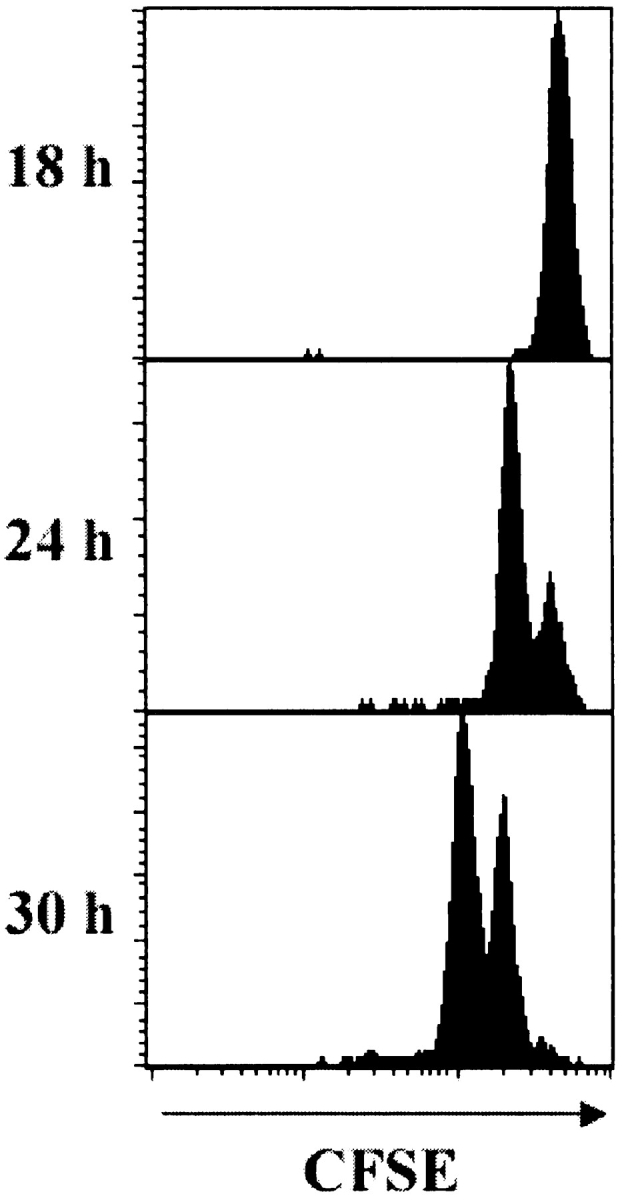
Naive T cells require a minimum 24-h lag between activation and commencement of proliferation. Mice receiving CFSE-labeled gBT-I.1 cells were immunized intravenously with gB498–505 peptide and the transgenic CD8+ T cells in the spleen analyzed 18, 24, and 30 h later for the presence of dividing cells. Histograms represent 5–10,000 live events.
In Vivo Antigen Presentation Correlates with CD8+ T Cell Activation.
To directly demonstrate this early activation, transgenic CD8+ T cells were analyzed for expression of the early activation marker CD69, which is expressed rapidly after TCR engagement (18). Specific up-regulation of this marker was observed 6 h after infection and further increased after 8 h (Fig. 4 A). This matches the timing of events predicted from the CFSE proliferation experiments described above.
Figure 4.
Activation of CD8+ T cells in the PLNs correlates with the appearance of specific antigen presentation. (A) Mice adoptively transferred with CFSE-labeled gBT-I.1 cells were killed at various times (2–8 h) after footpad infection with HSV-1 and CD8+CFSE+ cells analyzed for the expression of CD69. (B) PLNs from HSV-1–infected C57BL/6 mice were treated with collagenase to form a cell suspension. Graded amounts of these cells were placed into culture with HSV-2.3.2E2 lacZ-inducible hybridoma cells overnight. An X-Gal assay was then performed to stain responding hybridoma cells which were counted microscopically. Numbers presented represent the total number of lacZ + cells per separate PLN at various times (2–48 h) after infection, and error bars represent SD (n = 8–12).
The arrival of APCs presenting gB498–505, was determined using a lacZ-inducible hybridoma cell line, HSV-2.3.2E2 expressing the same receptor as the gBT-I.1 transgenic mice (9). Suspensions of cells from the PLNs of infected mice were treated with collagenase to isolate DCs and other APCs, and graded numbers of these cells were incubated with the LacZ-inducible hybridoma cells. Presentation of gB498–505 was first detected 4–6 h after cutaneous infection (Fig. 4 B) and continued to increase over the next 48 h.
