Abstract
Prostaglandin E (PGE)2 produced by osteoblasts acts as a potent stimulator of bone resorption. Inflammatory bone loss is accompanied by osteoclast formation induced by bone-resorbing cytokines, but the mechanism of PGE2 production and bone resorption in vivo is not fully understood. Using cytosolic phospholipase A2α (cPLA2α)-null mice, we examined the role of cPLA2α in PGE2 synthesis and bone resorption. In bone marrow cultures, interleukin (IL)-1 markedly stimulated PGE2 production and osteoclast formation in wild-type mice, but not in cPLA2α-null mice. Osteoblastic bone marrow stromal cells induced the expression of cyclooxygenase (COX)-2 and membrane-bound PGE2 synthase (mPGES) in response to IL-1 and lipopolysaccharide (LPS) to produce PGE2. Osteoblastic stromal cells collected from cPLA2α-null mice also induced the expression of COX-2 and mPGES by IL-1 and LPS, but could not produce PGE2 due to the lack of arachidonic acid release. LPS administration to wild-type mice reduced femoral bone mineral density by increased bone resorption. In cPLA2α-null mice, however, LPS-induced bone loss could not be observed at all. Here, we show that cPLA2α plays a key role in PGE production by osteoblasts and in osteoclastic bone resorption, and suggest a new approach to inflammatory bone disease by inhibiting cPLA2α.
Keywords: cPLA2α, bone loss, osteoclast, osteoblast, lipopolysaccharide
Introduction
Prostaglandin E (PGE)*2 is produced in bone mainly by osteoblasts and acts as a potent stimulator of bone resorption (1–3). The production of PGE2 by osteoblasts is regulated by several cytokines, including IL-1 and IL-6. We have reported that PGE2 stimulated adenylate cyclase in mouse osteoblasts to accumulate cellular cAMP, induced osteoclast formation in mouse bone marrow cultures, and stimulated bone resorption in mouse calvarial cultures (4–6). The addition of indomethacin, an inhibitor of prostaglandin (PG) synthesis, to mouse bone marrow cultures strikingly suppressed osteoclast formation induced by IL-1, indicating that PGE2 production by osteoblasts is involved in the mechanism of bone resorption induced by IL-1 (5, 6).
PGE2 synthesis is regulated by three metabolic steps: the release of arachidonic acid from the membranous phospholipids by phospholipase A2 (PLA2), the conversion of arachidonic acid to PGH2 by cyclooxygenase (COX), and the synthesis of PGE2 by PGE synthase (PGES; 7–13). We have previously reported that cytosolic PLA2α (cPLA2α) is constitutively expressed in mouse osteoblasts and is a key enzyme in IL-1–induced PGE2 synthesis after its activation by platelet-derived growth factor (6). Although both constitutive COX (COX-1) and inducible COX (COX-2) are expressed in mouse osteoblasts, the expression of COX-2 is markedly induced by several bone-resorbing factors, including IL-1 in osteoblasts. The addition of NS-398, a selective inhibitor of COX-2, inhibits the IL-1–induced PGE2 synthesis (5, 6). Two PGES, membrane-bound PGES (mPGES) and cytosolic PGES have been recently cloned (11–13). The expression of mPGES is greatly induced by LPS in rat peritoneal macrophages and by IL-1/TNF in rat osteoblasts. Therefore, cPLA2α, COX-2, and mPGES might be requisite for the regulation of PGE2 synthesis in response to IL-1 in mouse osteoblasts and bone marrow stromal cells.
It is known that the administration of LPS not only induces inflammation with fever but also osteoclastic bone resorption (14, 15). In macrophages, LPS binds to LPS receptors, called toll-like receptor (TLR)4, and induces the production of cytokines such as IL-1 and TNFα, and the COX-2–mediated PG synthesis (16, 17). However, it is not known whether LPS acts on osteoblasts to elicit PGE2 production. Because LPS could induce bone loss in vivo, LPS-induced PGE2 production by osteoblasts might be involved in the mechanism of bone resorption associated with inflammation.
Although PLA2 is a key enzyme in eicosanoid production, mammalian cells contain structurally diverse forms of PLA2 including secretory PLA2, calcium-independent PLA2, and cPLA2α (18, 19). To clarify the role of cPLA2α in eicosanoid production and its biological significance, two groups, at the same time, have developed cPLA2α-deficient mice by targeted disruption of the cPLA2α gene. We generated cPLA2α-null mice and reported that the allergic responses in the bronchopulmonary system were significantly reduced in cPLA2α-null mice (20). Bonventre et al. (21) showed a decrease in pathophysiological neuronal death after transient focal cerebral ischemia in cPLA2α-null mice. In either case, stimulated peritoneal macrophages from cPLA2α-null mice failed to produce eicosanoids such as PGE2, leukotriene B4, or leukotriene C4, and platelet-activating factor, and the female cPLA2α-null mice failed to deliver offspring due to defective reproductive systems including pregnancy and labor (20, 21).
We examined the role of cPLA2α in PG synthesis and bone resorption associated with inflammation both in vitro and in vivo. To achieve our aim, using cPLA2α-null and wild-type mice, PGE2 production and osteoclast formation were determined in bone marrow cultures treated with IL-1 in vitro, and LPS-induced bone resorption was analyzed in vivo.
Materials and Methods
Animals and Reagents.
cPLA2α-null mice were established by gene targeting (20). Mice heterozygous for the cPLA2α mutant allele with the genetic background of the C57BL/6J × 129/Ola hybrid were mated and the genotypes of the mice were determined by PCR using genomic DNA isolated from the tail tip as previously reported (20). At 6–8 wk of age, cPLA2α-null and wild-type mice were used. Both female and male littermates were used and the mice were matched for sex in each group. In some experiments, mice were injected with LPS (5 mg/kg of body weight) intraperitoneally on days 0 and 4 and the femurs were collected on day 8. Mice in the control group were injected with PBS. There was no significant difference in body weight between cPLA2α-null mice and wild-type mice. All procedures were performed in accordance with institutional guideline for animal research at the Tokyo University of Pharmacy and Life Science. Recombinant human IL-1α was purchased from Genzyme. LPS (Escherichia coli 055:B5) was purchased from Difco Laboratories.
