Abstract
Caspase activation is a central event in numerous forms of apoptosis and results in the proteolytic degradation of multiple substrate proteins that contribute to the apoptotic phenotype. An important route to caspase activation proceeds via assembly of the “apoptosome” as a result of the cell stress–associated release of mitochondrial cytochrome c. Previous studies have shown that primary neutrophils are largely incapable of mitochondrial respiration, suggesting that these cells either lack functional mitochondria or possess a defective respiratory chain. This prompted us to examine whether neutrophils retain an intact cytochrome c/apoptotic protease-activating factor 1 (Apaf-1) pathway to caspase activation and apoptosis. We show that primary human neutrophils contain barely detectable levels of cytochrome c as well as other mitochondrial proteins. Surprisingly, neutrophil cell–free extracts readily supported Apaf-1–dependent caspase activation, suggesting that these cells may assemble cytochrome c–independent apoptosomes. However, further analysis revealed that the trace amount of cytochrome c present in neutrophils is both necessary and sufficient for Apaf-1–dependent caspase activation in these cells. Thus, neutrophils have a lowered threshold requirement for cytochrome c in the Apaf-1–dependent cell death pathway. These observations suggest that neutrophils retain cytochrome c for the purpose of assembling functional apoptosomes rather than for oxidative phosphorylation.
Keywords: apoptosis, XIAP, caspases, cytochrome c, neutrophil
Introduction
Neutrophils are granulocytes that have relatively short lifespans of 18–24 h and therefore undergo constant turnover in vivo. It is well established that senescent neutrophils undergo caspase-dependent apoptosis that can be inhibited by cytokines or proinflammatory stimuli, thereby prolonging the function of these cells at inflammatory sites (1–3). However, neutrophil apoptosis remains relatively poorly defined at a molecular level and the trigger for senescence-associated neutrophil apoptosis remains to be established. Much of what we know concerning the molecular control of apoptosis is derived from studies on transformed or proliferating cells. However, it remains unclear whether the well-established pathways to apoptosis are accessible in all cells or whether cell type–specific pathways exist. For example, although specific caspases have been established to act as initiators of apoptosis in many situations, it is not clear whether the caspase repertoire differs substantially between distinct cell types. The absence of certain initiator caspases or other key components of the cell death machinery may render certain cell death pathways inaccessible in some cell types.
Caspases are cysteine proteases that play an evolutionary conserved role in apoptosis from nematodes to man (4–7). Three major routes to caspase activation and apoptosis have been defined at present. One route, the death receptor pathway, is initiated through interactions between certain cytokines of the TNF/nerve growth factor superfamily and their cognate receptors (e.g., TNFR, CD95R, and TNF-related apoptosis-inducing ligand receptor). Upon engagement, the latter receptors recruit adaptor molecules, such as TNF receptor type I–associated death domain and Fas-associated protein with a death domain, which can directly bind to specific caspases and promote their activation (8, 9). The second major pathway to caspase activation is more recently described and is triggered by the release of mitochondrial cytochrome c into the cytosol in response to cellular damage or stress (10–12). Diverse cytotoxic drugs, as well as radiation and heat shock, can engage the latter pathway. Upon entry into the cytosol, cytochrome c interacts with apoptotic protease-activating factor 1 (Apaf-1),*which promotes oligomerization of the latter and permits recruitment of caspase-9 molecules into the complex (13). Recruitment of several caspase-9 molecules into the Apaf-1 apoptosome permits autoprocessing and activation of this caspase that can then process additional caspases downstream (14). In the third major pathway to caspase activation, CTL/NK cells can engage the cell death machinery through direct proteolytic processing of caspases via the CTL/NK granule component, granzyme B (15, 16).
It is well established that neutrophils, as well as other granulocytes, are capable of engaging the death receptor pathway to apoptosis (17, 18). In addition, many cytotoxic drugs that are considered to engage the mitochondrial apoptosome pathway also promote neutrophil apoptosis, suggesting that this pathway is also intact in these cells (19). However, the presence of functional mitochondria in neutrophils has been the subject of debate. Recent reports indicate that primary human neutrophils are essentially incapable of oxidative phosphorylation, suggesting that these cells contain few mitochondria or that neutrophils may lack key respiratory chain components (20). Mitochondrial-like structures have been observed in neutrophils and other granulocytes using fluorescent dyes that preferentially label mitochondria (21, 22). However, these structures tend to be relatively few in number and have not been confirmed to contain cytochrome c or other proteins that participate in the regulation of apoptosis.
