Abstract
Pathogen attachment is a crucial early step in mucosal infections. This step is mediated by important virulence factors called adhesins. To exert these functions, adhesins are typically surface-exposed, although, surprisingly, some are also released into the extracellular milieu, the relevance of which has previously not been studied. To address the role of adhesin release in pathogenesis, we used Bordetella pertussis as a model, since its major adhesin, filamentous hemagglutinin (FHA), partitions between the bacterial surface and the extracellular milieu. FHA release depends on its maturation by the specific B. pertussis protease SphB1. We constructed SphB1-deficient mutants and found that they were strongly affected in their ability to colonize the mouse respiratory tract, although they adhered even better to host cells in vitro than their wild-type parent strain. The defect in colonization could be overcome by prior nasal instillation of purified FHA or by coinfection with FHA-releasing B. pertussis strains, but not with SphB1-producing FHA-deficient strains, ruling out a nonspecific effect of SphB1. These results indicate that the release of FHA is important for colonization, as it may facilitate the dispersal of bacteria from microcolonies and the binding to new sites in the respiratory tract.
Keywords: Bordetella pertussis, filamentous hemagglutinin, maturation protease, colonization, adhesin
Introduction
The expression of virulence by bacterial pathogens often requires the production and action of toxins and adhesins. Whereas toxins are generally released by the pathogens into the extracellular milieu and can thus act at distant sites, adhesins typically remain associated with the bacterial surface, allowing the microorganisms to adhere to host determinants such as glycolipids, proteoglycans, surface, and matrix proteins (1–5).
More recently, it has become apparent that some bacterial adhesins are, in part, released from the cell surface into the extracellular milieu (6–11). However, adhesin release has essentially been observed in vitro. Whether it has any relevance for the expression of virulence, or whether it even takes place in vivo, in apparent contrast with adhesins' established functions, has not been addressed so far.
To investigate the significance of adhesin release during bacterial infection, we used Bordetella pertussis, the whooping cough agent, as a model. Respiratory epithelial cells and phagocytic cells are the primary targets for adherence by B. pertussis, which produces several adhesins thought to act synergistically in the colonization of the human respiratory tract (12, 13). One of the B. pertussis major adhesins, filamentous hemagglutinin (FHA),* partitions in vitro between the cell surface and the extracellular milieu (14–16). As a multifunctional attachment protein, it has several ligands, including lactosyl ceramides of ciliated respiratory cells (17, 18), sulphated sugars at the surface of epithelial cells and in the extracellular matrix (19), and two leukocyte integrins (20, 21). In addition, and similar to other adhesins in many other pathogenic microorganisms, surface-associated FHA induces auto-agglutination (22).
FHA derives from a 370-kD precursor called FhaB (14) and corresponds to the NH2-terminal two-thirds of the precursor. The maturation of FhaB is necessary for extracellular FHA release, and the protease responsible for this maturation has been identified recently and named SphB1 (23). SphB1 is a surface protein of the subtilisin superfamily (24), and the expression of its gene is coordinately regulated with that of the FHA structural gene (25, 26). In SphB1-deficient bacteria, virtually no FHA is released into the surrounding milieu, although large amounts of FHA-related polypeptides are found at the cell surface (23).
To assess the role of FHA release in B. pertussis colonization, we took advantage of the recently discovered “redundancy” between FHA and pertussis toxin (PTX) in a mouse model of colonization (27). B. pertussis strains lacking either PTX or FHA colonized the mouse respiratory tract nearly as efficiently as the isogenic parent strain, while mutants lacking both factors colonized substantially less well (27). In that model, colonization in the absence of FHA is not due to the adhesive properties of PTX, but to its ADP-ribosylation activity, causing a strong inflammation of the mouse respiratory tract and thus obscuring the role of FHA (27). In contrast, mutants deficient in PTX production induce only mild inflammation, and require FHA for murine respiratory tract colonization. We thus produced a B. pertussis double mutant, deficient for both SphB1 and PTX. The analysis of this strain in that murine respiratory model of infection indicated that the maturation of FhaB and the release of FHA into the extracellular milieu are necessary for efficient colonization.
