Abstract
The steps involved in lymphocyte homing to the white pulp cords of the spleen are poorly understood. We demonstrate here that the integrins lymphocyte function associated (LFA)-1 and α4β1 make essential and mostly overlapping contributions necessary for B cell migration into white pulp cords. T cell entry to the white pulp is also reduced by blockade of LFA-1 and α4β1. The LFA-1 ligand, intercellular adhesion molecule 1 is critical for lymphocyte entry and both hematopoietic cells and radiation-resistant cells contribute to this requirement. Vascular cell adhesion molecule 1 contributes to the α4β1 ligand requirement and a second ligand, possibly fibronectin, also plays a role. By contrast with the entry requirements, antigen-induced movement of B cells from follicles to the outer T zone is not prevented by integrin blocking antibodies. Comparison of the distribution of integrin-blocked B cells and B cells treated with the Gαi inhibitor, pertussis toxin, early after transfer reveals in both cases reduced accumulation in the inner marginal zone. These observations suggest that chemokine receptor signaling and the integrins LFA-1 and α4β1 function together to promote lymphocyte transit from the marginal zone into white pulp cords.
Keywords: splenic white pulp, LFA-1, α4β1, lymphocyte homing, marginal zone
Introduction
The spleen is a major secondary lymphoid tissue important in host protection against many types of pathogen, especially encapsulated bacteria (1). The lymphoid regions of the spleen, known as white pulp cords, are organized into inner T cell zones and outer B cell follicles. The follicular areas associated with large white pulp cords are surrounded by a region termed the marginal zone, which consists of loosely associated B cells, macrophages, and reticular cells and forms the border between the white pulp and the red pulp (2). Many small follicular arterioles terminate in the marginal zone in a region known as the marginal sinus whereas a smaller number of penicillar arterioles terminate directly in the red pulp (3, 4). Although there is some controversy regarding whether a marginal sinus exists in the human spleen, corrosion casting experiments established that large amounts of blood are released into sinuses present within the marginal zone in all species examined, including humans and rodents (4, 5). Many of the lymphocytes entering the spleen are released in the marginal zone (6–8). Some of these cells, together with the nonlymphocytes, pass to the outer region of the marginal zone and then to the red pulp or directly into venous sinuses. A fraction of the lymphocytes take a different route and quickly begin appearing within the B and T cell areas of the white pulp cords (7, 8).
Extensive studies of the molecular mechanisms controlling lymphocyte entry into lymph nodes and Peyer's patches have established a cascade model of entry. First, lymphocytes undergo rolling interactions, principally mediated by L-selectin binding to ligands on the high endothelial venules (HEVs;* references 9 and 10). Subsequently, a chemokine-mediated triggering event occurs that causes integrin activation and adhesion. LFA-1 (αLβ2 or CD11a/CD18), α4β7, and α4β1 contribute to differing extents to this integrin adhesion requirement (10, 11).
Despite the detailed understanding of the steps involved in lymphocyte entry to lymph nodes and Peyer's patches, relatively little is known about how the cells enter white pulp cords in the spleen (12, 13). In particular, whether integrins are required has not been established, although some experiments have suggested that they may not be critical (14–16). However, our recent finding that marginal zone B cell adhesion within the splenic marginal zone involves redundant contributions by the integrins LFA-1 and α4β1 (17) led us to test the combined contribution of these integrins in lymphocyte entry to white pulp cords.
Materials and Methods
Mice and Adoptive Cell Transfer.
C57BL/6 (B6) mice were from Charles River Laboratories or a colony maintained at University of California San Francisco. Igha (Ig heavy chain of a) Thy1a GPI1a B6 mice (termed Igha B6 mice) were from The Jackson Laboratory. β2−/− (18), β7−/− (19), and intercellular adhesion molecule (ICAM)-1–deficient mice (20) were obtained on a B6 background from The Jackson Laboratory. IgHEL transgenic B6 mice express IgMa and IgDa specific for hen egg lysozyme (HEL). Mice were treated with antibodies by intraperitoneal injection in 300 uL PBS. Bone marrow chimeras were made as previously described (17). For adoptive transfers, mice were injected with ∼3 × 107 spleen cells in 0.3 ml medium. In some experiments, cells were labeled before transfer with 5(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) as previously described (21).
Antibodies and Treatments.
The anti-αL (clone M17/4, rat IgG2a) hybridoma was from American Type Culture Collection and the anti-α4 (clone PS/2, rat IgG2b) hybridoma was provided by David Erle (University of California San Francisco, San Francisco, CA). Anti–vascular cell adhesion molecule (VCAM)-1 (clone M/K-2, rat IgG1) was obtained from Southern Biotechnology Associates, Inc. Anti–mucosal addressin cell adhesion molecule (MAdCAM)-1 (clone MECA-367, rat IgG2A) and anti-β1 (clone HA2/5, Armenian hamster IgM) were obtained from BD Biosciences. Antibodies were administered intraperitoneally at 100 (anti-αL and anti-α4) or 200 ug (anti-β1, anti–MAdCAM-1, and anti–VCAM-1) doses approximately 1 h before intravenous cell transfer unless otherwise indicated. Cells were treated with PBS, 200 ng/ml oligomer B (List Biologicals), or 200 ng/ml pertussis toxin (PTX; Sigma-Aldrich) as previously described (12).
Flow Cytometric Analysis.
Transferred B cells were identified flow cytometrically by staining for B220, IgMa, and IgDa or B220, IgMb, and IgDb, or in the case of CFSE-labeled cells, by B220 staining alone. Transferred T cells were identified by staining for Thy1a, CD4, and CD8 or as CFSE-labeled CD3+ cells. Integrin saturation (anti-αL and anti-α4) on transferred and endogenous cells was detected by isolating splenocytes from antibody-treated animals and adding anti–rat-IgG PE (Jackson ImmunoResearch Laboratories) directly to the cells or after incubation with excess of the anti-integrin antibodies to achieve in vitro saturation. Splenocytes from a PBS-treated animal served as the negative control. Analysis 1 h after antibody injection showed that endogenous spleen cells were saturated with the integrin-neutralizing antibodies.
