Summary. The evasion and subversion of immune responses during infection has elucidated many interesting and ingenious pathways used by pathogens to survive, expand, and eventually be transmitted to new hosts. These immune evasion mechanisms are of interest as they frequently suggest ways to prevent pathogens from causing infection or disease. More rarely, they make us consider or evaluate the whole strategy of vaccine or disease prevention.
The Plasmodium species that cause malaria have classical immune evasion strategies through apparently immunologically distinct replication cycles and sites with the host and polymorphic and clonally variant antigens (1, 2). Recently, the human parasite Plasmodium falciparum was shown to modulate host defences more directly by altering the function of antigen-presenting cells (3, 4). The paper in this issue by Ocaña-Morgner et al. (5) describing inhibition of CD8+ T cell responses during murine malaria infection extends these observations and not only suggests a novel scheme of immune subversion by the parasite, but also poses important questions for the existing strategies to develop a vaccine against malaria.
Dendritic Cells (DCs).
The role of DCs in orchestrating immune responses has become apparent in recent years and is encapsulated in their subtitle of professional antigen-presenting cells (for review see reference 6). Subtypes of myeloid, plasmacytotoid, and Langerhans DCs appear to have some different functions in different tissues or sites. Nevertheless, a common feature of DCs is their ability to phagocytose antigens, undergo a process of maturation in response to exogenous or endogenous signals, and up-regulate the requisite molecules to stimulate lymphocytes including memory and naive T, B, and NK cells. Myeloid DCs in humans can activate CD4+ T cells to proliferate and secrete Th1- or Th2-type cytokines and also cross-present exogenous antigens to cytotoxic CD8+ T cells. Given the pivotal role of DCs in the stimulation of innate and acquired immune responses, it is reasonable to suggest that these cells play a significant role in the host's defense against P. falciparum malaria.
Malaria.
Most morbidity and mortality from malaria is caused by infection with P. falciparum. Falciparum malaria is not simply a major public health problem but it is even considered to restrain economic growth in many parts of the world (7). The mainstay of disease control is the detection and treatment of cases. However, the rise of drug-resistant parasites and the cost of effective therapy have made the development of a simple, cheap, and effective vaccine imperative.
The life cycle of the parasite appears complex but in essence has a liver phase of preamplification of a few to many thousand of infective forms in hepatocytes. This obligatory phase liver stage is the prelude to logarithmic growth in the blood as parasites enter and multiply within erythrocytes. The blood parasitemia might be substantial and accompanied by severe clinical symptoms including coma, metabolic acidosis and signs of severe anemia in children, and multisystem failure in nonimmune adults (Fig. 1 ; reference 8).
Figure 1.
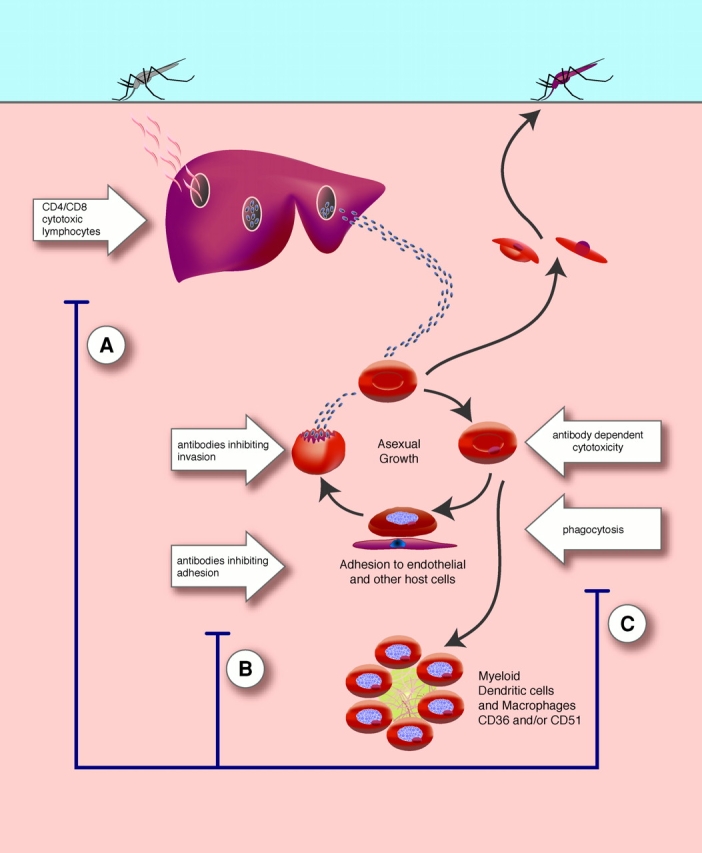
The life cycle of falciparum malaria Sporozoites, from female Anopheles mosquitoes taking a blood meal, enter the circulation and invade hepatocytes. ∼10,000 merozoites are formed by days 10–14. After rupture of the hepatocyte, the infective merozoites are released and invade erythrocytes. Here, the blood stage parasites can multiply approximately eightfold every 2 d and therefore within a short time a high proportion of erythrocytes might be infected comprising up to several grams of foreign antigens. During the second half of the erythrocytic cycle, PfEMP-1 or the variant antigen is expressed on the surface of infected erythrocytes. This family of proteins, and maybe other adhesive proteins, mediate the adhesion of infected erythrocytes to host receptors on endothelium, uninfected erythrocytes, and platelets (for reviews see references 38 and 39). Asexual parasites may differentiate into sexual forms or gametocytes. The parasite life cycle is completed within the female Anopheles mosquito after the