Abstract
Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) is an essential negative regulator of T cell activation. Recent evidence suggests that CTLA-4 association with the immunological synapse during contact with antigen-presenting cells is important for its inhibitory function. In the present study, we observed a direct interaction of CTLA-4 with the phosphorylated form of T cell receptor (TCR)ζ within the glycolipid-enriched microdomains associated with the T cell signaling complex. In this setting, CTLA-4 regulated the accumulation/retention of TCRζ in the signaling complex, as the lipid raft fractions from CTLA-4KO T cells contained significantly higher amounts of the TCR components when compared with wild-type littermates. In contrast, coligation of CTLA-4 with the TCR during T cell activation selectively decreased the amount of TCRζ that accumulated in the rafts. These results suggest that CTLA-4 functions to regulate T cell signaling by controlling TCR accumulation and/or retention within this a critical component of the immunological synapse.
Keywords: costimulation, T cells, GEM, immunological synapse, negative signal
Introduction
T cell activation is regulated by a balance between positive and negative signals mediated by a series of costimulatory ligand–receptor pairs. While costimulatory pathways involving molecules such as CD28, inducible costimulator (ICOS), 4–1BB, and CD40L are essential coactivators of proliferation, cytokine production, and migration, CTLA-4 and PD-1, homologues of CD28 family of cell surface receptors, provide strong negative signals (1). In particular, CTLA-4 coligation with TCR has been shown to inhibit IL-2 production, cell cycle progression, and proliferation. In vivo, CTLA-4 knockout mice manifest fatal lymphoproliferative phenotype and die within 4 wk of life (2, 3). In spite of the importance of CTLA-4 in regulation of the immune system, however, the molecular basis for this inhibitory function is still largely unknown.
The interface between the T cell and APC membranes form the hot spot for T cell activation, a highly organized ultra structure which is called the immunological synapse (4, 5). The membrane lipid raft is biochemically characterized as a detergent insoluble glycosphingolipid enriched microdomain that is considered as an essential component of the immunologic synapse (6). After TCR engagement, molecules critical for mediating activation signals, such as Lck, Fyn, protein kinase C (PKC)θ, phospholipase C (PLC)γ, and linker for activation of T cells (LAT), are all recruited to the raft aggregates at the T cell–APC contact area. More importantly, the TCR itself dynamically associates with raft upon receptor cross-linking, allowing the accessibility of signaling molecules to TCR facilitating the biochemical signals of activation. As such, pharmacological disruption of the rafts abrogate early signal events in T cell activation such as calcium influx, supporting the essential role of raft integrity for T cell activation (7).
Recently, Darlington et al. (8) reported that CTLA-4 is recruited to the raft during negative signaling. However, the functional importance of this association was unclear. In the present study, we analyzed the biochemical consequences of the colocalization of CTLA-4 to the rafts. We report that CTLA-4 forms a molecular complex with phosphorylated TCRζ within the rafts. Furthermore, the overall levels of TCRζ, most prominently phosphorylated TCRζ, in the rafts is regulated by CTLA-4 as assessed in CTLA4KO T cells and wild-type T cells after CTLA-4 cross-linking. Together, these results support a proximal role for CTLA-4 in attenuating TCR-mediated signal transduction which emphasizing that both positive and negative signaling events impact on the early events of T cell activation.
Materials and Methods
Antibodies and Reagents.
Hamster anti–mouse CD3ɛ (145–2C11), hamster anti–mouse CTLA-4 (UC10–4F10), anti–mouse CD28 (PV-1), hamster anti–mouse γδ TCR (GL-3), hamster anti–mouse class I (10H3), anti–B7–1(16–10A1), anti–B7–2(GL-2), and rabbit polyclonal anti–CTLA-4 (9) were prepared in our lab. Anti–mouse TCRζ (6B10.2: Santa Cruz Biotechnology, Inc.), anti-Fyn (Fyn3; Santa Cruz Biotechnology), anti-phosphotyrosine (4G10: Upstate Biotechnology), goat anti–hamster (Cappel), peroxidase-conjugated cholera toxin B subunit (Sigma-Aldrich), and FITC-conjugated cholera toxin B subunit (Sigma-Aldrich) were purchased. For mice T cell culture, DMEM (Gibco) supplemented with 10% fetal calf serum, 10 mM HEPES, nonessential amino acids (Biosource International), 55 μM 2-mercaptoethanol, 100 μg/ml penicillin, and 100 U/ml streptomycin was used as media.
Jurkat Cell Culture and Transfection of CTLA-4.
Wild-type murine CTLA-4 cDNA was subcloned into the expression vector pBSRαEN and stably transfected into Jurkat E6.1 cell line. Cells were maintained at 37°C in a 5% CO2 incubator in complete RPMI medium (University of California at San Francisco Cell Culture Facility), supplemented with 10% fetal calf serum and 2 mg/ml G418 (Life Technologies) for drug selection.
Animals.