Immunohistochemical Analysis and Cell Enumeration.
5–7-μm cryostat sections were fixed and stained as previously described (21) using the following antibodies: rat anti–IgMa-biotin, IgDa-biotin, B220-FITC, Thy1a-biotin, IgMb-biotin or FITC, IgDb-biotin or FITC, MAdCAM-1 (BD Biosciences), rabbit anti–rat fibronectin (Invitrogen), and rabbit anti–HEL-biotin (Rockland Immunochemicals). Rat IgG antibodies were detected using streptavidin alkaline phosphatase (AP), anti-FITC horseradish peroxidase (HRP), or anti–rat HRP. Rabbit IgG antibodies were detected using anti–rabbit HRP or streptavidin AP. Triple staining for IgMb plus IgDb, HEL, and MAdCAM-1 was accomplished by staining for IgMb-FITC plus IgDb-FITC and MAdCAM-1 as described above, heat inactivating the AP at 65°C for 15 min, and then proceeding to stain for HEL. Enzyme reactions were developed with conventional substrates for peroxidases (diaminobenzidine/H2O2; Sigma-Aldrich) and AP (Fast Blue/Fast Red; Sigma-Aldrich). Cell enumeration was performed by locating well-defined B220-stained white pulp cords (follicular areas encompassing a central T zone) and counting transferred B or T cells within at least three (mostly five to eight) white pulp cords per mouse. For experiments tracking WT cells in control, antibody-treated, or ICAM-1−/− recipients, Igha B6 donor cells were used and transferred B cells were identified using a combination of anti-IgMa and anti-IgDa antibodies. For experiments with β2−/− and β7−/− donors, Igha B6 recipients were used and transferred cells were identified by using a combination of anti-IgMb and anti-IgDb antibodies. In some transfers of WT Igha cells to B6 recipients, T cells were identified using anti-Thy1a antibodies, but in many cases this approach was unsuccessful due to diffusion of the stain. Additional T cell enumeration data was obtained by transfer of AUTOMACS (Miltenyi)-purified CFSE-labeled T cells and detecting the T cells with anti-FITC AP. Statistical analysis was performed using Student's t test.
Results and Discussion
LFA-1 and α4β1 Function in B and T Cell Entry to the Splenic White Pulp.
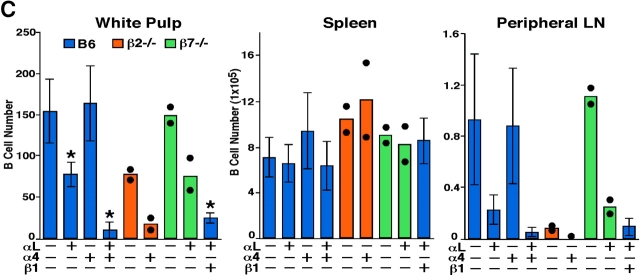
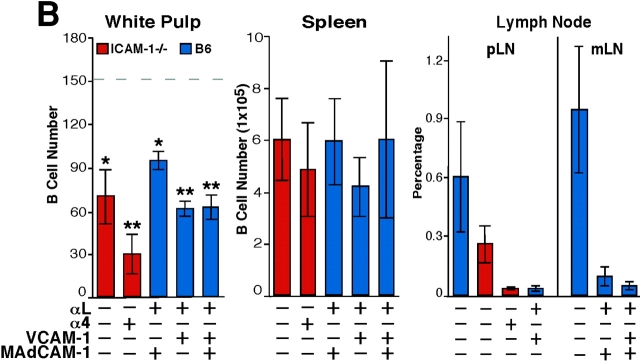
To test the possibility that LFA-1 and α4-containing integrins make overlapping contributions to lymphocyte entry into the splenic white pulp, WT mice were treated with a combination of LFA-1–and α4 blocking antibodies and the subsequent trafficking of transferred lymphocytes was examined. Strikingly, in mice given the combined treatment, B cell entry to white pulp cords was reduced by ∼90% (Fig. 1, A and C) and T cell entry was reduced by 50% (Fig. 1 B). As expected (17), the combined antibody treatment also led to displacement of marginal zone B cells from around the white pulp cords (Fig. 1, A and B) whereas marginal metallophilic macrophages and marginal zone macrophages were not displaced (unpublished data). The total number of transferred lymphocytes in the spleen was not greatly affected by the antibody treatment whereas entry into lymph nodes was strongly inhibited (Fig. 1 C), as expected (10, 11). The lack of an effect on total spleen cell numbers despite the inhibition in cell entry to white pulp cords is similar to the previous findings for cells treated with PTX (12) and most likely reflects the presence of greater numbers of cells in the red pulp due to the lymphocytosis caused by the block in entry to lymph nodes. Analysis of the effect of blocking LFA-1 alone revealed ∼50% inhibition in white pulp accumulation of B cells (Fig. 1 C) and ∼30% inhibition for T cells (not depicted). In a recent study, cells from LFA-1–deficient mice were reported to show a small (∼20%) reduction in lymphocyte homing to white pulp cords (16). The differences in the extent of inhibition observed might reflect our direct enumeration of cells within white pulp cords versus Nolte et al.'s (22) approach of measuring cells flow cytometrically in enzymatically isolated spleen preparations enriched for white pulp cord cells. Treatment with α4 blocking antibodies alone had no measurable effect on the number of B or T lymphocytes accumulating in the white pulp over the 3-h period (Fig. 1). Thus, the contribution of α4-containing integrins to lymphocyte homing to the white pulp appears to be fully redundant to LFA-1 whereas the contribution of LFA-1 is partially redundant with the α4-containing integrins.
Figure 1.