Materials and Methods
Construction of B. pertussis Mutant Strains.
The B. pertussis strains used in this study were derived from BPSM (reference 28; Table I). BPRA harbors a deletion of the entire ptx operon and BPDR harbors deletions of the entire gene coding for FHA, fhaB, and of the entire ptx operon (27, 29). BPLC6 is a BPRA-derived sphB1 null mutant obtained by homologous recombination (30) as follows. The two gene fragments flanking a ∼600 bp internal region of sphB1 were inserted into pJQ200mp18rpsL (31) as described earlier (23). This construct was used for allelic exchange in BPRA, yielding BPLC6. BPLC8 and BPLC9 were obtained by genetic inactivation of the first gene of the ptx operon, ptx-S1, with the pFUS2 suicide vector as described previously (25). The oligonucleotides 5′-TATAAAGCTTAACAGCGCTTTCGTCTCCAC-3′ and 5′-TATAGGTACCCATGCAAGCGCCTATCACC-3′ were used to amplify the 5′ portion of the ptx-S1 gene of the PTX operon. The PCR product was digested by HindIII and KpnI and inserted into the same sites of pFUS2. The resulting vector was used to inactivate the ptx operon in BPLC7 (23) and BPLC5 (23) by homologous recombination, yielding respectively BPLC8 and BPLC9. These strains thus carry a gentamycin-resistant plasmid stably inserted into ptx-S1, with a polar effect on the rest of the operon.
Table I.
Relevant Genotypes and Phenotypes of the B. pertussis Strains Used in this Study and Their Parental Strains
| Phenotypes
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strains | Parental strains |
Genotypes | Released FHA |
Cell- associated FHA |
SphB1 | PTX | Marker | Reference | |
| PTX-proficient strains | BPSM | + | + | + | + | 28 | |||
| BPLC5 | BPSM | ΔsphB1 | − | + | − | + | 23 | ||
| BPLC7 | BPSM | sphB1S412→A | − | + | Inactive | + | 23 | ||
| PTX-deficient strains | BPRA | BPSM | Δptx | + | + | + | − | 29 | |
| BPDR | BPRA | Δptx, ΔfhaB | − | − | + | − | 27 | ||
| BPLC6 | BPRA | Δptx, ΔsphB1 | − | + | − | − | This study | ||
| BPLC8 | BPLC7 | sphB1S412→A, ptx-S1 :: pFUS2 | − | + | Inactive | − | G | This study | |
| BPLC9 | BPLC5 | ΔsphB1, ptx-S1 :: pFUS2 | − | + | − | − | G | This study | |
G, gentamycin resistance cassette.
The absence of PTX in culture supernatants was verified by immunoblotting using antibodies against the S1 subunit of PTX, as described (23). All B. pertussis strains were grown on Bordet-Gengou agar (BG) or in liquid modified Stainer-Scholte medium as described before (32). The culture media were supplemented with 100 μg/ml streptomycin or 10 μg/ml gentamycin where appropriate.
Protein Preparations and Analyses.
Proteins were prepared and analyzed as described (23). A mixture of the anti-FHA monoclonal antibodies F1, F4, and F5 was used for immunoblotting (23). The antiserum against SphB1 was described previously (23). FHA was purified from BPRA culture supernatants as described (33).
Flow Cytometry.
B. pertussis cells were prepared as described (23). The bacterial suspensions were incubated for one hour with the anti-FHA monoclonal antibodies 55.4G9 or 55.6C4 (34) diluted 50-fold in PBS containing 5% glycerol (PBSG). The bacteria were washed three times in PBSG and incubated at room temperature for 1 h with FITC-conjugated anti–mouse IgG (Dako) diluted 300-fold in PBSG. After three washes, bacteria-associated fluorescence was quantified by flow cytometry using an EPICS Elite cytometer (Beckman Coulter).
Cell Adherence Assay.
