Abstract
To ensure that homeostasis of the immune system is maintained, the sensitivity of lymphocytes to Fas-mediated apoptosis is differentially regulated during their activation. The molecular mechanisms that link the activation program of lymphocytes to changes in sensitivity to Fas-mediated apoptosis have, however, not been fully characterized. In these studies, we have investigated whether Fas-mediated apoptosis can be regulated by interferon regulatory factor 4 (IRF-4), a lymphoid-restricted member of the IRF family of transcription factors. IRF-4 expression is upregulated during lymphocyte activation and IRF-4–deficient mice have defects in both lymphocyte activation and homeostasis. Here, we show that stable expression of IRF-4 in a human lymphoid cell line that normally lacks IRF-4 leads to a significantly enhanced apoptotic response on Fas receptor engagement. A systematic examination of the downstream effectors of Fas signaling in IRF-4–transfected cells demonstrates an increased activation of caspase-8, as well as an increase in Fas receptor polarization. We demonstrate that IRF-4–deficient mice display defects in activation-induced cell death, as well as superantigen-induced deletion, and that these defects are accompanied by impairments in Fas receptor polarization. These data suggest that IRF-4, by modulating the efficiency of the Fas-mediated death signal, is a novel participant in the regulation of lymphoid cell apoptosis.
Keywords: SEB, Fas, polarization, AICD, caspase-8
Introduction
Apoptosis plays a crucial role in maintaining lymphocyte homeostasis both by deleting auto-reactive cells in the periphery and by downsizing the pool of activated lymphocytes that have expanded during an immune response (1). Engagement of the cell surface receptor Fas, a member of the TNF receptor family, by its ligand, FasL (2, 3) constitutes one of the critical steps in the regulation of lymphocyte apoptosis as demonstrated by the fact that mutations in either Fas or Fas ligand (FasL)* can result in severe lymphoproliferation and loss of homeostatic control in mice as well as humans. Binding of the FasL to Fas leads to the clustering of pre-assembled Fas trimers, resulting in a high local concentration of Fas and in the transmission of a strong death signal (4). Transduction of this signal requires the assembly of a death-inducing signaling complex (DISC), which involves the recruitment of the Fas-associated death domain (FADD) adaptor protein and of caspase-8, a critical initiator caspase to the cytoplasmic portion of the Fas receptor (5). Interaction of FADD with caspase-8 stimulates activation of caspase-8, which activates effector caspases, such as caspase-9 and -3 (6). Activation of effector caspases by caspase-8 can either occur directly or can occur via a mitochondrial amplification step in which release of cytochrome c is required for the activation of caspase-9 and -3 (7). The sequential activation of initiator and effector caspases eventually culminates in the full execution of the apoptotic process.
Although activated T cells express both Fas and Fas ligand, their ability to undergo Fas-mediated apoptosis is differentially regulated as they progress along their activation program. Indeed, early after activation, T cells are insensitive to Fas-mediated apoptosis and undergo clonal expansion (8). However, as this expansion proceeds, T cells become increasingly more sensitive to Fas-induced death and can undergo what has been termed activation-induced cell death (AICD; reference 9). Two major mechanisms have been shown to control the differential sensitivity of T cells to Fas-mediated apoptosis during the course of their activation. The presence of intracellular inhibitors like FLICE/caspase-8 inhibitory protein (FLIP) represents a powerful regulatory step in this process. Recruitment of FLIP to the DISC inhibits the activation of caspase-8 (10–14) and changes in FLIP expression levels have been correlated with sensitization of T cells to AICD (15, 16). However, recent studies have uncovered that Fas signaling can also be controlled by the extent of polarization (or capping) of the Fas receptor within a cell (17, 18) and that the ability of the Fas receptor to undergo polarization in T cells depends on the state of activation of these cells. Indeed, after 1 d of activation, T cells are resistant to Fas-induced apoptosis and are unable to undergo Fas capping. In contrast, T cells that have been activated for 6 d and have acquired sensitivity to Fas-mediated apoptosis can effectively redistribute the Fas receptor to one pole of the cell. Polarization of the Fas receptor is mediated by cytoskeletal reorganization and interaction of Fas with the ERM protein, ezrin (18). The localization of Fas receptor in caps is believed to enhance the transduction of the death signal, thus increasing the sensitivity of lymphocytes to AICD. Although these studies have provided insights into the mechanisms used by T cells to modulate their susceptibility to Fas-mediated death, the molecular machinery that is responsible for implementing these processes has not been fully characterized.
IFN regulatory factor (IRF) 4 is a member of the IRF family of transcriptional regulators whose expression increases upon activation of T cells with mitogens or anti-CD3 antibodies (19–21). The expression of IRF-4 is primarily confined to lymphocytes, and it has been proposed that one of the distinctive roles of IRF-4 may be to confer lineage specificity to lymphocyte responses (22). Genetic studies have indicated that IRF-4 is a critical component of the activation program of mature T cells (23). In addition to profound defects in mature T cell function, IRF-4–deficient mice also display a lymphoproliferative disorder with a progressive accumulation of T and B cells in the spleen and lymph nodes upon aging (23). This phenotype suggests that IRF-4 may be crucial not only in regulating the activation, but also in controlling the apoptosis of lymphocytes. To investigate this possibility in more detail, we have examined the Fas signaling pathway in Jurkat T cells, which have been stably transfected with IRF-4. In these studies, we show that expression of IRF-4 in these cells enhances their sensitivity to Fas-mediated apoptosis. A systematic dissection of the Fas signaling pathway in these transfectants demonstrates that expression of IRF-4 leads to the enhanced activation of the initiator caspase-8. Interestingly, the increased sensitivity of these IRF-4–transfected cells to Fas-dependent apoptosis is associated with an increased polarization of the Fas receptor but not with changes in FLIP levels. Consistent with these results, an examination of primary T cells from IRF-4–deficient mice reveals that these lymphocytes not only display defects in their ability to undergo AICD, but also exhibit diminished Fas receptor capping. Thus, our data support a model in which IRF-4 may serve as a critical link between the activation and the apoptotic programs of lymphocytes.
Materials and Methods
Antibodies and Reagents.
The rabbit polyclonal antiserum against IRF-4 was obtained from Santa Cruz Biotechnology, Inc. The mAbs against cytochrome c, Fas, FasL, CD4, CD62L, CD45RB, Vβ8, and rabbit polyclonal antisera against human caspase-8, -9, and -3, were purchased from BD Biosciences. Agonistic anti–human Fas mAb (clone CH11) used to induce apoptosis was purchased from Upstate Biotechnology. The mAb against FLIP antiserum (Dave-2) and the FLIPlong cDNA were purchased from Qbiogene. Etoposide and staphylococcal enterotoxin B (SEB) were purchased from Sigma-Aldrich, and the caspase-8 inhibitor, z-IEDT-fmk, was purchased from Calbiochem. MitoTracker X-rhodamine mitochondrion-selective probe and Alexa Fluor® 568–conjugated antibodies were purchased from Molecular Probes.
Cell Lines, Stable Transfectants, and Mice.
The Jurkat (human T cell leukemia) cell line was obtained from American Type Culture Collection and maintained in IMDM supplemented with 10% FBS (Atlanta Biologicals) and 1% penicillin-streptomycin. For preparing IRF-4 stable transfectants, the Jurkat T cells were transfected by electroporation (960 µF, 260 V) using a BTX electroporator with either a control vector (pIRES-GFP) or an IRF-4 expression vector (pIRES-GFP-IRF-4-c-myc). The transfectants were selected in IMDM containing 1.5 mg/ml G418 (Promega). IRF-4 knockout mice on a C57BL6 background were obtained from Dr. T. Mak (University of Toronto, Toronto, Canada and Amgen Institute, Thousand Oaks, CA). C57/BL6 mice were used as controls. Mice were maintained under specific pathogen-free conditions.
Apoptosis Detection.
Jurkat cells plated at 106 cells/ml in IMDM were cultured in the presence of the agonist anti-Fas mAb (CH-11) at concentrations ranging from 0–1,000 ng/ml as indicated for each experiment. Apoptosis was measured by cytofluorometric analysis by costaining with annexin V conjugate and AAD (BD Biosciences) according to the manufacturer's instructions. Apoptosis was measured using a FACSCalibur™ cytometer (Becton Dickinson) and further analyzed using CellQuest software. The number of apoptotic cells was calculated according to the following formula: [(Percent experimental apoptosis − percent spontaneous apoptosis)/(100 − percent spontaneous apoptosis)] × 100. Apoptosis-associated alterations in mitochondria were assessed by flow cytometry using the MitoTracker X-rhodamine mitochondrion-selective dye. Results were analyzed using CellQuest software.
Cell Extracts and Western Blotting.
To quantify cytochrome c released during apoptosis, mitochondrial and cytosolic preparations were performed as described previously (24). Extracts were loaded onto 15% SDS–polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted with an anti–cytochrome c mAb. Western blotting for FLIP and caspase-8, -9, and -3 were performed as described previously (25).
Reverse Transcriptase PCR.
Total RNA was extracted using TRIzol® (GIBCO BRL). The first strand cDNA synthesis was synthesized from 5 μg of total RNA by Superscript II reverse transcriptase (GIBCO BRL) using oligo (dT) primer. The PCR primers specific for human FLIPlong and FasL were as follows: forward primer for FLIPlong 5′-GATGTCTGCTGAAGTCATCCATCA-3′; reverse primer for FLIPlong, 5′-CACTACGCCCAGCCTTTTGG-3′ (26); forward primer for FasL 5′-ATGTTTCAGCTCTTCCACCTACAGA-3′; and reverse primer for FasL 5′-CCAGAGAGAGCTCAGATACGTTGAC-3′ (27). The PCR reaction was carried out with Taq DNA polymerase (GIBCO BRL). Amplification was performed for 30 cycles (30-s denaturing at 94°C, 30-s annealing at 55°C, and 90-s extension at 72°C) in a thermal cycler. A human GAPDH fragment was amplified under parallel conditions as a control. The 1,470-bp FLIPlong and 300-bp GAPDH products were run on a 1.2% agarose gel and transferred to nylon membrane. The blot was UV cross-linked and probed with a radiolabeled human FLIPlong cDNA (Qbiogene) or GAPDH cDNA probe.