Mouse Bone Marrow Cultures.
Bone marrow cells were isolated from 6-wk-old cPLA2α-null and wild-type mice and cultured in 0.5 ml αMEM containing 10% FCS at 106 cells/well in 24-well plates. The cultures were fed every 3 d by replacing 0.4 ml of the old medium with fresh medium. After being cultured for 9 d, the cultured medium was collected for the measurement of PGE2 and the cells adhering to the well surface were stained for tartrate-resistant acid phosphatase (TRAP). The TRAP+ multinucleated cells containing three or more nuclei/cell were counted as osteoclasts.
Measurement of PGE2 Content.
The concentrations of PGE2 in the cultured medium and the bone marrow fluid collected from the mouse femurs and tibiae were determined using an enzyme immunoassay (EIA; Amersham Biosciences). The antibody had the following cross-reactivity when calculated by the bound/free ratio: PGE2, 100%; PGE1, 7.0%; 6-keto-PGF1α, 5.4%; PGF2α, 4.3%; and PGD2, 1.0%.
Culture of Osteoblastic Stromal Cells and RT-PCR Analysis.
To obtain osteoblastic stromal cells, mouse bone marrow cells were isolated from the 6–8-wk-old cPLA2α-null and wild-type mice and individually cultured for 2 wk in αMEM containing 10% FCS. For RT-PCR analysis, total RNA was extracted from the cells. cDNA was synthesized from 5 μg total RNA by reverse transcriptase (Superscript II Preamplification System; Life Technologies) and amplified using PCR. The primers used in PCR for the mouse COX-2 gene were 5′-TCAGCCAGGCAG CAAATCCTTG-3′ (sense) and 5′-TAGTCTCTCCTATGAGTATGAGTC-3′ (anti-sense). The reaction conditions for PCR were 26 cycles, denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. The primers used in PCR for the mouse mPGES gene were 5′-ATGCCTTCCCCGGGCCTG-3′ (sense) and 5′-TCACAGATGGTGGGCCAC-3′ (anti-sense). The reaction conditions for PCR were 26 cycles, denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. The primers used in PCR for the mouse GAPDH gene were 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ (sense) and 5′-CATGTAGGCCATGAGGTCCACCAC-3′ (anti-sense). The reaction conditions for PCR were 30 cycles, denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. The PCR product was run on a 1.5% agarose gel and stained with ethidium bromide.
Bone Marrow Fluid.
6-wk-old cPLA2α-null and wild-type mice were injected with LPS (5 mg/Kg of body weight) intraperitoneally and the femurs and tibiae were collected 1 h after the injection. To obtain the bone marrow fluid, bone marrow cells and cancellous bone fragments in the femurs and tibiae were individually collected with 1 ml αMEM, as previously reported (22). After centrifugation to remove the cells and bone fragments, the supernatant was collected as bone marrow fluid for measurement of PGE2.
Radiographic Analysis of the Femur.
Radiographs of the femurs were taken by soft X ray (model CMB-2; SOFTEX). The bone mineral density (BMD) of the femurs was measured by dual X-ray absorptiometry (model DCS-600R; Aloka) as previously reported (23). The bone mineral content of the femurs was closely correlated with the ash weight (23). The BMD was calculated by dividing the bone mineral content of the measured area by the area. The scanned area was divided into three parts: proximal femur, midshaft, and distal femur.
Histological Analysis of the Femoral Cancellous Bone.
The distal metaphysis of the femur was fixed with 70% ethanol and embedded in glycol methacrylate, and undecalcified 3-μm sections were prepared and stained for TRAP as previously reported (23). The trabecular bone volume density (bone volume/tissue volume [BV/TV]), the mean number of osteoclasts in each millimeter of the trabecular bone surface (osteoclast number/bone surface, mm−), trabecular separation (Tb.Sp), and trabecular thickness (Tb.Th) were determined in the cancellous bone tissue at the secondary spongiosa of the distal metaphysis (23).
Statistical Analysis.
The data are expressed as the means ± SEM. The significance of differences was analyzed using Student's t test.
Results
PGE2 Synthesis and Osteoclast Formation in the Bone Marrow Cultures.