All the mice in this study were kept in a specific pathogen-free animal barrier facility at the University of California at San Francisco. C57BL/6 (B6) mice were purchased from Charles Rivers. CTLA-4 knockout mice, on the B6 background, were maintained as F1 heterozygotes which were interbred to generate the CTLA-4KO mice used in experiments. The mice were screened within 24 h of birth by PCR of tail DNA using the oligonucleotide primers for CTLA-4 (5′-ATGGTGTTGGCTAGCAGCCATG and 3′-TTGGATGGTGAGGTTCACTC) and the neomycin resistance gene (5′-ATTGAACAAGATGGATTGCAC and 3′-CGTCCAGATCATCCTGATC). The constitutive CTLA-4 Y201V-expressing transgenic mice (10), a gift of Dr. Ellen Chuang, Cornell University School of Medicine, New York, NY) were maintained on a mixed background.
Activation of CTLA-4 Knockout Mouse T Cells.
CTLA-4 knockout mice were treated by anti–B7–1 (100 μg) and anti–B7–2 (100 μg) antibody on days 8, 11, and 14 after their birth to inhibit T cell activation and maintain the T cells in a naive state. The mice were killed at day 21, and single cell suspensions of lymph nodes were stimulated by plate-bound anti-CD3 and anti-CD28 (1 μg/ml each) in culture media for 48 h. Cells were then transferred to T-175 culture flasks with fresh medium supplemented with 2 U/ml recombinant human IL-2. Live cells were enriched by Ficoll density gradient 24 h later and used for experiments.
In Vitro Stimulation by Antibody Cross-linking.
Single cell suspensions of mouse lymph nodes were stimulated by plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) for 60 to 70 h in culture media, harvested, washed, and replated in T-175 culture flasks in the presence of fresh medium and 20 U/ml recombinant human IL-2. After 6 d, live cells were enriched by Ficoll density gradient. An aliquot of 108 cells was used for each stimulation condition. Washed cells were resuspended in serum-free media at the density of 2 × 108 /ml. Cells were incubated with a mixture of anti-CD3 in the presence of either anti–CTLA-4 or control (anti-γδ TCR) mAbs as indicated in figure legends. After incubation on ice for 20 min, surface bound antibodies were cross-linked by adding anti–hamster antibodies at a final dilution of 1:100 for an additional 5 min. The reaction was stopped by adding ice cold PBS containing 1 mM sodium orthovanadate.
Isolation of the Lipid Raft by Sucrose Density Gradient.
T cells were stimulated as described above, pelleted, and resuspended with 500 μl of ice-cold lysis buffer (final concentration: 0.2% Triton X-100, 50 mM Tris [pH 7.6], 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM PMSF) on ice. The lysates were disrupted with 10 strokes using a Dounce cell homogenizer and mixed with an equal volume of sucrose as an 85% solution in TNE buffer (20 mM Tris [pH 8.0], 150 mM NaCl, 50 mM EDTA). The suspension was transferred into an ultracentrifuge tube (Kendro Laboratory Products) where it was overlaid with 3 ml of 30% sucrose and 1 ml of 5% sucrose both suspended in TNE. The tube was then filled to the top with TNE buffer. These gradients were spun for 12 to 16 h at 40,000 rpm in a SW-40 Ti swinging bucket rotor at 4°C in a Beckman ultracentrifuge. After removing top TNE buffer, the gradients were harvested into six 0.8-ml fractions from top to bottom. The GEMs were identified by dot blot analysis of the cholera toxin B subunit as described (11). The individual fractions were mixed with 0.1 volume of 0.6 M n-octyl β-D-galactopyranoside (final 60 mM), incubated for 2 h to solubilize the rafts, and subjected to immunoprecipitation and Western blot analyses.
Results and Discussion
CTLA-4 Localizes to Rafts Where It Selectively Interacts with Accumulated Phosphorylated TCRζ.
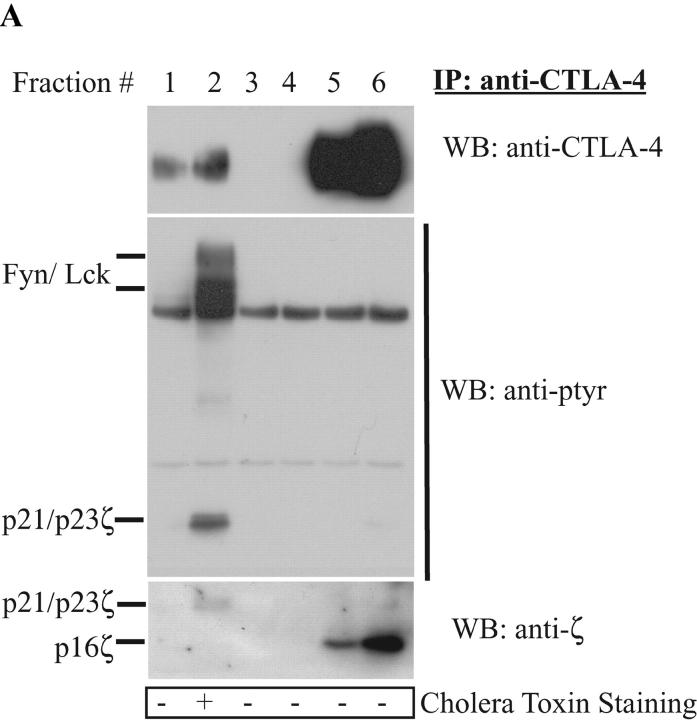
