Abstract
Mycobacterium tuberculosis represents a world-wide health risk and immunosuppression is a particular problem in M. tuberculosis infections. Although macrophages are primarily infected, dendritic cells (DCs) are important in inducing cellular immune responses against M. tuberculosis. We hypothesized that DCs represent a target for M. tuberculosis and that the observed immuno-suppression results from modulation of DC functions. We demonstrate that the DC-specific C-type lectin DC-SIGN is an important receptor on DCs that captures and internalizes intact Mycobacterium bovis bacillus Calmette-Guérin (BCG) through the mycobacterial cell wall component ManLAM. Antibodies against DC-SIGN block M. bovis BCG infection of DCs. ManLAM is also secreted by M. tuberculosis–infected macrophages and has been implicated as a virulence factor. Strikingly, ManLAM binding to DC-SIGN prevents mycobacteria- or LPS-induced DC maturation. Both mycobacteria and LPS induce DC maturation through Toll-like receptor (TLR) signaling, suggesting that DC-SIGN, upon binding of ManLAM, interferes with TLR-mediated signals. Blocking antibodies against DC-SIGN reverse the ManLAM-mediated immunosuppressive effects. Our results suggest that M. tuberculosis targets DC-SIGN both to infect DCs and to down-regulate DC-mediated immune responses. Moreover, we demonstrate that DC-SIGN has a broader pathogen recognition profile than previously shown, suggesting that DC-SIGN may represent a molecular target for clinical intervention in infections other than HIV-1.
Keywords: DC-SIGN, Toll-like receptors, Mycobacterium tuberculosis, ManLAM, immunosuppression
Introduction
Tuberculosis has been a major world-wide cause of death for centuries. One third of the world's population is infected with Mycobacterium tuberculosis, which causes 2 million deaths per year. Macrophages are the primary targets for M. tuberculosis, and the mycobacteria survive within so called phagosomes of the infected macrophages. Initially, innate immune responses against mycobacteria predominate and are directed by activated macrophages (for a review, see reference 1). However, subsequent recruitment of cellular responses that restrict mycobacterial infections, are mediated by dendritic cells (DCs)* (2). Immature DCs are seeded throughout peripheral tissues to act as sentinels against invading pathogens. Upon pathogen capture, DCs are activated, process pathogens into antigenic peptides for presentation in association with either MHC II or nonclassical MHC-like molecules such as CD1, and migrate to the secondary lymphoid organs where they activate naive T cells to initiate adaptive immune responses (3). Indeed, immature DCs internalize M. tuberculosis–derived lipoarabinomannans and present these structures via the CD1b-presentation pathway to lipoarabinomannan-specific T cells (4).
After the initial reactive phase, M. tuberculosis infections are restricted by cellular immune responses and enter a chronic latent phase in the host. However, latent infections have the potential to reactivate and cause clinical tuberculosis (5, 6). The ability of M. tuberculosis to exist as a latent infection of the host suggests that mycobacteria are able to suppress cellular immune responses. Although DCs are not the primary targets for infection by mycobacteria, the specific function of DCs in the cellular immune response seems to be modulated by mycobacteria (7). Thus, knowledge about the interaction of DCs with mycobacteria and mycobacterial components is essential to fully understand and combat M. tuberculosis infections.
The interaction of mycobacteria with macrophages has been extensively investigated and the mannose receptor (MR), CD11b, and CD11c have been demonstrated to act as receptors on macrophages for mycobacteria (8, 9). Although these receptors also have been implicated in the interaction of mycobacteria with DCs (4, 7), little is known about the actual cell-surface receptors on DCs that are involved in DC–mycobacteria interactions. We have recently identified the DC-specific C-type lectin DC-SIGN that plays a key role in the dissemination of HIV-1 by DCs through HIV-1 gp120 binding (10). DC-SIGN has a high affinity for mannose-containing carbohydrates (11, 12) and we hypothesized that, based on its carbohydrate recognition profile, DC-SIGN might function as a receptor for pathogens other than HIV-1.
We have investigated the interaction of DC-SIGN with mycobacteria and demonstrate that DC-SIGN is an important receptor on DCs for viable mycobacteria, such as M. tuberculosis and M. bovis bacillus Calmette-Guérin (BCG), and ManLAM, despite the presence of the other reported mycobacterial receptors. We demonstrate that mycobacteria specifically target DC-SIGN through ManLAM to impair DC maturation and to induce production of the antiinflammatory cytokine IL-10. These conditions promote immunosuppression and may contribute to the survival of M. tuberculosis. These results imply that clinical strategies targeting DC-SIGN function could succeed in combating M. tuberculosis infections by shifting the precarious balance between immune activation and suppression to favor the elimination of mycobacteria.
Materials and Methods
Antibodies.
The following antibodies were used: anti-MR (Clone 19; BD Biosciences), CD11b (bear-1; reference 13), CD11c (SHCL3; reference 14), anti-DC-SIGN (AZN-D1, AZN-D2 [12], CSRD [15]), LAMP-1 (H4A3; BD Biosiences), and the PE-conjugated antibodies CD80, CD86, HLA-DR (BD Biosciences), and CD83 (Beckman Coulter).
Cells.
Immature DCs were cultured from monocytes in the presence of IL-4 and GM-CSF (500 and 800 U/ml, respectively; Schering-Plough; reference 16). K562 transfectants expressing wild-type DC-SIGN were generated by transfection of K562 cells with 10 μg pRc/CMV-DC-SIGN plasmid by electroporation as described previously (10).
Mycobacteria.
M. smegmatis, M. bovis BCG (Pasteur), M. tuberculosis strains H37Ra and H37Rv, and M. paratuberculosis were gifts from A. Kolk (Royal Tropical Institute, Amsterdam, Netherlands). M. bovis BCG was cultured in vitro using Middelbrook 7H9 broth supplemented with 0.05% Tween 80 and albumin-dextrose-catalase. The glycolipids ManLAM and AraLAM were obtained from J. Belisle, Colorado State University, Fort Collins, CO, and the National Institutes of Health, Bethesda, MD (contract NO1 AI-75320). DCs were infected with mycobacteria by coculturing them at an appropriate multiplicity of infection (MOI) as indicated in the figure legends.
Capture and internalization of mycobacteria by cells was evaluated using FITC-conjugated M. bovis BCG. Bacteria (109/ml) were labeled by incubation of 0.5 mg FITC per ml in phosphate-buffered saline (pH 7.4) at room temperature for 1 h. The FITC-pulsed bacteria were washed three times to remove unbound FITC. Capture was determined by measuring the percentage of cells that bound FITC-conjugated bacteria using flow cytometry (FACSCalibur™; Becton Dickinson). Phagocytosis was determined using a fluorescence-quenching technique as reported previously (17). In brief, quenching of noninternalized membrane-bound FITC-conjugated M. bovis BCG was achieved by treating the cells with 0.05% trypan blue for 5 min.
Fluorescent Bead Adhesion Assay.
Carboxylate-modified TransFluorSpheres (488/645 nm, 1.0 μm; Molecular Probes) were coated with the glycolipid forms of lipoarabinomannan (LAM). Streptavidin-coated beads (18) were incubated with biotinylated F(ab′)2 fragment goat anti–mouse IgG (6 μg/ml; Jackson ImmunoResearch Laboratories) followed by an overnight incubation with mouse-anti-LAM antibody (F30.5) at 4°C. The beads were washed and incubated with 250 ng/ml purified glycolipid LAM overnight at 4°C. The fluorescent beads adhesion assay was performed as described by Geijtenbeek et al. (18).
Soluble DC-SIGN-Fc Adhesion Assay.