Because of the importance of the mitochondrion as a conduit for numerous death-promoting stimuli, we were prompted to explore whether neutrophils retained an intact mitochondrial apoptosome pathway to apoptosis. Here, we report that primary human neutrophils contain very minimal amounts of cytochrome c compared with many transformed cell lines, but that these levels were still sufficient to engage the apoptosome pathway to caspase activation. In line with this observation, neutrophil apoptosomes became active at much lower cytochrome c concentrations than were required to initiate caspase activation in other cell types. Thus, neutrophils have a lowered threshold requirement for engagement of the Apaf-1 pathway by cytochrome c. These observations suggest that neutrophils retain cytochrome c for the purpose of assembling functional apoptosomes rather than for mitochondrial respiration.
Materials and Methods
Reagents.
Anti–cytochrome c, anti–caspase-2, anti–caspase-3, anti–caspase-7, anti–X-linked inhibitor of apoptosis protein (XIAP), anti-BRUCE, and anti-gelsolin antibodies were purchased from BD Biosciences; anti–caspase-1 rabbit polyclonal antibody was purchased from Santa Cruz Biotechnology, Inc.; anti–caspase-6 and anti–caspase-9 antibodies were purchased from Upstate Biotechnology; anti–cIAP-1 and anti-survivin antibodies were from R&D Systems; anti–manganese superoxidase dismutase and anti–heat shock protein 60 (HSP-60) antibodies were from StressGen Biotechnologies; anti–caspase-9 antibodies were purchased from Oncogene Research Products; anti–caspase-8 mAb was provided by M. Peter (Ben May Institute for Cancer Research, Chicago, IL); anti–cytochrome c oxidase, subunit I, was purchased from Molecular Probes; second mitochondrial activator of caspases (Smac) polyclonal antibody was generated by immunizing rabbits with recombinant polyhistidine-tagged Smac and has been described previously (23); antibodies selectively recognizing active caspase-3 were purchased from New England Biolabs, Inc.; and anti-actin and anti-tubulin antibodies were from ICN Biomedicals. Unless otherwise indicated, all other reagents were purchased from Sigma-Aldrich.
Neutrophil Isolation.
Neutrophils were isolated from healthy volunteers by dextran (3%) sedimentation followed by centrifugation through a discontinuous ficoll gradient. Neutrophil pellets were resuspended in DMEM supplemented with 10% fetal calf serum, 1% penicillin/streptomycin solution, and fungizone at a concentration of 106 cells/ml. Cells were incubated in polypropylene tubes (Falcon; Becton Dickinson) to prevent adherence. Neutrophil purity, as assessed by size and granularity on flow cytometry, was consistently >95%.
Quantification of Apoptosis.
Spontaneous apoptosis of neutrophils was quantified by flow cytometry as the percent of cells with hypodiploid DNA at 24 h. 106 cells were centrifuged at 400 g for 5 min and then gently resuspended in 400 μl hypotonic fluorochrome solution (50 μg/ml propidium iodide, 3.4 mM sodium citrate, 1 mM Tris, 0.1 mM EDTA, 0.1% Triton X-100 in PBS, pH 7.2) followed by incubation at 4°C for 10 min. Cells were analyzed (minimum of 5,000 events per sample) using a Coulter Epics XL-mcl cytofluorimeter and all measurements were performed under the same instrument settings. Apoptotic nuclei were distinguished from normal nuclei by their hypodiploid DNA content.
Preparation of Cell-free Extracts.
Cell-free extracts of primary human neutrophils were prepared as previously described (14, 24). In brief, 5 × 108 cells (from four to five pooled donors) were packed into a 2-ml homogenizer and an equal volume of ice cold cell extract buffer was added (CEB: 20 mM Hepes-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 100 μM PMSF, 10 μg/ml leupeptin, 2 μg/ml aprotinin). Cells were allowed to swell on ice for 20–30 min in the CEB and then were lysed by homogenization with ∼20–30 strokes of a B-type pestle. Crude extracts were then centrifuged for 30 min at 15,000 g to remove nuclei, unbroken cells, and other debris. Postnuclear extracts were then used for immunodepletions as described below.
Immunodepletion of Extracts.
Cell extracts were immunodepleted of specific caspases or cytochrome c as previously described (14, 24). In brief, 40 μl of a 50% slurry of protein A/G agarose beads (Santa Cruz Biotechnology, Inc.) were coated with 5 μg depletion antibody by continual rotation for 3 h at 4°C in PBS, pH 7.2 (final volume of 200 μl). Purified rabbit or mouse IgG (Sigma-Aldrich) was used as a control. Antibody-coated beads were then washed twice with PBS, pH 7.2, before adding to cell extracts. 100 μl cell extracts were immunodepleted overnight at 4°C under constant rotation followed by removal of antibody-coated beads by centrifugation. Depleted extracts were used immediately and were not refrozen.
Assembly of Cell-free Reactions.