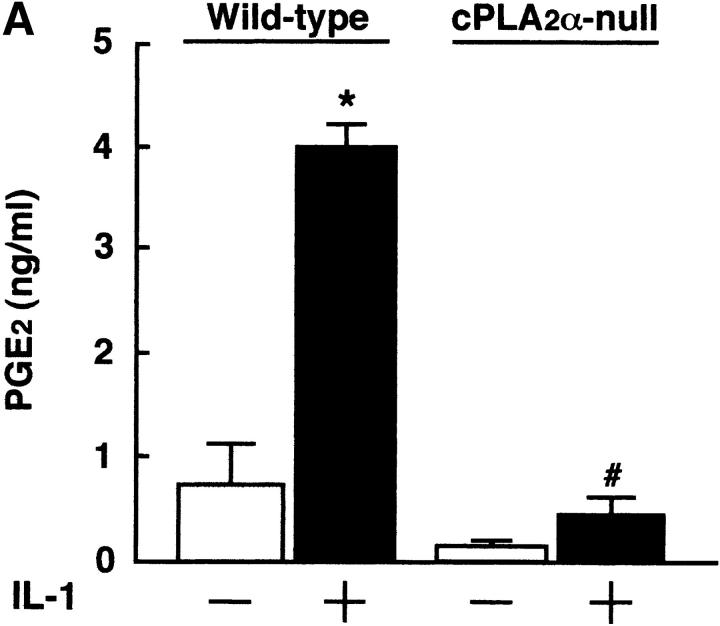
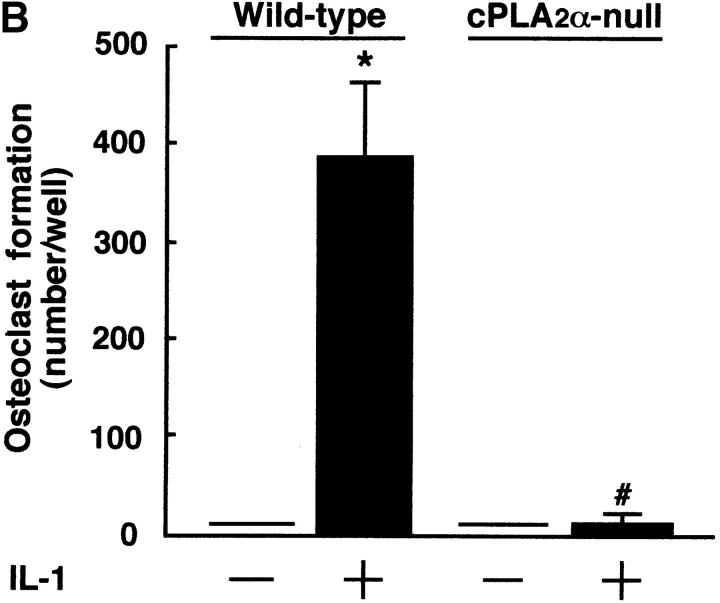
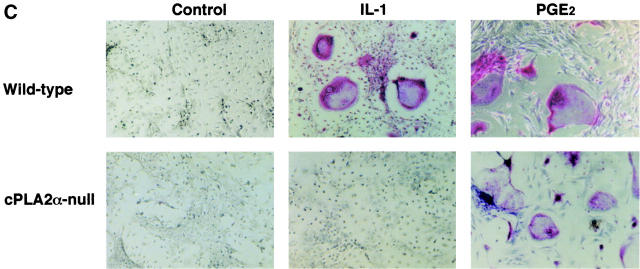
IL-1 acts on osteoblasts to induce PGE2 synthesis and promotes osteoclast formation in mouse bone marrow cultures (1–5). We have previously reported that cPLA2 is expressed in mouse osteoblasts and plays an important role in the process of PGE2 production by osteoblasts (6). To identify the role of cPLA2 in PGE2 production and osteoclast formation, bone marrow cells were collected from cPLA2α-null and wild-type mice and cultured for 9 d with IL-1. In wild-type mice, the addition of IL-1 to the bone marrow cultures markedly induced the production of PGE2 and the formation of TRAP+ osteoclast-like cells (Fig. 1, A and B) . In cPLA2α-null mice, however, the PGE2 level in the conditioned medium of the control cultures without IL-1 was lower than that in wild-type mice and the IL-1–induced PGE2 synthesis could not be detected in the bone marrow cultures (Fig. 1 A). In addition, the osteoclast formation induced by IL-1 was not detected in the bone marrow cultures from cPLA2α-null mice (Fig. 1, B and C). When PGE2 was added to the bone marrow cells, the osteoclast formation was greatly induced in cPLA2α-null mice and the level was similar to that in wild-type mice (Fig. 1 C). Therefore, cPLA2α is essential for PGE2 synthesis and osteoclast formation induced by IL-1 in mouse bone marrow cultures.
Figure 1.
PGE2 production and osteoclast formation in mouse bone marrow cultures treated with IL-1. (A) The bone marrow cells were collected from cPLA2α-null and wild-type mice and cultured for 9 d with or without 2 ng/ml IL-1. The concentration of PGE2 in the cultured medium was determined using an EIA as described in Materials and Methods. Data are expressed as the means ± SEM of three to five mice. *, P < 0.001 versus control without IL-1; #, P < 0.001 versus IL-1–treated wild-type group. (B) The bone marrow cells were cultured under the conditions described above and the cells were stained for TRAP, a specific marker for osteoclasts. The number of TRAP+ multinucleated cells containing three or more nuclei was counted. Data are expressed as the means ± SEM of three to five mice. *, P < 0.001 versus control without IL-1; #, P < 0.001 versus IL-1–treated wild-type group. (C) The bone marrow cells were collected from cPLA2α-null and wild-type mice and cultured for 9 d with 2 ng/ml IL-1 or 1 μM PGE2. The cells were stained for TRAP to detect osteoclasts.
Production of PGE2 by Osteoblastic Bone Marrow Stromal Cells.