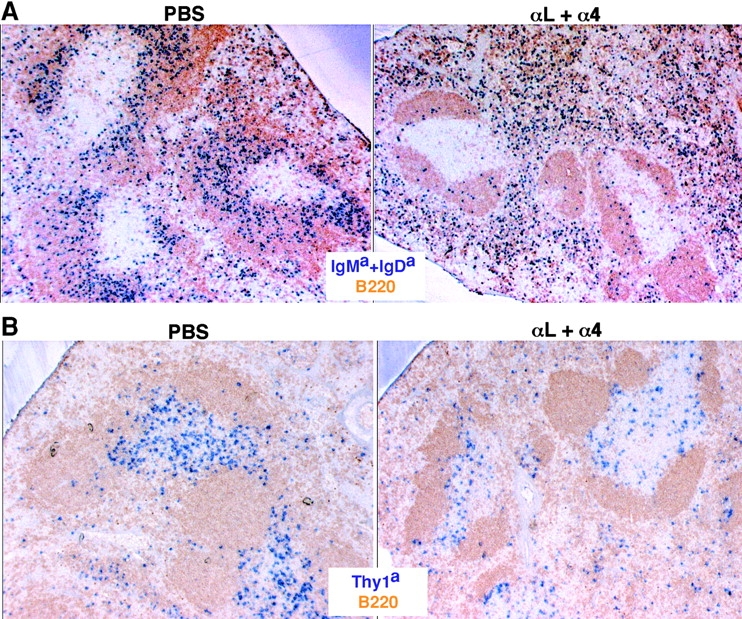
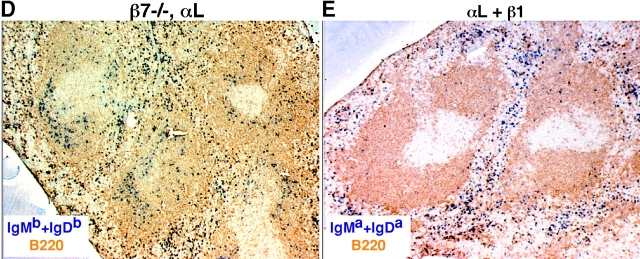
LFA-1 and α4β1 function in B and T cell entry into splenic white pulp cords. (A and B) Immunohistochemical analysis of spleen sections from B6 mice that had received WT Igha Thy1a spleen cells 3 h before and had been pretreated with PBS or αL and α4 neutralizing antibodies, as indicated, 1 h before cell transfer. Transferred B cells were detected by IgMa plus IgDa staining (A, blue), transferred T cells by Thy1a staining (B, blue), and endogenous B cells by B220 staining (brown). ×5. (C) Summary of B cell homing data showing the average number of transferred B cells per white pulp cross section (left), per one fifth of spleen (middle), and in a pool of inguinal and brachial lymph nodes (right). Donor cells were from WT, β2−/−, or β7−/− mice as indicated. Each bar shows the average (±SD) value for data from at least four animals of each type except for the β2−/− and β7−/− transfers where the individual data points are denoted by •. *, P < 0.05 compared with untreated WT controls. Similar enumeration was performed for T cells and the following average number of cells were detected per white pulp cord: PBS-treated, 218 ± 11 (n = 3); α4-treated, 242 (n = 1); αL-treated, 152 ± 13 (n = 3); αL plus α4-treated, 118 ± 14 (n = 4). (D and E) Immunohistochemical analysis of spleen sections from WT Igha B6 mice that had received β7−/− Ighb cells (D) or B6 mice that had received WT Igha spleen cells (E) and had been treated with integrin neutralizing antibodies, as indicated. Transferred B cells were detected by staining IgMb plus IgDb (D) or IgMa plus IgDa (E).
As B cell homing was more strongly inhibited by integrin neutralization than T cell homing, we focused our subsequent experiments on B cells. To further test the contribution of β2-containing integrins and dissect the contributions of α4β1 and α4β7 to white pulp homing of B cells, transfer experiments were performed with spleen cells from β2−/− (18) or β7−/ − (19) mice. In the absence of antibody treatment, β2-deficient B cells showed a twofold decrease in white pulp homing compared with WT cells, consistent with the LFA-1 blocking experiments (Fig. 1 C). When β2-deficient cells were transferred to mice that had been pretreated with α4 blocking antibodies, homing was inhibited to the same extent as in mice treated with LFA-1 and α4 blocking antibodies (Fig. 1 C), indicating that the few remaining cells that continue to enter white pulp cords in LFA-1– and α4-blocked animals was not due to incomplete neutralization of LFA-1 or to contributions by other β2-containing integrins. Furthermore, as treatment with anti-α4 alone does not cause displacement of marginal zone B cells (17), this experiment indicates that the diminished white pulp homing is not due to effects on marginal zone B cells. When β7-deficient cells were transferred to WT mice, no defects in cell entry to splenic white pulp cords were observed, consistent with previous observations in mice treated with α4β7 blocking antibodies (15). In addition, when β7-deficient cells were transferred to mice pretreated with anti–LFA-1, B cell homing was no more affected than was the case for WT cells treated with anti–LFA-1, further indicating that α4β7 is not critical for lymphocyte trafficking into white pulp cords (Fig. 1, C and D). This observation implicates α4β1 as the key α4-containing integrin involved and the finding that combined treatment with β1 and LFA-1 blocking antibodies inhibited B cell homing to white pulp cords to the same extent as combined treatment with α4 and LFA-1 blocking antibodies confirms this conclusion (Fig. 1, C and E).
Integrin Ligand Requirements for Lymphocyte Entry to the White Pulp.