DC-SIGN-Fc consists of the extracellular portion of DC-SIGN (amino acid residues 64–404) fused at the COOH terminus to a human IgG1-Fc fragment (19). The soluble DC-SIGN adhesion assay was performed as follows. Soluble ligands were coated onto ELISA plates (1 μg/well for purified proteins and 5 μg/well for lysates) for 18 h at room temperature, followed by blocking with 1% BSA for 2 h at 37°C. Soluble DC-SIGN-Fc supernatant was added for 30 min at 37°C. Unbound DC-SIGN-Fc was washed away and binding was determined by anti-IgG1 ELISA. Specificity was determined in the presence of either 50 μg/ml blocking antibodies, 50 μg/ml mannan or 5 mM EGTA.
DC Activation.
Immature DCs (2 × 106 cells/ml) were cultured for 24 h in the presence of IL-4 (500 U/ml; Schering-Plough), GM-CSF (800 U/m; Schering-Plough), and either LPS (10 ng/ml) or LAM glycolipids (15 μg/ml). The effect of LAM on LPS-induced activation was determined by preincubating immature DCs (300,000 cells) with AZN-D2 (40 μg/ml) for 30 min, and subsequently with LPS in the presence of LAM (15 μg/ml) for 18 h. LAM glycolipids were obtained from J. Belisle (Colorado State University and the National Institutes of Health [contract NO1 AI-75320]) and contained <5 ng/mg endotoxin. Activation was determined by cell-surface expression of MHC class II (HLA-DR) and the costimulatory molecules CD80, CD83, and CD86 using PE-conjugated antibodies.
Cytokine Production.
For the detection of cytokines, culture supernatants were harvested at day 1 and frozen at −80°C until analysis. The supernatants were analyzed for the presence of IL-10 and IL-12p40 by ELISA (Biosource International).
Results
DC-SIGN Interacts with M. tuberculosis through ManLAM Glycolipids.
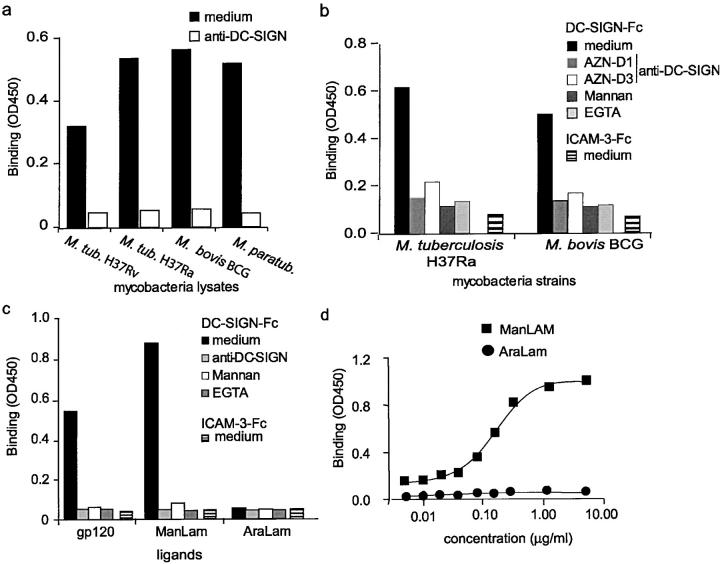
The interaction of DC-SIGN with different mycobacteria strains was investigated using the DC-SIGN-Fc binding assay (19). DC-SIGN-Fc specifically interacted with the lysates from both virulent and avirulent M. tuberculosis strains, as the interaction was inhibited with blocking antibodies against DC-SIGN (Fig. 1 a). Moreover, DC-SIGN also interacted with the lysates from other mycobacteria strains, such as M. bovis BCG and M. paratuberculosis (Fig. 1 a). Next, we investigated whether DC-SIGN-Fc also interacts with viable mycobacteria. Therefore, viable M. tuberculosis H37Ra and M. bovis BCG were coated and the interaction of DC-SIGN with these pathogens was analyzed using the DC-SIGN-Fc binding assay (19). M. bovis BCG is a tuberculosis vaccine strain that is mildly virulent but nonpathogenic, yet retains some immunological properties of tuberculosis (20). DC-SIGN-Fc interacted specifically with both M. tuberculosis H37Ra and M. bovis BCG, as the interaction was inhibited with blocking DC-SIGN–specific antibodies (Fig. 1 b). Moreover, an irrelevant Fc chimera, intercellular adhesion molecule (ICAM)-3-Fc, did not interact with the mycobacteria (Fig. 1 b). The interaction is mediated by the C-type lectin domain of DC-SIGN, as binding to both M. tuberculosis and M. bovis BCG was inhibited by either the Ca2+-chelator EGTA, mannan, or the DC-SIGN–specific antibody AZN-D1 that recognizes the lectin domain (19; Fig. 1 b). We next investigated the binding of DC-SIGN to purified mycobacterial cell wall lipoarabinomannan (LAM), as DC-SIGN has a high affinity for mannose-containing carbohydrates (11, 12) and LAM comprises a mannose-rich polysaccharide core, containing highly branched arabinofuranosyl side chains, and a GPI anchor (21). LAM isolated from M. tuberculosis contains mannose residues consisting exclusively of mono-, di-, and trimers of α-d-mannoses directly linked to the arabinofuranosyl-termini and is called ManLAM, whereas LAM isolated from the fast growing M. smegmatis is not mannose-capped and is called AraLAM (21). Strikingly, purified ManLAM was efficiently bound by DC-SIGN, in contrast to AraLAM (Fig. 1 c), demonstrating that DC-SIGN specifically interacts with the mono-, di-, and trimers of α-d-mannoses of ManLAM. Even at high concentrations, DC-SIGN did not bind AraLAM, demonstrating a high specificity for ManLAM and its mannose-cap (Fig. 1 d). The interaction of DC-SIGN with ManLAM is specific, as the binding was inhibited by antibodies against DC-SIGN, whereas an irrelevant Fc chimera did not interact with ManLAM (Fig. 1 c). These data suggest that DC-SIGN interacts with various mycobacteria strains that contain ManLAM, whereas it may not bind mycobacteria strains that contain LAM without the mannose-cap, such as M. smegmatis.
Figure 1.
DC-SIGN specifically binds ManLAM, a cell wall component of M. tuberculosis. (a) DC-SIGN interacts with several mycobacteria strains. DC-SIGN-Fc binding to mycobacteria (lysates; 5 μg) was determined by a Fc-specific ELISA. Specificity was determined by measuring binding in the presence of blocking DC-SIGN–specific antibodies (AZN-D1). Standard deviation <0.02 OD450. One representative experiment out of three is shown. (b) DC-SIGN interacts with viable mycobacteria strains. DC-SIGN-Fc binding to viable mycobacteria (5 × 105 bacteria) was determined by a Fc-specific ELISA. Specificity was determined by measuring binding in the presence of blocking DC-SIGN–specific antibodies (AZN-D1 or AZN-D3) and mannan. EGTA was used to determine the calcium dependency of the DC-SIGN-Fc–mediated binding. ICAM-3-Fc binding to mycobacteria was also measured to exclude nonspecific binding by the Fc domain. Standard deviation <0.02 OD450. One representative experiment out of three is shown. (c) The mannosylated-lipoarabinomannan ManLAM, in contrast to the nonmannosylated AraLAM, is specifically bound by DC-SIGN. The anti–DC-SIGN antibody AZN-D1 was used to determine specificity. The DC-SIGN-Fc binding assay was performed as described for panel a. Standard deviation <0.02 OD450. One representative experiment out of three is shown. (d) DC-SIGN does not interact with AraLAM. The DC-SIGN-Fc binding assay was performed as described for panel a. One representative experiment out of three is shown.
Both Mycobacteria and ManLAM Interact with the Primary Binding Site of DC-SIGN.