T Cell Purification and AICD.
Naive CD4+ T cells were isolated from splenocytes of IRF-4–deficient and control mice by negative selection using the naive CD4+-specific T cell enrichment columns (R&D Systems). The purity of naive CD4+ cells was assessed by flow cytometry and was found to be >90% pure. AICD was determined as per previously published protocols (28). In brief, naive purified CD4+ T cells were cultured in 6-well plates coated with anti-CD3 mAb (BD Biosciences) at 1 μg/ml. Murine IL-2 (BD Biosciences) was added at 10 ng/ml. After 72 h, cells were collected and subjected to Ficoll density centrifugation. Cells were washed and cultured in duplicate wells with 10 ng/ml IL-2 in the presence or absence of plate-bound anti-CD3 mAb. Cells were collected 24 h later, washed, and fixed in 70% ethanol at 4°C for 24 h. Cells were stained with propidium iodide (PI) staining solution (PBS containing 50 μg/ml PI, 100 U/ml RNase A, and 1 mg/ml glucose) for 30 min at room temperature before FACS® analysis. Apoptosis was determined by quantification of the sub-G0 population. Three independent experiments were performed and treatments were done in duplicate for each experiment. Surface expression of Fas and FasL was determined by FACS® analysis on naive CD4+ T cells as described previously (28).
Immunofluorescence.
The visualization of Fas on the surface of transfected Jurkats was performed as described previously (17). In brief, 5 × 105 cells were incubated with 1 μg/ml anti-Fas antibody (CH-11) for 60 min at 4°C followed by incubation with Alexa Fluor® 568 goat anti–mouse IgM at 1:100 dilution for 60 min at 4°C. Cells were incubated at 37°C to induce capping for 30 s, 2 min, 4 min, and 30 min, and immediately fixed with 3.7% formaldehyde for 20 min on ice. Purified naive CD4+ T cells were incubated with 20 μg/ml antimurine Fas mAb (Jo-2) for 60 min at 4°C followed by incubation with Alexa Fluor® 568 goat anti–hamster IgG 20 μg/ml for 60 min at 4°C. Cells were incubated at 37°C to induce capping for 3 min and immediately fixed with 3.7% formaldehyde for 10 min on ice. Control cells were exposed to primary anti-Fas antibody and secondary antibody at 4°C, but no capping was induced at 37°C. Visualization of Jurkat T cells and images were collected with a Axioplan II microscope (Carl Zeiss MicroImaging, Inc.) using a Plan-Apochromat 100×/1.4 NA objective lens, and a cooled CCD camera (model Orca-100; Hamamatsu Photonics). Light output from the 100-W mercury arc lamp was controlled using a shutter driver (model Uniblitz D122; Vincent Associates) and attenuated using neutral density filters (Omega Optical Corporation). IP Lab software (Scanalytics) was used to control the shutter driver and capture images. Primary T cells were examined by a laser scanning confocal microscope (model LSM 510; Carl Zeiss MicroImaging, Inc.) with a 100×/1.3 Plan-Neofluor objective lens. Optical section thickness was ∼1 μm. Image enhancement and analysis were performed using the public domain program NIH Image 1.6 and Adobe® Photoshop 6.0. The percentage of cells displaying caps, in which the Alexa Fluor® condenses onto <25% of the cell surface, was determined by counting 150–250 cells.
Superantigen Treatment.
Adult mice were injected intraperitoneally with 150 μg SEB (Sigma-Aldrich) or with PBS. 10 d after injection, lymph node cells were harvested and stained with antibodies to Vβ8 and CD4+ or to Vβ6+ and CD4+ (BD Biosciences) to evaluate the percentage of SEB-responsive (Vβ8+) or SEB-unresponsive (Vβ6+) CD4+ T cells that were present by flow cytometry.
Results
Expression of IRF-4 in Jurkat T Cells Leads to an Enhancement in Fas-mediated Apoptosis.
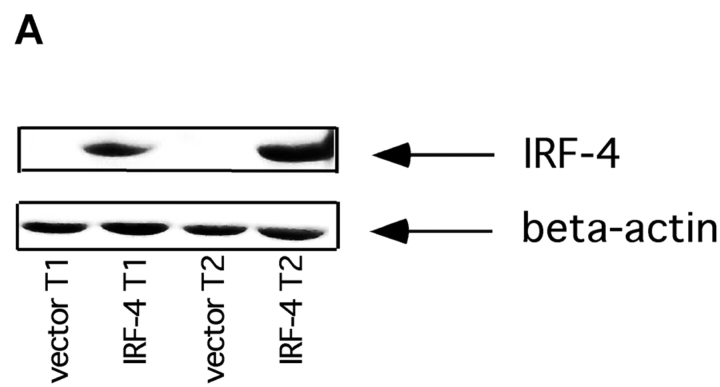
Genetic studies have demonstrated the lack of IRF-4 results in lymphoproliferation and altered homeostasis (23). Given the critical role played by the Fas pathway in lymphocyte survival, we hypothesized that IRF-4 might control the ability of lymphocytes to undergo Fas-mediated apoptosis. To start testing this hypothesis, IRF-4 was stably expressed in Jurkat cells, a human T cell line that normally lacks endogenous IRF-4. Independent sets of stable transfectants were obtained, and two of them (termed T1 and T2) were used in the following experiments. Western blot analysis demonstrated that both sets of IRF-4 transfectants displayed good expression of IRF-4, whereas no IRF-4 was detected in cells transfected with an empty vector (Fig. 1 A).
Figure 1.
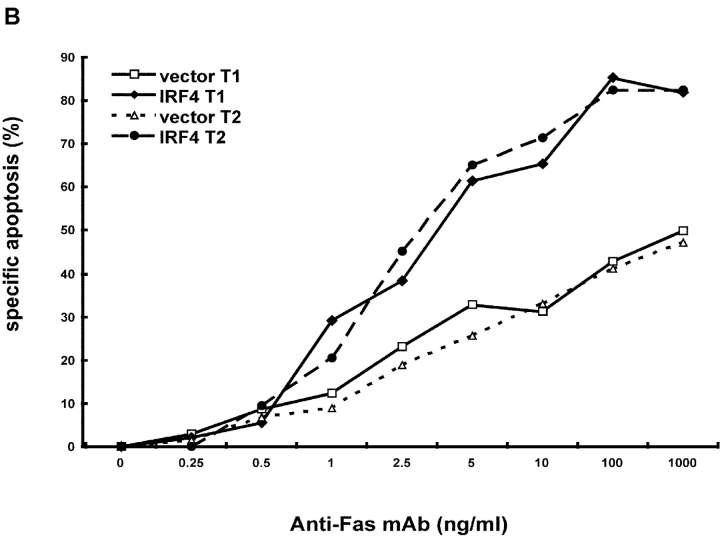
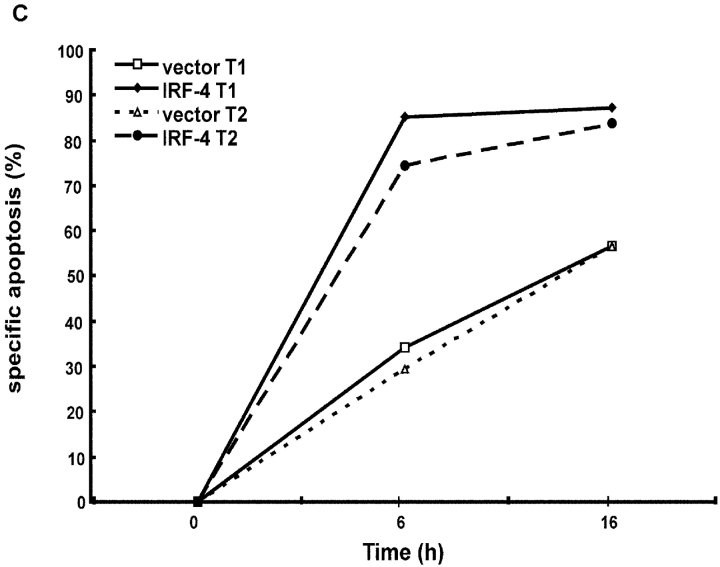
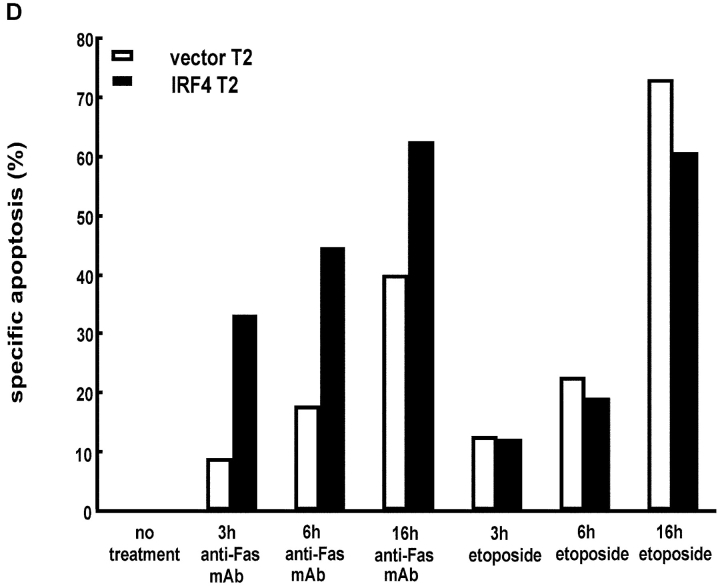
Expression of IRF-4 enhances Fas-mediated apoptosis. (A) Western blot analysis of IRF-4 in whole cell lysates of Jurkat cells stably transfected with an either empty vector or an IRF-4 expression vector. The blot was first analyzed using an anti–IRF-4 antibody (top), it was later stripped and reprobed with a β-actin antibody to ensure for equal loading (bottom). (B) Jurkat-transfected cells were incubated for 16 h with the indicated concentrations of an anti-Fas mAb (CH11). After this incubation, cells were harvested and analyzed by flow cytometry for apoptosis by annexin V and AAD staining. (B–D) Data shown are representative of three independent experiments and were performed on two independent sets of transfectants. Transfected Jurkat cells were cultured with 100 ng/ml anti-Fas mAb for 0, 6, or 16 h. Cells were collected at the distinct time points and apoptosis was measured as described in B. The data shown are representative of three independent experiments and were performed on two independent sets of transfectants. Transfected Jurkat cells were cultured with 100 ng/ml anti-Fas mAb or with 10 μg/ml etoposide for 0, 3, 6, or 16 h. After this incubation, cells were harvested and apoptosis was measured as described in B. Data are representative of three independent experiments (open bars). Control Jurkat transfectants; (solid bars) IRF-4 Jurkat transfectants.