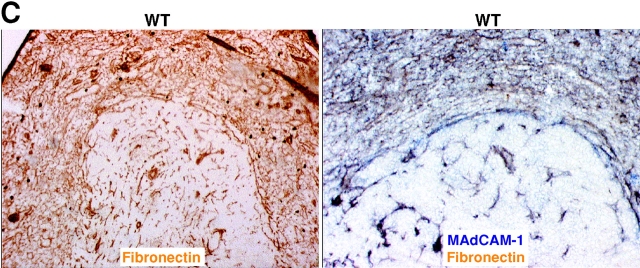
The best-defined ligands for LFA-1 are ICAM-1, ICAM-2, and ICAM-3 whereas key α4β1 ligands are VCAM-1, MAdCAM-1, and fibronectin (10, 23, 24). Many of the lymphocytes entering the spleen are released in the marginal zone and ICAM-1 and VCAM-1 expression has been detected throughout this zone (5, 16, 17). MAdCAM-1 is also expressed within the marginal zone (5) and in mice the expression is mostly limited to cells associated with the follicular side of the marginal sinus (15). We first tested the contribution of ICAM-1 to B cell migration into white pulp cords by using ICAM-1–deficient mice (20). B cell homing was partially reduced in these animals and when they had been treated with anti-α4 blocking antibodies, homing was inhibited to an extent similar to experiments in which mice were treated with a combination of LFA-1 and α4 blocking antibodies (Fig. 2, A and B) . Analysis of α4β1 ligand involvement was performed by treating mice with VCAM-1 or MAdCAM-1 blocking antibodies together with the LFA-1 neutralizing antibody. No contribution could be identified for MAdCAM-1 despite the expected block in lymphocyte homing to mucosal lymph nodes (Fig. 2 B). By contrast, inhibition of VCAM-1 function was associated with a reduction in B cell homing to the white pulp compared with animals treated with LFA-1 blocking antibodies alone (Fig. 2, A and B). However, the block in homing by the combination of anti–VCAM-1 and anti–LFA-1 was less severe than that observed after anti-α4 plus anti–LFA-1 treatment or anti-β1 plus anti–LFA-1 treatment (Fig. 1), indicating that an additional α4β1 ligand participates in B cell homing into white pulp cords. Combined treatment with neutralizing antibodies specific for VCAM-1, MAdCAM-1, and LFA-1 did not cause any greater inhibition than observed by VCAM-1 and LFA-1 blocking (Fig. 2 B). Although no reagents are available to selectively inhibit α4β1–fibronectin interactions in the mouse, these findings are consistent with the possibility that fibronectin functions as an adhesion molecule for B cell migration into white pulp cords. However, our experiments do not rule out possible contributions by other molecules that can bind α4β1, such as von Willebrand factor and thrombospondin (24). Favoring a role for fibronectin, immunohistochemical analysis revealed that this extracellular matrix protein is expressed at a high level throughout the marginal zone as well as in the red pulp (Fig. 2 C). Analysis of sections double stained for fibronectin and MAdCAM-1 indicated an overlap in the staining pattern consistent with fibronectin being associated with marginal sinus lining cells (Fig. 2 C). Fibronectin has also been identified within the marginal zone of human spleen (25).
Figure 2.
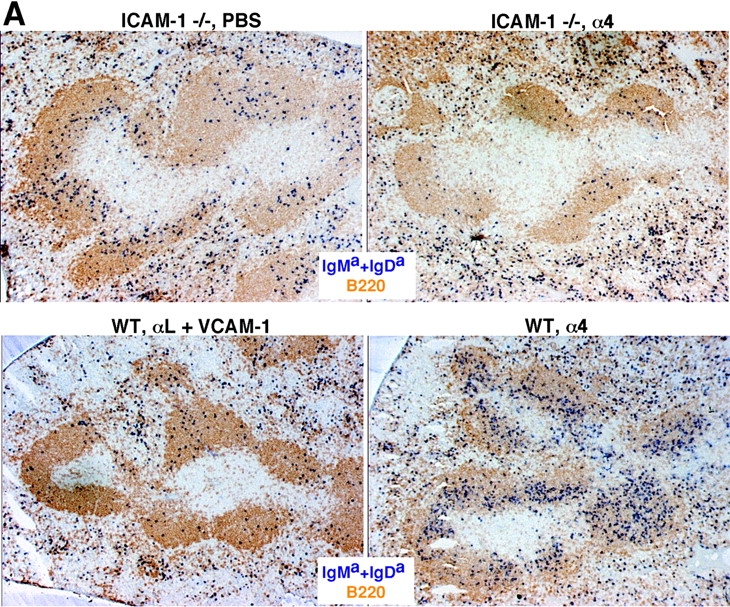
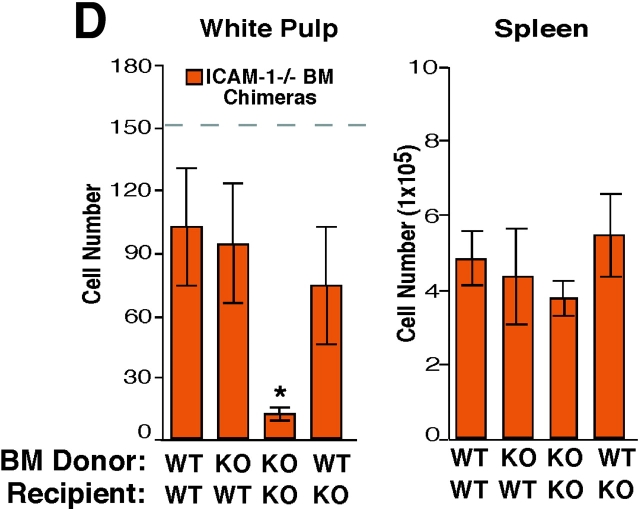
ICAM-1 and VCAM-1 function in B cell entry into splenic white pulp cords. (A) Immunohistochemical analysis of spleen sections from ICAM-1−/− or WT mice that had received Igha B6 spleen cells 3 h before and had been pretreated as indicated 1 h before cell transfer. Transferred cells were detected as described in Fig. 1. ×5. (B) Summary of transferred B cell homing data showing the average number of B cells per white pulp cross section (left), per one fifth of spleen (middle), and the frequency in a pool of inguinal and brachial lymph nodes (pLN) or in mesenteric lymph nodes (mLN) as indicated (right). Donor cells were from WT mice and the genotype of the recipient animals is indicated. The dashed line in the White Pulp panel indicates the average number of transferred WT cells reaching the white pulp in untreated controls as shown in Fig. 1. *, P < 0.05 compared with untreated WT controls; **, P < 0.05 compared with ICAM-1−/− or anti-αL–treated mice. (C) Fibronectin expression pattern in mouse spleen. Spleen sections from WT mice were stained to detect fibronectin alone (left, brown) or together with MAdCAM-1 (right, blue). (D) Summary of B cell homing data in ICAM-1−/− or control bone marrow chimeras treated with α4 blocking antibody. Recipient ICAM-1−/− or WT mice were reconstituted with ICAM-1−/− or WT bone marrow (BM), as indicated, treated with anti-α4, and transferred with WT cells. *, P < 0.05 compared with WT bone marrow chimeras. Cell number was enumerated as described in Materials and Methods. Each bar shows the average (±SD) value for data from at least four animals per group in B and three animals in D.