Cell-free reactions were typically set up in 10–100-μl reaction volumes. For 100-μl scale reactions, 50 μl cell extract (∼30 mg/ml) was brought to a final volume of 100 μl in CEB. Caspase activation was initiated by the addition of 50 μg/ml bovine heart cytochrome c and 1 mM dATP. 10–20 μl samples of cell-free reactions were removed at various times and frozen at −70°C for subsequent Western blot analysis of caspase activation.
Preparation of Subcellular Fractions.
Cytosolic and mitochondrial-enriched fractions of neutrophils and Jurkat cells were generated using a digitonin-based subcellular fractionation technique essentially as previously described (23). In brief, 107 cells were harvested by centrifugation at 800 g, washed in PBS, pH 7.2, and recentrifuged. Cells were digitonin permeabilized for 5 min on ice at a density of 3 × 107/ml in cytosolic extraction buffer (250 mM sucrose, 70 mM KCl, 137 mM NaCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.2, 100 μM PMSF, 10 μg/ml leupeptin, 2 μg/ml aprotinin, containing 250 μg/ml digitonin). Plasma membrane permeabilization of cells was confirmed by staining in a 0.2% trypan blue solution. Cells were then centrifuged at 1,000 g for 5 min at 4°C. The supernatants (cytosolic fractions) were saved and the pellets solubilized in the same volume of mitochondrial lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.2% Triton X-100, 0.3% NP-40, 100 μM PMSF, 10 μg/ml leupeptin, 2 μg/ml aprotinin) followed by centrifugation at 10,000 g for 10 min at 4°C. For the detection of MnSOD or cytochrome c, equal volumes (32 μl) of cytosolic and pellet fractions were supplemented with 5× SDS-PAGE loading buffer and subjected to standard 12% SDS-PAGE.
Immunoprecipitation (IP) of Cytochrome c.
Cell lysates were prepared by resuspending cells in 800 μl IP lysis buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP-40) containing 100 μM PMSF, 10 μg/ml leupeptin, and 2 μg/ml aprotinin. After centrifugation, clarified lysates were subjected to IP using 1 μg anti–cytochrome c antibody (BD Biosciences) and 30 μl agarose-coupled protein A/G (Santa Cruz Biotechnology, Inc.). Samples were incubated under rotation for 3–6 h at 4°C and complexes were washed three to four times in IP lysis buffer containing 0.1% NP-40.
Immunostaining.
Freshly isolated primary human neutrophils were loaded with Mitotracker CMXRos (Molecular Probes) by incubation with 50 nM Mitotracker CMXRos for 45 min followed by washing. Neutrophils were then cytospun onto glass slides at a density of 5 × 105 cells/ml followed by immediate fixation in 3% paraformaldehyde/PBS for 10 min. Fixed cells were then washed three times in PBS and permeabilized in 0.15% Triton X-100 in PBS for 15 min. Cells were blocked for 30 min in 2% BSA/PBS and probed with a 1:40 dilution (25 μg/ml final concentration) of anti–cytochrome c (clone 6H2.B4; BD Biosciences) for 2 h at room temperature. After incubation with primary antibody, cells were washed three times (10 min per wash) in 2% BSA/PBS followed by probing for 1 h with a 1:500 dilution (4 μg/ml final concentration) of Alexafluor 488–conjugated anti–mouse antibodies (Molecular Probes). Immunostained cells were washed three times in PBS, briefly stained with 20 μM Hoechst 33358 in PBS, and mounted in Slowfade Light Antifade mounting medium (Molecular Probes).
Results and Discussion
Neutrophil Mitochondria Are Deficient in Cytochrome c.
Mitochondria have been implicated as important sensors of cellular damage in diverse cell types. However, previous reports suggest that neutrophils are incapable of oxidative phosphorylation, suggesting that these cells contain very few or defective mitochondria (20). Mitotracker staining studies indicate that primary neutrophils do contain mitochondrial structures (21), which led us to explore whether these mitochondria contain cytochrome c and possess an intact apoptosome pathway to caspase activation. Initially, we prepared total cell lysates from primary human neutrophils and explored the relative levels of the mitochondrial proteins: cytochrome c, cytochrome c oxidase (subunit I), manganese superoxide dismutase (MnSOD), Smac/DIABLO, and HSP-60 in neutrophils versus other cell types. As controls, we also probed the same cell lysates for cytoplasmic proteins. As illustrated in Fig. 1 , although neutrophils contained readily detectable levels of MnSOD, they appeared devoid of the other mitochondrial proteins examined (cytochrome c, cytochrome c oxidase, and HSP-60). In contrast, caspase-1, -3, -8, and actin were readily detectable in the same lysates. Even prolonged overexposure of the blots failed to detect cytochrome c in lysates from neutrophils whereas this protein was readily detectable in all other cells types tested (not depicted).