Fas-mediated apoptosis can be induced in Jurkat cells by exposure to a Fas agonist monoclonal antibody (CH-11). Thus, the different transfectants were cultured in the presence of increasing concentrations of this agonist anti-Fas antibody and apoptosis was measured by flow cytometry (Fig. 1 B). Starting at doses as low as 1 ng/ml anti-Fas antibody, the cells transfected with IRF-4 displayed a higher susceptibility to apoptosis as compared to cells transfected with an empty vector. Thereafter, at all doses tested, the IRF-4 transfectants contained at least twice as many apoptotic cells as the control transfectants. Kinetic studies further demonstrated that the IRF-4–transfected cells underwent apoptosis faster than the control transfectants because, by 6 h, ∼80% of the IRF-4–transfected cells had already undergone apoptosis as compared to ∼30% of the control cells (Fig. 1 C). The level of apoptosis observed in the control transfectants was found to be comparable to that exhibited by untransfected Jurkat cells, indicating that the differences observed between the IRF-4 and the control transfectants are not due to a defect in the ability of the control transfectants to undergo apoptosis (unpublished data). Thus, these experiments demonstrate that the presence of IRF-4 leads to a markedly enhanced sensitivity of lymphoid cells to Fas-mediated apoptosis.
To determine whether the presence of IRF-4 was globally affecting the susceptibility of Jurkat cells to apoptosis, the stable transfectants were exposed to different apoptotic inducers. One major inducer used in these studies was etoposide, a DNA-damaging agent that targets the mitochondria to release cytochrome c in a caspase-8–independent pathway (29). As shown in Fig. 1 D, in contrast to the effects obtained upon engagement of the Fas receptor, treatment with etoposide led to similar levels of apoptosis in both control as well as IRF-4 transfectants at all time points tested. Furthermore, no difference in apoptosis induction was detected between control and IRF-4–transfected cells upon culturing them with TNF-α and cycloheximide, a combination known to induce apoptosis in Jurkat cells (30; unpublished data). Thus, the enhanced apoptosis displayed by the IRF-4 transfectants is selective for the Fas signaling pathway.
Increased Activation of Caspase-8 in IRF-4 Transfectants.
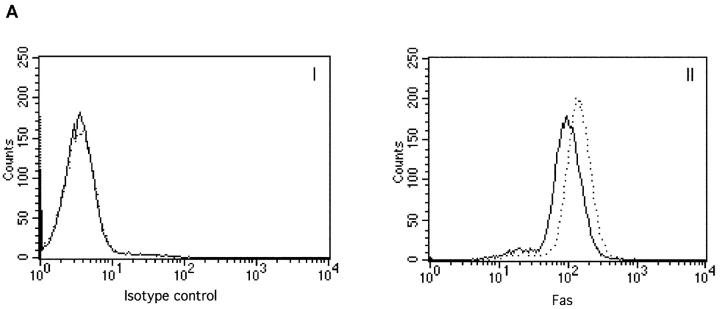
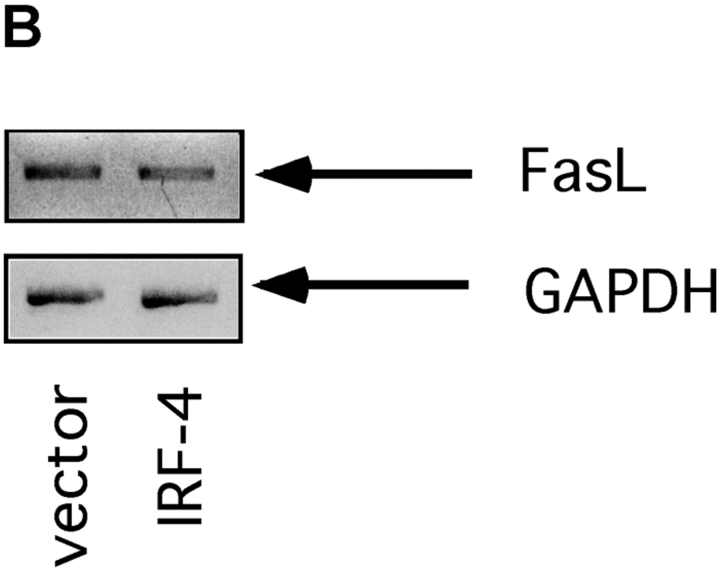
To start elucidating the mechanisms used by IRF-4 to modulate Fas-dependent apoptosis, we performed a systematic dissection of the different components of the Fas signaling pathway in these transfectants. We first investigated the expression levels of Fas and FasL. As shown in Fig. 2 A, FACS® analysis demonstrated that IRF-4 transfectants exhibit a slight increase in Fas expression as compared to vector control. No significant differences in FasL levels between control and IRF-4 transfectants were noted either by semi-quantitative PCR (Fig. 2 B) or by Western blotting (unpublished data). Thus, the presence of IRF-4 does not significantly alter FasL expression and exerts only minimal effects on Fas receptor levels in these cells, suggesting that an alternative mechanism may account for the striking increase in Fas-induced apoptosis observed in the IRF-4–transfected cells.
Figure 2.
Expression of Fas and FasL in control and IRF-4 transfectants. (A) Surface expression of the Fas receptor in Jurkat transfectants. Cells from control and IRF-4–transfected cells were stained with either a PE-labeled isotype-matched control Ab (I) or a PE-labeled anti-Fas IgG1 antibody (II). Cells were subsequently analyzed by flow cytometry. Control vector transfectants (solid lines); IRF-4 transfectants (dotted lines). (B) RT-PCR analysis for FasL expression in IRF-4 Jurkat transfectants. Total RNA was isolated from control and IRF-4–transfected cells, and RT-PCR was performed using primers specific for FasL (top) or GAPDH (bottom). PCR products were separated by electrophoresis on a 2% agarose gel.
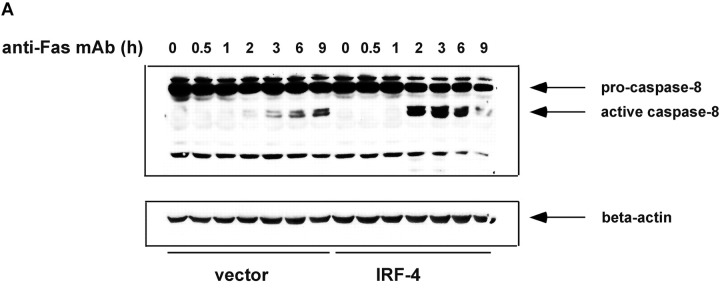
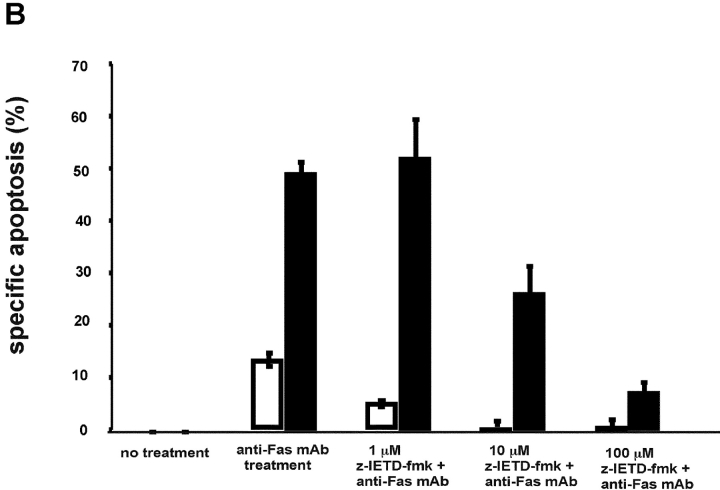
Given that caspase-8 acts as a critical initiator of the Fas-mediated signaling cascade, the activation of this caspase was examined next (5, 31). Jurkat transfectants were exposed to 100 ng/ml anti-Fas mAb for various durations, whole cell extracts were prepared and activation of specific caspases was examined by Western blot analysis (Fig. 3 A). These experiments demonstrated that in the IRF-4 transfectants, there is a striking increase in caspase-8 activation as compared to the control. This increased activation was most noticeable at 2–3 h. After 6 h of exposure to the anti-Fas antibody, the IRF-4 transfectants showed a decrease in caspase-8 activation most likely due to the fact that by this time the great majority of IRF-4–transfected cells had already undergone apoptosis (Fig. 1 C). To confirm the requirement for caspase-8 in the IRF-4–mediated apoptotic process, cells were cultured with a caspase-8–specific inhibitor, z-IEDT–fmk (32). As shown in Fig. 3 B, pretreatment with the caspase-8 inhibitor blocked Fas-mediated apoptosis in both IRF-4 and control transfectants in a dose-dependent manner. Not surprisingly, given the higher level of caspase-8 activation displayed by the IRF-4–transfected cells, a higher dose of the caspase-8 inhibitor was required to reach optimal inhibition of Fas-mediated apoptosis in the IRF-4 transfectants. Thus, these results indicate that enhanced activation of caspase-8 is a critical step in the increased susceptibility to the Fas-mediated cell death exhibited by IRF-4 transfectants.