To determine whether the ICAM-1 necessary for B cell homing in the spleen was made by hematopoietic or nonhematopoietic cells, radiation bone marrow chimeras were generated. After reconstitution, these animals were treated with α4 blocking antibodies and then transferred with WT lymphocytes (Fig. 2). For reasons that are unclear, the homing of B cells to white pulp cords in control bone marrow chimeras (WT bone marrow → WT recipients) was reduced compared with homing in nonirradiated WT controls (Fig. 2 D), indicating that there was some alteration in B cell trafficking due to the effects of irradiation. However, compared with this control group, homing in ICAM-1–deficient chimeras (ICAM-1−/− bone marrow → ICAM-1−/− recipients) was severely inhibited (Fig. 2 D). By contrast, in mice where ICAM-1 expression was restricted to radiation-resistant cells (ICAM-1−/− bone marrow → WT recipients), B cell entry to white pulp cords was only weakly reduced compared with the WT controls. Similarly, when ICAM-1 expression was restricted to radiation-sensitive hematopoietic cells (WT bone marrow → ICAM-1−/− recipients), homing was only weakly affected (Fig. 2 D). Therefore, the ICAM-1 requirement for B cell homing into splenic white pulp cords involves contributions by both radiation-sensitive hematopoietic cells and radiation-resistant cells. Consistent with a hematopoietic cell type playing a role in guiding lymphocyte homing in the spleen, ablation of marginal zone macrophages was associated with a reduction in the efficiency of lymphocyte homing into white pulp cords (26). The nature of the ICAM-1–expressing radiation-resistant cells in the marginal zone is unclear but may include the marginal sinus lining cells and marginal zone reticular cells (2, 5, 17).
Relocalization of Antigen-engaged B Cells from Follicles to T Zones Is Not Blocked by Integrin Neutralizing Antibodies.
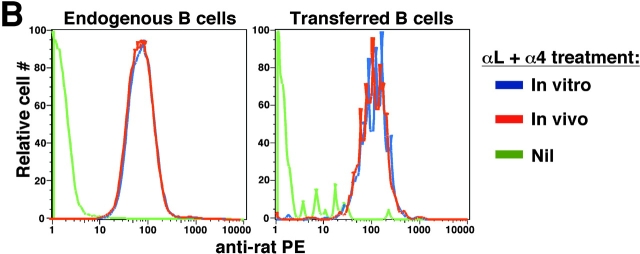
To examine whether integrins are also critical for B cell movement within the white pulp, we tested the effect of LFA-1 and α4 blocking antibodies on the migration of antigen-engaged B cells from follicles to the outer T zone (21). Previous experiments have established that this redistribution process is complete within 6 h of antigen exposure. Therefore, IgHEL transgenic B cells specific for HEL were transferred to WT mice and after allowing 1 d for the cells to become distributed in lymphoid follicles, mice were treated with LFA-1 and α4 neutralizing antibodies and then exposed to HEL antigen. Immunohistochemical analysis of spleens isolated 6 h later demonstrated that B cells had redistributed from the follicles to T cell areas in a manner similar to mice that did not receive integrin blocking antibodies (Fig. 3 A). Samples of the tissue from each animal were used to generate cell suspensions and these were tested by flow cytometric analysis to confirm that the integrins on both the endogenous and transferred B cells were saturated by the neutralizing antibodies (Fig. 3 B). These findings indicate that movement of B cells inside the splenic white pulp can occur when LFA-1 and α4β1 are blocked, favoring the conclusion that the integrin requirement for homing into the white pulp reflects a role during an early entry step. Additional approaches will be needed to determine whether integrins contribute to the basal motility of naive lymphocytes within the white pulp and to identify what other types of adhesive interactions are used by migrating white pulp cells.
Figure 3.
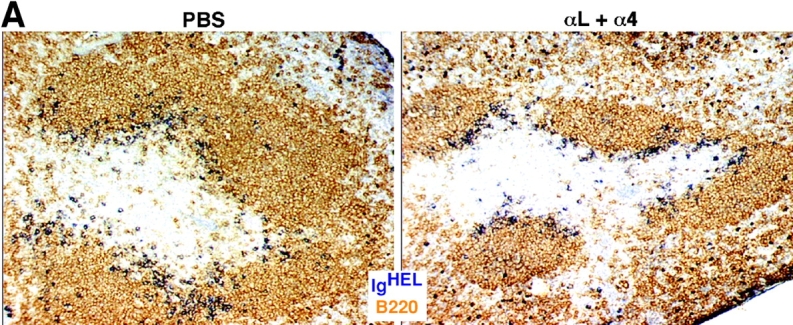
Inhibition of LFA-1 and α4 function does not prevent antigen-induced relocalization of B cells from follicles to the outer T zone. (A) Immunohistochemical analysis of spleen sections from mice that had received IgHEL transgenic B cells 1 d before, anti–LFA-1 and anti-α4 antibodies or PBS 8 h before, and 1 mg HEL antigen 6 h before. Transgenic B cells were detected by staining for HEL binding (blue) and endogenous B cells were detected with anti-B220 (brown). ×20. (B) Flow cytometric analysis to detect amounts of integrin blocking antibodies on the surface of B cells. Spleen cells from mice treated as described in A were either directly stained with anti–rat IgG (In vivo, red line), or after additional incubation in vitro with saturating amounts of anti–LFA-1 and anti-α4 (In vitro, blue line), and with antibodies to detect B220 and HEL. Levels of anti–rat IgG staining on B220+ HEL− endogenous cells (left) or B220+ HEL+ transferred cells (right) are shown. Cells from mice that had not been injected with anti-integrin antibodies served as a negative control (Nil, green line).