Figure 1.
Neutrophils are deficient in cytochrome c and other mitochondrial proteins. Lysates (107 cells/ml) were prepared from primary human neutrophils (neutrophils were pooled from five donors) and the indicated cell lines. Equal amounts of protein (∼100 μg) were separated by SDS-PAGE followed by probing replicate blots with antibodies against the indicated proteins.
To explore further whether neutrophil mitochondria contain cytochrome c, we prepared concentrated mitochondrial and cytosolic fractions from neutrophils using standard methods. As a control, we prepared similar fractions from Jurkat T cells, a cell line that contains relatively low levels of cytochrome c (Fig. 1). Numerous studies have shown that cytochrome c enters the cytosol during apoptosis initiated by cytotoxic drugs such as staurosporine and actinomycin D. However, whereas cytochrome c was readily detectable in the mitochondrial fractions prepared from staurosporine- or actinomycin D–treated Jurkat cells (Fig. 2 A), mitochondrial cytochrome c failed to be detected in similar fractions prepared from primary human neutrophils (Fig. 2 B). MnSOD was readily detected in neutrophil as well as Jurkat mitochondrial preparations confirming that these preparations do contain mitochondria.
Figure 2.
Apoptosis-associated mitochondrial cytochrome c releases in Jurkat but not neutrophils. (A) Jurkat cells were induced to undergo apoptosis by treated with 500 nM staurosporine or 20 μM actinomycin D and mitochondrial and cytosolic fractions were prepared at the indicated times as described in Materials and Methods. Cell fractions were probed with the indicated antibodies. (B) Primary human neutrophils and Jurkat cells were treated with 500 nM staurosporine or 20 μM actinomycin D followed by preparation of mitochondrial and cytosolic fractions. Cell fractions were then probed for cytochrome c and MnSOD as indicated.
Neutrophils Retain an Intact Cytochrome c/Apaf-1 Pathway to Caspase Activation.
To date, cytochrome c is the only protein known to be capable of promoting assembly of the Apaf-1 apoptosome. Thus, the apparent absence of cytochrome c from neutrophils was provocative and suggested either that primary human neutrophils do not require cytochrome c for engagement of the apoptosome or that these cells cannot engage this pathway. To explore these alternatives, we generated cell-free extracts of neutrophils (devoid of mitochondria) and explored whether these extracts were capable of supporting caspase activation in response to the addition of exogenous cytochrome c/dATP. For comparative purposes, we also generated cell-free extracts from Jurkat and HEK293 cells which are known to possess an intact cytochrome c/Apaf-1 pathway.
Because little is known concerning the caspase repertoire in primary neutrophils, we initially compared the relative abundance of caspase-1, -2, -3, -4, -6, -7, -8, -9, and -14 in neutrophils versus Jurkat and HE293T cells. As shown in Fig. 3 A, neutrophils were found to express essentially all of these caspases with the notable exception of caspase-2. Interestingly, highly elevated levels of caspase-1 were also detected in neutrophils, which is consistent with the role of these cells in inflammatory responses and their ability to produce mature IL-1β. Previous studies using cell-free extracts of Jurkat, Hela, and HEK293 cells have shown that the addition of cytochrome c and dATP to these extracts is sufficient to trigger assembly of the Apaf-1 apoptosome, which recruits and activates caspase-9. The latter event has been shown to propagate a well-defined cascade of caspase activation events involving caspase-9, -3, -7, -6, -2, -8, and -10 (Fig. 3 B; reference 14). As illustrated in Fig. 3 B, the addition of cytochrome c and dATP to neutrophil cell-free extracts also triggered the processing of a similar repertoire of caspases (with the exception of caspase-2, which was undetectable in neutrophil lysates), suggesting that the Apaf-1–dependent caspase activation pathway was indeed intact in these cells.
Figure 3.
The cytochrome c–initiated caspase activation cascade is intact in neutrophils. (A) Cell-free extracts were prepared from Jurkat, HEK293, and primary neutrophils as described in Materials and Methods. Equal amounts (125 μg) of protein were separated by SDS-PAGE followed by probing replicate blots with antibodies against the indicated proteins. Note the absence of caspase-2 in neutrophil cell-free extracts. (B) Kinetics of cytochrome c/dATP-inducible caspase activation in Jurkat, HEK293, and neutrophil cell-free extracts. Cell-free reactions were assembled as described in Materials and Methods and incubated at 37°C for the indicated times. Data are representative of at least four to five independent experiments.
Neutrophil Cell-free Extracts Do not Require the Addition of Exogenous Cytochrome c to Initiate Apaf-1–dependent Caspase Activation.