We used K562 transfectants stably expressing DC-SIGN to investigate the binding of cell-surface–expressed DC-SIGN to M. bovis BCG, M. smegmatis, and the mycobacterial component ManLAM. These cells do not express the previously reported mycobacterial receptors MR, CD11b, and CD11c (8, 9; Fig. 2 a). K562 transfectants express high levels of DC-SIGN (Fig. 2 a) and bind strongly to both viable M. bovis BCG and ManLAM, in contrast to mock transfected K562 cells (Fig. 2 b). The interaction is blocked by DC-SIGN–specific antibodies (Fig. 2 b). DC-SIGN did not bind to viable M. smegmatis, which contains uncapped AraLAM (Fig. 2 b). This supports the hypothesis that DC-SIGN binds ManLAM on M. bovis BCG. The interaction of DC-SIGN with both M. bovis BCG and ManLAM is similar to that of the other DC-SIGN ligands ICAM-3 and HIV-1 (Fig. 2 b). DC-SIGN expressed by K562 transfectants did not interact with AraLAM (unpublished data). Thus, cellular DC-SIGN specifically binds to both M. bovis BCG and ManLAM, as was observed with recombinant DC-SIGN-Fc (Fig. 1 c).
Figure 2.

Cellular DC-SIGN binds strongly to both viable mycobacteria and the mycobacterial component ManLAM through its primary binding site. (a) K562-DC-SIGN transfectants express high levels of DC-SIGN but lack expression of the other reported ManLAM receptors MR, CD11b, and CD11c. Transfectants were generated as described previously (reference 12). Open histograms represent the isotype controls, and filled histograms indicate the specific antibody staining. (b) DC-SIGN, expressed by K562 transfectants, binds strongly to intact M. bovis BCG and the mycobacterial component ManLAM but not to M. smegmatis and AraLAM. The adhesion of cells to the LAM glycans was determined using the fluorescent bead adhesion assay. Binding to viable mycobacteria was determined by measuring the binding of K562 transfectants to FITC-conjugated mycobacteria (MOI 20) using flow cytometry. Specificity was determined by measuring binding in the presence of blocking antibodies against DC-SIGN. Standard deviation for the fluorescent bead adhesion assay and the mycobacteria binding assay was <5 and <2%, respectively. One representative experiment out of three is shown. (c) The Val351 amino acid residue is not essential for the interaction of DC-SIGN with M. bovis BCG and ManLAM, similar to HIV-1 gp120, whereas it is essential for ICAM-3 binding. Binding to the V351G DC-SIGN mutant expressed by K562 cells was measured as described for panel b. Specificity was determined by measuring binding in the presence of blocking antibodies against DC-SIGN, mannan or EGTA. Standard deviation <5% (fluorescent bead adhesion assay) and <2% (mycobacteria binding assay). One representative experiment out of three is shown.
The C-type lectin domain of DC-SIGN contains two calcium ions (12), and the amino acid residues that are in close contact with Ca2+ at site 2 (Glu347, Asn349, Glu354, and Asn365) form the core of the ligand binding site (19, 22). Changing in DC-SIGN either Glu347 into Gln (E347Q), or Asn349 and Asn365 into Asp, resulted in complete loss of binding to viable mycobacteria and ManLAM (Fig. 2 b, and unpublished data), as was previously shown for both ICAM-3 and HIV-1 gp120 (Fig. 2 b, and reference 19). The Ca2+ at site 1, the so-called auxiliary site, coordinates the correct positioning of the primary binding site (19, 22), and loss of this Ca2+ by mutating Asp320, Glu324 (E324A), Asn350, or Asp355 into Ala residues resulted in complete loss of both M. bovis BCG and ManLAM binding (Fig. 2 b, and unpublished data).
Recently, we demonstrated that the binding site of DC-SIGN for its cellular ligand ICAM-3 is distinct from that of HIV-1 gp120 (19), as a specific mutation in DC-SIGN (V351G) abrogated ICAM-3, but not HIV-1 gp120 binding (Fig. 2 c, and reference 19). Strikingly, the DC-SIGN V351G mutant also interacts with M. bovis BCG as well as ManLAM (Fig. 2 c), demonstrating that both HIV-1 and mycobacteria bind similarly to DC-SIGN at a distinct site from the cellular ligand ICAM-3.
DC-SIGN Is an Important Receptor for Mycobacteria on DCs.
Immature DCs express, besides high levels of DC-SIGN, high levels of the receptors MR, CD11b, and CD11c (Fig. 3 a), which have previously been reported to mediate binding of mycobacteria by macrophages (8, 9). We used blocking antibodies against these receptors to evaluate their contributions to ManLAM binding by DCs. Immature DCs bind strongly to ManLAM, but not to AraLAM, and the interaction was inhibited by the DC-SIGN–specific antibody, but strikingly not by any of the antibodies against MR, CD11b, or CD11c (Fig. 3 b). Both EGTA and the C-type lectin-specific inhibitor mannan block ManLAM binding by DCs to a similar extent as the DC-SIGN–specific antibodies, demonstrating that DC-SIGN is an important ManLAM-binding C-type lectin on immature DCs (Fig. 3 b).
Figure 3.
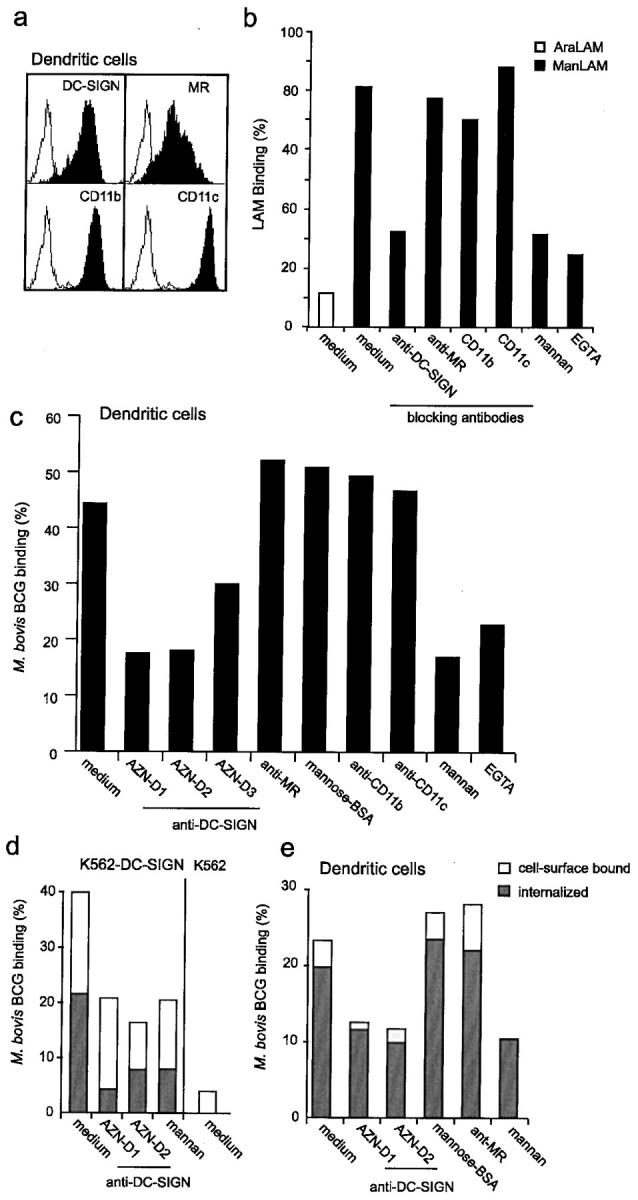
DC-SIGN is an important receptor for both ManLAM and mycobacteria on DCs. (a) Immature DCs express high levels of DC-SIGN and the other reported LAM receptors MR, CD11b, and CD11c. Open histograms represent isotype control and filled histograms indicate specific antibody staining. (b) Immature DCs bind strongly to ManLAM via DC-SIGN. Binding was determined using the fluorescent bead adhesion assay. Specificity was determined by measuring binding in the presence of mannan, EGTA or blocking antibodies against DC-SIGN (AZN-D2), MR (Clone 19), CD11b (bear-1), or CD11c (SHCL3). Standard deviation <5%. One representative experiment out of three is shown. (c) DC-SIGN mediates capture of M. bovis BCG by immature DCs. Binding was determined by flow cytometry using FITC-conjugated mycobacteria. Specificity was determined by measuring binding in the presence of antibodies against DC-SIGN (AZN-D1, AZN-D2, and AZN-D3), MR (Clone 19), CD11b (bear-1), and CD11c (SHCL3). Binding was also measured in the presence of the C-type lectin inhibitors mannan and EGTA, whereas a known MR ligand, mannose-BSA, was used to determine the contribution of the MR receptor. Standard deviation <2%. One representative experiment out of three is shown. (d) DC-SIGN mediates capture and internalization of M. bovis BCG by K562 cells. K562 transfectants were incubated with FITC-conjugated M. bovis BCG (MOI 20). Cells were washed, and surface FITC was quenched by exposure to trypan blue. Phagocytosis was determined by comparing the FITC emission before and after quenching using flow cytometry. Surface bound bacteria are represented by open bars, internalized by closed bars. Standard deviation <4%. One representative experiment out of three is shown. (e) Immature DCs rapidly phagocytose mycobacteria through DC-SIGN. The internalization was determined as described for panel b. Surface bound bacteria are represented by open bars, internalized by closed bars. Standard deviation <5%. One representative experiment out of three is shown.