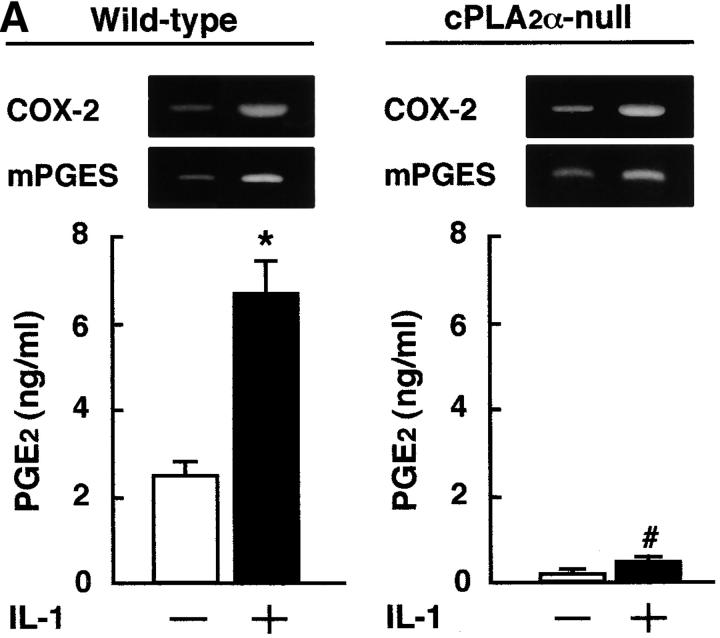
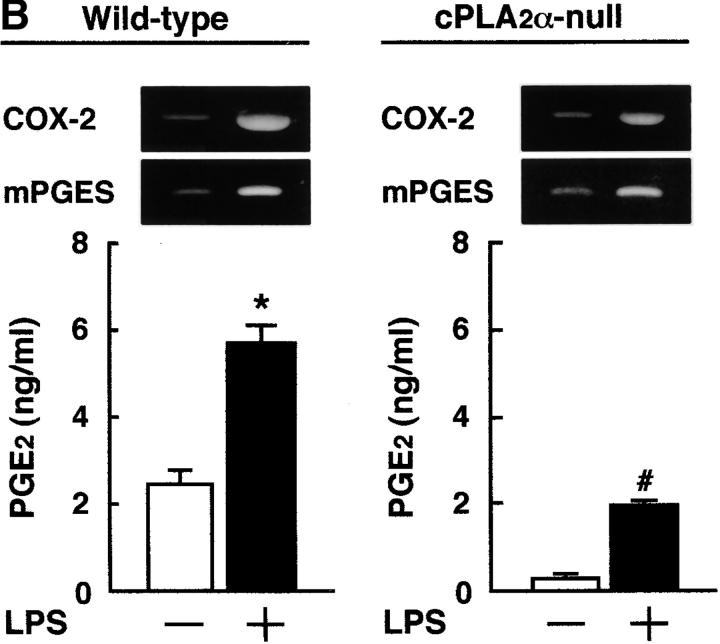
It has been reported that bone marrow stromal cells exhibit osteoblastic phenotype and support the differentiation of osteoclast progenitor to mature osteoclasts (1, 24). To examine PGE2 synthesis by osteoblastic cells, the bone marrow cells were collected from cPLA2α-null and wild-type mice and cultured for 2 wk to obtain the osteoblastic stromal cells. When the stromal cells derived from wild-type mice were treated with IL-1 and LPS, the expression of COX-2 and mPGES mRNAs was markedly induced to produce PGE2. However, in the stromal cells derived from cPLA2α-null mice, the PGE2 production induced by IL-1 and LPS was significantly attenuated compared with wild-type (Fig. 2) . The expression of COX-2 and mPGES was elevated by IL-1 and LPS in osteoblastic stromal cells not only collected from wild-type mice but also from cPLA2α-null mice (Fig. 2). The expression of GAPDH mRNA was similarly detected in all conditions (not depicted). These results indicate that the impaired PGE2 production in cPLA2α-null mice is due to the lack of the release of arachidonic acid from the membranous phospholipids in osteoblastic stromal cells.
Figure 2.
Expression of COX-2 and mPGES mRNA and PGE2 production in osteoblastic bone marrow stromal cells treated with IL-1 and LPS. (A) The bone marrow stromal cells were isolated from cPLA2α-null and wild-type mice. Cells were cultured for 3 h with or without 2 ng/ml IL-1 to detect COX-2 and mPGES mRNA by RT-PCR. To measure the levels of PGE2 in conditioned medium, cells were cultured for 24 h with or without IL-1. (B) The bone marrow stromal cells were cultured for 3 h with or without 10 ng/ml LPS to detect COX-2 and mPGES mRNA by RT-PCR. To measure the levels of PGE2 in conditioned medium, cells were cultured for 24 h with or without LPS. The concentration of PGE2 in the cultured medium was determined using an EIA. Data are expressed as the means ± SEM of three to four wells. *, P < 0.01 versus control without IL-1 or LPS; #, P < 0.001 versus IL-1– or LPS-treated wild-type group.
LPS-induced PGE2 Synthesis in Mouse Bone Marrow.
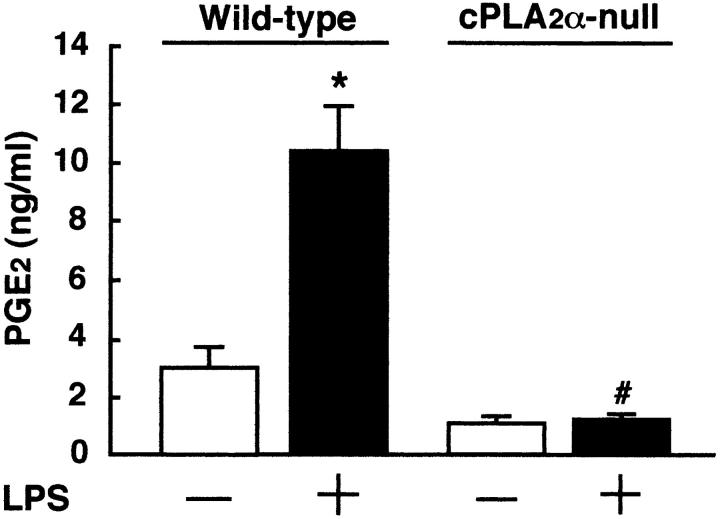
The administration of LPS induces not only inflammation with fever but also osteoclastic bone resorption in vivo. PGE2 produced by various cells, such as macrophages and mast cells, is thought to be involved in the mechanism of LPS-elicited inflammation. To examine the LPS-induced production of PGE2 in the bone and bone marrow in mice, bone marrow fluid was collected from the femurs and tibiae 1 h after LPS administration. The level of PGE2 in the bone marrow fluid was detectable and markedly elevated by LPS administration in wild-type mice (Fig. 3) . In cPLA2α-null mice, the administration of LPS did not enhance the level of PGE2 in the mouse bone marrow (Fig. 3), indicating a key role of cPLA2α in PGE2 production by bone and bone marrow cells in vivo.
Figure 3.