PTX and Integrin Blocking Antibodies Inhibit Splenic B Cell Migration at a Similar Step.
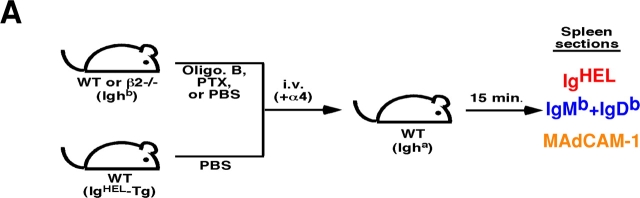
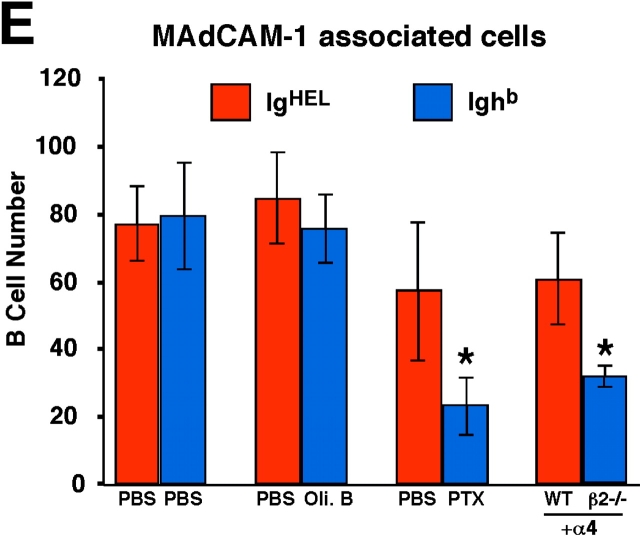
Previous experiments have demonstrated that PTX-treated lymphocytes fail to enter white pulp cords (12, 13). To examine whether the requirements for Gαi signaling and integrin function occur at a similar step, short-term transfer experiments were performed in which mice received cotransfers of treated and control cells (Fig. 4 A). At early time points after transfer of untreated cells, many cells were present within the marginal zone and, similar to a previous report (7), a fraction of the cells appeared on the inner (follicular) edge of the marginal zone associated with the MAdCAM-1+ cells (Fig. 4 B). Comparison of the distribution of PTX-treated cells and control cells revealed that fewer of the treated cells were located along this boundary whereas treatment with the inactive oligomer B subunit of PTX had no effect (Fig. 4, B, C and E). A similar analysis was attempted to test the role of integrins at this step by transferring cells that had been pretreated with integrin blocking antibodies together with untreated cells, but no differences were observed in the distribution of treated and untreated cells (unpublished data). However, flow cytometric analysis of the pretreated cells isolated 10 min after transfer revealed that ∼50% of the surface integrins were no longer occupied by neutralizing antibody despite presaturation before transfer (not depicted). Therefore, we used an alternative approach where β2-deficient and WT cells were cotransferred to mice that had been pretreated with α4 blocking antibodies. Although all the cells would have their α4 integrins neutralized, the experiments described above established that this does not measurably affect passage of WT lymphocytes into white pulp cords (Fig. 1). Analysis of the distribution of β2−/− versus control cells 15 min after transfer revealed a deficiency in β2−/− cells near the inner edge of the marginal zone (Fig. 4, D and E). Therefore, treatment with PTX and combined inhibition of α4β1 and LFA-1 both disrupt attachment or accumulation of B cells on the inner side of the marginal zone, as well as prevent their subsequent appearance within the white pulp cords.
Figure 4.
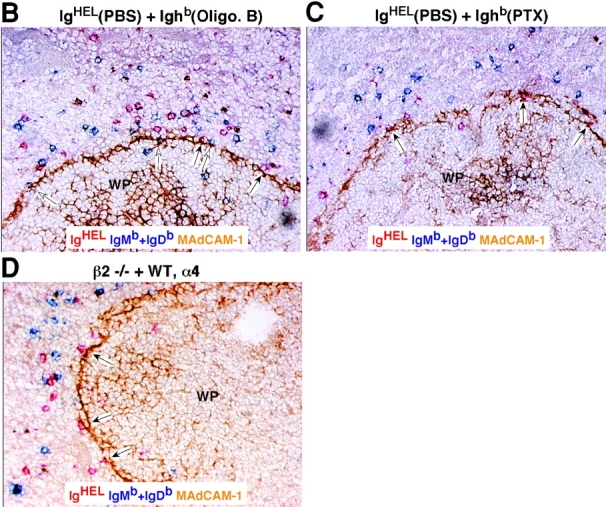
PTX and integrin blocking affect B cell entry into white pulp cords at a similar early step. (A) Mixed adoptive transfers were performed using spleen cells from WT Ighb mice that were treated in vitro with PBS, oligomer B (Oli. B), or PTX and transferred intravenously to WT Igha recipients together with equal numbers of PBS-treated WT IgHEL transgenic cells. Alternatively, β2−/− Ighb spleen cells were mixed with WT IgHEL transgenic cells and transferred to anti-α4 antibody–treated recipients. 15 min after transfer, recipient spleens were isolated and subjected to immunohistochemical analysis as indicated. (B–D) Three color immunohistochemical analysis of spleen sections from mice that had received a mixture of oligomer B– (B) or PTX–treated (C) spleen cells and PBS-treated IgHEL transgenic internal control cells, or from mice that had been pretreated with anti-α4 antibody and received a mixture of β2−/− and IgHEL transgenic cells (D). Sections were stained for IgMb plus IgDb (blue) to detect treated or β2−/− B cells, HEL (red) to detect IgHEL transgenic B cells, and MAdCAM-1 (brown) to highlight the boundary between the marginal zone and white pulp. Filled arrows identify control IgHEL B cells (red) and open arrows identify treated or β2−/− B cells (blue) in the inner marginal zone (defined by physical association with the MAdCAM-1–stained cells). WP, white pulp. ×20. (E) Summary of data showing inhibitory effect of PTX treatment and integrin blocking on B cell localization in the inner marginal zone. Transferred cells were identified as indicated in A and the number of cells in the inner marginal zone was enumerated as exemplified by the cells indicated by arrows in B–D. More than 12 separate cross sections from each spleen were enumerated and between 2 and 4 spleens of each type were analyzed. *, P < 0.05 compared with IgHEL transgenic internal control cells.