The major contribution of DC-SIGN to the interaction of immature DCs with ManLAM prompted us to investigate whether DC-SIGN was also important in the interaction of immature DCs to viable mycobacteria. Immature DCs interacted strongly with M. bovis BCG (Fig. 3 c). Strikingly, DC-SIGN is an important receptor for M. bovis BCG, as the antibodies against DC-SIGN strongly inhibited the infection of immature DCs with M. bovis BCG (Fig. 3 c). Antibodies against MR, CD11b, and CD11c did not inhibit the infection, whereas the C-type lectin inhibitor mannan blocked the infection to a similar level as the DC-SIGN antibodies (Fig. 3 c). Moreover, the MR-ligand mannose-BSA did not inhibit the interaction of DCs with M. bovis BCG (Fig. 3 c) demonstrating that the C-type lectin domains of MR are not involved in M. bovis BCG infection of DCs. Both the anti-MR antibody and mannose-BSA are inhibitors of MR function as they block binding of another MR-ligand, dextran, to DCs (unpublished data). These results demonstrate that DC-SIGN is an important C-type lectin on DCs that functions as a receptor for M. bovis BCG. Other nonlectin receptors may contribute to the interaction, as the infection was not completely inhibited by antibodies against DC-SIGN (Fig. 3 c).
Next, we investigated whether DC-SIGN mediates internalization of M. bovis BCG by using the trypan blue method to quench surface FITC-conjugated mycobacteria. Mock transfected K562 cells did not phagocytose M. bovis BCG, whereas 50% of the K562 transfectant, expressing DC-SIGN, had internalized M. bovis BCG within 45 min (Fig. 3 d). Both mannan and antibodies against DC-SIGN blocked the internalization of M. bovis BCG (Fig. 3 d).
Immature DCs are highly phagocytoic cells and indeed within 45 min >90% of the DCs that bound viable M. bovis BCG, had also internalized the mycobacteria (Fig. 3 e). As was observed for the binding of M. bovis BCG by DCs (Fig. 3c), phagocytosis of M. bovis BCG is partly blocked by antibodies against DC-SIGN, whereas both anti-MR antibodies and the MR-ligand mannose-BSA did not inhibit the observed phagocytosis (Fig. 3 e). Both anti-MR antibodies and the MR-ligand mannose–BSA blocked internalization of dextran by immature DC (unpublished data). These results demonstrate that DC-SIGN enables the capture and internalization of M. bovis BCG by immature DCs through binding of the mycobacterial cell wall component ManLAM.
Mycobacteria and ManLAM Are Internalized by DC-SIGN and Targeted to Lysosomes.
Recently, we have demonstrated that DC-SIGN can function as an antigen receptor that internalizes antigens and targets them to lysosomal compartments for presentation on MHC class II (15). Therefore, the fate of the captured M. bovis BCG by immature DCs was followed by immunofluorescence analyses. Immature DCs were infected with FITC-conjugated M. bovis BCG for 2 h and both DC-SIGN and the lysosomal marker LAMP-1/CD107a were stained (Fig. 4 a). The observed colocalization of DC-SIGN with FITC-conjugated M. bovis BCG further supports a role for DC-SIGN in the capture and internalization of mycobacteria (Fig. 4 a). Phagocytosed mycobacteria were targeted to lysosomes, as the internalized FITC-conjugated mycobacteria colocalized with LAMP-1 staining (Fig. 4 a). Similarly, ManLAM was also captured and internalized by DC-SIGN on immature DCs, as ManLAM staining colocalized with DC-SIGN (Fig. 4 a) whereas AraLAM was not internalized by DCs (unpublished data). Internalized ManLAM colocalized with LAMP-1 in immature DC (Fig. 4 a) indicating that internalized ManLAM is targeted to lysosomes.
Figure 4.
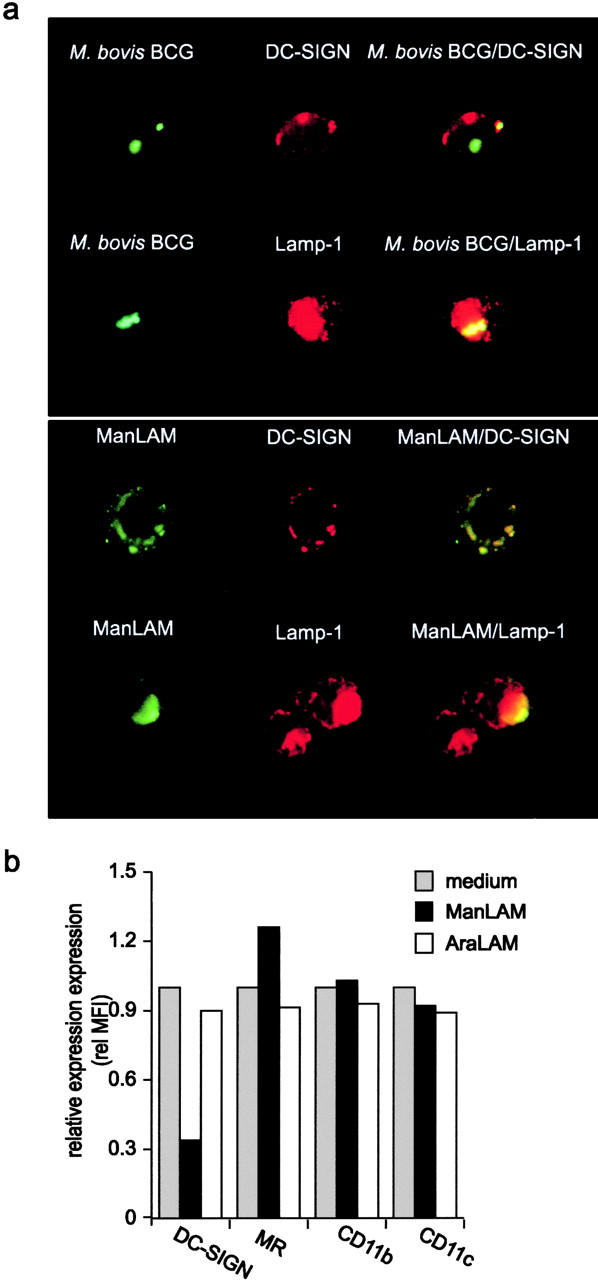
DC-SIGN mediates internalization of captured mycobacteria and ManLAM. (a) M. bovis BCG and ManLAM are internalized by DC-SIGN on immature DCs and targeted to the lysosomes. The fate of captured mycobacteria was followed by analyzing immature DCs pulsed with FITC-conjugated M. bovis BCG (MOI 20) for 2 h using immunofluorescence microscopy (magnification 200×). ManLAM was followed by incubating DCs with ManLAM (10 μg/ml) for 1 h. DC-SIGN, ManLAM, and CD207a/LAMP-1 were stained with AZN-D1, F30.5, and H4A3, respectively. One representative experiment out of three is shown. (b) ManLAM induces down-regulation of DC-SIGN, but not of MR, CD11b, and CD11c. Immature DCs were incubated with 15 μg/ml of ManLAM or AraLAM for 18 h, and then DC-SIGN expression was determined by flow cytometry. One representative experiment out of three is shown.