The level of PGE2 in the bone marrow fluid collected from cPLA2α-null and wild-type mice injected with LPS. To obtain the bone marrow fluid, cPLA2α-null and wild-type mice were injected with LPS or PBS intraperitoneally and the femurs and tibiae were collected 1 h after the injection. Thereafter, the bone marrow fluid was prepared using 1 ml αMEM as described in Materials and Methods, and the concentration of PGE2 in the bone marrow fluid was determined using an EIA. Data are expressed as the means ± SEM of three to four mice. *, P < 0.01 versus control without LPS; #, P < 0.01 versus LPS-treated wild-type group.
LPS-induced Bone Loss by Increased Bone Resorption.
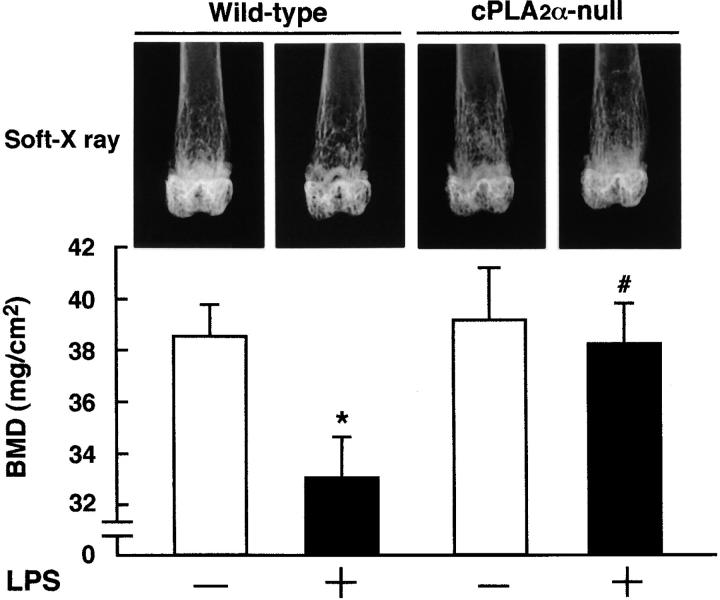
To determine the influence of cPLA2α-dependent PGE2 synthesis in bone loss induced by LPS administration, cPLA2α-null and wild-type mice were injected with LPS and the femurs were collected on day 8 for X-ray analysis. The X-ray analysis showed that the LPS administration had caused a marked loss of mineralized cancellous bone, especially in the distal metaphysis of the femur in wild-type mice (Fig. 4) . In cPLA2α-null mice, the loss of cancellous bone by the LPS administration could not be seen in the X-ray analysis (Fig. 4). To determine the change of bone mass due to LPS administration, we measured the BMD at a distal region of the femur using cPLA2α-null and wild-type mice. The BMD was significantly reduced by the LPS administration at the distal metaphysis in wild-type mice, but it was not changed at all by LPS in cPLA2α-null mice (Fig. 4).
Figure 4.
Lack of inflammatory bone loss induced by LPS administration in cPLA2α-null mice. Measurement of femoral BMD and soft X-ray analysis in cPLA2α-null and wild-type mice injected with LPS. To measure the femoral BMD, cPLA2α-null and wild-type mice were injected with LPS or PBS intraperitoneally on days 0 and 4, and the femurs were collected on day 8 after the first injection. The BMD was measured at the region of distal metaphysis of the excised femurs. Data are expressed as the means ± SEM of five to six mice. *, P < 0.05 versus control without LPS; #, P < 0.05 versus LPS-treated wild-type group. The top shows the radiograms of the femurs collected from the animals of each group. The region of femoral distal metaphysis was determined by soft X-ray analysis. Note that marked bone loss occurred in the femoral cancellous bone in the LPS-injected wild-type mice, but not in cPLA2α-null mice.
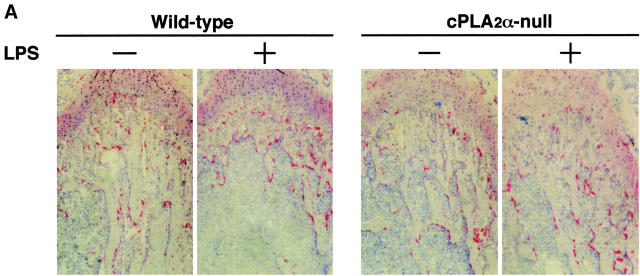
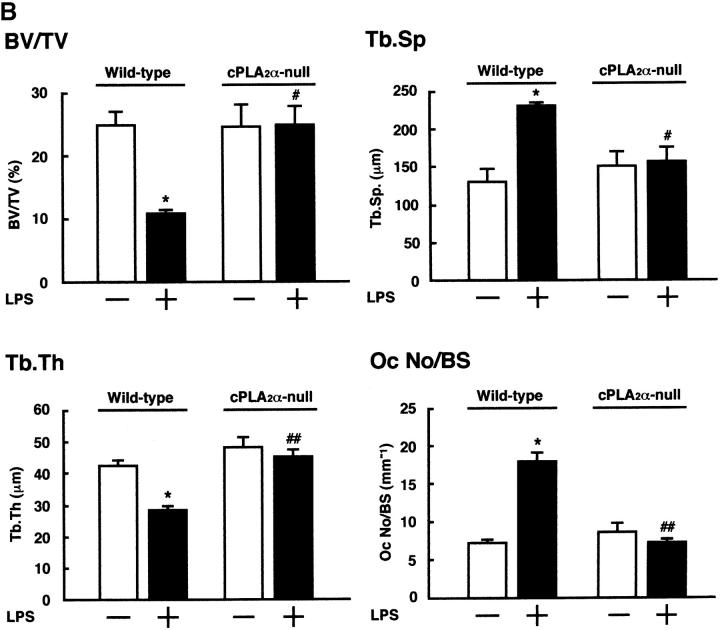
To define the mechanism of bone loss due to LPS administration, histological sections of the distal femoral metaphysis were prepared and stained for TRAP (Fig. 5 A). In wild-type mice, the bone density (BV/TV) of the trabecular bone and the Tb.Th were significantly reduced whereas the Tb.Sp increased with the LPS administration (Fig. 5 B). The increase in the Tb.Sp indicates that the osteoclastic bone resorption was stimulated, resulting in enhanced intertrabecular space. In fact, the number of TRAP+ multinucleated osteoclasts significantly increased in the trabecular bone obtained from wild-type mice treated with LPS in vivo (Fig. 5, A and B). In cPLA2α-null mice, however, the LPS administration did not influence the BV/TV, Tb.Sp, Tb.Th, and the number of osteoclasts at all (Fig. 5, A and B). These results indicate that cPLA2α is crucial for LPS-induced osteoclastic bone resorption, which results in bone loss associated with PGE2-mediated inflammation.