Concluding Remarks.
The findings described above demonstrate that LFA-1 and α4β1 and their ligands play critical roles in lymphocyte entry into the splenic white pulp, revealing closer parallels than previously thought between this process and entry into lymph nodes and Peyer's patches. The integrin entry requirement is stronger for B than T cells, and LFA-1 appears to make the dominant contribution to the homing requirement for both cell types. Overlapping contributions of LFA-1 and α4β1 have previously been observed for lymphocyte attachment to HEVs and accumulation in the bone marrow (11) and for marginal zone B cell lodgement in the marginal zone (17). Our findings indicate that the key ligands involved in B cell entry are ICAM-1, VCAM-1, and a second α4β1 ligand. As fibronectin is present in large amounts within the marginal zone, we suggest that this α4β1 ligand also contributes to the entry process. Although MAdCAM-1 is expressed in an appropriate location to participate in entry (15), experiments using MAdCAM-1 blocking antibodies and β7-deficient cells failed to reveal a contribution for this adhesion molecule even when the contributions of LFA-1/ICAM-1 were blocked. Consistent with MAdCAM-1 not having a critical role, this ligand has not been detected in rat spleen (27). The requirement of α4β1 and LFA-1 both for cell entry into the white pulp and for long-term adherence of cells in the marginal zone might be explained at least in part by marginal zone B cells expressing higher levels of functional integrins and adhering more strongly to ICAM-1 and VCAM-1 than follicular cells (17). It may also indicate that other differences between marginal zone and follicular B cells contribute to positioning, such as differences in chemokine responsiveness.
Although our experiments indicate that Gαi signaling and integrins participate at a similar early step in white pulp entry, we have not yet been able to determine the order of their requirement. Our findings are consistent with the possibility that integrins function both upstream and downstream of chemokine signals during the entry process. Selectins do not appear to be required for lymphocyte entry to the splenic white pulp (16) perhaps because the shear forces operating on lymphocytes released from splenic arterioles are weaker than those occurring in HEVs. However, some tethering mechanism might be necessary to prevent the cells from being flushed into the red pulp and α4β1 and LFA-1 may contribute to this process in a similar way to their role in helping tether lymphocytes to mucosal HEVs (10) and neutrophils to inflamed endothelium (28). This step may also involve lectins (other than selectins) and their glycoprotein ligands (15, 29). In addition, integrins may function downstream of chemokine signals to facilitate transit of cells from the marginal zone into the white pulp cords. Whether this step involves passage across an endothelial-type barrier is not known although it seems possible that this is the case as the marginal sinus lining cells in the mouse have properties similar to endothelial cells (2). Chemokine receptor requirements for entry into splenic follicles and T zones include CXCR5 and CCR7, respectively (see references in 10 and 21), although contributions by other Gαi-coupled receptors have not been ruled out. Finally, the basis for the differing requirements of B and T cells for LFA-1 and α4β1 to enter white pulp cords is unclear. In addition to entry after their release in the marginal zone, some cells are believed to migrate to white pulp cords after release at terminal arterioles in the red pulp (8, 30). It seems possible that the integrin requirements for entry by this pathway will be distinct and that T and B cells may differ in the degree to which they use these routes to enter the white pulp cords.
Acknowledgments
We thank the Bluestone lab for use of the AUTOMACS and Eric Brown, James Lo, Sanjiv Luther, and Takarahu Okada for comments on the manuscript.
C.G. Lo is supported by the University of California San Francisco Boyer Program in the Biological Sciences, T.T. Lu was supported by a National Institute of Child Health and Human Development (NICHD) fellowship of the Pediatric Scientist Development Program (NICHD grant award K12-HD00850), and J.G. Cyster is a Packard Fellow and a Howard Hughes Medical Institute Assistant Investigator. This work was supported by National Institutes of Health grants AI40098 and AI45073.
Footnotes
Abbreviations used in this paper: AP, alkaline phosphatase; CFSE, 5(and 6)-carboxyfluorescein diacetate succinimidyl ester; HEL, hen egg lysozyme; HEV, high endothelial venule; HRP, horseradish peroxidase; ICAM, intercellular adhesion molecule; MAdCAM, mucosal addressin cell adhesion molecule; PTX, pertussis toxin; VCAM, vascular cell adhesion molecule.