Several reports have suggested that the lipid rafts are critical during T cell activation (for a review, see reference 6). However, the basis for negative regulation of these complexes remains unclear. A recent publication by Darlington et al. (8) reported that CTLA-4 localizes within the raft. Given our previous studies demonstrating the interaction of CTLA-4 with the TCRζ chain, we hypothesized that CTLA-4 may negatively regulate lipid raft signaling. As a first step in addressing this issue, we examined the distribution of CTLA-4 within the rafts. The Jurkat T cell line was used for these studies. Lysates from Jurkat cells, stably transfected with murine CTLA-4, were subjected to sucrose gradient fractionation. CTLA-4 was immunoprecipitated from each fraction. As seen in Fig. 1 A, a small fraction of CTLA-4 was constitutively found within the rafts. An anti-phosphotyrosine Western blot showed that the raft-associated CTLA-4 coprecipitated tyrosine phosphorylated bands migrating at 59, 56, 23, and 21 kD (Fig. 1 A, second panel). The identity of the 59 and 56 kD bands as Fyn and Lck, respectively, were confirmed by reprobing with kinase-specific antibodies (unpublished data). This observation is novel and suggests that CTLA-4 interacts within the lipid rafts as part of a complex that regulates T cell receptor–mediated signal transduction. The observation builds on previous work demonstrating the ability of lck and fyn to phosphorylate CTLA-4 and TCRζ (12, 13, 14). Interestingly, reprobing of the membrane with anti-TCRζ antibody (Fig. 1 A, bottom panel). showed that the p21 and p23 observed with the anti-pTyr detected the phosphorylated form of TCRζ only in the lipid raft fractions while the immunoblot demonstrated that CTLA-4 precipitated predominantly the nonphosphorylated form of TCRζ from the non-lipid raft bottom fractions. It is important to note that the fractions were solubilized by n-octyl β-D-galactopyranoside to ensure that associations were due to protein–protein interactions and not copresence within membrane vesicles. In addition, several control hamster antibodies were tested to ensure the specificity of the immunoprecipitations (unpublished data).
Figure 1.
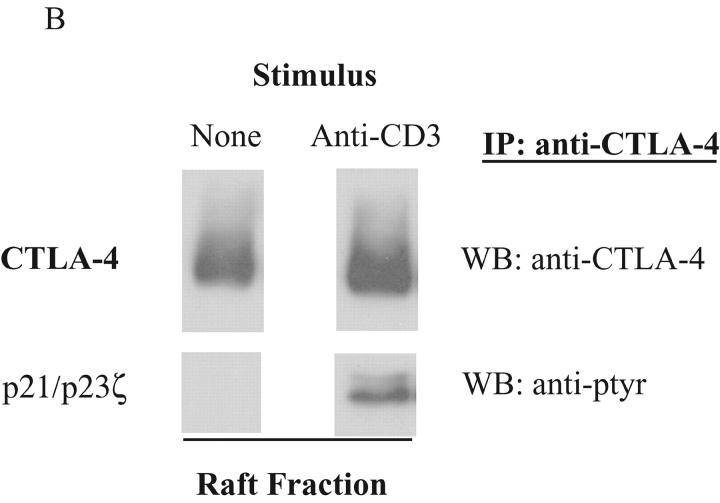
CTLA-4 associates with tyrosine phosphorylated TCRζ in the lipid raft. (A) 108 Jurkat T cells, stably transfected with wild-type murine CTLA-4, were lysed and subjected to sucrose gradient centrifugation. CTLA-4 was immunoprecipitated (IP) from each fraction and detected by Western blotting (WB) using an anti-CTLA-4 antibody (1st panel). At the same time, the membrane was probed with an anti-phosphotyrosine antibody (anti-ptyr: 2nd panel). The blot was stripped and reprobed by an anti-TCRζ antibody (3rd panel). The bottom panel depicts the result from cholera toxin horse radish peroxidase dot blot from each fraction. (B) 108 activated mouse T cells were left untreated or restimulated with anti-CD3 (3 μg/ml) antibody. After 5 min incubation, the cells were lysed and fractionated by sucrose density gradient. CTLA-4 in the raft fraction was immunoprecipitated and detected by polyclonal CTLA-4 antibody (top panels). TCRζ association was detected by anti-phosphotyrosine antibody (bottom panels).
As Jurkat cells are tumor cells in a quasi activation state, the physiologic role of TCR stimulation in CTLA-4/raft interactions was examined using previously activated murine T cells. CTLA-4 protein was precipitated from the rafts of nonstimulated and anti-CD3–stimulated T cells and the association of CTLA-4 with TCRζ was examined. Upon cross-linking of TCR, the amount of CTLA-4 in the raft was slightly increased (Fig. 1 B, top right and top left panel), consistent with the previous findings (8). No coprecipitation of phosphorylated bands around 21 and 23 kD was observed in the resting T cells (Fig. 1 B, bottom left panel). However, upon TCR cross-linking, CTLA-4 coprecipitated phosphorylated TCRζ (Fig. 1 B, bottom right panel). Although 10 times more CTLA-4 protein was observed outside the rafts, the nonraft CTLA-4 associated predominantly with nonphosphorylated TCRζ (unpublished data). These results were identical to that observed in the Jurkat cells. Neither the amount of CTLA-4 protein nor TCRζ associated CTLA-4 changed upon cross-linking of TCR (unpublished data). Thus, CTLA-4 interacts with TCRζ both inside and outside the raft. However, the relative proportion of the CTLA-4 protein complexed with phosphorylated TCRζ within the rafts was significantly greater than the phosphoTCRζ/CTLA-4 complexes outside the rafts. These results suggest that the CTLA-4/phosphoTCRζ interaction may regulate T cell signaling at the immunologic synapse.