Figure 5.
Histological analyses of the femoral trabecular bone collected from cPLA2α-null and wild-type mice injected with LPS. (A) Wild-type and cPLA2α-null mice were injected with LPS or PBS intraperitoneally on days 0 and 4, and the femurs were collected on day 8 after the first injection to prepare the sections of femoral distal metaphysis. The sections were stained for TRAP to detect osteoclasts. (B) Using the TRAP-stained section shown in A, histomorphometric analyses were performed to calculate BV/TV, Tb.Sp, Tb.Th, and osteoclast number/bone surface as described in Materials and Methods. Data are expressed as the means ± SEM of three to four mice. *, P < 0.001 versus control without LPS; #, P < 0.01; ##, P < 0.001 versus LPS-treated wild-type group.
Discussion
Previous studies have shown that IL-1 acts on osteoblasts and stromal cells to synthesize PGE2 by an increased expression of COX-2 and that the production of PGE2 is essential for osteoclast formation induced by IL-1 in mouse bone marrow cultures (1–5, 25). In cPLA2α-null mice, both PGE2 production and osteoclast formation was not induced by IL-1 in the bone marrow cultures (Fig. 1). Therefore, the cPLA2α gene is crucial for PGE2 synthesis by initiating the release of arachidonic acid from membranous phospholipids and regulating osteoclast formation. The addition of PGE2 to the bone marrow cells collected from cPLA2α-null mice markedly induced osteoclast formation (Fig. 1 C), indicating that the processes for osteoclast differentiation followed by PGE2 production are normal in cPLA2α-null mice. The expression of COX-2 and mPGES was elevated by IL-1 and LPS in osteoblastic stromal cells not only collected from wild-type mice but also from cPLA2α-null mice (Fig. 2). When osteoblastic stromal cells collected from cPLA2α-null mice were pretreated for 24 h with IL-1 and cultured for 8 h with or without 10 μM arachidonic acid, the level of PGE2 in the conditioned medium was significantly elevated by the addition of arachidonic acid (control 0.12 ± 0.01 ng/ml; arachidonic acid 1.25 ± 0.13 ng/ml, P < 0.01). These results indicate that COX-2 and mPGES induced by IL-1 convert exogenous arachidonic acid into PGE2 via PGH2 in the stromal cells from cPLA2α-null mice, and that cPLA2α is essential for the release of arachidonic acid from membranous phospholipids in osteoblastic stromal cells.
Recently, a key molecule for osteoclast formation was cloned and named as a receptor activator of nuclear factor κB ligand (RANKL; 26–29). RANKL is expressed on the surface of osteoblasts and bone marrow stromal cells treated with bone-resorbing factors such as PTH, 1α,25(OH)2D3, PGE2, and IL-1. As IL-1 enhances RANKL expression in the osteoblasts, it might be possible that IL-1 directly induces osteoclast formation. However, IL-1–induced osteoclast formation is highly dependent on the production of PGE2 in the bone marrow cultures. The continuous production and accumulation of PGE2 may powerfully induce RANKL in bone marrow cells treated with IL-1.
We found that the LPS administration markedly enhanced the level of PGE2 in the bone marrow fluid collected from wild-type mice, but not from cPLA2α-null mice (Fig. 3). Previous studies have indicated that macrophages and mast cells collected from cPLA2α-null mice could not produce PGs after various stimuli (20, 21, 30). Bone marrow fluid was collected from the bone marrow cavity and distal cancellous bone area of femurs and tibiae, indicating that PGE2 in the fluids is derived from bone marrow cells and osteoblasts. Therefore, cPLA2α seems to play a key role in PGE2 production by bone and bone marrow cells in vivo.
As previously reported, cPLA2α-null mice grow normally (20) and no apparent defects nor abnormalities are detected in the bone by soft X-ray analysis (unpublished data). Bone loss after LPS administration was observed in the area of distal cancellous bone of the femurs in wild-type mice but not in cPLA2α-null mice (Figs. 4 and 5), suggesting that increased PGE2 in bone and bone marrow is involved in bone resorption elicited by LPS. Abu-Amer et al. (15) reported that TNFα was involved in the osteoclast formation induced by LPS. The production of cytokines, including IL-1, IL-6, and TNFα might be enhanced by LPS treatment in macrophages. Most of these cytokines induce PGE2 production in osteoblasts and stromal cells, and osteoclast formation induced by the cytokines is dependent on PGE2 synthesis in mouse bone marrow cultures. Although we cannot exclude the possibility of PGE2-independent action of these cytokines in osteoclast formation, cPLA2α-mediated PGE2 production is largely involved in bone loss induced by LPS in vivo.
Previous studies have shown that LPS binds to LPS receptors, called TLR4, in macrophages and induces COX-2–mediated PG synthesis (16, 17). In this study, LPS greatly induced cPLA2α-dependent PGE2 production by osteoblastic stromal cells (Fig. 2 B). In osteoblasts collected from mouse calvaria, LPS induced COX-2– and mPGES-mediated PGE2 production in normal C3H/HeN mice, but not in TLR4-mutated C3H/HeJ mice (unpublished data). Therefore, LPS may stimulate the PGE2 production in the various cells, including macrophages, mast cells, and osteoblasts by the TLR4-mediated mechanism.