sexual forms of the parasite are ingested in a blood meal. Immune responses against the plasmodium parasites are distinct for liver and blood stage parasites. Cytotoxic lymphocytes can recognize and destroy intrahepatic parasites and secretion of IFN-γ by both CD4+ and CD8+ T cells is associated with protective immune responses. Specific immunity against blood stage antigens includes antibodies directed against parasite molecules required for invasion of erythrocytes and antibodies recognizing the adhesive and variant proteins (PfEMP-1) expressed on the surface of erythrocytes. Infected erythrocytes may also be phagocytosed by splenic macrophages and/or destroyed by antibody-dependent cytotoxicity. Other effector mechanisms are poorly defined. Immune responses might be subverted during the blood stage of infection. (A) In this issue, Ocaña-Morgner et al. (reference 5) describe the inhibition of CD8+ T cell responses during murine malaria infection. (B) Adhesion of infected erythrocytes to DCs via CD36 and CD51 (αv integrin of the αvβ3 or αvβ5 heterodimer) may modulate DC function. These interactions reduce the ability of myeloid DCs to stimulate primary and secondary CD4+ T cell responses (references 3 and 4). (C) Adhesion of infected erythrocytes to macrophages may reduce secretion of inflammatory cytokines including TNF-α (reference 40).
Specific host effector responses against the falciparum parasites appear to be distinct for liver and blood stage parasites. There is substantial evidence that cytotoxic lymphocytes can recognize and destroy intrahepatic parasites (9, 10). More importantly, the secretion of IFN-γ by both CD4+ and CD8+ T cells has been associated with protective immune responses (9, 11–13). Indirect evidence for the presence of effective clinical immunity against liver stage parasites includes the association of specific class I allotypes with protection from malaria in a large case-control study in the Gambia (14) and the frequency of nonsynonymous to synonymous base substitution in the coding sequences for liver stage antigens circumsporozoite protein, liver stage antigen 1, and thrombospondin-related anonymous protein. (15–17).
Specific immunity against blood stage antigens includes antibodies directed against parasite molecules required for invasion of erythrocytes and antibodies able to stimulate antibody-dependent cytotoxicity (18, 19). Other antibodies recognizing the adhesive and variant proteins (P. falciparum membrane protein 1 [PfEMP-1]) expressed on the surface of erythrocytes may inhibit adhesion of infected erythrocytes to host ligands and/or enhance their phagocytosis of infected erythrocytes (Fig. 1; references 20 and 21).
Malaria Vaccines.
Several strategies are being followed to develop a vaccine against P. falciparum and are aimed at the liver stage, blood stage, and at “toxins” (including the malaria-specific glycosylphosphatidyl-inositol linkage) that may provoke host cell damage. Blood stage and anti-toxin vaccine programs have identified several potential candidate antigens that are being evaluated in animal models of malaria (22, 23).
Although there is ample evidence for the presence of anti-liver stage immune responses in individuals living in endemic areas, these effector mechanisms are not capable of complete elimination of parasites as people continue to suffer from successful liver stage infection and thus blood stage infection throughout their lives. Ongoing attempts to develop vaccines are focusing on the induction of cellular responses against the liver stage of the parasite based on the observation that potent protection against liver stages was achieved by inoculation with irradiated but not live sporozoites (24, 25).
It is hoped that strong cellular responses can be induced against liver stage antigens to destroy a proportion of the parasites developing within hepatocytes so that the number of parasites entering the blood would be reduced (for reviews see references 26 and 27). This would increase the time for a clinically significant parasitemia to develop and might allow the defences against blood stage parasites to be more effective.
New Evidence of Inhibition of Liver Stage Responses by Malaria Parasites.