Because our earlier experiments failed to detect cytochrome c in neutrophil mitochondrial preparations, the possibility remained that neutrophils do not require cytochrome c to engage the apoptosome pathway to caspase activation. To explore whether this was the case, we performed an experiment in which dATP was added to the extracts over a range of concentrations in the absence of exogenous cytochrome c. As expected, cell-free extracts derived from Jurkat or HEK293 cells failed to support dATP-inducible caspase activation in the absence of exogenously added cytochrome c (Fig. 4 A). Rather surprisingly, neutrophil cell-free extracts exhibited robust activation of caspase-3, which did not require the addition of exogenous cytochrome c, suggesting that these cells might be able to assemble apoptosomes in the absence of this cofactor (Fig. 4 A). Caspase-3 activation was seen over a wide range of dATP concentrations but was abolished in the absence of this nucleotide.
Figure 4.

Neutrophil cell-free extracts contain undetectable amounts of cytochrome c but support robust dATP-inducible caspase activation. (A) Jurkat, HEK293, and primary neutrophil cell-free extracts were incubated in the presence or absence of 50 μg/ml cytochrome c along with the indicated amounts of dATP. Reactions were stopped after 60 min followed by analysis of caspase-3 activation status in the extracts. (B) Equal amounts (120 μg) of protein from Jurkat, HEK293, or neutrophil cell-free extracts were separated by SDS-PAGE followed by probing for cytochrome c. Data are representative of at least three independent experiments.
To exclude the possibility that this observation was due to contamination of neutrophil cell-free extracts by cytochrome c (as a result of mitochondrial rupture) during their preparation, we assessed the relative levels of cytochrome c in the extracts by Western blotting. As Fig. 4 B illustrates and in line with our earlier observations, cytochrome c was undetectable in neutrophil cell-free extracts but trace amounts were found in extracts generated from Jurkat and HEK293 cells. Thus, despite the fact that both Jurkat and HEK293 cell extracts contained detectable amounts of endogenous cytochrome c, these cell extracts still required the addition of exogenous cytochrome c for assembly of the apoptosome and caspase activation. In contrast, neutrophil extracts that appeared devoid of cytochrome c were still capable of supporting dATP-inducible caspase activation.
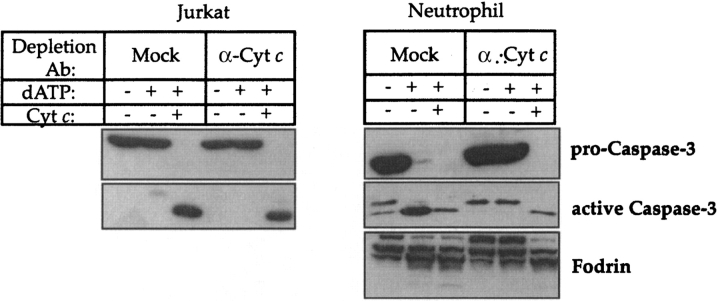
To confirm that the dATP-inducible caspase activating complex in neutrophils was indeed composed of Apaf-1 and caspase-9, we generated cell-free extracts depleted of both of these constituents. As a control, similar depletions were performed with Jurkat cell-free extracts. As Fig. 5 shows, although mock-immunodepleted neutrophil extracts were capable of supporting dATP-inducible caspase-3 activation, extracts depleted of Apaf-1 and caspase-9 were not, confirming that the dATP-inducible caspase-activating complex observed in neutrophil cell-free extracts was indeed comprised of Apaf-1 and caspase-9.
Figure 5.
dATP-inducible caspase activation in neutrophil cell-free extracts is Apaf-1 and caspase-9 dependent. Jurkat and neutrophil cell-free extracts were immunodepleted of Apaf-1 and caspase-9 as described in Materials and Methods. Mock-depleted or Apaf-1/caspase-9–depleted extracts were assessed for their ability to support dATP- or cytochrome c/dATP-inducible caspase activation by incubation for 2 h at 37°C as indicated (dATP was added to 1 mM and cytochrome c to 50 μg/ml final concentrations). Note that depletion of Apaf-1/caspase-9 completely abrogated dATP-inducible caspase activation in both cases. Data are representative of two independent experiments.
Immunodepletion of Cytochrome c Arrests dATP-inducible Caspase Activation.