Recently, we demonstrated that binding of soluble ligands or antibodies to DC-SIGN triggers internalization of the DC-SIGN–ligand complex to late endosomes/lysosomes, and results in down-regulation of DC-SIGN from the surface (15). Therefore, we investigated whether DC-SIGN was internalized after ManLAM binding by measuring the cell-surface expression of DC-SIGN after ManLAM binding using a specific antibody against DC-SIGN (15). Indeed, binding of ManLAM, but not AraLAM, to DC-SIGN resulted in down-regulation of DC-SIGN (Fig. 4 b), demonstrating that DC-SIGN on DCs binds ManLAM and mediates the internalization of ManLAM to LAMP-1+ lysosomes. However, other mycobacteria receptors such as MR, CD11b, and CD11c were not down-regulated (Fig. 4 b). Thus, ManLAM binding to DC-SIGN triggers internalization of the DC-SIGN/ManLAM complex to lysosomes and may enable antigen processing of ManLAM by DCs.
ManLAM Modulates Cytokine Production by DCs through DC-SIGN Binding.
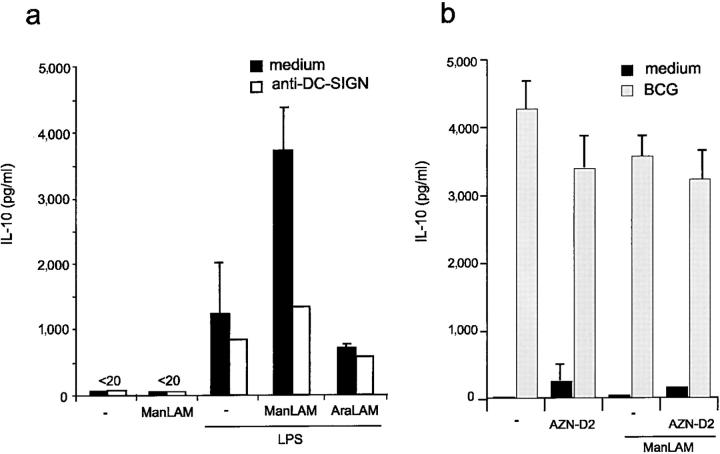
ManLAM is present not only a mycobacterial cell wall component but is also secreted from phagosomes after macrophage ingestion of M. tuberculosis (21, 23, 24). Potentially, mycobacteria within infected macrophages can influence bystander immune cells and modulate the immune response through secretion of ManLAM. The cytokine IL-10 is a potent immunosuppressive factor induced in macrophages by some intracellular bacteria to dampen down host immune responses and promote their survival (25). We investigated the influence of ManLAM binding to DC-SIGN in IL-10 production by DCs. ManLAM alone did not induce IL-10 production by immature DC (Fig. 5 a). Strikingly, ManLAM, but not AraLAM, strongly induced IL-10 production by DCs, when they received simultaneously an activation signal, such as LPS (Fig. 5 a). This IL-10 induction was completely inhibited by DC-SIGN–specific antibodies to the level of LPS-activated DCs alone (Fig. 5 a). The findings that only ManLAM could induce IL-10 production, which could be blocked by DC-SIGN–specific antibodies indicates that the IL-10 induction is specific for the ManLAM/DC-SIGN interaction. Antibodies against DC-SIGN alone did not induce IL-10 production by LPS-activated DCs (Fig. 5 a), demonstrating that ligation of DC-SIGN alone is not sufficient for IL-10 induction. Thus, the binding of ManLAM to DC-SIGN triggers intracellular signals that induce IL-10 production by DCs, indicating that mycobacteria target DC-SIGN to suppress the immune response and promote their survival in the host. Both immature and LPS-activated DCs, alone or in combination with ManLAM, produced very low amounts of IL-12p70 (<5 pg/ml). Infection of immature DCs with M. bovis BCG induced a strong production of IL-10 that was not inhibited by antibodies against DC-SIGN (Fig. 5 b). No differences were observed in the presence of ManLAM (Fig. 5 b). These results suggest that mycobacteria induce IL-10 production by direct infection as well as by secreting ManLAM.
Figure 5.
Mycobacteria induce IL-10 production by DCs through ManLAM and direct infection. (a) ManLAM induces IL-10 production of LPS-matured DCs. Immature DCs were incubated with 15 μg/ml of either ManLAM or AraLAM in the presence of LPS (10 ng/ml). The specificity was determined in the presence of blocking antibodies against DC-SIGN (AZN-D2; 20 μg/ml). Supernatants were harvested after 18 h and the IL-10 production was measured by ELISA. Values are the means ± standard deviations of triplicate determinations. One representative experiment out of three is shown. (b) M. bovis BCG infection of immature DCs induces IL-10 production. Immature DCs were infected with M. bovis BCG (MOI 4), and the experiment was performed as described for panel a. Values are the means ± standard deviations of triplicate determinations. One representative experiment out of three is shown.
ManLAM Inhibits DC Activation through DC-SIGN.
Immature DCs are highly efficient in antigen capture and processing, whereas mature DCs are specialized in the naive T cell activation necessary for cellular immune responses (3). Immature DCs mature in response to specific ‘danger’ signals such as bacterial components (LPS) or inflammatory cytokines (TNFα, PGE2). We investigated the effect of ManLAM on the maturation of DCs. Neither ManLAM nor AraLAM induced DC maturation, as both ManLAM and AraLAM, in contrast to LPS that triggers Toll-like receptor (TLR)4, did not up-regulate expression of the activation markers CD80, CD83, CD86, or HLA-DR (Fig. 6 a).
Figure 6.
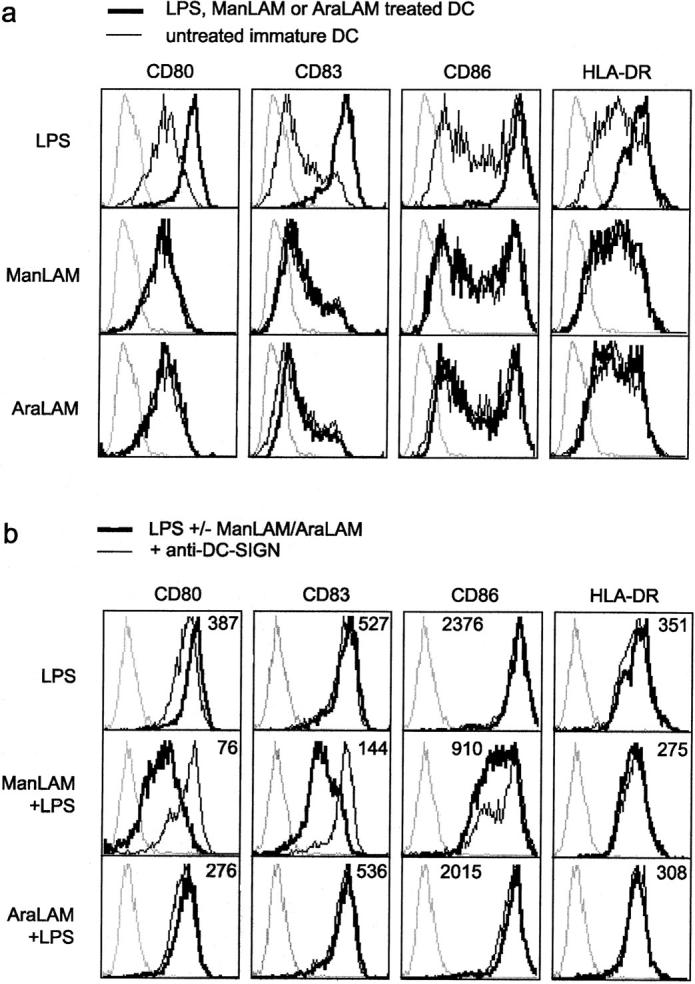
ManLAM inhibits LPS-induced DC activation through DC-SIGN binding. (a) ManLAM does not induce activation of immature DCs. Immature DCs were incubated with ManLAM, AraLAM, or LPS for 18 h, and activation was determined by measuring the expression of CD80, CD86, CD83, and HLA-DR. Dotted lines represent isotype controls, the thin lines indicate expression levels of immature DCs, and the thick line represents immature DCs that have been treated with either LPS (10 ng/ml), ManLAM (15 μg/ml), or AraLAM (15 μg/ml). One representative experiment out of three is shown. (b) LPS-induced activation of DCs is blocked by ManLAM. Immature DCs were cocultured with LPS alone, or together with either ManLAM or AraLAM for 18 h. Dotted lines represent isotype controls. Thick lines, and the mean fluorescence values in the histograms, represent the expression levels after treatment with LPS alone, or in combination with either ManLAM or AraLAM. Thin lines indicate the presence of antibodies against DC-SIGN throughout the incubation. One representative experiment out of three is shown.