The data provided by Ocaña-Morgner et al. (5) in this issue now provide an explanation for the discrepancy in the immunogenicity of live and irradiated sporozoites and indeed natural exposure to liver stages of Plasmodium. Using a rodent model of malaria, they present compelling evidence for the modulation of myeloid DCs during blood stage Plasmodium yoelii infection resulting in the inhibition of CD8+ T cell responses.
First, they show that irradiated sporozoites of P. yoelii in BALB/c mice induce a CD8+ T cell response against an epitope of the circumsporozoite protein strongly expressed on the sporozoite surface. This CD8+ T cell response is abrogated either by the simultaneous injection of viable nonirradiated sporozoites that go on to develop blood stage infection or by direct inoculation of blood stage forms themselves. The inhibitory effect of nonirradiated sporozoites is abolished by chemotherapy directed at blood stage parasites. Second, they show that the presence of low levels of P. yoelii–infected erythrocytes suppress the priming of CD8+ T cell responses normally induced by irradiated sporozoites.
It appears that inhibition of CD8+ T cell responses is mediated by DCs. Ocaña-Morgner et al. go on to show that maturation of bone marrow–derived DCs in vitro and ex vivo is inhibited and the survival of the DCs in vitro is increased after their exposure to blood stage parasites. Furthermore, after exposure to blood stage parasites the pattern of cytokine secretion by DCs is profoundly modified. IL-12 secretion is reduced and Il-10 secretion increased in response to LPS. Finally, after exposure to blood stage parasites either in vivo or in vivo, DCs fail to activate a CD8+ T cell clone. Thus, isolated live sporozoites do not inhibit DCs directly.
Their findings are consistent with the observations made with P. falciparum parasites in vitro where myeloid DCs were modulated by the adhesion of infection erythrocytes (3). Here, there was good evidence that CD4+ T cells were rendered functionally unresponsive and although adhesion of infected erythrocytes to DCs increased IL-10 secretion, inhibition of primary and recall CD4+ T cells was not obviously mediated by this cytokine (4).
By contrast, modulation of DC function was not observed with erythrocytes infected with the rodent parasite Plasmodium chabaudi chabaudi (28). Here, bone marrow–derived DCs produce TNF-α, IFN-γ, and IL-12 and mature normally in response to LPS when exposed to the parasite although their ability to support T cell activation was not investigated. Although another group also observed normal DC maturation in response to P. yoelii–infected erythrocytes, they nevertheless established that these DCs inhibited T cell proliferation and IL-2 secretion (29).
The data from Ocaña-Morgner et al. support the suggestion that the ability of parasites to modulate the function of immune system of their vertebrate host is not a singular feature of P. falciparum, but a more general feature of Plasmodium spp (3, 30). The precise mechanism involving cytoadhesion or soluble factors, or indeed both, may vary between parasite strains that have adopted to their particular host. These mechanism(s), including cytoadhesion, may simply serve to protect the more vulnerable stages of the parasitic life cycle, i.e., sporozoites and gametocytes.
Clearly, the suppression of liver stage immune responses has to be confirmed in humans. The epidemiology of the disease, with the failure of natural immunity to liver stage to prevent continued reinfection even in the most heavily exposed individuals, suggests immunity to the liver stage cannot provide sterile immunity. Indeed, direct observation of CD8+ T cell responses during malaria infection in endemic areas exposed to malaria parasites have yielded lower responses than might be expected (31). Previous explanations for the ability of fresh infection to establish and flourish the liver would have included polymorphic T cell epitopes, T cell antagonism by altered peptide ligands, and stochastic events allowing a few parasites to evade and escape detection (32). However, active suppression of immunity to liver stage parasites during blood stage infection adds a new dimension to the dynamics of parasite survival within the host.
Regulation of CD8+ T Cell Responses During Malaria Infection.
The suppression of CD8+ T cell responses during blood stage infection has wider consequences for the immune response not only to malaria but also to other infectious agents. Indeed, the suppression of CD8+ T cell responses is consistent with the association of Burkitt's lymphoma and endemic malaria (33). It has been suggested that malaria must induce loss of control of EBV-infected B lymphocytes (34) or provide a mitogen or mechanism that stimulates B cell growth (35). The observations by Ocaña-Morgner et al. suggest a mechanism for the failure of CD8+ lymphocytes to control EBV-infected lymphocytes.
The dysregulation of CD8+ T cells as a result of malaria infection might be even more extensive. Suppression of specific CD8+ T cell responses during blood stage malaria may explain the higher viral load in HIV+ individuals not only during but after infection with P. falciparum (36). There is the potential for malaria infection to accelerate the progression of HIV, although a recent systematic review of the area suggested that there is no convincing evidence for an interaction between malaria and HIV with the possible exception of an interaction between placental malaria and HIV infection (37). The overall significance of CD8+ T cell suppression during P. falciparum infection will clearly require study in the clinic and the laboratory and may have far reaching consequences for the implementation of public health measures.