Figure 3.
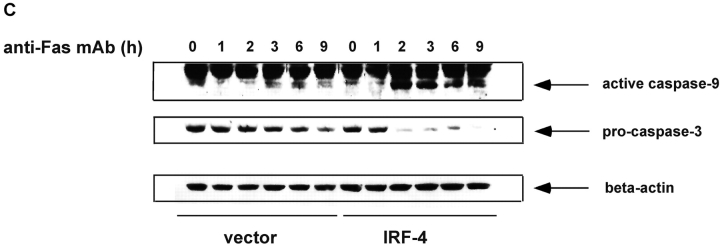
Stable expression of IRF-4 leads to increased activation of caspase-8 and of downstream caspases upon engagement of the Fas receptor. (A) Cells from control and IRF-4 transfectants were cultured with the anti-Fas mAb CH-11 at 100 ng/ml and harvested after the indicated periods of incubation. Total cell lysates were separated by 7% SDS–polyacrylamide gel electrophoresis, and caspase-8 processing was detected by immunoblotting with an anti–human caspase-8 Ab (top). Vector refers to the control Jurkat transfectants, whereas IRF-4 refers to the IRF-4 stable Jurkat transfectants. The procaspase-8 form at 55 kD and the active caspase-8 at 40 kD are indicated by arrows. Blots were stripped and reprobed with a β-actin Ab as a loading control (bottom). (B) Open bars: control Jurkat transfectants; solid bar: IRF-4 Jurkat transfectants. Vector control and IRF-4 transfectants were cultured with or without z-IETD-fmk, a caspase-8 inhibitor, for 1 h at 37°C at 1-, 10-, or 100-μM concentration followed by the addition of 100 ng/ml anti-Fas mAb for 6 h. After this incubation, cells were harvested and analyzed by flow cytometry for apoptosis as indicated in Fig. 1 B. Addition of z-IETD fmk alone had no effect on the viability of the cells (unpublished data). (C) Cells were incubated with 100 ng/ml anti-Fas mAb CH-11 and harvested after the indicated periods of incubation. Total cell lysates were separated by SDS–polyacrylamide gel electrophoresis, and caspase-9 and -3 processing was detected by immunoblotting with antibodies against either active human caspase-9 (top) or procaspase-3 (middle). The active caspase-9 form at 37 kD and the procaspase-3 form are indicated by arrows. Blots were stripped and reprobed with a β-actin Ab as a loading control (bottom).
To confirm that the enhanced activation of caspase-8 observed in IRF-4 transfectants led to an increase in the activation of downstream caspases, we examined the activation of caspase-9 and -3. Caspase-9 is cleaved in the mitochondrial pathway downstream of caspase-8 (33, 34), and caspase-3 can be cleaved by both caspase-8 and -9 (35). As shown by Western blot analysis, caspase-9 activation is significantly increased at 2 h in IRF-4–transfected cells as compared to control cells (Fig. 3 C). Again, due to the overall increased cell death observed in the IRF-4 transfectants after 6 h of treatment with the anti-Fas mAb, a decrease in the activation of caspase-9 in the IRF-4 transfectants was noted at this time point. A significant decrease in procaspase-3 was also observed by Western blot, suggesting that caspase-3 activation was similarly enhanced (Fig. 3 C).
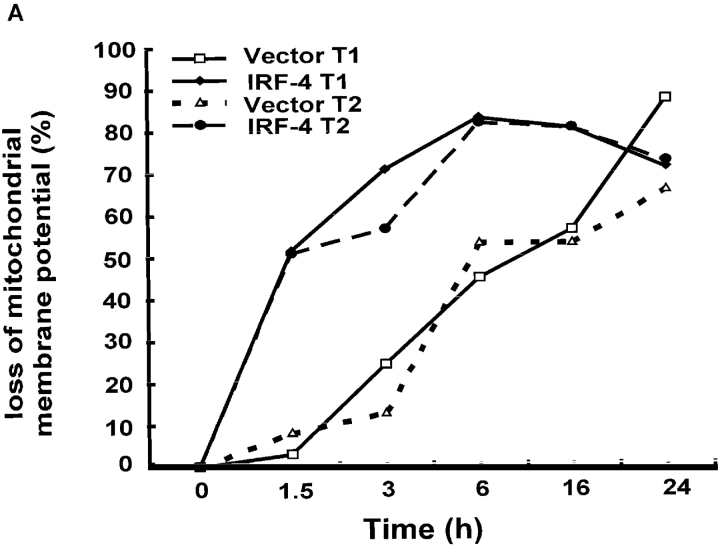
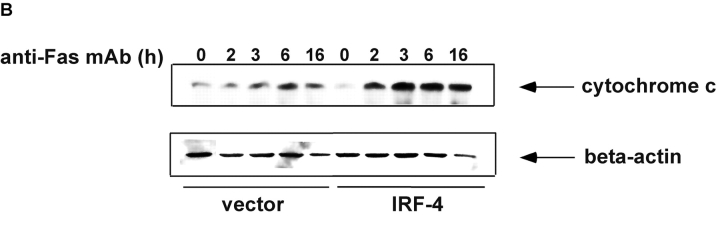
Numerous studies have shown that in Jurkat cells, caspase-8 activation is followed by disruption of mitochondrial function (36–38) and subsequent cytochrome c release into the cytosol. Given that the activation of caspase-8 is greatly enhanced in IRF-4–transfected cells, the integrity of the mitochondria after Fas stimulation of these cells was assessed by measuring the transmembrane potential and the release of cytochrome c. FACS® analysis using Mitotracker, a dye whose incorporation into the cell depends on mitochondrial integrity, demonstrated that mitochondrial membrane potential decreased upon incubation of the cells with the anti-Fas antibody (Fig. 4 A). The loss of mitochondrial membrane potential occurred faster in the IRF-4 transfectants as compared to control cells. This finding was confirmed by examining the release of cytochrome c from the mitochondria into the cytosol after engagement of the Fas receptor. As shown in Fig. 4 B, Western blot analysis with a cytochrome c antibody revealed that the IRF-4–transfected cells displayed a greater release of cytochrome c into the cytosol after anti-Fas antibody treatment as compared to control cells. Therefore, all these studies indicate that the presence of IRF-4 leads to a significant increase in caspase-8 activation, which, in turn, markedly enhances the activation of further downstream effectors of the Fas signaling pathway.
Figure 4.
Mitochondrial dysfunction after engagement of the Fas receptor is enhanced in the presence of IRF-4. (A) Jurkat control and IRF-4–transfected cells were incubated with the anti-Fas mAb at 100 ng/ml for the indicated times. After this incubation, cells were harvested, stained with the MitoTracker X-rhodamine mitochondrion-selective dye, and analyzed by flow cytometry for loss of mitochondrial membrane potential. Cells that lost staining with the mitochondrial-specific dye were considered as cells with loss of mitochondrial membrane potential. (B) Cells were cultured with 100 ng/ml anti-Fas mAb CH-11 and harvested after the indicated periods of incubation. Cytosolic extracts were separated by SDS–polyacrylamide gel electrophoresis and cytochrome c release was detected by immunoblotting with an antibody against human cytochrome c (top). The position of cytochrome c at 15 kD is indicated by arrows. Blots were stripped and reprobed with a β-actin Ab as a loading control (bottom).
Presence of IRF-4 Does Not Significantly Affect FLIP Levels but Leads to an Increased Polarization of the Fas Receptor.
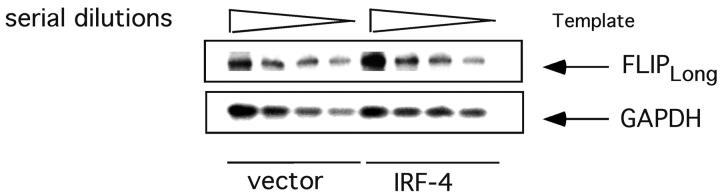
Recent studies have demonstrated that FLIP, which can complex with caspase-8 and prevent its activation (11, 12), is a critical regulator of the sensitivity of lymphocytes to Fas-mediated apoptosis. Given the enhanced caspase-8 activation exhibited by the IRF-4 transfectants, we decided to investigate whether the presence of IRF-4 could affect the expression of FLIP. As shown in Fig. 5 , semi-quantitative RT-PCR failed to demonstrate significant differences in the mRNA levels of one of the major isoforms of FLIP, FLIPlong, between the IRF-4 and the control transfectants. Similar results were obtained for the other major FLIP isoform, FLIPshort (unpublished data). Western blotting with an antibody against FLIP further confirmed these results (unpublished data). Therefore, IRF-4 does not significantly affect the expression of FLIP in Jurkat cells.
Figure 5.
Expression of IRF-4 does not significantly affect FLIP levels. Total RNA from control and IRF-4 stable transfectants was isolated and reverse transcribed using oligo dT primers. PCR was performed on twofold serial dilutions of the first-strand cDNA using primers specific for FLIPlong or GAPDH. The PCR products were electrophoresed on a 2% agarose gel and transferred to a nylon membrane. The blot was probed with 32P-labeled full-length FLIP cDNA (top) followed by probing with GAPDH cDNA (bottom).