The human pulmonary epithelial cell line A549 and the murine alveolar macrophage-like cell line MH-S were cultured as described (27). For 2 d in 24-well plates, 2 × 105 cells per well were cultured. The bacterial strains were grown and labeled as described (27). Mammalian cells were washed once with RPMI 1640 (GIBCO BRL) before the addition of 4 × 106 35S-labeled bacteria per well and incubation for 1 h 30 min at 37°C with 5% CO2. After three washes with RPMI to remove nonadherent bacteria, the mammalian cells were lysed with 0.5% sodium dodecyl sulfate. Whole lysates were quantified by liquid scintillation counting. The experiments were performed three times independently in triplicate.
Intranasal Infection of Mice.
B. pertussis grown on BG agar plates was suspended in sterile PBS and adjusted to a concentration of ∼5 × 107 CFU/ml, 5 × 106 CFU/ml, or 5 × 105 CFU/ml depending on the experiments as indicated in the figure legends. Infections were performed by the intranasal route with 20 μl of the bacterial suspensions deposited into the nostrils of 4-wk-old female BALB/c inbred mice (Iffa Credo) lightly anesthetized with a cocktail of physiological water containing 20% (vol/vol) Imalgen 1000 (Merial), 10% Atropine (Aguettant), and 6% Valium (Roche), given intraperitoneally (150 μl per 20 g of body weight; reference 27). This mode of inoculation prevents swallowing of the inoculum, while causing no significant respiratory compromise (35). At the indicated time points, serial dilutions from individual lungs were plated onto BG agar, and the numbers of CFU were determined after 3 d of incubation at 37°C. Four BALB/c mice per time point and per group were assessed, and the experiments were performed at least twice independently. All animal studies were performed under the guidelines of the Institut Pasteur animal study board.
Statistical Analysis.
The results were analyzed using the unpaired Student's t test. Differences were considered significant at a P value of <0.05 for in vivo experiments and P < 0.01 for in vitro experiments.
Results
Construction and Characterization of B. pertussis Strains.
B. pertussis strains that differ with respect to the expression of fhaB and sphB1 were constructed by allelic exchange for use in a murine respiratory model. All the B. pertussis strains used in this study have a PTX− phenotype, because this model allows us to analyze specifically the role of FHA, which is otherwise obscured by the strong PTX-induced inflammation (27).
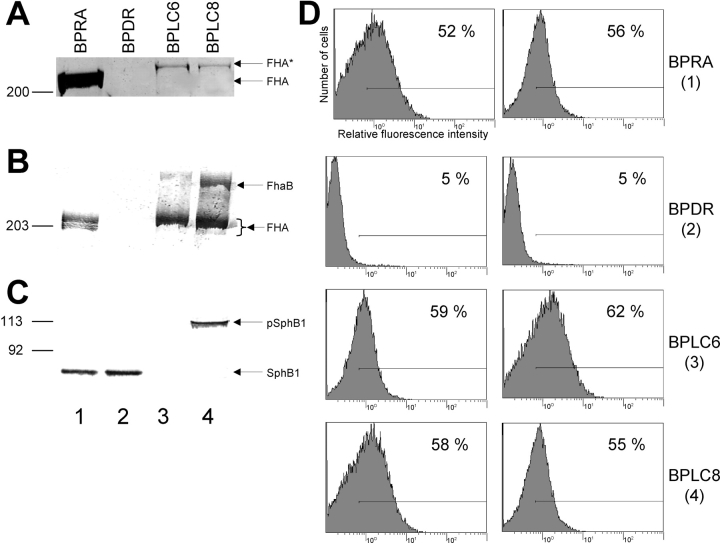
The parental strain, BPRA, both produces cell-associated FHA and releases substantial amounts of FHA into the extracellular milieu (Fig. 1, A and B , lane 1), whereas the FHA-deficient strain BPDR does not (Fig. 1, A and B, lane 2). Both strains produce SphB1 (Fig. 1 C, lanes 1 and 2), and BPRA presents surface-bound FHA, as evidenced by flow cytometry, whereas BPDR does not (Fig. 1 D, 1 and 2). The sphB1 null mutant BPLC6 produces no SphB1 (Fig. 1 C, lane 3) and does not release FHA into the extracellular milieu (Fig. 1 A, lane 3), although it produces cell-associated FHA-related polypeptides (Fig. 1 B, lane 3) that are surface-exposed (Fig. 1 D, 3). Only low amounts of an FHA-related protein 14 kD larger than FHA and resulting from an alternative maturation of FhaB by an undetermined protease (FHA* in Fig. 1 A, lane 3; reference 23) were found in culture supernatants of BPLC6. Similar to BPLC6, BPLC8, which produces inactive SphB1 because of the substitution of the catalytic serine by an alanine (Ser412→Ala; reference 23), produces cell-associated FHA-related polypeptides (Fig. 1 B, lane 4) that are surface-exposed (Fig. 1 D, 4) and only releases small amounts of FHA* into the extracellular milieu (Fig. 1 A, lane 4). As SphB1 is responsible for its own proteolytic maturation (23), only the precursor form of SphB1 was found in the BPLC8 cell extracts (Fig. 1 C, lane 4).