The combined data shows that synthesis of gB, transport to the draining lymph nodes, and presentation occurs within hours of infection. The actual identity of the APCs involved remains unknown, although recent experiments using bone marrow chimeric mice has shown that these are of haemopoietic origin (data not shown). Therefore these are likely to be professional APCs probably of DC origin (1). It remains unclear whether presentation involves the migration of antigen-bearing APCs or the simple transfer of cell-independent antigen to the draining lymph nodes. Tracking studies have demonstrated the migration of DCs from the skin to lymph nodes as early as 30 min after contact sensitization (2). In addition, gB has been shown to be expressed within 2–3 h of infection (19–21). Taken together, there is sufficient time for viral infection and protein synthesis in the epithelium, followed by transport of antigen via APCs to the PLNs, consistent with the timing of events described above.
Antigen Presentation and T Cell Activation Does Not Require the Presence of Virus in the Draining Lymph Nodes.
Inoculation of mice with HSV-1 via the footpad involves a subcutaneous injection that may drain to the PLNs via the lymphatics. It was formally possible that direct infection of APCs in the lymph nodes may have accounted for the rapid antigen presentation and swift T cell activation after infection. We attempted to amplify viral DNA from the PLNs by PCR using HSV-1–specific oligonucleotides (13). Specific bands were amplified from the PLNs, indicating the presence of significant amounts of viral DNA within 2 h of infection (Fig. 5 A). However, the intensity of bands amplified 6 and 24 h after infection demonstrate a rapid decrease in the amount of HSV-1 in the lymph nodes, and by 48 h we were unable to detect viral DNA within the PLNs. Moreover, this lack of viral DNA at 48 h did not correlate with detectable antigen presentation, which continued to increase over this time period, suggesting that direct APC infection was not necessary for gB-specific T cell activation.
Figure 5.
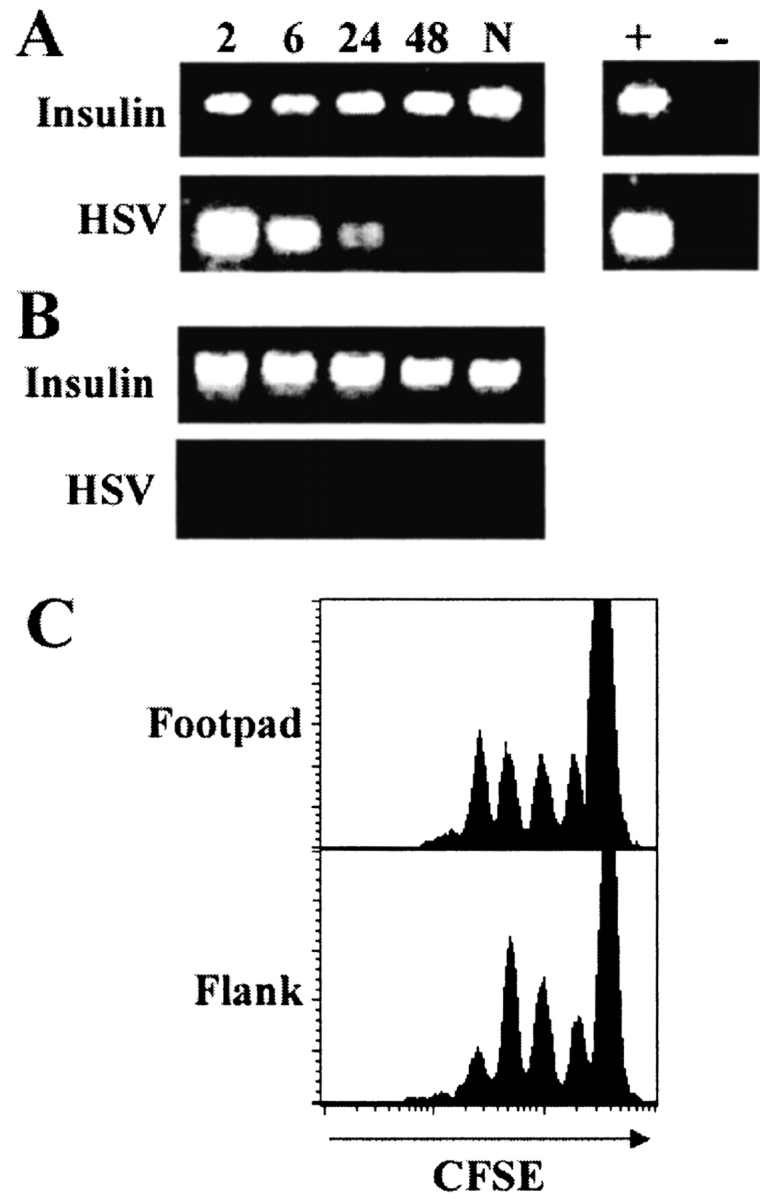
Activation of the gB-specific T cells does not require the presence of viral DNA in the draining lymph nodes. Mice were killed at various times (2–48 h; N, naive) after footpad infection (A), or after flank scarification (B) and DNA isolated from the draining PLNs or pooled axillary and inguinal lymph nodes, respectively. 100 ng of DNA was amplified by PCR using HSV-1 or insulin-specific primers. DNA from the footpad of a mouse infected 24 h earlier was used as a positive control (+). A no DNA control was included to rule out contamination (−). (C) Mice receiving CFSE-labeled gBT-I.1 cells were infected with HSV-1 by footpad injection or flank scarification, and CD8+ T cells from the PLNs or pooled axillary and inguinal lymph nodes, respectively, were analyzed for the presence of proliferating cells via CFSE intensity at 48 h after infection. Histograms represent 5–10,000 live events.