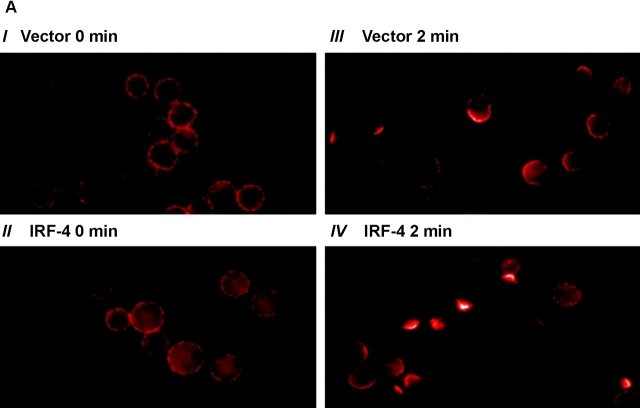
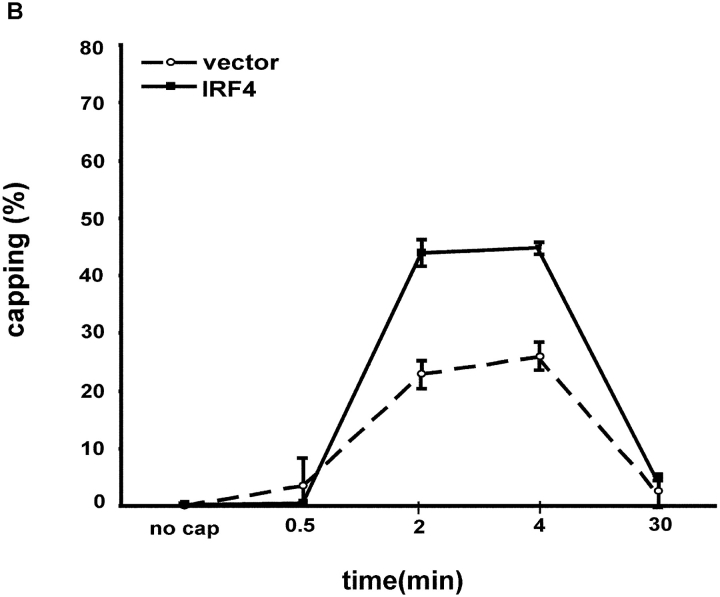
The polarization (or capping) of the Fas receptor has recently been proposed to function as a novel regulatory mechanism for controlling the sensitivity of activated lymphocytes to Fas-mediated apoptosis (18). The ability of the IRF-4 transfectants to undergo Fas capping by cross-linking with an anti-Fas antibody was examined. Before induction of capping, Fas was uniformly distributed around the cell surface (Fig. 6 A). Both control and IRF-4 transfectants displayed a similar staining pattern under these conditions. However, once cross-linking of Fas was induced, the IRF-4 transfectants displayed a markedly enhanced ability to undergo Fas capping as compared to the control cells (Fig. 6 A). This enhancement was noted at two distinct time points, 2 and 4 min (Fig. 6 B). After 4 min of cross-linking, the Fas receptor was internalized into the cells. Thus, these data suggest that the presence of IRF-4 enhances the ability of the Fas receptor to undergo polarization, thus possibly allowing for a more efficient and organized transduction of the Fas-mediated death signal.
Figure 6.
Stable expression of IRF-4 in Jurkat cells leads to enhanced polarization of the Fas receptor. (A) 106 Jurkat transfectants were exposed first to 1 μg/ml anti-Fas Ab at 4°C for 60 min, and then to Alexa Fluor® 568–conjugated secondary antibody. Cells were warmed at 37°C for 30 s, 2 min, 4 min, and 30 min, harvested, fixed with 3.7% formaldehyde, and mounted onto slides. Unstimulated control cells (no cap) were exposed to the anti-Fas Ab and the secondary antibody at 4°C only. Cells were examined with a microscope using a Plan-Apochromat 100×/1.4 NA objective lens, and a cooled CCD camera. Photos were taken of no capping samples and of the maximum capping time point of 2 min in both control and IRF-4 transfectants. Panels I and II are no capping controls of vector and IRF-4, respectively. Panels III and IV are representative of control and IRF-4 transfectant cells, respectively, that have undergone Fas capping for 2 min. (B) Quantitation of Fas capping in the distinct Jurkat transfectants. Fas capping was quantitated as described in Materials and Methods. 150–250 cells were analyzed at each time point.
Lymphocytes from IRF-4–deficient Mice Demonstrate a Decreased Sensitivity to AICD Coupled with Impairments in the Polarization of the Fas Receptor.
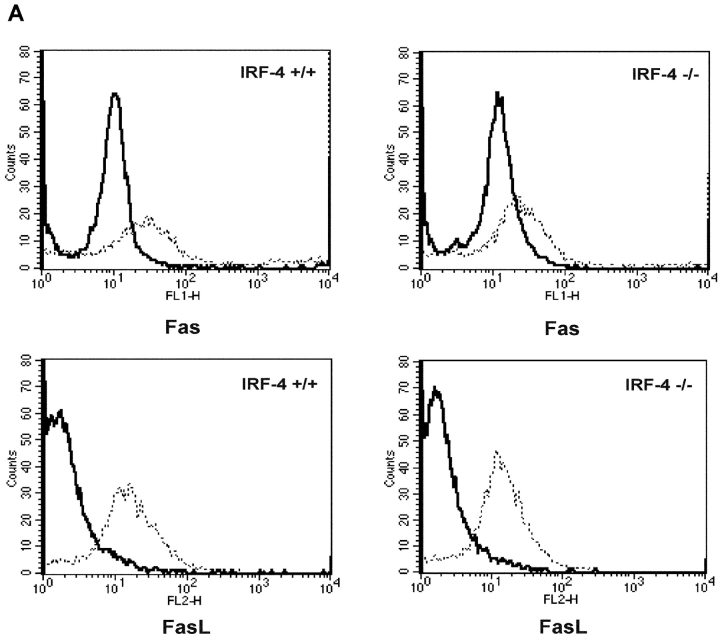
To ascertain the physiological contribution of the IRF-4–mediated effects of lymphocyte apoptosis, we next investigated the Fas pathway in lymphocytes from IRF-4–deficient mice. Purified naive CD4+ T cells from control and IRF-4–deficient mice were activated for 72 h with anti-CD3 and anti-CD28 in the presence of IL-2. Fas and FasL levels on unstimulated as well as stimulated cells were measured by FACS® analysis. As shown in Fig. 7 A, no significant alterations in the basal or inducible levels of FasL in T cells from IRF-4–deficient mice were noted. Furthermore, the lack of IRF-4 resulted only in a slight decrease in the inducibility of the Fas receptor.
Figure 7.
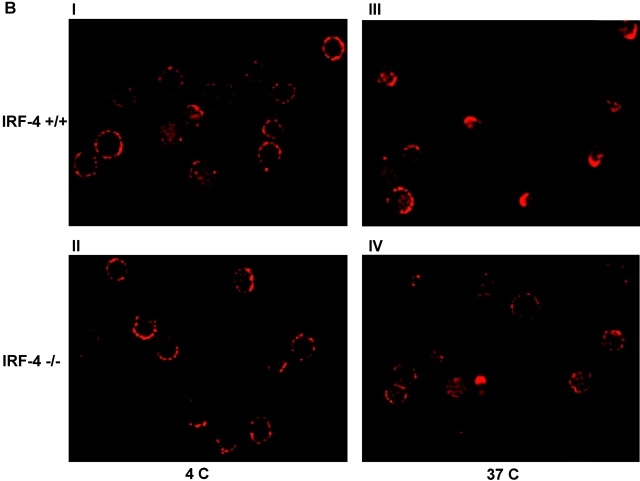
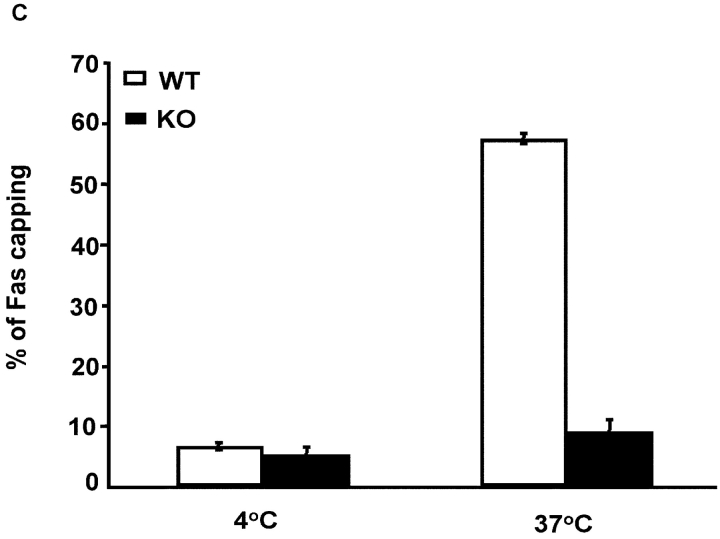
CD4+ T cells from IRF-4–deficient mice exhibit normal Fas and FasL expression but reduced polarization of the Fas receptor. (A) FACS® analysis of Fas and FasL expression in purified CD4+ T cells from wild-type (IRF-4 +/+) or IRF-4–deficient (IRF-4 −/−) mice, which were either left unstimulated (solid lines) or stimulated with 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28 Abs plus 10 ng/ml IL-2 for 3 d (dotted lines). No differences were noted by staining with isotype-matched controls performed in parallel samples (unpublished data). (B) I and II are no capping controls of control and IRF-4–deficient T cells, respectively. III and IV are representative of control and IRF-4–deficient T cells, respectively, that have undergone Fas capping for 3 min. Fas capping. After activation, 106 purified naive CD4+ T cells were exposed first to 20 μg/ml anti-Fas at 4°C for 60 min, and then to Alexa Fluor® 568–conjugated secondary antibody. Cells were warmed at 37°C for 3 min, harvested, fixed with 3.7% formaldehyde, and mounted onto slides. No capping control cells were exposed to anti-Fas and secondary antibody at 4°C only. Cells were examined using a laser scanning confocal microscope with a 100×/1.3 Plan-Neofluor objective lens. Optical section thickness was ∼1 μm. Representative images were taken of no capping samples and of the maximum capping time point of 3 min. Capped T cells are indicated by arrows, whereas partially capped or patched T cells are indicated by asterisks. (C) Quantitation of Fas capping. Cells were stimulated as described in B. Fas capping was quantified using a fluorescent microscope as described in Fig. 6 B. Cells were considered to have Fas caps if the staining pattern showed polarization that condensed to <25% of the cell surface. The experiment is representative of three separate experiments, which were conducted on 5–8-wk-old mice. Isotype-matched controls were also performed and were comparable to control no capped staining (unpublished data).