Figure 1.
Secretion of FHA and detection of cell-associated FHA and SphB1 in B. pertussis parental and mutant strains. (A) Identical volumes of nonconcentrated supernatants from each culture at the late logarithmic phase of growth were analyzed by SDS-PAGE using an 8% polyacrylamide gel. The gel was stained with Coomassie Blue. Only the gel portion corresponding to high molecular masses is shown. BPRA (FHA+ SphB1+), BPDR (FHA− SphB1+), BPLC6 (FHA+ SphB1−), and BPLC8 (FHA+ S412→A SphB1). FHA indicates the position of the mature protein and FHA* that of a slightly larger FHA-related polypeptide. (B) Cellular lysates were subjected to SDS-PAGE using a 6% polyacrylamide gel and immunoblotting with anti-FHA antibodies. FhaB and FHA represent the precursor and mature forms of FHA, respectively. (C) Cellular lysates were subjected to SDS-PAGE using an 8% polyacrylamide gel and immunoblotting with an anti-SphB1 antiserum. pSphB1 and SphB1 are the precursor and mature forms of the protease SphB1, respectively. (D) BPRA (1), BPDR (2), BPLC6 (3), and BPLC8 (4) were incubated with monoclonal anti-FHA antibodies 55.4G9 (left row) or 55.6.C4 (right row), followed by anti–mouse FITC conjugate. The fluorescent cells were detected by flow cytometry, with 20,000 events counted for each sample. The fluorescence threshold (left end of the horizontal bar) was set such that 98% of nonlabeled cells had intensities of autofluorescence below the threshold value. A representative experiment is shown, with percentages of fluorescent cells indicated in each panel.
Similar to BPLC6, BPLC9 produces no SphB1 and has cell-associated, surface-exposed FHA-related polypeptides (data not depicted), but in addition it carries a chromosomal resistance marker useful in coinfection experiments (see below). BPRA, BPDR, BPLC6, BPLC8, and BPLC9 do not produce PTX, as evidenced by immunoblotting of culture supernatants using antibodies against the S1 subunit of PTX (data not depicted).
The relevant features of the strains are summarized in Table I. The growth rates in the exponential phase of liquid cultures were similar for all strains, irrespective of the presence of the gentamycin marker or the SphB1 defect.
In Vitro Adherence of the Recombinant Strains.