To verify that the virus did not need to be present in lymph nodes to initiate a response we infected mice via scarification of the flank (11). Unlike footpad infection, scarification removes only the top layer of skin thereby confining the infection to the upper epithelial layers and restricting direct viral entry into the dermal lymphatics (22). Therefore, only antigen transported via APCs should reach the draining lymph nodes. As predicted, mice infected in the flank displayed no detectable viral DNA in the draining axillary and inguinal lymph nodes at any time over the 48 h period after infection (Fig. 5 B). Nonetheless, flank inoculation resulted in effective T cell activation and the same kinetics of proliferation as found using footpad injection. This was shown by comparing CD8+ T cell proliferation 48 h after cutaneous footpad infection or flank scarification. The number of CFSE+ peaks in the draining lymph nodes indicated that the gB-specific T cells were activated at the same time after both routes of cutaneous infection (Fig. 5 C). Thus, despite the discrepancy in the presence of viral DNA between the two peripheral infection routes, both showed the same relatively short lag between infection and activation of the gB-specific T cells.
Overall, our results suggest that the presence of viral DNA in the draining PLNs is largely immunologically irrelevant, as activation of the gB-specific T cells appears to occur independent of detectable viral DNA. While HSV-1 can infect DCs, this has been associated with downmodulation of costimulatory molecule expression as well as severe reduction in the T cell–stimulatory capacity of these cells (23). Therefore direct DC presentation of HSV-1 antigens may prove inefficient at T cell priming. Cross-priming, which involves the transfer of antigen from infected cells to professional APCs (24), represents an effective means of achieving T cell activation circumventing HSV-mediated immune evasion (25). In addition, cross-presentation of antigen at a site remote from the actual infection would explain the absence of HSV-1 DNA within the draining axillary and inguinal lymph nodes. The rapidity with which antigen transfer occurs so soon after cutaneous infection suggests that this is a very efficient event. As shown here, antigen presentation appears in draining lymph nodes within 6 h of infection. Most importantly, even at this time, presentation is at levels clearly capable of initiating an effective CTL response.
Acknowledgments
The authors would like to thank Drs. Andrew Brooks and Gabrielle Belz for critical review of the manuscript.
This work was supported in part by grants from the National Health and Medical Research Council (NHMRC) of Australia (W.R. Heath and F.R. Carbone), the National Institutes of Health (AI43347 to W.R. Heath) and a Howard Hughes Medical Institute International Fellowship (W.R. Heath).