Given that expression of IRF-4 in Jurkat cells significantly enhanced the ability of the Fas receptor to undergo capping, we tested whether naive CD4+ T cells from IRF-4–deficient mice display impairments in the polarization of the Fas receptor (Fig. 7 B). Before cross-linking the Fas receptor, both control and IRF-4–deficient T cells displayed a similar uniform pattern of surface staining of the Fas receptor. However, upon cross-linking of the Fas receptor, ∼60% of T cells from control mice displayed capping of the Fas receptor, whereas only ∼10% of T cells from IRF-4–deficient mice were able to polarize the Fas receptor (Fig. 7, B and C). Thus, consistent with our results in the Jurkat transfectants, the lack of IRF-4 affects the ability of T cells to redistribute the Fas receptor upon cross-linking.
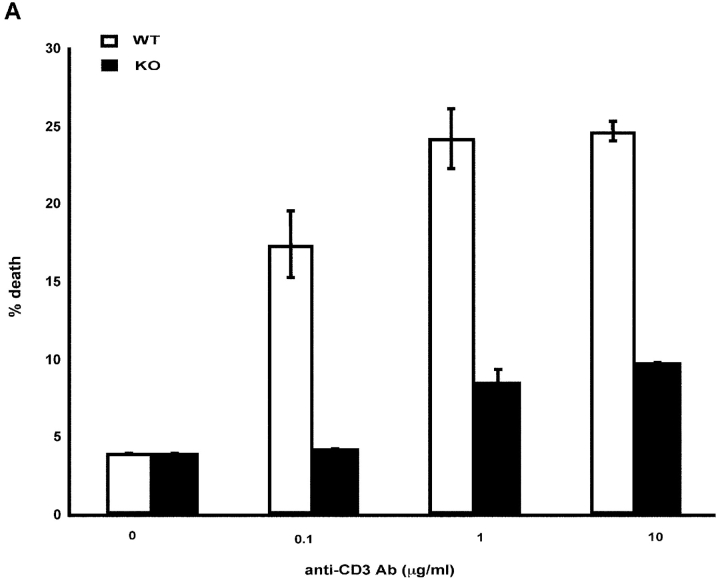
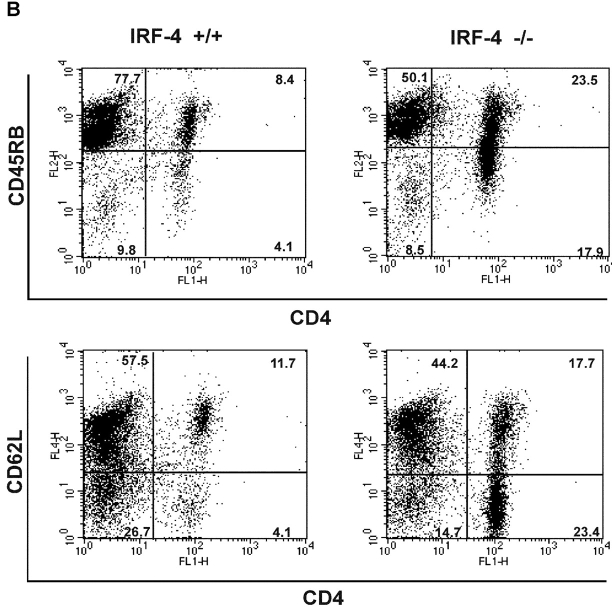
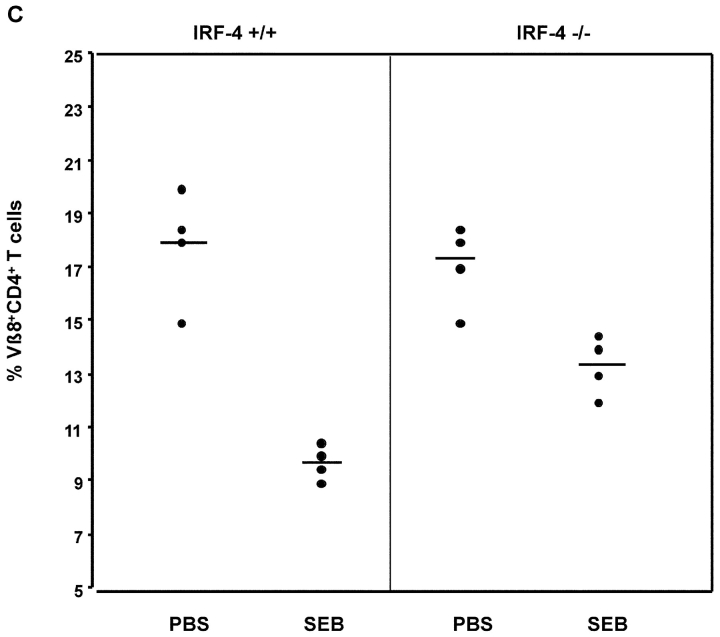
To determine whether the defect in Fas polarization was coupled to a differential susceptibility to undergo apoptosis, we examined whether naive CD4+ T cells from IRF-4–deficient mice were resistant to AICD. As shown in Fig. 8 A, CD4+ T cells from IRF-4–deficient mice exhibited a markedly diminished ability to undergo AICD (Fig. 8 A). In agreement with these findings, an examination of the CD4+ T cells accumulating in the lymphoid tissues of aging IRF-4–deficient mice revealed an increased number of T cells bearing the phenotype of previously experienced T cells (CD44high, CD45RBlow, and CD62Llow) (Fig. 8 B and unpublished data). Furthermore, we treated mice with the superantigen, SEB, which leads to the elimination of Vβ8+ (superantigen responsive) T cells (39) in a Fas-dependent manner (40, 41). As shown in Fig. 8 C, upon SEB injection, IRF-4–deficient mice displayed a decreased ability to eliminate Vβ8+CD4+-responsive cells as compared to wild-type mice. As expected, SEB injection did not lead to the elimination of the Vβ6+CD4+ T cell population (which is not responsive to SEB) in either wild-type or IRF-4–deficient mice (unpublished data). Thus, these data support the notion that IRF-4 plays a physiologic role in the ability of the immune system to eliminate activated cells.
Figure 8.
IRF-4–deficient mice exhibit impaired AICD and an accumulation of CD4+ T cells with an “experienced” phenotype upon aging. (A) AICD. Purified naive CD4+ T cells were activated with 1 μg/ml anti-CD3 plus IL-2 for 3 d, and then harvested. The cells were restimulated with either IL-2 alone or IL-2 + anti-CD3 at the indicated doses for 24 h. The percentage of apoptotic cells was determined by quantification of the sub-G0 population by FACS®. Each assay was conducted in duplicate. The experiment is representative of three separate experiments. (B) Flow cytometric analysis of spleen cells from 12–15-wk-old wild-type and IRF-4–deficient mice. Single cell suspensions of splenocytes were stained with anti-CD4 versus anti-CD45RB (top) or anti-CD4 versus CD62L (bottom). Percentages of positive cells within each quadrant are indicated. (C) Mice (four per treatment) were injected with either PBS or SEB. After 10 d, the percentage of Vβ8+CD4+ (SEB responsive) T cells in lymph nodes was determined by flow cytometry. Each symbol represents an individual mouse. Bars represent the mean of each group of four mice. The number of Vβ6+CD4+ (SEB unresponsive) T cells was also determined by flow cytometry as a control, and it was found not to be affected by SEB treatment in either wild-type or IRF-4–deficient mice (unpublished data).
Discussion
The Fas signaling pathway has been shown to be critical in helping the immune system maintain a state of equilibrium (9). Because mice deficient in IRF-4 display disturbances in lymphocyte homeostasis (23), in these studies we have investigated whether IRF-4 regulates the ability of lymphocytes to undergo Fas-dependent apoptosis. Our results indicate that expression of IRF-4 enhances the susceptibility of lymphocytes to undergo Fas-induced cell death. Consistent with these findings, lymphocytes from IRF-4–deficient mice exhibit an impaired ability to undergo AICD, a well-known Fas-mediated event, as well as defects in the SEB-mediated elimination of Vβ8+CD4+ T cells (42–44). The IRF-4–mediated effect is selective for the Fas pathway because expression of IRF-4 does not alter the sensitivity of Jurkat cells to other apoptotic inducers. The critical target of the IRF-4–mediated effects is the activation of caspase-8, a key initiator caspase in the Fas signaling pathway (5, 31). Indeed, stable expression of IRF-4 leads to a marked increase in caspase-8 activation upon Fas engagement and the addition of a caspase-8 inhibitor blocks Fas-mediated cell death in IRF-4–transfected cells. The increased caspase-8 activation detected in the IRF-4 stable transfectants is accompanied by a commensurate increase in mitochondrial dysfunction and in the activation of effector caspases, i.e., in steps known to be downstream of caspase-8 activation. Together, these data indicate that IRF-4 can control lymphocyte apoptosis by modulating one of the critical initiating events in the Fas signaling cascade.