Pharmacological study for the blockades of PGE is closely related to the metabolic steps of PGs, which are regulated by PLA2, COX, and PGES. Various COX inhibitors have been made previously, but no effective cPLA2 inhibitor is currently available. The expression of mPGES is greatly induced by LPS in rat peritoneal macrophages and by IL-1/TNF in rat osteoblasts (13). Uematsu et al. (31) have generated mPGES-null mice and showed the impaired PGE production induced by LPS. Therefore, mPGES inhibitor might be a powerful way of blocking PGE synthesis. In addition, the PGE receptors, divided into four subtypes (EP1, EP2, EP3, and EP4), are important in regulating PGE action in the target cells. Using knockout mice of respective EP and specific agonists, we reported that PGE2 stimulates bone resorption mainly by EP4 (32, 33). Therefore, EP4 inhibitor is a candidate for the blockade of PGE2 action. These trials are necessary for the development of drugs regulating several diseases associated with inflammation.
Using cPLA2α-null mice, previous studies have shown the role of cPLA2α in the allergic response and injury in the lung (20, 34). Acute lung injury in a murine model of the adult respiratory distress syndrome was significantly reduced in cPLA2α-null mice (34). Here, we showed a principal role of cPLA2α in PGE2 production by osteoblastic cells and inflammatory bone loss due to the increased bone resorption. In an accompanying paper, Hegen et al. (35) have shown that cPLA2α-null mice are resistant to collagen-induced arthritis. Therefore, cPLA2α might be involved in bone destruction followed by rheumatoid arthritis. These findings suggest that the inhibition of cPLA2α-initiated pathway might provide a possible therapeutic approach to inflammatory bone destruction in rheumatoid arthritis and periodontal diseases.
Acknowledgments
This work was supported in part by a Human Science Research Grant to C. Miyaura from the Ministry of Health and Welfare, Japan, by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T. Shimizu, and by ONO Medical Research Foundation to N. Uozumi.
Footnotes
Abbreviations used in this paper: BMD, bone mineral density; BV/TV, bone volume/tissue volume; COX, cyclooxygenase; cPLA2α, cytosolic phospholipase A2α; EIA, enzyme immunoassay; mPGES, membrane-bound prostaglandin E synthase; PG, prostaglandin; PGE, prostaglandin E; PGES, PGE synthase; PLA2, phospholipase A2; RANKL, receptor activator of nuclear factor κB ligand; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TLR, Toll-like receptor; TRAP, tartrate-resistant acid phosphatase.
References
- 1.Suda, T., N. Takahashi, and T.J. Martin. 1992. Modulation of osteoclast differentiation. Endocr. Rev. 13:66–80. [DOI] [PubMed] [Google Scholar]
- 2.Raisz, L.G., J.Y. Vanderhoek, H.A. Simmons, B.E. Kream, and K.C. Nicolaou. 1979. Prostaglandin synthesis by fetal rat bone in vitro: evidence for a role of prostacyclin. Prostaglandins. 17:905–914. [DOI] [PubMed] [Google Scholar]
- 3.Sato, K., Y. Fujii, K. Kasono, M. Saji, T. Tsushima, and K. Shizume. 1986. Stimulation of prostaglandin bone resorption by recombinant human interleukin 1 alpha in fetal mouse bone. Biochem. Biophys. Res. Commun. 138:618–624. [DOI] [PubMed] [Google Scholar]
- 4.Akatsu, T., N. Takahashi, N. Udagawa, K. Imamura, A. Yamaguchi, K. Sato, N. Nagata, and T. Suda. 1991. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J. Bone Miner. Res. 6:183–190. [DOI] [PubMed] [Google Scholar]
- 5.Tai, H., C. Miyaura, C.C. Pilbeam, T. Tamura, Y. Ohsugi, Y. Koishihara, N. Kubodera, H. Kawaguchi, L.G. Raisz, and T. Suda. 1997. Transcriptional induction of cyclooxygenase-2 in osteoblasts is involved in interleukin-6-induced osteoclast formation. Endocrinology. 138:2372–2379. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Q.R., C. Miyaura, S. Higashi, M. Murakami, I. Kudo, S. Saito, T. Hiraide, Y. Shibasaki, and T. Suda. 1997. Activation of cytosolic phospholipase A2 by platelet-derived growth factor is essential for cyclooxygenase-2-dependent prostaglandin E2 synthesis in mouse osteoblasts cultured with interleukin-1. J. Biol. Chem. 272:5952–5958. [DOI] [PubMed] [Google Scholar]
- 7.Kudo, I., M. Murakami, S. Hara, and K. Inoue. 1993. Mammalian non-pancreatic phospholipase A2. Biochim. Biophys. Acta. 117:217–231. [DOI] [PubMed] [Google Scholar]
- 8.Smith, W.L., and D.L. DeWitt. 1996. Prostaglandin endoperoxide H synthase-1 and –2. Adv. Immunol. 62:167–215. [DOI] [PubMed] [Google Scholar]
- 9.Clark, J.D., L.L. Lin, R.W. Kriz, C.S. Ramesha, L.A. Sultzman, A.Y. Lin, N. Milona, and J.L. Knopf. 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+- dependent translocation domain with homology to PKC and GAP. Cell. 65:1043–1051. [DOI] [PubMed] [Google Scholar]
- 10.Sharp, J.D., D.L. White, X.G. Chiou, T. Goodson, G.C. Gamboa, D. McClure, S. Burgett, J. Hoskins, P.L. Skatrud, and J.R. Sportsman. 1991. Molecular cloning and expression of human Ca(2+)-sensitive cytosolic phospholipase A2. J. Biol. Chem. 266:14850–14853. [PubMed] [Google Scholar]
- 11.Jakobsson, P.-J., S. Thoren, R. Morgenstern, and B. Samuelsson. 1999. Identification of human prostaglandin E synthase: A microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA. 96:7220–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanioka, T., Y. Nakatani, N. Semmyo, M. Murakami, and I. Kudo. 2000. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 275:32775–32782. [DOI] [PubMed] [Google Scholar]