It remained formally possible that neutrophil cell-free extracts contained trace amounts of cytochrome c below the detection threshold of direct Western blot analysis, but sufficient to support dATP-inducible caspase activation. Although we considered this possibility unlikely, we performed an immunodepletion experiment with anti-cytochrome c antibodies to assess whether this would abolish Apaf-1–dependent caspase activation in these extracts. Neutrophil and Jurkat cell-free extracts were subjected to overnight depletion with anti–cytochrome c antibodies and were then assessed for their ability to support dATP-inducible caspase activation. Surprisingly, this procedure completely abolished dATP-triggered caspase activation in neutrophil extracts, which was readily restored by the addition of exogenous cytochrome c (Fig. 6) . The latter result suggested that despite our inability to detect cytochrome c by direct Western blot analysis of neutrophil cell lysates or mitochondrial fractions, these cells do retain trace amounts of this mitochondrial protein that remains necessary for apoptosome assembly.
Figure 6.
dATP-inducible caspase activation in neutrophil cell-free extracts is cytochrome c dependent. Jurkat and neutrophil cell-free extracts were immunodepleted of cytochrome c as described in Materials and Methods. Mock- or cytochrome c–depleted extracts were assessed for their ability to support dATP- or cytochrome c/dATP-inducible caspase activation by incubation for 1 h at 37°C as indicated (dATP was added to 1 mM and cytochrome c to 50 μg/ml final concentrations). Data are representative of three independent experiments.
Neutrophils Contain Trace Amounts of Cytochrome c.
In an effort to detect cytochrome c in neutrophils, we conducted extensive Western blot analysis of neutrophil lysates in comparison with lysates prepared from Jurkat and HEK293 cells. Although cytochrome c was readily detectable in lysates prepared from as few as 5 × 104 Jurkat or HEK293 cells, this protein remained below the detection threshold in lysates prepared from 5 × 105 neutrophils (Fig. 7 A). In contrast, MnSOD was readily detectable in neutrophil lysates and appeared almost as abundant as in Jurkat and HEK293 cells. In a further effort to detect cytochrome c in neutrophils, IP analysis was performed. This approach succeeded in detecting cytochrome c in neutrophils, although ∼10 times the number of neutrophils (as compared with Jurkat or HEK293 cells) were required to saturate the same amount of antibody used for IP (Fig. 7 B). Thus, it appears that primary human neutrophils contain a fraction (<10%) of the cytochrome c that is contained in the other cell types examined. Despite this, cytochrome c was still evidently required for apoptosome assembly in these cells (Fig. 6).
Figure 7.
Relative levels of cytochrome c in primary neutrophils, Jurkat, and HEK293 cells. (A) Total protein lysates were prepared from equal numbers of Jurkat, HEK293, and neutrophils (107 cells/ml). The indicated cell equivalents were loaded on 15% SDS-PAGE gels followed by Western blot analysis of cytochrome c and MnSOD levels in the lysates. The indicated amounts of bovine cytochrome c were loaded as a control and to enable comparisons between different blots. (B) Cytochrome c was immunoprecipitated from lysates prepared from the indicated numbers of Jurkat or primary neutrophils. IPs were performed as described in Materials and Methods.
To exclude the possibility that only a minor subset of neutrophils (or contaminating cells in the neutrophil preparations) expressed cytochrome c, we also conducted immunofluorescence analysis (Fig. 8) . Initially, we incubated primary neutrophils with Mitotracker Red to assess whether this dye would become incorporated into mitochondrial structures as is readily observed in other cell types. As illustrated in Fig. 8 A, neutrophils exhibited significant uptake of Mitotracker dye into punctate cytoplasmic structures as previously reported (21). Immunostaining of neutrophils for cytochrome c also resulted in a punctate pattern that colocalized well with Mitotracker-positive areas of the cell, confirming that primary neutrophils do indeed contain cytochrome c (Fig. 8 B).
Figure 8.
Immunolocalization of cytochrome c in primary neutrophils. (A) Purified human neutrophils were incubated for 45 min at 37°C in Mitotracker Red followed by fixation and staining with Hoechst dye to visualize nuclei. (B) Neutrophils were labeled with Mitotracker Red followed by immunostaining for cytochrome c as described in Materials and Methods.
Neutrophil Cell-free Extracts Display a Lowered Threshold for Cytochrome c–inducible Caspase Activation.
Because neutrophils contain minimal cytochrome c but can readily support dATP-inducible caspase activation, this suggested that these cells may have a lowered threshold requirement for cytochrome c to initiate Apaf-1–dependent caspase activation. To explore whether this was the case, we used cytochrome c–depleted neutrophil extracts to determine the minimum concentration of exogenous cytochrome c required to support dATP-inducible caspase activation. As a control, cytochrome c–depleted Jurkat and HEK293 extracts were used for comparison. Using this approach, we found that neutrophil cell-free lysates were capable of supporting caspase activation at cytochrome c concentrations well below (∼5%) those required to achieve caspase activation in Jurkat and HEK293 cell-free lysates (Fig. 9) .