References
- 1.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Macatonia, S.E., S.C. Knight, A.J. Edwards, S. Griffiths, and P. Fryer. 1987. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J. Exp. Med. 166:1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cose, S.C., C.M. Jones, M.E. Wallace, W.R. Heath, and F.R. Carbone. 1997. Antigen-specific CD8+ T cell subset distribution in lymph nodes draining the site of herpes simplex virus infection. Eur. J. Immunol. 27:2310–2316. [DOI] [PubMed] [Google Scholar]
- 4.McNally, J.M., D. Dempsey, R.M. Wolcott, R. Chervenak, and S.R. Jennings. 1999. Phenotypic identification of antigen-dependent and antigen-independent CD8 CTL precursors in the draining lymph node during acute cutaneous herpes simplex virus type 1 infection. J. Immunol. 163:675–681. [PubMed] [Google Scholar]
- 5.Coles, R.M., S.N. Mueller, W.R. Heath, F.R. Carbone, and A.G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localised infection with HSV-1. J. Immunol. 168:834–838. [DOI] [PubMed] [Google Scholar]
- 6.Hou, S., and P.C. Doherty. 1993. Partitioning of responder CD8+ T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J. Immunol. 150:5494–5500. [PubMed] [Google Scholar]
- 7.Usherwood, E.J., T.L. Hogg, and D.L. Woodland. 1999. Enumeration of antigen-presenting cells in mice infected with Sendai virus. J. Immunol. 162:3350–3355. [PubMed] [Google Scholar]
- 8.Sano, G., J.C. Hafalla, A. Morrot, R. Abe, J.J. Lafaille, and F. Zavala. 2001. Swift development of protective effector functions in naive CD8+ T cells against malaria liver stages. J. Exp. Med. 194:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller, S.N., W.R. Heath, F.R. Carbone, and C.M. Jones. 2002. The characterisation of two transgenic mice specific for herpes simplex virus. Immunol. Cell Biol. 80:156–163. [DOI] [PubMed] [Google Scholar]
- 10.Desai, P., F.L. Homa, S. Person, and J.C. Glorioso. 1994. A genetic selection method for the transfer of HSV-1 glycoprotein B mutations from plasmid to the viral genome: preliminary characterization of transdominance and entry kinetics of mutant viruses. Virology. 204:312–322. [DOI] [PubMed] [Google Scholar]
- 11.Simmons, A., and A.A. Nash. 1984. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J. Virol. 52:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson, S., and N. Shastri. 1994. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 6:369–376. [DOI] [PubMed] [Google Scholar]
- 13.Lucotte, G., C. Bathelier, V. Lespiaux, C. Bali, and T. Champenois. 1995. Detection and genotyping of herpes simplex virus types 1 and 2 by polymerase chain reaction. Mol. Cell. Probes. 9:287–290. [DOI] [PubMed] [Google Scholar]
- 14.Lyons, A.B. 1999. Divided we stand: tracking cell proliferation with carboxyfluorescein diacetate succinimidyl ester. Immunol. Cell Biol. 77:509–515. [DOI] [PubMed] [Google Scholar]
- 15.Gett, A.V., and P.D. Hodgkin. 2000. A cellular calculus for signal integration by T cells. Nat. Immunol. 1:239–244. [DOI] [PubMed] [Google Scholar]
- 16.Oehen, S., and K. Brduscha-Riem. 1998. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 161:5338–5346. [PubMed] [Google Scholar]
- 17.Aichele, P., K. Brduscha-Riem, S. Oehen, B. Odermatt, R.M. Zinkernagel, H. Hengartner, and H. Pircher. 1997. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity. 6:519–529. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama, W.M., F. Koning, P.J. Kehn, G.M. Pereira, G. Stingl, J.E. Coligan, and E.M. Shevach. 1988. Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J. Immunol. 141:369–376. [PubMed] [Google Scholar]
- 19.Pederson, N.E., S. Person, and F.L. Homa. 1992. Analysis of the gB promoter of herpes simplex virus type 1: high-level expression requires both an 89-base-pair promoter fragment and a nontranslated leader sequence. J. Virol. 66:6226–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice, S.A., and D.M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, I.L., M.A. Hardwicke, and R.M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 186:74–86. [DOI] [PubMed] [Google Scholar]
- 22.Weeks, B.S., R.S. Ramchandran, J.J. Hopkins, and H.M. Friedman. 2000. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Arch. Virol. 145:385–396. [DOI] [PubMed] [Google Scholar]
- 23.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245–3253. [DOI] [PubMed] [Google Scholar]
- 24.Sigal, L.J., S. Crotty, R. Andino, and K.L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 398:77–80. [DOI] [PubMed] [Google Scholar]
- 25.Heath, W.R., and F.R. Carbone. 2001. Cross-presentation in viral immunity and self-tolerance. Nature Rev. Immunol. 1:126–134. [DOI] [PubMed] [Google Scholar]