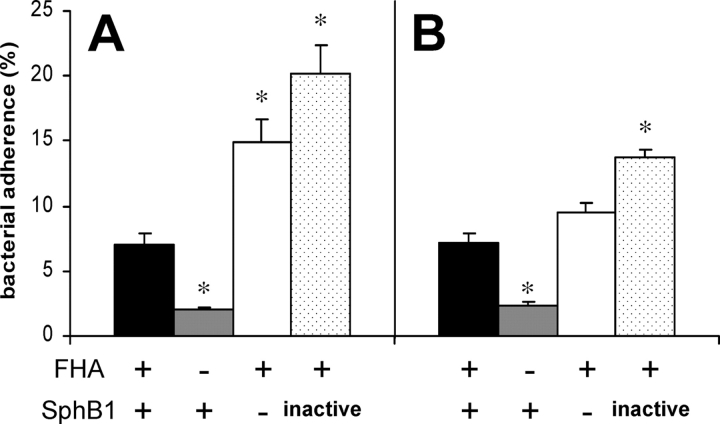
To determine whether the absence of FhaB maturation affects the adherence properties of B. pertussis, we compared the in vitro attachment of SphB1-deficient bacteria to eukaryotic cells, with those of FHA− and FHA+ isogenic strains, using the human pulmonary epithelial cell line A549 and the murine alveolar macrophage-like cell line MH-S (Fig. 2) . The absence of FHA significantly affected the adherence of B. pertussis to both cell lines, as reported previously (27). In contrast, the absence of SphB1 or the presence of the inactive protease appeared to actually increase the levels of in vitro attachment relative to those of parental BPRA (FHA+ SphB1+), indicating that SphB1-deficient B. pertussis presenting FHA-related polypeptides at the cell surface are competent for adherence to the host cells. Since surface-associated FHA has been shown to induce auto-agglutination (22), the increased levels of adherence of the SphB1-deficient strains might reflect the tendency to self-aggregation of these strains, as was observed in the late exponential phase of liquid cultures (data not depicted).
Figure 2.
Adherence of B. pertussis to cell lines in vitro. Human pulmonary epithelial cells A549 (A) and murine alveolar macrophage-like MH-S cells (B) were incubated with the indicated 35S-labeled bacteria at a multiplicity of infection of 20. After washing, adherence was assessed by scintillation counting. The results are expressed as percentages of counts per minute relative to the counts per minute present in the inoculum. Black bars, BPRA, gray bars, BPDR, white bars, BPLC6 and stippled bars, BPLC8. The data represent averages and standard deviations for triplicate experiments. *P < 0.01 relative to values obtained for BPRA.
Importance of FHA Release for Optimal Colonization by B. pertussis.
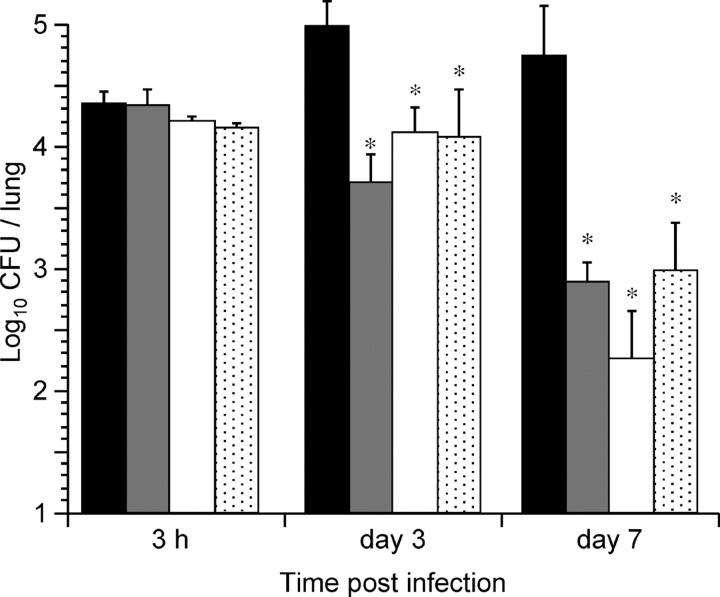
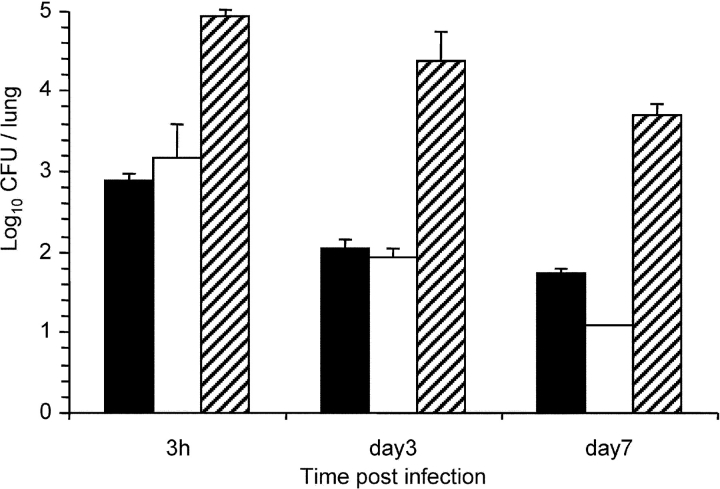