Regulation of CD8+ T Cell Responses and Vaccine Development.
What would the implications be for vaccine development for malaria if these or functionally similar mechanisms of CD8+ T cell dysregulation occurred in human infection? There has been an unspoken assumption that immunity to liver and blood stage parasites are independent. However, we may not be able to regard these apparently distinct immune responses in isolation in natural infection.
For example, we now have to consider that the T cell responses might be reduced as a result of patent blood stage infection. One immediate implication that the levels of T cell responses measured during a clinical infection may not necessarily indicate the level of those responses before infection. Thus, the association between T cell responses and clinical protection could only reliably be established by prospective studies.
Of more pressing concern is the finding by Ocaña-Morgner et al. that blood stage parasites can inhibit established CD8+ T cell responses against liver stage parasites. If this also occurs in human infection in endemic areas, then the effectiveness of any liver stage vaccine would be less than that expected by challenge studies where significant blood stage infections do not develop. Vaccine development is somewhat empirical even with ample preliminary data and it might be that such concerns are unfounded by virtue of the innate difference between mice and men or by the immune responses induced by specific vaccination protocols.
On a more positive note, the interdependence of immunity to liver and blood stages suggests that there might be synergy between the control of parasite multiplication in the liver and the blood. Thus, vaccines stimulating the appropriate immune responses against liver or blood stage antigens or epitopes, and giving partial protection against the respective stages, may have a greater than additive effect when given together.
Of course it is “too early to tell” the significance of inhibition of CD8+ T cell responses by malaria infection. At the very least, the studies by Ocaña-Morgner et al. will stimulate thought and experiment not only in malaria but also in many areas of immunology. The results of these studies may influence the strategies to develop malaria vaccines.
Acknowledgments
B.C. Urban is supported by the Wellcome Trust. D.J. Roberts is supported by the National Blood Service, the University of Oxford, and the Howard Hughes Memorial Institute.
References
- 1.Roberts, D.J., A.G. Craig, A.R. Berendt, R. Pinches, G. Nash, K. Marsh, and C.I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 357:689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolad, A., and K. Berzins. 2000. Antigenic diversity of Plasmodium falciparum and antibody-mediated parasite neutralization. Scand. J. Immunol. 52:233–239. [DOI] [PubMed] [Google Scholar]
- 3.Urban, B.C., D.J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J.M. Austyn, and D.J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 400:73–77. [DOI] [PubMed] [Google Scholar]
- 4.Urban, B.C., N. Willcox, and D.J. Roberts. 2001. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA. 98:8750–8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocana-Morgner, C., M.M. Mota, and A. Rodriguez. 2002. Malaria blood-stage suppression of liver-stage immunity by dendritic cells. J. Exp. Med. 197:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 7.Breman, J.G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64:1–2. (Suppl):1–11. [DOI] [PubMed] [Google Scholar]
- 8.Newton, C.R., and S. Krishna. 1998. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol. Ther. 79:1–53. [DOI] [PubMed] [Google Scholar]
- 9.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 330:664–666. [DOI] [PubMed] [Google Scholar]
- 10.Romero, P., J.L. Maryanski, G. Corradin, R.S. Nussenzweig, V. Nussenzweig, and F. Zavala. 1989. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 341:323–325. [DOI] [PubMed] [Google Scholar]
- 11.Moreno, A., P. Clavijo, R. Edelman, J. Davis, M. Sztein, D. Herrington, and E. Nardin. 1991. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int. Immunol. 3:997–1003. [DOI] [PubMed] [Google Scholar]
- 12.Seguin, M.C., F.W. Klotz, I. Schneider, J.P. Weir, M. Goodbary, M. Slayter, J.J. Raney, J.U. Aniagolu, and S.J. Green. 1994. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J. Exp. Med. 180:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doolan, D.L., and S.L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453–1462. [DOI] [PubMed] [Google Scholar]