Acute mycobacterial infections represent sites of inflammation that attract and induce DC maturation through the presence of maturation components. Therefore, we investigated the effect of ManLAM and AraLAM in combination with the stimulatory bacterial LPS. TLR4 interaction with LPS generates intracellular signaling, most notably via the transcription factor nuclear factor (NF)-κB, that results in DC activation/maturation. Indeed, DCs efficiently mature in the presence of LPS alone (Fig. 6 b). Strikingly, this LPS-induced activation is inhibited in the presence of ManLAM, as the expression levels of the activation markers CD80, CD83, and CD86 were considerably lower than those of LPS-activated DCs (Fig. 6 b). The observed inhibition of DC activation/maturation is specific for ManLAM, as AraLAM did not inhibit DC activation (Fig. 6 b). This is further supported by the ability of antibodies against DC-SIGN, that inhibit ManLAM binding (Fig. 3 b), to completely restore LPS-induced maturation in the presence of ManLAM (Fig. 6 b). These results indicate that ManLAM binding to DC-SIGN generates intracellular signals that interfere with the TLR4-mediated activation of DCs. Moreover, this process is specific for the ManLAM-DC-SIGN interaction and DC-SIGN ligation alone is not sufficient, as antibodies against DC-SIGN did not block LPS-induced DC activation/maturation (Fig. 6 b).
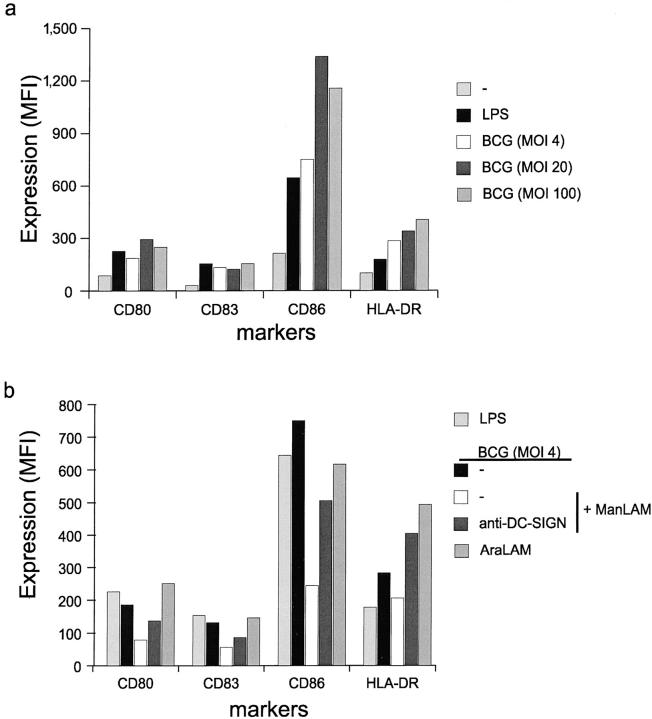
Both M. tuberculosis and M. bovis BCG are able to induce DC maturation through their cell-wall components via TLR2- and TLR4-mediated signaling (26–29). Indeed, M. bovis BCG infection of immature DCs results in DC maturation, as demonstrated by the increased expression of MHC class II and the costimulatory molecules CD80, CD83, and CD86 after M. bovis BCG infection (Fig. 7 a). Next, we investigated whether ManLAM binding to DC-SIGN prevented M. bovis BCG-induced DC maturation, as immature DCs attracted to sites of mycobacterial infection will encounter both secreted ManLAM and intact mycobacteria. Strikingly, the M. bovis BCG-induced DC maturation is strongly inhibited by ManLAM (Fig. 7 b). The expression of MHC class II, CD80, CD83, and CD86 on M. bovis BCG-infected DCs in the presence of ManLAM was considerably lower than on M. bovis BCG-infected DCs (Fig. 7 b). Moreover, the maturation was mostly restored when DCs were preincubated with the blocking DC-SIGN–specific antibody (Fig. 7 b), demonstrating that the ManLAM interaction with DC-SIGN prevents DC maturation by M. bovis BCG. Moreover, AraLAM did not block the DC maturation by M. bovis BCG (Fig. 6 c), as the costimulatory molecules, CD80, CD83, and CD86, are expressed at similar levels on both infected DCs and AraLAM-treated infected DCs. This indicates that the DC-SIGN–ManLAM interaction blocks the maturation of DCs induced by LPS as well as M. bovis BCG.
Figure 7.
M. bovis BCG induces maturation and ManLAM inhibits the induced DC activation through DC-SIGN binding. (a) M. bovis BCG induces maturation of immature DCs. Immature DCs were incubated with LPS or viable M. bovis BCG (MOI 4, 20, and 100) for 18 h, and activation was determined by measuring the expression of CD80, CD86, CD83, and HLA-DR. The mean fluorescence intensity (MFI) of the difference between specific antibody and isotype control is depicted. One representative experiment out of three is shown. (b) M. bovis BCG-induced activation of DCs is blocked by ManLAM. Immature DCs were infected with M. bovis BCG (MOI 4). Cells were preincubated with 15 μg/ml of either ManLAM or AraLAM and the expression of the markers was measured after 18 h as described for panel a. Specificity was determined by preincubating cells with blocking antibodies against DC-SIGN (AZN-D2; 20 μg/ml). One representative experiment out of three is shown.
Discussion
DCs are essential for the containment of M. tuberculosis infections by inducing cellular immune responses against mycobacteria (30). However, M. tuberculosis infections remain latent throughout the host lifetime, demonstrating that mycobacteria have developed evasive mechanisms to suppress immune responses and to survive within the host. The unique function of DCs in eliciting and directing cellular immune responses may be modulated by mycobacteria. We demonstrate here that the DC-specific C-type lectin DC-SIGN is an important receptor on DCs for mycobacteria that express ManLAM, and that mycobacteria target DC-SIGN on DCs through secreted ManLAM to block maturation of mycobacteria-infected DCs and to induce the immunosuppressive cytokine IL-10.