References
- 1.Bohnsack, J.F., and E.J. Brown. 1986. The role of the spleen in resistance to infection. Annu. Rev. Med. 37:49–59. [DOI] [PubMed] [Google Scholar]
- 2.Kraal, G. 1992. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132:31–73. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto, K., T. Kobayashi, and T. Murakami. 1982. Arterial terminals in the rat spleen as demonstrated by scanning electron microscopy of vascular casts. Scan. Electron Microsc. 1:455–458. [PubMed] [Google Scholar]
- 4.Schmidt, E.E., I.C. MacDonald, and A.C. Groom. 1993. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc. 7:613–628. [PubMed] [Google Scholar]
- 5.Steiniger, B., P. Barth, and A. Hellinger. 2001. The perifollicular and marginal zones of the human splenic white pulp: do fibroblasts guide lymphocyte immigration? Am. J. Pathol. 159:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford, W.L. 1969. The kinetics of lymphocyte recirculation within the rat spleen. Cell Tissue Kinet. 2:171–191. [Google Scholar]
- 7.Brelinska, R., and C. Pilgrim. 1982. The significance of the subcompartments of the marginal zone for directing lymphocyte traffic within the splenic pulp of the rat. Cell Tissue Res. 226:155–165. [DOI] [PubMed] [Google Scholar]
- 8.van Ewijk, W., and P. Nieuwenhuis. 1985. Compartments, domains and migration pathways of lymphoid cells in the splenic pulp. Experientia. 41:199–208. [DOI] [PubMed] [Google Scholar]
- 9.Springer, T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. [DOI] [PubMed] [Google Scholar]
- 10.Butcher, E.C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209–253. [DOI] [PubMed] [Google Scholar]
- 11.Berlin-Rufenach, C., F. Otto, M. Mathies, J. Westermann, M.J. Owen, A. Hamann, and N. Hogg. 1999. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1–deficient mice. J. Exp. Med. 189:1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cyster, J.G., and C.C. Goodnow. 1995. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J. Exp. Med. 182:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons, A.B., and C.R. Parish. 1995. Are murine marginal-zone macrophages the splenic white pulp analog of high endothelial venules? Eur. J. Immunol. 25:3165–3172. [DOI] [PubMed] [Google Scholar]
- 14.Saito, S., N. Kuwashima, H. Koizumi, T. Nomura, H. Yagita, K. Okumura, A. Sonoda, T. Tadakuma, and H. Tanaka. 1995. In vivo function of homing receptors participating in lymphocyte recirculation: transfer analysis in SCID mice. Pathobiology. 63:305–313. [DOI] [PubMed] [Google Scholar]
- 15.Kraal, G., K. Schornagel, P.R. Streeter, B. Holzmann, and E.C. Butcher. 1995. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am. J. Pathol. 147:763–771. [PMC free article] [PubMed] [Google Scholar]
- 16.Nolte, M.A., A. Hamann, G. Kraal, and R.E. Mebius. 2002. The strict regulation of lymphocyte migration to splenic white pulp does not involve common homing receptors. Immunology. 106:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu, T.T., and J.G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 297:409–412. [DOI] [PubMed] [Google Scholar]
- 18.Scharffetter-Kochanek, K., H. Lu, K. Norman, N. van Nood, F. Munoz, S. Grabbe, M. McArthur, I. Lorenzo, S. Kaplan, K. Ley, et al. 1998. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 188:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner, N., J. Lohler, E.J. Kunkel, K. Ley, E. Leung, G. Krissansen, K. Rajewsky, and W. Muller. 1996. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 382:366–370. [DOI] [PubMed] [Google Scholar]
- 20.Xu, H., J.A. Gonzalo, Y. St Pierre, I.R. Williams, T.S. Kupper, R.S. Cotran, T.A. Springer, and J.C. Gutierrez-Ramos. 1994. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1–deficient mice. J. Exp. Med. 180:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reif, K., E.H. Ekland, L. Ohl, H. Nakano, M. Lipp, R. Forster, and J.G. Cyster. 2002. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 416:94–99. [DOI] [PubMed] [Google Scholar]
- 22.Nolte, M.A., E.N. Hoen, A. van Stijn, G. Kraal, and R.E. Mebius. 2000. Isolation of the intact white pulp. Quantitative and qualitative analysis of the cellular composition of the splenic compartments. Eur. J. Immunol. 30:626–634. [DOI] [PubMed] [Google Scholar]
- 23.Strauch, U.G., A. Lifka, U. Gosslar, P.J. Kilshaw, J. Clements, and B. Holzmann. 1994. Distinct binding specificities of integrins alpha 4 beta 7 (LPAM-1), alpha 4 beta 1 (VLA-4), and alpha IEL beta 7. Int. Immunol. 6:263–275. [DOI] [PubMed] [Google Scholar]
- 24.Yin, Z., E. Giacomello, E. Gabriele, L. Zardi, S. Aota, K.M. Yamada, B. Skerlavaji, R. Doliana, A. Colombatti, and R. Perris. 1999. Cooperative activity of alpha4beta1 and alpha4beta7 integrins in mediating human B-cell lymphoma adhesion and chemotaxis on fibronectin through recognition of multiple synergizing binding sites within the central cell-binding domain. Blood. 93:1221–1230. [PubMed] [Google Scholar]
- 25.Liakka, K.A., and H.I. Autio-Harmainen. 1992. Distribution of the extracellular matrix proteins tenascin, fibronectin, and vitronectin in fetal, infant, and adult human spleens. J. Histochem. Cytochem. 40:1203–1210. [DOI] [PubMed] [Google Scholar]
- 26.Kraal, G., H. Rodrigues, K. Hoeben, and N. Van-Rooijen. 1989. Lymphocyte migration in the spleen: the effect of macrophage elimination. Immunology. 68:227–232. [PMC free article] [PubMed] [Google Scholar]
- 27.Iizuka, T., T. Tanaka, M. Suematsu, S. Miura, T. Watanabe, R. Koike, Y. Ishimura, H. Ishii, N. Miyasaka, and M. Miyasaka. 2000. Stage-specific expression of mucosal addressin cell adhesion molecule-1 during embryogenesis in rats. J. Immunol. 164:2463–2471. [DOI] [PubMed] [Google Scholar]
- 28.Henderson, R.B., L.H. Lim, P.A. Tessier, F.N. Gavins, M. Mathies, M. Perretti, and N. Hogg. 2001. The use of lymphocyte function-associated antigen (LFA)-1–deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J. Exp. Med. 194:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenan, M., and C.R. Parish. 1986. Modification of lymphocyte migration by sulfated polysaccharides. Eur. J. Immunol. 16:423–430. [DOI] [PubMed] [Google Scholar]
- 30.Brelinska, R., C. Pilgrim, and I. Reisert. 1984. Pathways of lymphocyte migration within the periarterial lymphoid sheath of rat spleen. Cell Tissue Res. 236:661–667. [DOI] [PubMed] [Google Scholar]