- 13.Murakami, M., H. Naraba, T. Tanioka, N. Semmyo, Y. Nakatani, F. Kojima, T. Ikeda, M. Fueki, A. Ueno, S. Oh, and I. Kudo. 2000. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275:32783–32792. [DOI] [PubMed] [Google Scholar]
- 14.Ushikubi, F., E. Segi, Y. Sugimoto, T. Murata, T. Matsuoka, T. Kobayashi, H. Hizaki, K. Tuboi, M. Katsuyama, A. Ichikawa, et al. 1998. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 395:281–284. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Amer, Y., F.P. Ross, J. Edwards, and S.L. Teitelbaum. 1997. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J. Clin. Invest. 100:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuguchi, T., T. Musikacharoen, T. Ogawa, and Y. Yoshikai. 2000. Gene expression of Toll-like receptor 2, but not toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J. Immunol. 165:5767–5772. [DOI] [PubMed] [Google Scholar]
- 17.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in tlr 4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 18.Dennis, E.A. 2000. Phospholipase A2 in eicosanoid generation. Am. J. Respir. Crit. Care Med. 161:S32–S35. [DOI] [PubMed] [Google Scholar]
- 19.Leslie, C.C. 1997. Properties and regulation of cytosolic phospholipase A2. J. Biol. Chem. 272:16709–16712. [DOI] [PubMed] [Google Scholar]
- 20.Uozumi, N., K. Kume, T. Nagase, N. Nakatani, S. Ishii, F. Tashiro, Y. Komagata, K. Maki, K. Ikuta, Y. Ouchi, et al. 1997. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 390:618–622. [DOI] [PubMed] [Google Scholar]
- 21.Bonventre, J.V., Z. Huang, M.R. Taheri, E. O'Leary, E. Li, M.A. Moskowitz, and A. Sapirstein. 1997. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 390:622–625. [DOI] [PubMed] [Google Scholar]
- 22.Miyaura, C., K. Kusano, T. Masuzawa, O. Chaki, Y. Onoe, M. Aoyagi, T. Sasaki, T. Tamura, Y. Koishihara, Y. Ohsugi, et al. 1995. Endogenous bone-resorbing factors in estrogen deficiency: cooperative effects of IL-1 and IL-6. J. Bone Miner. Res. 10:1365–1373. [DOI] [PubMed] [Google Scholar]
- 23.Miyaura, C., Y. Onoe, M. Inada, K. Maki, K. Ikuta, M. Ito, and T. Suda. 1997. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc. Natl. Acad. Sci. USA. 94:9360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udagawa, N., N. Takahashi, T. Akatsu, T. Sasaki, A. Yamaguchi, H. Kodama, T.J. Martin, and T. Suda. 1989. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteocalst-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 125:1805–1813. [DOI] [PubMed] [Google Scholar]
- 25.Sato, T., I. Morita, K. Sakaguchi, K.I. Nakahama, W.L. Smith, D.L. Dewitt, and S. Murota. 1996. Involvement of prostaglandin endoperoxide H synthase-2 in osteoclast-like cell formation induced by interleukin-1 beta. J. Bone Miner. Res. 11:392–400. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, et al. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA. 95:3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson, D.M., E. Maraskovsky, W.L. Billingsley, W.C. Dougall, M.E. Tometsko, E.R. Roux, M.C. Teepe, R.F. DuBose, D. Cosman, and L. Galibert. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 390:175–179. [DOI] [PubMed] [Google Scholar]
- 28.Wong, B.R., J. Rho, J. Arron, E. Robinson, J. Orlinick, M. Chao, S. Kalachikov, E. Cayani, F.S. Bartlett, W.N. Frankel, et al. 1997. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 272:25190–25194. [DOI] [PubMed] [Google Scholar]
- 29.Lacey, D.L., E. Timms, H.L. Tan, M.J. Kelley, C.R. Dunstan, T. Burgess, R. Elliott, A. Colombero, G. Elliott, S. Scully, et al. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 93:165–176. [DOI] [PubMed] [Google Scholar]
- 30.Fujishima, H., M.R.O. Sanchez, C.O. Bingham, B.K. Lam, A. Sapirstein, J.V. Bonventre, K.F. Austen, and J.P. Arm. 1999. Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc. Natl. Acad. Sci. USA. 96:4803–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uematsu, S., M. Matsumoto, K. Takeda, and S. Akira. 2002. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J. Immunol. 168:5811–5816. [DOI] [PubMed] [Google Scholar]
- 32.Miyaura, C., M. Inada, T. Suzawa, Y. Sugimoto, F. Ushikubi, A. Ichikawa, S. Narumiya, and T. Suda. 2000. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J. Biol. Chem. 275:19819–19823. [DOI] [PubMed] [Google Scholar]
- 33.Suzawa, T., C. Miyaura, M. Inada, T. Maruyama, Y. Sugimoto, F. Ushikubi, A. Ichikawa, S. Narumiya, and T. Suda. 2000. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3 and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 141:1554–1559. [DOI] [PubMed] [Google Scholar]
- 34.Nagase, T., N. Uozumi, S. Ishii, K. Kume, T. Izumi, Y. Ouchi, and T. Shimizu. 2000. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat. Immunol. 1:42–46. [DOI] [PubMed] [Google Scholar]
- 35.Hegen, M., L. Sun, N. Uozumi, K. Kume, M.E. Goad, C.L. Nickerson-Nutter, T. Shimizu, and J.D. Clark. 2003. Cytosolic phospholipase A2α–deficient mice are resistant to collagen-induced arthritis. J. Exp. Med. 197:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]