Soluble as well as cellular DC-SIGN bind strongly to viable mycobacteria such as M. tuberculosis and M. bovis BCG (Fig. 1 and 2), but not to M. smegmatis (Fig. 2). A major cell wall component of these mycobacteria strains is ManLAM, a mannose-containing phosphorylated glycolipid implicated as a virulence factor (21). The LAM from pathogenic M. tuberculosis strains is between 40–70% mannose-capped (21). Strikingly, DC-SIGN binds strongly to ManLAM, but not to uncapped AraLAM. ManLAM, in contrast to AraLAM, contains a mannose-cap consisting exclusively of mono-, di-, and trimers of α-d-mannoses directly linked to the arabinofuranosyl-termini (21). Thus, DC-SIGN binds specifically to the exterior α-d-mannose residues of ManLAM, but not to the mannose-containing core of ManLAM, which is shared with AraLAM. Accessibility and the spacing of the mannose-containing termini on ManLAM may contribute to the strong interaction. Both viable mycobacteria, containing ManLAM, and ManLAM alone bind specifically to the primary binding site of DC-SIGN, similarly to the other DC-SIGN ligands ICAM-3 and HIV-1 gp120 (Fig. 2). ICAM-3 and gp120 make distinct additional contacts with DC-SIGN, and Val351 at the edge of the binding pocket denotes the difference between these ligands. The DC-SIGN V351G mutant does not bind ICAM-3, whereas HIV-1 gp120 is still bound by this mutant (Fig. 2 c, and reference 19). Both M. bovis BCG and ManLAM are bound by the DC-SIGN V351G mutant (Fig. 2), indicating that they have the same binding site. The cellular ligand ICAM-3 contains N-linked glycosylations consisting of high mannose-type oligosaccharides that are essential for DC-SIGN binding, whereas the binding of HIV-1 gp120 is not exclusively mediated by high mannose-polycarbohydrates (19). These data indicate that the DC-SIGN V351G mutant is unable to bind complex high-mannose structures but remains able to bind linear mannose residues such as those present in ManLAM. Thus, the pathogen structures ManLAM and HIV-1 gp120 occupy a similar binding pocket in DC-SIGN, that is distinct from that of the cellular ligand ICAM-3. These observations suggest that DC-SIGN may distinguish between different types of ligand and may tailor its responses specifically. These results further support the hypothesis that DC-SIGN interacts with viable mycobacteria through ManLAM. Indeed, cellular DC-SIGN did not interact with viable M. smegmatis, demonstrating that DC-SIGN does not interact with mycobacteria that do not contain ManLAM.
DC-SIGN is an important receptor on DCs for both M. bovis BCG and ManLAM (Fig. 3), even though DCs also express the receptors MR, CD11b, and CD11c that have previously been implicated in the interactions of mycobacteria with both macrophages and DCs (8, 9, 31). DC-SIGN–specific antibodies, in contrast to MR-specific antibodies, block the interaction of DCs with both M. bovis BCG and ManLAM (Fig. 3). Involvement of MR in ManLAM binding by DCs has previously been inferred by inhibition studies using mannan (4, 7), as MR has a high affinity for this polycarbohydrate. However, mannan also inhibits the functions of other C-type lectins on DCs, such as DC-SIGN (for a review, see reference 32), demonstrating that specific blocking antibodies against particular receptors are necessary to accurately determine the involvement of a receptor. Indeed, mannan inhibited the interaction of DCs with ManLAM to a similar extent as DC-SIGN–specific antibodies, whereas MR-specific antibodies did not block ManLAM binding. Moreover, the MR-ligand mannose-BSA did not inhibit the interaction of DC with M. bovis BCG (Fig. 3 c), demonstrating that the mannose-specific C-type lectin domains of MR may not be involved in M. bovis BCG infection of DCs. These results demonstrate that DC-SIGN is an important C-type lectin on DCs that interacts with the glycolipid ManLAM. Moreover, captured ManLAM is rapidly internalized by DCs upon DC-SIGN binding and targeted to CD107a/LAMP-1+ lysosomes (Fig. 4). DC-SIGN, in contrast to MR, CD11b, and CD11c, was down-regulated after ManLAM binding, which supports a role of DC-SIGN in ManLAM internalization (Fig. 4). Uptake of M. tuberculosis LAM by DCs results in presentation by CD1b to specific T cells (4) and our results suggest that DC-SIGN may be responsible for the delivery of ManLAM to late endosomes/lysosomes for presentation by CD1b. This is supported by the recent findings that DC-SIGN also functions as an antigen receptor that targets internalized antigen to late endosomes/lysosomes for presentation to T cells (15). In contrast to our data, Prigozy et al. (4) suggested a role for MR in the uptake of LAM, as the uptake of LAM by immature DCs was inhibited by mannan and LAM presentation was inhibited by polyclonal antibodies against MR. The earlier observed block of LAM presentation (4) could be due to other domains in MR than the C-type lectin domains, as we used monoclonal antibodies that block the C-type lectin-mediated activity of MR. This is further supported by our results that both mannan and anti–DC-SIGN antibodies do not completely inhibit the ManLAM interaction mediated by DCs (Fig. 3). Future experiments comparing the DC-SIGN and MR function should reveal whether both are involved in CD1b presentation of ManLAM.
Rapid internalization of ManLAM by DC-SIGN suggests that DC-SIGN may also be involved in mycobacterial uptake by DCs, as was shown for HIV-1 (10). Indeed, blocking antibodies against DC-SIGN inhibit the internalization of viable M. bovis BCG by immature DCs (Fig. 3). Moreover, the erythroleukemic cell line K562 is unable to internalize M. bovis BCG, whereas the K562 transfectant expressing DC-SIGN internalizes viable M. bovis BCG. Thus, DC-SIGN mediates both capture and internalization of mycobacteria such as M. bovis BCG, which is supported by the colocalization of DC-SIGN staining and FITC-conjugated mycobacteria (Fig. 4). Moreover, internalized FITC-conjugated mycobacteria were targeted to the lysosomes, as they colocalized with LAMP-1 staining (Fig. 4). Recently, it was shown that murine DCs can act as a reservoir in vivo for mycobacteria (33) and we cannot exclude that bacilli escaped the lysosomal pathway to productively infect human DCs.
LAM glycolipids are present in the mycobacterial cell wall but are also secreted from phagosomes following macrophage ingestion of M. tuberculosis (21, 23, 24). The presence of anti-LAM antibodies in sera of tuberculosis patients suggests that LAM is released in vivo (34). Thus, mycobacteria within macrophages can affect bystander immune cells and modulate the immune response mediated by DCs. We demonstrate here that secreted ManLAM targets DC-SIGN on DCs to suppress DC functions (Figs. 5–7). Triggering of TLR on DCs induces the release of cytokines and the up-regulation of accessory molecules for efficient stimulation of T lymphocytes (35, 36) and DC maturation by LPS is mediated through TLR4, which generates intracellular signaling most notably via the transcription factor NF-κB (37). Mycobacteria such as M. bovis BCG induce DC maturation (29; Fig. 7) and M. bovis BCG can mediate the observed maturation through TR2 and TLR4 signaling (29). Strikingly, both M. bovis BCG- and LPS-induced maturation of DCs was specifically blocked by ManLAM but not by AraLAM (Figs. 6 and 7). This inhibition by ManLAM is mediated through DC-SIGN, as antibodies against DC-SIGN abrogated this effect and restored strong DC maturation by both M. bovis BCG and LPS. These results suggest that DC-SIGN, upon binding ManLAM, delivers a signal that interferes with the M. bovis BCG-induced signals presumably generated by TLR4. Our results demonstrate for the first time that pathogen-binding to DC-SIGN may mediate intracellular signaling. Ligation of DC-SIGN with antibodies alone is not sufficient, as antibodies against DC-SIGN that trigger internalization similarly to ManLAM (15; Fig. 4 b) did not prevent DC activation (Figs. 6 and 7) nor induce IL-10 production (Fig. 5). The ManLAM-induced production of IL-10 could contribute to the virulence of mycobacteria, as IL-10 impairs the ability of DCs to generate Th1 responses by blocking up-regulation of costimulatory molecules and IL-12 production (25). Moreover, M. bovis BCG-infected DCs produced high levels of IL-10 (Fig. 5 b), demonstrating that mycobacteria induce IL-10 through both direct infection and by influencing bystander DCs by ManLAM secretion. Recently, it was demonstrated that ManLAM inhibits the IL-12 production by LPS-matured DCs (7). The authors suggested that MR is involved in ManLAM binding, as a similar IL-12 down-regulation was observed with anti-MR antibodies alone (7). Cross-linking of MR may induce signals that block the LPS-induced IL-12 up-regulation, and although we were unable to block the interaction of ManLAM to DCs with the same anti-MR monoclonal antibody (Fig. 3 b), it is possible that other domains of MR are involved in ManLAM binding. The authors hypothesized that pathogen receptors could interfere with TLR signaling upon pathogen recognition, modulating the cellular immune responses against pathogens (7). Our data further support this hypothesis, and this may be a general principle by which pathogens suppress the immune response (38).