One of the most interesting features of our work is that the IRF-4–mediated effects on lymphocyte apoptosis are associated with changes in the ability of the Fas receptor to undergo polarization (or cap). These effects were noted both in the Jurkat IRF-4 transfectants (Fig. 6) as well as in primary T cells from IRF-4–deficient mice (Fig. 7). Polarization of Fas is believed to enhance lymphocyte susceptibility to Fas-mediated death by facilitating Fas clustering and enhancing the transmission of the Fas signal (17, 18). The exact mechanism by which IRF-4 promotes Fas polarization is presently under investigation. Redistribution of Fas on the cell surface has been shown to require association of Fas with the cytoskeleton via interaction with ezrin, a protein known to link plasma membrane proteins to the actin cytoskeleton (18). Thus, it is conceivable that IRF-4 may control the expression of specific cytoskeletal components that are required for this process. Indeed, preliminary results from a microarray analysis performed in our laboratory suggest that the presence of IRF-4 can modulate the expression of a number of genes involved in cytoskeletal rearrangement (unpublished observations). Furthermore, such a mechanism would be consistent with recent studies on IFN consensus sequence-binding protein, another member of the IRF family highly homologous to IRF-4. ICSBP, which is critical for myeloid cell differentiation and function, was shown to control the expression of genes involved in the regulation of macrophage spreading and adhesion (45). Thus, it is tempting to speculate that these two members of the IRF family, IRF-4 and ICSBP, play a distinctive role in the control of the cytoskeletal organization, respectively, of lymphocytes and macrophages.
Our current findings support the notion that the susceptibility of lymphocytes to AICD can be determined not only by the levels of expression of Fas and its ligand, but also by the precise spatial arrangement of the Fas receptor within the cell surface. It is indeed becoming increasingly evident that efficient and sustained signaling by many receptors requires the clustering of these receptors together with associated signaling molecules in precisely organized molecular complexes. This has been most notably described for the TCR (46), which upon contact with the appropriate MHC–peptide complex undergoes a dynamic redistribution to form what has been termed an immunological synapse (47, 48). Interestingly, formation of this immunological synapse is profoundly dependent on cytoskeletal reorganization (49–53); similarly, Fas polarization requires the activity of the ERM protein ezrin (54–56). Given that clustering of both Fas and T cell receptors may involve common cytoskeletal components, it will be interesting to determine whether the presence or absence of IRF-4 affects the redistribution of the TCR and/or of additional receptor complexes.
Although our studies indicate that presence of IRF-4 enhances the susceptibility of lymphocytes to Fas-mediated death, the role of IRF-4 in this process is likely to be under complex regulation because a large proportion of T and B lymphoid malignancies express IRF-4 (57-61). Because resistance to Fas-mediated apoptosis is a common mechanism used by tumors to evade the immune system, it will be interesting to determine whether the IRF-4–mediated effects on the susceptibility to Fas-mediated death are selectively bypassed in IRF-4–expressing tumors. Interestingly, we have reported previously that IRF-4 function can be modulated by the presence of BCL-6 (25), a Krüppel-type zinc finger transcriptional repressor, whose deregulation is a common event in non-Hodgkin's lymphoma (62). Given that coexpression of IRF-4 and BCL-6 has been detected in 50% of non-Hodgkin's lymphomas (63, 64), it is possible that BCL-6 may interfere with the IRF-4–mediated effects on Fas-dependent apoptosis thus potentially leading to pathophysiological consequences. Further research will be needed to elucidate the full implications of the IRF-4–BCL-6 interaction in lymphocyte apoptosis.
Our work is consistent with a model whereby IRF-4 serves as a molecular link between the activation program of lymphocytes and their ability to undergo AICD. Such a role might explain why the lack of IRF-4 results not only in defects in lymphocyte activation, but also in progressive lymphoaccumulation (23). The critical question remains whether the dual role of IRF-4 in the activation and death of lymphocytes can be differentially modulated. Given that IRF-4 is well-known to interact with additional cofactors (65–68), an attractive hypothesis is that the presence or absence of specific partners may modulate the ability of IRF-4 to function in the two pathways. We have proposed previously that one of the distinctive features of IRF-4 may be to function as an “integrator” in the course of lymphocyte activation (22). Such an integrator, possibly via its ability to associate with different cofactors, may help lymphocytes to summate the information provided to them by distinct incoming signals and may thus be ideally positioned to play a central role in the life and death decisions made by lymphocytes.
Acknowledgments
We would like to thank Dr. Sanjay Gupta and Andrea Lee for significant input and discussions, Dr. Aida Cremesti for her help with the Fas capping, and Drs. G. Siu and C. Schindler for their critical reading of the manuscript.
The research was supported by a grant from the American Cancer Society and by the National Institutes of Health grant R01 HL-62215.
Footnotes
Abbreviations used in this paper: AICD, activation-induced cell death; FasL, Fas ligand; FLIP, FLICE/caspase-8 inhibitory protein; IRF-4, interferon regulatory factor 4; SEB, staphylococcal enterotoxin B.
References
- 1.Thome, M., and J. Tschopp. 2001. Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 1:50–58. [DOI] [PubMed] [Google Scholar]
- 2.Nagata, S., and P. Golstein. 1995. The Fas death factor. Science. 267:1449–1456. [DOI] [PubMed] [Google Scholar]
- 3.Schulze-Osthoff, K., D. Ferrari, M. Los, S. Wesselborg, and M.E. Peter. 1998. Apoptosis signaling by death receptors. Eur. J. Biochem. 254:439–459. [DOI] [PubMed] [Google Scholar]
- 4.Golstein, P. 2000. Signal transduction. FasL binds preassembled Fas. Science. 288:2328–2329. [DOI] [PubMed] [Google Scholar]
- 5.Muzio, M., A.M. Chinnaiyan, F.C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J.D. Bretz, M. Zhang, R. Gentz, et al. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 85:817–827. [DOI] [PubMed] [Google Scholar]
- 6.Stennicke, H.R., J.M. Jurgensmeier, H. Shin, Q. Deveraux, B.B. Wolf, X. Yang, Q. Zhou, H.M. Ellerby, L.M. Ellerby, D. Bredesen, et al. 1998. Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273:27084–27090. [DOI] [PubMed] [Google Scholar]
- 7.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K.J. Tomaselli, K.M. Debatin, P.H. Krammer, and M.E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inaba, M., K. Kurasawa, M. Mamura, K. Kumano, Y. Saito, and I. Iwamoto. 1999. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J. Immunol. 163:1315–1320. [PubMed] [Google Scholar]
- 9.Krammer, P.H. 2000. CD95's deadly mission in the immune system. Nature. 407:789–795. [DOI] [PubMed] [Google Scholar]
- 10.Inohara, N., T. Koseki, Y. Hu, S. Chen, and G. Nunez. 1997. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc. Natl. Acad. Sci. USA. 94:10717–10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J.L. Bodmer, M. Schroter, K. Burns, C. Mattmann, et al. 1997. Inhibition of death receptor signals by cellular FLIP. Nature. 388:190–195. [DOI] [PubMed] [Google Scholar]
- 12.Hu, S., C. Vincenz, J. Ni, R. Gentz, and V.M. Dixit. 1997. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J. Biol. Chem. 272:17255–17257. [DOI] [PubMed] [Google Scholar]
- 13.Scaffidi, C., I. Schmitz, J. Zha, S.J. Korsmeyer, P.H. Krammer, and M.E. Peter. 1999. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274:22532–22538. [DOI] [PubMed] [Google Scholar]
- 14.Krueger, A., I. Schmitz, S. Baumann, P.H. Krammer, and S. Kirchhoff. 2001. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276:20633–20640. [DOI] [PubMed] [Google Scholar]
- 15.Algeciras-Schimnich, A., T.S. Griffith, D.H. Lynch, and C.V. Paya. 1999. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J. Immunol. 162:5205–5211. [PubMed] [Google Scholar]
- 16.Refaeli, Y., L. Van Parijs, C.A. London, J. Tschopp, and A.K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 8:615–623. [DOI] [PubMed] [Google Scholar]
- 17.Cremesti, A., F. Paris, H. Grassme, N. Holler, J. Tschopp, Z. Fuks, E. Gulbins, and R. Kolesnick. 2001. Ceramide enables fas to cap and kill. J. Biol. Chem. 276:23954–23961. [DOI] [PubMed] [Google Scholar]
- 18.Parlato, S., A.M. Giammarioli, M. Logozzi, F. Lozupone, P. Matarrese, F. Luciani, M. Falchi, W. Malorni, and S. Fais. 2000. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 19:5123–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenbeis, C.F., H. Singh, and U. Storb. 1995. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 9:1377–1387. [DOI] [PubMed] [Google Scholar]