Presence of CTLA-4 Alters TCRζ Accumulation within Rafts.
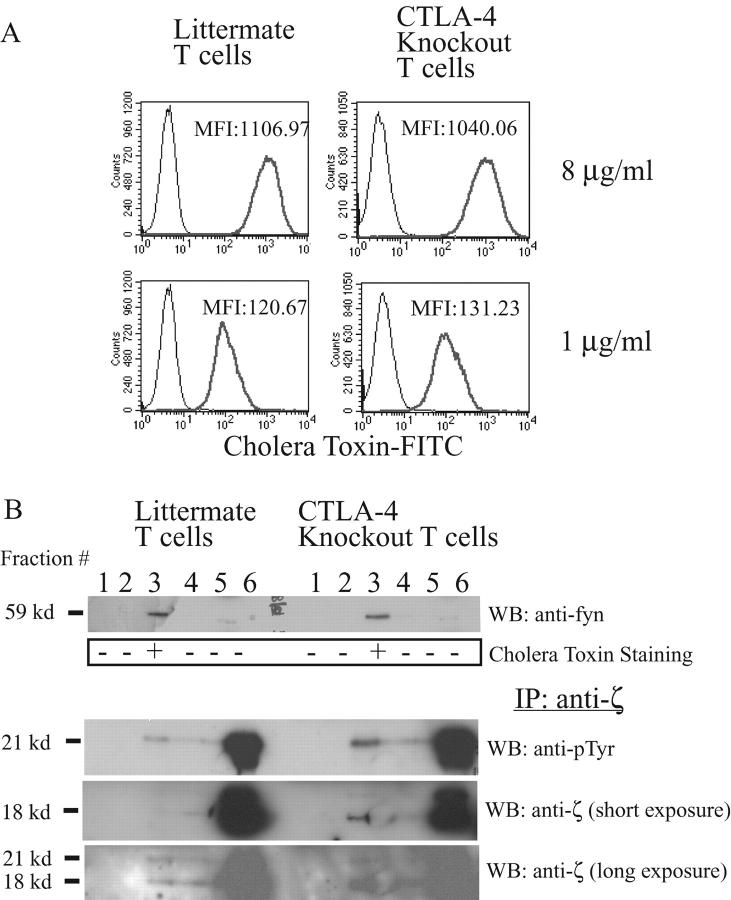
Given the results above, we hypothesized that signaling through CTLA-4 in the raft might affect TCR phosphorylation or TCR recruitment. Lipid rafts from CTLA-4 knockout mice versus wild-type littermates were compared with directly examine the function of CTLA-4 on signaling. To ensure that the T cell populations used in these studies were naive, CTLA-4 knockout mice were treated by anti-B7 antibodies to block essential CD28/B7 interactions critical for early activation. Previous studies have shown that the conditions used in this paper result in T cell populations that are comparable in terms of cell surface phenotype, cytokine production, and proliferative capacity (15). Indeed, at the time of sacrifice, knockout mice looked completely healthy and their lymph nodes and spleens looked the same as of their littermates in terms of size (unpublished data). Flow cytometric analyses confirmed that the majority of the T cells in this setting were CD69−CD25−CD62Lhi (unpublished data). Isolated lymph node cells were activated by anti-CD3 and anti-CD28 antibody for 2 d. After 24 h of cell expansion in the presence of IL-2, the cell surface raft expression was compared. The staining of activated T cells with FITC-conjugated cholera toxin B subunit, as a marker for the surface rafts, demonstrated that the amount of cell surface raft, ganglioside GM1, was equivalent in each cell population (Fig. 2 A). The fact that CTLA-4 expression on the activated T cells did not influence the raft expression is in contrast to previous reports that CTLA-4 controls the cell surface raft expression during primary T cell activation (16). These results suggest that CTLA-4 inhibits T cell activation in primary T cells that is essential for raft formation.
Figure 2.