Murine DCs are infected by mycobacteria both in vitro and in vivo (33, 39) and the infected DCs rapidly lost their antigen presentation ability upon infection by M. bovis BCG in vivo (33). These observations support an important role for DCs as hosts for mycobacteria. Moreover, our observed inhibition of DC maturation and induction of IL-10 by ManLAM–DC-SIGN demonstrates that in human M. tuberculosis may target DC-SIGN to suppress cellular immune responses since both immature DCs and IL-10–treated DCs are not only less efficient at stimulating T cell responses but can also induce a state of antigen-specific tolerance (40, 41). The results obtained with the mildly virulent M. bovis BCG strain indicate that the mechanism of immunosuppression may not directly contribute to the virulence and persistence of M. tuberculosis strains. However, differences between the interaction of DC-SIGN with virulent and avirulent mycobacteria strains may account for differences in persistence and will have to be investigated in more detail.
Our results suggest that DC-SIGN is important in the pathogenesis of M. tuberculosis, as was also demonstrated for HIV-1 (10). DC-SIGN may be a prime target for pathogens such as HIV-1 and M. tuberculosis to manipulate the DC function. Therefore, DC-SIGN could be an important target for clinical intervention in M. tuberculosis and HIV-1 infections. The hypothesis of distinct binding sites in DC-SIGN for cellular ligands and pathogen structures could provide the molecular basis for the design of strategies to inhibit DC-SIGN/pathogen interactions without affecting the immunological function of DC-SIGN.
Acknowledgments
We thank L. Colledge for helpful suggestions and editing of the manuscript.
E.A. Koppel is supported by the Dutch Scientific Research (NWO; contract no. 908-02-004).
S.J. van Vliet and E.A. Koppel contributed equally to this work.
Footnotes
Abbreviations used in this paper: BCG, bacillus Calmette-Guérin; DC, dendritic cell; ICAM, intercellular adhesion molecule; LAM, lipoarabinomannan; MOI, multiplicity of infection; MR, mannose receptor; TLR, Toll-like receptor.
References
- 1.Fenton, M.J., and M.W. Vermeulen. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demangel, C., and W.J. Britton. 2000. Interaction of dendritic cells with mycobacteria: where the action starts. Immunol. Cell Biol. 78:318–324. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 4.Prigozy, T.I., P.A. Sieling, D. Clemens, P.L. Stewart, S.M. Behar, S.A. Porcelli, M.B. Brenner, R.L. Modlin, and M. Kronenberg. 1997. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 6:187–197. [DOI] [PubMed] [Google Scholar]
- 5.Manabe, Y.C., and W.R. Bishai. 2000. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat. Med. 6:1327–1329. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann, S.H. 2000. Is the development of a new tuberculosis vaccine possible? Nat. Med. 6:955–960. [DOI] [PubMed] [Google Scholar]
- 7.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477–7485. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger, L.S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920–2930. [PubMed] [Google Scholar]
- 9.Schlesinger, L.S., S.R. Hull, and T.M. Kaufman. 1994. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 152:4070–4079. [PubMed] [Google Scholar]
- 10.Geijtenbeek, T.B.H., D.S. Kwon, R. Torensma, S.J. van Vliet, G.C.F. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. KewalRamani, D.R. Littman, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587–597. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell, D.A., A.J. Fadden, and K. Drickamer. 2001. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 276:28939–28945. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T.B.H., R. Torensma, S.J. van Vliet, G.C.F. van Duijnhoven, G.J. Adema, Y. van Kooyk, and C.G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 100:575–585. [DOI] [PubMed] [Google Scholar]
- 13.Keizer, G.D., J. Borst, C.G. Figdor, H. Spits, F. Miedema, C. Terhorst, and J.E. De Vries. 1985. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur. J. Immunol. 15:1142–1148. [DOI] [PubMed] [Google Scholar]
- 14.Caligaris-Cappio, F., G. Pizzolo, M. Chilosi, L. Bergui, G. Semenzato, L. Tesio, L. Morittu, F. Malavasi, M. Gobbi, R. Schwarting, et al. 1985. Phorbol ester induces abnormal chronic lymphocytic leukemia cells to express features of hairy cell leukemia. Blood. 66:1035–1042. [PubMed] [Google Scholar]
- 15.Engering, A., T.B. Geijtenbeek, S.J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C.G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118–2126. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hed, J., G. Hallden, S.G. Johansson, and P. Larsson. 1987. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J. Immunol. Methods. 101:119–125. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T.B., Y. van Kooyk, S.J. van Vliet, M.H. Renes, R.A. Raymakers, and C.G. Figdor. 1999. High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood. 94:754–764. [PubMed] [Google Scholar]
- 19.Geijtenbeek, T.B., G.C. van Duijnhoven, S.J. van Vliet, E. Krieger, G. Vriend, C.G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell- specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314–11320. [DOI] [PubMed] [Google Scholar]
- 20.Kim, K.D., H.G. Lee, J.K. Kim, S.N. Park, I.S. Choe, Y.K. Choe, S.J. Kim, E. Lee, and J.S. Lim. 1999. Enhanced antigen-presenting activity and tumour necrosis factor-alpha- independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guerin. Immunology. 97:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee, D., and K.H. Khoo. 1998. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 8:113–120. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg, H., D.A. Mitchell, K. Drickamer, and W.I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 294:2163–2166. [DOI] [PubMed] [Google Scholar]
- 23.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D.G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568–2578. [PubMed] [Google Scholar]
- 24.Sturgill-Koszycki, S., P.H. Schlesinger, P. Chakraborty, P.L. Haddix, H.L. Collins, A.K. Fok, R.D. Allen, S.L. Gluck, J. Heuser, and D.G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 263:678–681. [DOI] [PubMed] [Google Scholar]
- 25.Redpath, S., P. Ghazal, and N.R. Gascoigne. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9:86–92. [DOI] [PubMed] [Google Scholar]
- 26.Demangel, C., A.G. Bean, E. Martin, C.G. Feng, A.T. Kamath, and W.J. Britton. 1999. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis Bacillus Calmette Guerin-infected dendritic cells. Eur. J. Immunol. 29:1972–1979. [DOI] [PubMed] [Google Scholar]
- 27.Henderson, R.A., S.C. Watkins, and J.L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635–643. [PubMed] [Google Scholar]
- 28.Inaba, K., M. Inaba, M. Naito, and R.M. Steinman. 1993. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect. Immun. 68:6883–6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray, P.J. 1999. Defining the requirements for immunological control of mycobacterial infections. Trends Microbiol. 7:366–372. [DOI] [PubMed] [Google Scholar]
- 31.Ehlers, M.R., and M. Daffe. 1998. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 6:328–335. [DOI] [PubMed] [Google Scholar]
- 32.Figdor, C.G., Y. van Kooyk, and G.J. Adema. 2002. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2:77–84. [DOI] [PubMed] [Google Scholar]
- 33.Jiao, X., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294–1301. [DOI] [PubMed] [Google Scholar]
- 34.Sada, E., P.J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28:2587–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 37.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 38.Engering, A., T.B. Geijtenbeek, and Y. Van Kooyk. 2002. Immune escape through C-type lectins on dendritic cells. Trends Immunol. 23:480–485. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Juarrero, M., and I.M. Orme. 2001. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect. Immun. 69:1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinbrink, K., H. Jonuleit, G. Muller, G. Schuler, J. Knop, and A.H. Enk. 1999. Interleukin-10-treated human dendritic cells induce a melanoma-antigen- specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood. 93:1634–1642. [PubMed] [Google Scholar]