Figure 9.
Neutrophil cell-free extracts exhibit a lowered threshold for cytochrome c–triggered caspase activation. Cell-free lysates prepared from primary neutrophils, Jurkat, or HEK293 cells (adjusted to the same final protein concentration) were immunodepleted of cytochrome c as described in Materials and Methods. The concentration of exogenous cytochrome c required to trigger Apaf-1–dependent caspase activation was then assessed by incubation of the extracts for 1 h at 37°C in the presence of 1 mM dATP as indicated.
Neutrophils Contain Elevated Apaf-1 Levels.
The lowered threshold for cytochrome c–initiated caspase activation in neutrophils could be due to multiple factors. However, possible reasons include elevated Apaf-1 levels or abnormally low levels of molecules that can antagonize Apaf-1 function such as the inhibitor of apoptosis proteins (IAPs). To explore these obvious possibilities, we assessed the relative levels of Apaf-1, XIAP, cIAP-1, survivin, and Bruce in Jurkat and HEK293 cells and primary neutrophils. As Fig. 10 illustrates, this analysis revealed that neutrophils possess highly elevated Apaf-1 levels and modest or undetectable amounts of the IAP proteins relative to the other cell types examined. One interpretation of these observations is that the paucity of cytochrome c in neutrophils is compensated for by elevated Apaf-1 levels in these cells. Increased abundance of Apaf-1 may facilitate efficient apoptosome assembly even in the context of very limiting amounts of cytochrome c.
Figure 10.

Neutrophils contain highly elevated Apaf-1 levels and reduced levels of IAPs. Lysates (107 cells/ml) were prepared from primary human neutrophils (neutrophils were pooled from five donors) and the indicated cell lines. (A) Equal amounts of protein (∼100 μg) were separated by SDS-PAGE followed by probing replicate blots with antibodies against Apaf-1. Recombinant Apaf-1 was obtained by transiently overexpressing an Apaf-1 expression plasmid in HEK293 cells. (B) Proteins were loaded as in A and were probed for the indicated IAP proteins.
The observations reported in this study suggest that neutrophils retain an intact apoptosome pathway to caspase activation despite possessing mitochondria that are incapable of significant mitochondrial respiration (20). This pathway involved a repertoire of caspases similar to that previously reported in other cell types with the exception of caspase-2, which was not detected in neutrophil lysates. The absence of caspase-2 at the mRNA and protein levels in primary neutrophils has also been previously reported (25). The order of cytochrome c/Apaf-1–initiated caspase activation events observed in neutrophils (unpublished data) was also identical to that previously reported for Jurkat cells (14).
Despite difficulties encountered in detecting cytochrome c in neutrophil lysates by direct Western blot analysis, this protein was detected by immunofluorescence as well as by IP analysis and was estimated to be present in neutrophils at levels that are a fraction (∼10% or less) of those observed in proliferating cell lines. Because cytochrome c is essential for electron transport within the mitochondrial respiratory chain, this observation may at least partly explain why neutrophils exhibit almost undetectable rates of mitochondrial respiration. Nonetheless, these levels of cytochrome c were both necessary and sufficient to support Apaf-1–dependent caspase activation in neutrophil cell-free extracts, but not in HEK293 nor Jurkat cell extracts. This suggests that neutrophils have a lowered threshold requirement for cytochrome c for engagement of the Apaf-1–dependent pathway to caspase activation, but the reason(s) for this remain unclear. Factors such as highly elevated Apaf-1 levels and decreased levels of the caspase inhibitory proteins, XIAP and cIAP1, may facilitate apoptosome assembly at the low concentrations of cytochrome c that are attainable in neutrophils.
In conclusion, although primary neutrophils contain few mitochondria that are most likely deficient in electron transport, these mitochondria retain sufficient cytochrome c to instigate the Apaf-1–dependent pathway to caspase activation and apoptosis. It remains unclear whether this pathway is engaged during neutrophil senescence-associated apoptosis or whether an alternative pathway to caspase activation is engaged during neutrophil turnover in vivo. However, because neutrophils undergo apoptosis in response to many stimuli that engage the Apaf-1–dependent pathway in other cell types, it is likely that this pathway represents an important physiological route to neutrophil apoptosis.
Acknowledgments
We thank The Health Research Board of Ireland (grant RP61/2000), The European Union (grant QLG1-1999-00739), and Science Foundation Ireland (grant PI1/B038) for their generous support of this work.
Footnotes
Abbreviations used in this paper: Apaf-1, apoptotic protease-activating factor 1; HSP-60, heat shock protein 60; IAP, inhibitor of apoptosis protein; IP, immunoprecipitation; MnSOD, manganese superoxide dismutase; Smac, second mitochondrial activator of caspases; XIAP, X-linked inhibitor of apoptosis protein.