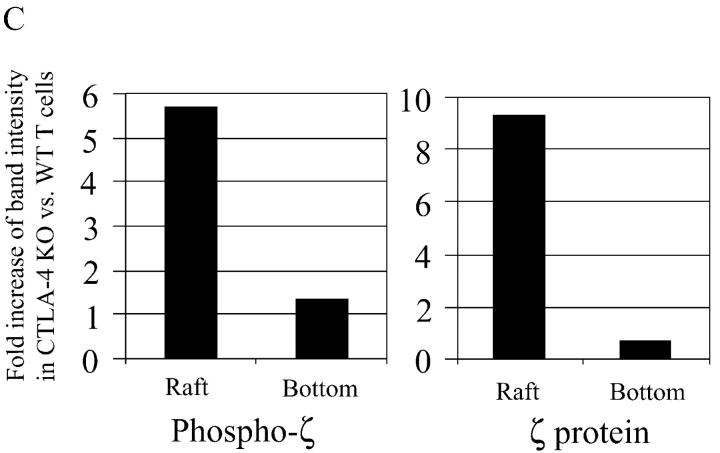
CTLA-4 knockout T cells have more TCR in the raft than their littermate T cells. (A) CTLA-4 knockout and heterozygote littermate T cells were activated by plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) for 2 d and rested for 1 d. 106 cells were stained using different concentrations of cholera toxin FITC (8 μg/ml and 1 μg/ml) and analyzed using a flow cytometry. MFI, mean fluorescent intensity. (B) 10 mg of each lysate from CTLA-4 knockout and littermate T cells, which have been activated with anti-CD3 plus anti-CD28 as described for panel A, were subjected to sucrose density gradient separation. The gradient was divided into six fractions that were analyzed for Fyn (1st panel) and cholera toxin horseradish peroxidase. TCRζ was IP from each fraction and analyzed by WB using anti-ptyr antibody (3rd panel). The membrane was stripped and reprobed using an anti-TCRζ antibody. The 4th and 5th panels depict short (1 min) and long (10 min) exposure of the membrane, respectively. (C) The band intensity of the phosphorylated TCRζ in the raft fraction from above experiments was measured by densitometry. The intensity of TCRζ in CTLA-4 knockout T cells is shown as the fold increase as compared with littermate T cells.
Cell lysates were prepared from wild-type and CTLA-4KO mice T cells activated with anti-CD3 plus anti-CD28 to compare raft composition using sucrose gradient fractionation and immunoblotting. An anti-Fyn Western blot (Fig. 2 B, top panel) as well as a peroxidase conjugated cholera toxin dot blot (Fig. 2 B, second panel) confirmed that the lipid rafts were successfully isolated in fraction 3 of cell lysates from knockout mice and littermates. As the raft fractions contained much less protein than nonraft detergent soluble fraction (17), the TCRζ proteins were enriched from each fraction by immunoprecipitation before subjecting to Western blotting. Anti-phosphotyrosine blotting of the anti-TCRζ immunoprecipitate demonstrated that there was significantly more phosphorylated TCRζ in the rafts from CTLA-4 knockout than littermate T cells (Fig. 2 B, third panel lane 3) while there was only a small difference in TCRζ in the soluble fraction from knockouts versus littermates (Fig. 2 B, third panel lane 6, and Fig. 2 C). We do not believe that the absence of CTLA-4 alters the overall level of protein in the raft fraction as can be seen in Fig. 2 B, the level of fyn in the raft fraction (using a whole cell lysate) is unchanged (as shown in Fig. 2 B, top panel). Similar results were observed when examining other, but not all, proteins using anti-pTyr blotting. Finally, as seen in Fig. 2 A the overall cholera toxin staining is not different between wild-type and knockout cells. Moreover, the results were not due to the increased phosphorylation of TCRζ within the rafts, as a direct comparison by probing with TCRζ-specific antibodies demonstrated significantly more TCRζ protein in the CTLA-4KO T cells (Fig. 2 B, fourth and fifth panel, and Fig. 2 C, right panel). These finding suggest that CTLA-4 negatively regulates TCRζ accumulation in the raft in fully activated T cells.
Functional Consequence of Increased CTLA-4/TCRζ Interaction with the Rafts of Activated T Cells.
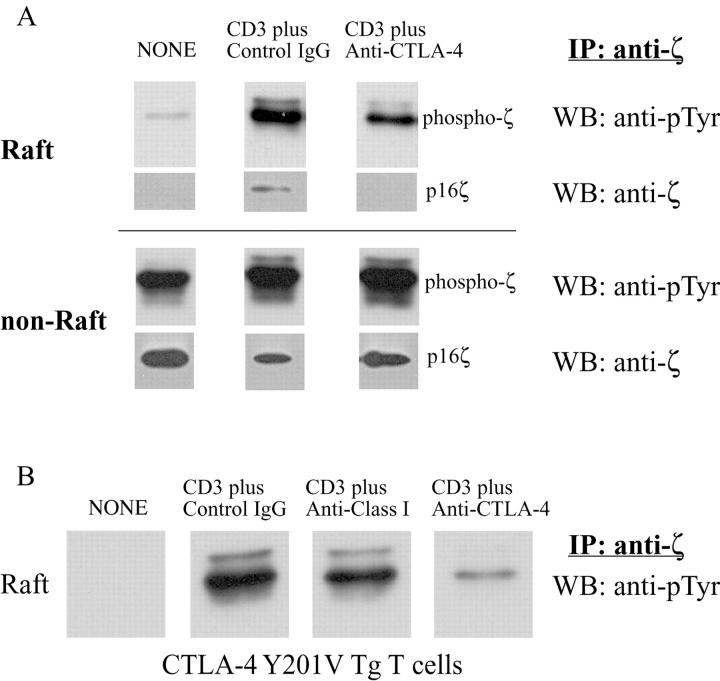
The function of CTLA-4 costimulation on the raft-associated TCR was examined by activating T cells with anti-CD3 in the presence of an anti–CTLA-4 mAb using a cross-linking anti–hamster antibody as described previously (18). Similar to previous studies (12), concomitant CD3 and CTLA-4 cross-linking resulted in significantly reduced tyrosine phosphorylation of TCRζ, CD3ɛ, and LAT as compared with CD3 cross-linking alone (unpublished data). Activated T cells from B6 mice were subjected to sucrose gradient separation to isolate rafts. TCRζ was immunoprecipitated from either the raft or nonraft fractions and subjected to Western blot analysis. As shown previously (6), TCRζ accumulation within the rafts was induced by TCR cross-linking. This accumulation was significantly inhibited by CTLA-4 co-cross-linking as determined by anti-phosphotyrosine blotting (Fig. 3 A, top three panels, and Fig. 3 C, left panel). Reprobing the membrane using a TCRζ-specific antibody showed that the p16ζ band disappeared after CTLA-4-cross-linking (Fig. 3 A, second three panels). These findings suggest that either the association of CTLA-4 with TCRζ in the nonraft fractions prevents ζ-chain phosphorylation and raft accumulation or that the association of the raft membrane-associated CTLA-4 alters the phosphorylation state of TCRζ resulting in dissociation of TCRζ from the signaling complex.