- 20.Matsuyama, T., A. Grossman, H.W. Mittrucker, D.P. Siderovski, F. Kiefer, T. Kawakami, C.D. Richardson, T. Taniguchi, S.K. Yoshinaga, and T.W. Mak. 1995. Molecular cloning of LSIRF, a lymphoid-specific member of the interferon regulatory factor family that binds the interferon-stimulated response element (ISRE). Nucleic Acids Res. 23:2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagata, T., J. Nishida, S. Tanaka, R. Sakai, K. Mitani, M. Yoshida, T. Taniguchi, Y. Yazaki, and H. Hirai. 1996. A novel interferon regulatory factor family transcription factor, ICSAT/Pip/LSIRF, that negatively regulates the activity of interferon-regulated genes. Mol. Cell. Biol. 16:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pernis, A.B. 2002. The role of IRF-4 in B and T cell activation and differentiation. J. Interferon Cytokine Res. 22:111–120. [DOI] [PubMed] [Google Scholar]
- 23.Mittrucker, H.W., T. Matsuyama, A. Grossman, T.M. Kundig, J. Potter, A. Shahinian, A. Wakeham, B. Patterson, P.S. Ohashi, and T.W. Mak. 1997. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 275:540–543. [DOI] [PubMed] [Google Scholar]
- 24.Yang, J., X. Liu, K. Bhalla, C.N. Kim, A.M. Ibrado, J. Cai, T.I. Peng, D.P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 275:1129–1132. [DOI] [PubMed] [Google Scholar]
- 25.Gupta, S., A. Anthony, and A.B. Pernis. 2001. Stage-specific modulation of IFN-regulatory factor 4 function by Kruppel-type zinc finger proteins. J. Immunol. 166:6104–6111. [DOI] [PubMed] [Google Scholar]
- 26.Tepper, C.G., and M.F. Seldin. 1999. Modulation of caspase-8 and FLICE-inhibitory protein expression as a potential mechanism of Epstein-Barr virus tumorigenesis in Burkitt's lymphoma. Blood. 94:1727–1737. [PubMed] [Google Scholar]
- 27.Fulda, S., E. Meyer, and K.M. Debatin. 2000. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 60:3947–3956. [PubMed] [Google Scholar]
- 28.Zheng, Y., F. Ouaaz, P. Bruzzo, V. Singh, S. Gerondakis, and A.A. Beg. 2001. NF-kappa B RelA (p65) is essential for TNF-alpha-induced fas expression but dispensable for both TCR-induced expression and activation-induced cell death. J. Immunol. 166:4949–4957. [DOI] [PubMed] [Google Scholar]
- 29.Robertson, J.D., V. Gogvadze, B. Zhivotovsky, and S. Orrenius. 2000. Distinct pathways for stimulation of cytochrome c release by etoposide. J. Biol. Chem. 275:32438–32443. [DOI] [PubMed] [Google Scholar]
- 30.Varghese, J., S. Chattopadhaya, and A. Sarin. 2001. Inhibition of p38 kinase reveals a TNF-alpha-mediated, caspase-dependent, apoptotic death pathway in a human myelomonocyte cell line. J. Immunol. 166:6570–6577. [DOI] [PubMed] [Google Scholar]
- 31.Waring, P., and A. Mullbacher. 1999. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 77:312–317. [DOI] [PubMed] [Google Scholar]
- 32.Seol, D.W., J. Li, M.H. Seol, S.Y. Park, R.V. Talanian, and T.R. Billiar. 2001. Signaling events triggered by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): caspase-8 is required for TRAIL-induced apoptosis. Cancer Res. 61:1138–1143. [PubMed] [Google Scholar]
- 33.Kuida, K., T.F. Haydar, C.Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M.S. Su, P. Rakic, and R.A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 94:325–337. [DOI] [PubMed] [Google Scholar]
- 34.Hakem, R., A. Hakem, G.S. Duncan, J.T. Henderson, M. Woo, M.S. Soengas, A. Elia, J.L. de la Pompa, D. Kagi, W. Khoo, et al. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 94:339–352. [DOI] [PubMed] [Google Scholar]
- 35.Li, P., D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, E.S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91:479–489. [DOI] [PubMed] [Google Scholar]
- 36.Gross, A., X.M. Yin, K. Wang, M.C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S.J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156–1163. [DOI] [PubMed] [Google Scholar]
- 37.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 94:481–490. [DOI] [PubMed] [Google Scholar]
- 38.Li, H., H. Zhu, C.J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 94:491–501. [DOI] [PubMed] [Google Scholar]
- 39.White, J., A. Herman, A.M. Pullen, R. Kubo, J.W. Kappler, and P. Marrack. 1989. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 56:27–35. [DOI] [PubMed] [Google Scholar]
- 40.Bonfoco, E., P.M. Stuart, T. Brunner, T. Lin, T.S. Griffith, Y. Gao, H. Nakajima, P.A. Henkart, T.A. Ferguson, and D.R. Green. 1998. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity. 9:711–720. [DOI] [PubMed] [Google Scholar]
- 41.Renno, T., M. Hahne, J. Tschopp, and H.R. MacDonald. 1996. Peripheral T cells undergoing superantigen-induced apoptosis in vivo express B220 and upregulate Fas and Fas ligand. J. Exp. Med. 183:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhein, J., H. Walczak, M.O. Westendorp, C. Baumler, K. Stricker, R. Frank, K.M. Debatin, and P.H. Krammer. 1995. Molecular mechanisms of APO-1/Fas(CD95)-mediated apoptosis in tolerance and AIDS. Behring Inst. Mitt. 96:13–20. [PubMed] [Google Scholar]
- 43.Brunner, T., R.J. Mogil, D. LaFace, N.J. Yoo, A. Mahboubi, F. Echeverri, S.J. Martin, W.R. Force, D.H. Lynch, C.F. Ware, and et al. 1995. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 373:441–444. [DOI] [PubMed] [Google Scholar]
- 44.Ju, S.T., D.J. Panka, H. Cui, R. Ettinger, M. el-Khatib, D.H. Sherr, B.Z. Stanger, and A. Marshak-Rothstein. 1995. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 373:444–448. [DOI] [PubMed] [Google Scholar]
- 45.Rosenbauer, F., A. Kallies, M. Scheller, K.P. Knobeloch, C.O. Rock, M. Schwieger, C. Stocking, and I. Horak. 2002. Disabled-2 is transcriptionally regulated by ICSBP and augments macrophage spreading and adhesion. EMBO J. 21:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bromley, S.K., W.R. Burack, K.G. Johnson, K. Somersalo, T.N. Sims, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. [DOI] [PubMed] [Google Scholar]
- 47.Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. [DOI] [PubMed] [Google Scholar]
- 48.Wulfing, C., M.D. Sjaastad, and M.M. Davis. 1998. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl. Acad. Sci. USA. 95:6302–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wulfing, C., and M.M. Davis. 1998. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 282:2266–2269. [DOI] [PubMed] [Google Scholar]
- 50.Grakoui, A., S.K. Bromley, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- 51.Penninger, J.M., and G.R. Crabtree. 1999. The actin cytoskeleton and lymphocyte activation. Cell. 96:9–12. [DOI] [PubMed] [Google Scholar]
- 52.Shaw, A.S. 2001. FERMing up the synapse. Immunity. 15:683–686. [DOI] [PubMed] [Google Scholar]
- 53.Dustin, M.L., and J.A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23–29. [DOI] [PubMed] [Google Scholar]
- 54.Roumier, A., J.C. Olivo-Marin, M. Arpin, F. Michel, M. Martin, P. Mangeat, O. Acuto, A. Dautry-Varsat, and A. Alcover. 2001. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 15:715–728. [DOI] [PubMed] [Google Scholar]
- 55.Allenspach, E.J., P. Cullinan, J. Tong, Q. Tang, A.G. Tesciuba, J.L. Cannon, S.M. Takahashi, R. Morgan, J.K. Burkhardt, and A.I. Sperling. 2001. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 15:739–750. [DOI] [PubMed] [Google Scholar]
- 56.Delon, J., K. Kaibuchi, and R.N. Germain. 2001. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 15:691–701. [DOI] [PubMed] [Google Scholar]
- 57.Falini, B., M. Fizzotti, A. Pucciarini, B. Bigerna, T. Marafioti, M. Gambacorta, R. Pacini, C. Alunni, L. Natali-Tanci, B. Ugolini, et al. 2000. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 95:2084–2092. [PubMed] [Google Scholar]
- 58.Natkunam, Y., and R.A. Warnke. 2001. Angiocentric lymphomas (lymphomatous vasculitis). Semin. Diagn. Pathol. 18:67–77. [PubMed] [Google Scholar]
- 59.Tsuboi, K., S. Iida, H. Inagaki, M. Kato, Y. Hayami, I. Hanamura, K. Miura, S. Harada, M. Kikuchi, H. Komatsu, et al. 2000. MUM1/IRF4 expression as a frequent event in mature lymphoid malignancies. Leukemia. 14:449–456. [DOI] [PubMed] [Google Scholar]
- 60.Alizadeh, A.A., M.B. Eisen, R.E. Davis, C. Ma, I.S. Lossos, A. Rosenwald, J.C. Boldrick, H. Sabet, T. Tran, X. Yu, et al. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 403:503–511. [DOI] [PubMed] [Google Scholar]
- 61.Iida, S., P.H. Rao, M. Butler, P. Corradini, M. Boccadoro, B. Klein, R.S. Chaganti, and R. Dalla-Favera. 1997. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat. Genet. 17:226–230. [DOI] [PubMed] [Google Scholar]
- 62.Ye, B.H., F. Lista, F. Lo Coco, D.M. Knowles, K. Offit, R.S. Chaganti, and R. Dalla-Favera. 1993. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 262:747–750. [DOI] [PubMed] [Google Scholar]
- 63.Carbone, A., A. Gloghini, L.M. Larocca, D. Capello, F. Pierconti, V. Canzonieri, U. Tirelli, R. Dalla-Favera, and G. Gaidano. 2001. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood. 97:744–751. [DOI] [PubMed] [Google Scholar]
- 64.Gaidano, G., and A. Carbone. 2000. MUM1: a step ahead toward the understanding of lymphoma histogenesis. Leukemia. 14:563–566. [DOI] [PubMed] [Google Scholar]
- 65.Su, G.H., H.S. Ip, B.S. Cobb, M.M. Lu, H.M. Chen, and M.C. Simon. 1996. The Ets protein Spi-B is expressed exclusively in B cells and T cells during development. J. Exp. Med. 184:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagulapalli, S., and M.L. Atchison. 1998. Transcription factor Pip can enhance DNA binding by E47, leading to transcriptional synergy involving multiple protein domains. Mol. Cell. Biol. 18:4639–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rengarajan, J., K.A. Mowen, K.D. McBride, E.D. Smith, H. Singh, and L.H. Glimcher. 2002. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 195:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu, C.M., S.Y. Jang, J.C. Fanzo, and A.B. Pernis. 2002. Modulation of T cell cytokine production by interferon regulatory factor-4. J. Biol. Chem. 277:49238–49246. [DOI] [PubMed] [Google Scholar]