References
- 1.Savill, J.S., A.H. Wyllie, J.E. Henson, M.J. Walport, P.M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee, A., M.K. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283–288. [PubMed] [Google Scholar]
- 3.Watson, R.W.G., O.D. Rotstein, J. Parodo, R. Bitar, and J.C. Marshall. 1998. The IL-1 beta-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1 beta. J. Immunol. 161:957–962. [PubMed] [Google Scholar]
- 4.Earnshaw, W.C., L.M. Martins, and S.H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383–424. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson, D.W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028–1042. [DOI] [PubMed] [Google Scholar]
- 6.Hengartner, M.O. 2000. The biochemistry of apoptosis. Nature. 407:770–776. [DOI] [PubMed] [Google Scholar]
- 7.Martin, S.J. 2002. Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell. 109:793–796. [DOI] [PubMed] [Google Scholar]
- 8.Boldin, M.P., T.M. Goncharov, Y.V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 85:803–815. [DOI] [PubMed] [Google Scholar]
- 9.Muzio, M., A.M. Chinnaiyan, F.C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J.D. Bretz, M. Zhang, R. Gentz, et al. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signalling complex. Cell. 85:817–827. [DOI] [PubMed] [Google Scholar]
- 10.Liu, X., C.N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 86:147–157. [DOI] [PubMed] [Google Scholar]
- 11.Kluck, R.M., E. Bossy-Wetzel, D.R. Green, and D.D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 275:1132–1136. [DOI] [PubMed] [Google Scholar]
- 12.Yang, J., X. Liu, K. Bhalla, C.N. Kim, A.M. Ibrado, J. Cai, T.I. Peng, D.P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 275:1129–1132. [DOI] [PubMed] [Google Scholar]
- 13.Li, P., D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, E.S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91:479–489. [DOI] [PubMed] [Google Scholar]
- 14.Slee, E.A., M.T. Harte, R.M. Kluck, B.B. Wolf, C.A. Casiano, D.D. Newmeyer, H.G. Wang, J.C. Reed, D.W. Nicholson, E.S. Alnemri, et al. 1999. Ordering the cytochrome c–initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9–dependent manner. J. Cell Biol. 144:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmon, A.J., D.W. Nicholson, and R.C. Bleackley. 1995. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 377:446–448. [DOI] [PubMed] [Google Scholar]
- 16.Martin, S.J., G.P. Amarante-Mendes, L. Shi, T.-H. Chuang, C.A. Casiano, G.A. O'Brien, P. Fitzgerald, E.M. Tan, G.M. Bokoch, A.H. Greenberg, et al. 1996. The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO J. 15:2407–2416. [PMC free article] [PubMed] [Google Scholar]
- 17.Liles, W.C., P.A. Kiener, J.A. Ledbetter, A. Aruffo, and S.J. Klebanoff. 1996. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J. Exp. Med. 184:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson, R.W.G., O.D. Rotstein, M. Jimenez, J. Parodo, and J.C. Marshall. 1997. Augmented intracellular glutathione inhibits Fas-triggered apoptosis of activated human neutrophils. Blood. 89:1175–1181. [PubMed] [Google Scholar]
- 19.Whyte, M.K., J. Savill, L.C. Meagher, A. Lee, and C. Haslett. 1997. Coupling of neutrophil apoptosis to recognition by macrophages: coordinated acceleration by protein synthesis inhibitors. J. Leukoc. Biol. 62:195–202. [DOI] [PubMed] [Google Scholar]
- 20.Peachman, K.K., D.S. Lyles, and D.A. Bass. 2001. Mitochondria in eosinophils: functional role in apoptosis but not respiration. Proc. Natl. Acad. Sci. USA. 98:1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maianski, N.A., F.P. Mul, J.D.van Buul, D. Roos, and T.W. Kuijpers. 2002. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 99:672–679. [DOI] [PubMed] [Google Scholar]
- 22.Nopp, A., J. Lundahl, and H. Stridh. 2002. Caspase activation in the absence of mitochondrial changes in granulocyte apoptosis. Clin. Exp. Immunol. 128:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adrain, C., E.M. Creagh, and S.J. Martin. 2001. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 20:6627–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slee, E.A., C. Adrain, and S.J. Martin. 2001. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 276:7320–7326. [DOI] [PubMed] [Google Scholar]
- 25.Santos-Beneit, A.M., and F. Mollinedo. 2000. Expression of genes involved in initiation, regulation, and execution of apoptosis in human neutrophils and during neutrophil differentiation of HL-60 cells. J. Leukoc. Biol. 67:712–724. [DOI] [PubMed] [Google Scholar]