Figure 3.
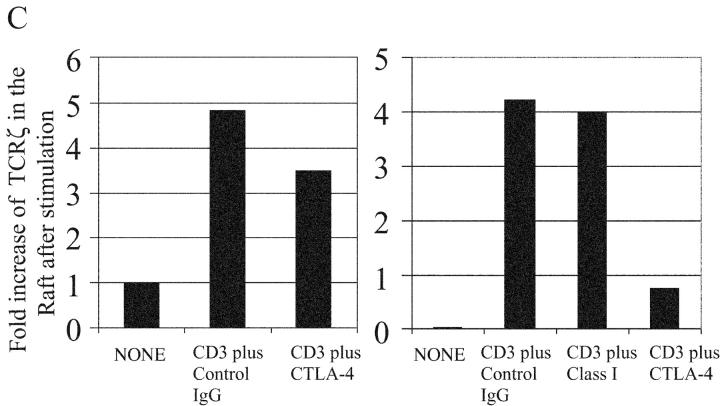
CTLA-4 inhibits TCR association to the lipid raft. (A) 108 activated mouse T cells were left untreated or stimulated with anti-CD3 (3 μg/ml) plus control hamster antibody (30 μg/ml) or anti-CTLA-4 antibodies (30 μg/ml) and the lysates were fractionated by sucrose density gradient centrifugation. TCRζ was IP from the raft fractions (top six panels) or from the detergent soluble nonraft fractions (bottom six panels and detected by WB by either anti-ptyr (top panels) or anti-TCRζ antibody. (B) The experiments described above were performed using CTLA-4 Y201V mutant Tg mice. Phosphotyrosine blot of the raft fractions is shown. Note that hamster anti–mouse class I antibody was included as a surface control. (C) The band intensity of the phosphorylated TCRζ in the raft fraction from above experiments was measured by densitometry. The data is normalized as the fold increase as compared with the background phosphorylation of ζ in B6 mice. The results were representative of eight independent experiments.
To confirm that CTLA-4 engagement inhibited TCRζ accumulation within the raft, T cells from transgenic (Tg) mice overexpressing CTLA-4 were compared with transgene negative littermates. Previous studies have shown that the mutation of amino acid 201 from tyrosine to valine results in a functional CTLA-4 molecule that is constitutively expressed on the T cell membrane (unpublished data, and reference 10). T cells from normal B6 or CTLA-4 Tg mice were activated with anti-CD3 alone or in the presence of CTLA-4 cross-linking. As can be seen in Fig. 3 B, there was dramatic inhibition of TCRζ accumulation in the raft in Tg-expressing T cells based on immunoblotting of anti-TCRζ precipitates with anti-phosphotyrosine. Cross-linking of anti–class I with anti-CD3 did not result in down-regulation of TCRζ in the rafts. Similar results were observed with anti-CD28 (unpublished data). In addition, although only the relevant bands are shown in the figure, there were no extraneous bands observed in the rest of the gel image with the exception of bands consistent with heavy and light chains of the immunoglobulin used for immunoprecipitation. Analysis of TCRζ demonstrated an 82% decrease in phosphoTCRζ accumulation in the raft when CTLA-4 was cross-linked with the TCR (Fig. 3 C, right panel). This compares to only a 28% decrease in the wild-type B6 T cells reflecting the lower CTLA-4 expression in this setting (Fig. 3 C, left panel). Interestingly, the amount of basal phospho-ζ in the rafts of unstimulated T cells was lower in Tg+ versus control wild-type T cells suggesting that overexpression of CTLA-4 reducing accumulation/retention of pTCRζ opposite what was observed in the CTLA-4KO T cells. Thus, CTLA-4–mediated inhibition of TCR signaling occurs preferentially within the rafts as a consequence of reduced TCR accumulation and TCRζ chain phosphorylation.