- 14.Hill, A.V., C.E. Allsopp, D. Kwiatkowski, N.M. Anstey, P. Twumasi, P.A. Rowe, S. Bennett, D. Brewster, A.J. McMichael, and B.M. Greenwood. 1991. Common west African HLA antigens are associated with protection from severe malaria. Nature. 352:595–600. [DOI] [PubMed] [Google Scholar]
- 15.Lockyer, M.J., K. Marsh, and C.I. Newbold. 1989. Wild isolates of Plasmodium falciparum show extensive polymorphism in T cell epitopes of the circumsporozoite protein. Mol. Biochem. Parasitol. 37:275–280. [DOI] [PubMed] [Google Scholar]
- 16.Robson, K.J., A. Dolo, I.R. Hackford, O. Doumbo, M.B. Richards, M.M. Keita, T. Sidibe, A. Bosman, D. Modiano, and A. Crisanti. 1998. Natural polymorphism in the thrombospondin-related adhesive protein of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 58:81–98. [DOI] [PubMed] [Google Scholar]
- 17.Escalante, A.A., A.A. Lal, and F.J. Ayala. 1998. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics. 149:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druilhe, P., and J.L. Perignon. 1994. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol. Lett. 41:115–120. [DOI] [PubMed] [Google Scholar]
- 19.Good, M.F., and D.L. Doolan. 1999. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11:412–419. [DOI] [PubMed] [Google Scholar]
- 20.Bull, P.C., B.S. Lowe, M. Kortok, C.S. Molyneux, C.I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bull, P.C., and K. Marsh. 2002. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 10:55–58. [DOI] [PubMed] [Google Scholar]
- 22.Good, M.F. 2001. Towards a blood-stage vaccine for malaria: are we following all the leads? Nat. Rev. Immunol. 1:117–125. [DOI] [PubMed] [Google Scholar]
- 23.Schofield, L., M.C. Hewitt, K. Evans, M.A. Siomos, and P.H. Seeberger. 2002. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature. 418:785–789. [DOI] [PubMed] [Google Scholar]
- 24.Nussenzweig, R.S., J.P. Vanderberg, H. Most, and C. Orton. 1969. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 222:488–489. [DOI] [PubMed] [Google Scholar]
- 25.Clyde, D.F. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24:397–401. [DOI] [PubMed] [Google Scholar]
- 26.Nardin, E., F. Zavala, V. Nussenzweig, and R.S. Nussenzweig. 1999. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia. 41:397–402. [PubMed] [Google Scholar]
- 27.Moorthy, V., and A.V. Hill. 2002. Malaria vaccines. Br. Med. Bull. 62:59–72. [DOI] [PubMed] [Google Scholar]
- 28.Seixas, E., C. Cross, S. Quin, and J. Langhorne. 2001. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur. J. Immunol. 31:2970–2978. [DOI] [PubMed] [Google Scholar]
- 29.Luyendyk, J., O.R. Olivas, L.A. Ginger, and A.C. Avery. 2002. Antigen-presenting cell function during Plasmodium yoelii infection. Infect. Immun. 70:2941–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban, B.C., and D.J. Roberts. 2002. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr. Opin. Immunol. 14:458–465. [DOI] [PubMed] [Google Scholar]
- 31.Lalvani, A., N. Hurt, M. Aidoo, P. Kibatala, M. Tanner, and A.V. Hill. 1996. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur. J. Immunol. 26:773–779. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert, S.C., M. Plebanski, S. Gupta, J. Morris, M. Cox, M. Aidoo, D. Kwiatkowski, B.M. Greenwood, H.C. Whittle, and A.V. Hill. 1998. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 279:1173–1177. [DOI] [PubMed] [Google Scholar]
- 33.Burkitt, D. 1958. A sarcoma of the jaw in African children. Br. J. Surg. 46:218–223. [DOI] [PubMed] [Google Scholar]
- 34.Whittle, H.C., J. Brown, K. Marsh, B.M. Greenwood, P. Seidelin, H. Tighe, and L. Wedderburn. 1984. T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum malaria. Nature. 312:449–450. [DOI] [PubMed] [Google Scholar]
- 35.Greenwood, B.M. 1974. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1:435–436. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman, I.F., C.S. Jere, T.E. Taylor, P. Munthali, J.R. Dyer, J.J. Wirima, S.J. Rogerson, N. Kumwenda, J.J. Eron, S.A. Fiscus, et al. 1999. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 13:487–494. [DOI] [PubMed] [Google Scholar]
- 37.Chandramohan, D., and B.M. Greenwood. 1998. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int. J. Epidemiol. 27:296–301. [DOI] [PubMed] [Google Scholar]
- 38.Craig, A., and A. Scherf. 2001. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol. Biochem. Parasitol. 115:129–143. [DOI] [PubMed] [Google Scholar]
- 39.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673–767. [DOI] [PubMed] [Google Scholar]
- 40.McGilvray, I.D., L. Serghides, A. Kapus, O.D. Rotstein, and K.C. Kain. 2000. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 96:3231–3240. [PubMed] [Google Scholar]