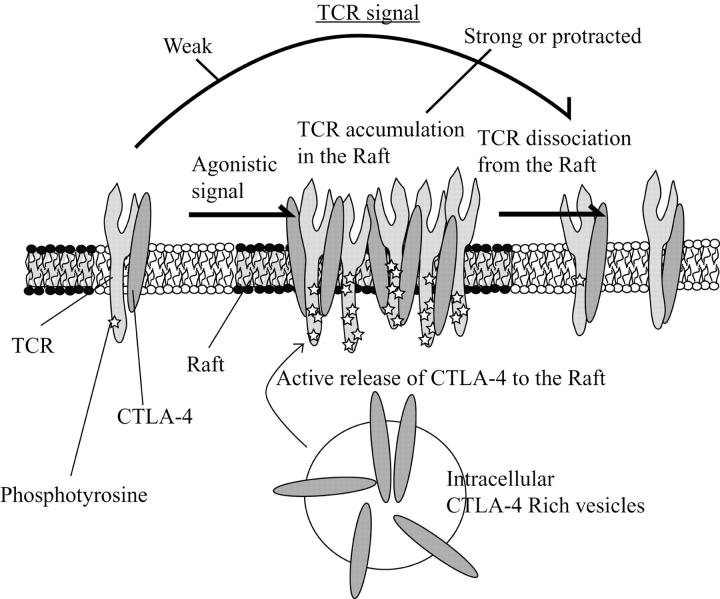
Studies designed to dissect the molecular mechanism of CTLA-4–mediated immune regulation have been conflicting. We proposed an “inhibition of proximal signal transduction model” based on the ability of CTLA-4 to interact directly with TCR complex proteins (12). However, others have shown more downstream effects of CTLA-4 engagement consistent with a distal role for CTLA-4 function (19). Some of the difficulty in dissecting the functional basis of CTLA-4 resides in its complex cell localization with the great majority residing in intracellular vesicles. However, the recent studies by several groups demonstrating the localization of CTLA-4 to the lipid rafts/immunological synapse after T cell activation provided a structural basis for the proximal signal inhibition model (8, 20). In this study, we have extended this observation to the functional level. Based on the results presented, we propose that after T cell activation, CTLA-4 is induced and translocates from the intracellular vesicles to the immunological synapse where it associates with TCRζ in the lipid rafts attenuating T cell signaling. This is most prominent after strong or protracted T cell agonist signaling as more CTLA-4 is recruited to the raft/synapse. The accumulation of CTLA-4 in the raft/synapse attenuates the sustained TCR signal by driving TCR out of raft complex where LAT and lck reside. In addition, CTLA-4 can associate with nonraft TCRζ where it increases the activation threshold by inhibiting TCR association to the raft (Fig. 4) . These effects are consistent with the proposed models of CTLA-4 inhibition (21).
Figure 4.
A model for CTLA-4–mediated inhibition of TCR association to the raft.
Finally, these results leave open the question of how CTLA-4 regulates the raft association of TCR. There are at least two possibilities. One is a potential role of CTLA-4–regulated tyrosine phosphatases to control T cell signaling. Two phosphatases, namely, SHP-1 and SHP-2, have been suggested in several studies to mediate CTLA-4–mediated inhibition either directly by dephosphorylating TCRζ (12, 22) or indirectly through dephosphorylation of the regulatory tyrosine of ZAP-70 (23). During CTLA-4 association to the rafts, the associated phosphatases might dephosphorylate these important signal components and subsequently cause dissociation of the raft associated molecules such as Lck, Fyn, LAT, and TCR chain. In fact, tyrosine phosphorylation of ZAP70 controls its association to LAT and Lck, while TCRζ phosphorylation is important for its interaction with Fyn, Lck, and ZAP70. It is also possible that CTLA-4 might influence the spatial localization of TCR and coreceptors. The crystal structure of the B7-CTLA-4 predicts that the intermembrane distance of the B7-CTLA-4 complex is very similar to that of MHC–TCR interaction (130–150 Å) but forms highly ordered oligomers due to their unique dimerization structures (for a review, see reference 24). Upon CTLA-4 association to the immunological synapse, the highly ordered CTLA-4-B7 complex may surround TCR complex in the c-SMAC and limit the accessibility of CD4-Lck complex. In fact, Lck-associated CD4 coreceptors and ZAP70-associated TCR differentially cluster during the immunological synapse formation (25). Understanding the spatial relationship of CTLA-4 and the proximal TCR signaling components will hopefully help elucidate the molecular mechanism by which CTLA-4 down-regulates T cell activation.
Acknowledgments
The authors wish to thank Drs. Anthony DeFranco for critical review of the manuscript as well as Drs. Helene Bour-Jordan, Qizhi Tang, and Arthur Weiss for helpful discussions.
This research was supported by National Institutes of Health grant USPHS P01 AI35297 and USPHS R01 AI26644.
References
- 1.Greenwald, R.J., Y.E. Latchman, and A.H. Sharpe. 2002. Negative co-receptors on lymphocytes. Curr. Opin. Immunol. 14:391–396. [DOI] [PubMed] [Google Scholar]
- 2.Waterhouse, P., J.M. Penninger, E. Timms, A. Wakeham, A. Shahinian, K.P. Lee, C.B. Thompson, H. Griesser, and T.W. Mak. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 270:985–988. [DOI] [PubMed] [Google Scholar]
- 3.Tivol, E.A., F. Borriello, A.N. Schweitzer, W.P. Lynch, J.A. Bluestone, and A.H. Sharpe. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 3:541–547. [DOI] [PubMed] [Google Scholar]
- 4.Krummel, M.F., and M.M. Davis. 2002. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr. Opin. Immunol. 14:66–74. [DOI] [PubMed] [Google Scholar]
- 5.Dustin, M.L., and A.C. Chan. 2000. Signaling takes shape in the immune system. Cell. 103:283–294. [DOI] [PubMed] [Google Scholar]
- 6.Cherukuri, A., M. Dykstra, and S.K. Pierce. 2001. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity. 14:657–660. [DOI] [PubMed] [Google Scholar]
- 7.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity. 8:723–732. [DOI] [PubMed] [Google Scholar]
- 8.Darlington, P.J., M.L. Baroja, T.A. Chau, E. Siu, V. Ling, B.M. Carreno, and J. Madrenas. 2002. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J. Exp. Med. 195:1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikuma, S., M. Murakami, K. Tanaka, and T. Uede. 2000. Janus kinase 2 is associated with a box 1-like motif and phosphorylates a critical tyrosine residue in the cytoplasmic region of cytotoxic T lymphocyte associated molecule-4. J. Cell. Biochem. 78:241–250. [PubMed] [Google Scholar]
- 10.Masteller, E.L., E. Chuang, A.C. Mullen, S.L. Reiner, and C.B. Thompson. 2000. Structural analysis of CTLA-4 function in vivo. J. Immunol. 164:5319–5327. [DOI] [PubMed] [Google Scholar]
- 11.Tang, Q., S.K. Subudhi, K.J. Henriksen, C.G. Long, F. Vives, and J.A. Bluestone. 2002. The Src family kinase Fyn mediates signals induced by TCR antagonists. J. Immunol. 168:4480–4487. [DOI] [PubMed] [Google Scholar]
- 12.Lee, K.M., E. Chuang, M. Griffin, R. Khattri, D.K. Hong, W. Zhang, D. Straus, L.E. Samelson, C.B. Thompson, and J.A. Bluestone. 1998. Molecular basis of T cell inactivation by CTLA-4. Science. 282:2263–2266. [DOI] [PubMed] [Google Scholar]
- 13.Samelson, L.E., A.F. Phillips, E.T. Luong, and R.D. Klausner. 1990. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc. Natl. Acad. Sci. USA. 87:4358–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang, E., K.M. Lee, M.D. Robbins, J.M. Duerr, M.L. Alegre, J.E. Hambor, M.J. Neveu, J.A. Bluestone, and C.B. Thompson. 1999. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J. Immunol. 162:1270–1277. [PubMed] [Google Scholar]
- 15.Khattri, R., J.A. Auger, M.D. Griffin, A.H. Sharpe, and J.A. Bluestone. 1999. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 162:5784–5791. [PubMed] [Google Scholar]
- 16.Martin, M., H. Schneider, A. Azouz, and C.E. Rudd. 2001. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J. Exp. Med. 194:1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katagiri, Y.U., T. Mori, H. Nakajima, C. Katagiri, T. Taguchi, T. Takeda, N. Kiyokawa, and J. Fujimoto. 1999. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 274:35278–35282. [DOI] [PubMed] [Google Scholar]
- 18.Schneider, H., S. da Rocha Dias, H. Hu, and C.E. Rudd. 2001. A regulatory role for cytoplasmic YVKM motif in CTLA-4 inhibition of TCR signaling. Eur. J. Immunol. 31:2042–2050. [DOI] [PubMed] [Google Scholar]
- 19.Calvo, C.R., D. Amsen, and A.M. Kruisbeek. 1997. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J. Exp. Med. 186:1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egen, J.G., and J.P. Allison. 2002. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 16:23–35. [DOI] [PubMed] [Google Scholar]
- 21.Egen, J.G., M.S. Kuhns, and J.P. Allison. 2002. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 3:611–618. [DOI] [PubMed] [Google Scholar]
- 22.Marengere, L.E., P. Waterhouse, G.S. Duncan, H.W. Mittrucker, G.S. Feng, and T.W. Mak. 1996. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 272:1170–1173. [DOI] [PubMed] [Google Scholar]
- 23.Guntermann, C., and D.R. Alexander. 2002. CTLA-4 suppresses proximal TCR signaling in resting human CD4(+) T cells by inhibiting ZAP-70 Tyr(319) phosphorylation: a potential role for tyrosine phosphatases. J. Immunol. 168:4420–4429. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, J.C., X. Zhang, S.G. Nathenson, and S.C. Almo. 2002. Structural mechanisms of costimulation. Nat. Immunol. 3:427–434. [DOI] [PubMed] [Google Scholar]
- 25.Krummel, M.F., M.D. Sjaastad, C. Wulfing, and M.M. Davis. 2000. Differential clustering of CD4 and CD3ζ during T cell recognition. Science. 289:1349–1352. [DOI] [PubMed] [Google Scholar]