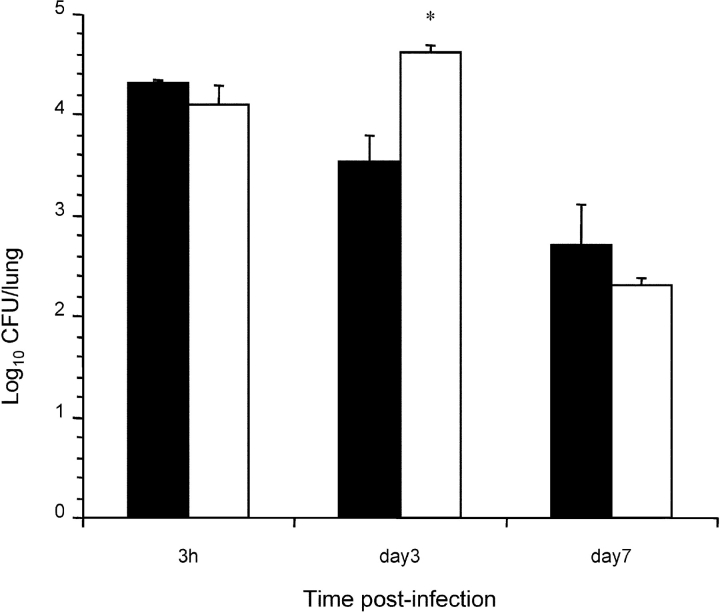
To assess the importance of FHA release for colonization in a mouse model of infection by B. pertussis, mice were infected intranasally with sphB1 null BPLC6, with BPLC8, producing enzymatically inactive SphB1, or with BPRA (FHA+) or BPDR (FHA−) used as controls. The numbers of CFU present in the lungs were determined 3 h, 3 d, and 7 d after infection. The lungs were chosen as endpoint because, compared with the tracheal colonization, the majority of the bacteria (∼90%) were found in the lungs with this mode of inoculation. After 3 d, BPRA had multiplied in the lungs, and it persisted at high numbers for at least 7 d after infection (Fig. 3) . In contrast, BPDR, BPLC6, and BPLC8 had not multiplied at day 3, and all three were cleared significantly faster than parental BPRA, as shown by the low numbers of bacteria recovered at day 7. The low levels of colonization in the absence of SphB1 or in the presence of inactive SphB1 suggest that the processing and release of FHA are necessary for optimal colonization of the mouse lungs by B. pertussis.
Figure 3.
Lung colonization by SphB1 mutant strains. Balb/c mice were infected intranasally with ∼105 CFU of B. pertussis BPRA (black bars), BPLC6 (white bars), BPLC8 (stippled bars), or BPDR (gray bars). At the indicated time points, mice were killed, and the viable bacteria present in the lungs were counted. Four mice were analyzed per time point for each group. *P < 0.05 relative to the BPRA values.
Role of Free FHA in Colonization by B. pertussis.
To rule out the possibility that the colonization defect of the SphB1-deficient bacteria arises from protease functions other than FHA maturation, cocolonization experiments were performed with the following rationale. If, as is the case for some mucosal pathogens, the protease degrades host proteins, which may facilitate colonization (36), bacteria producing SphB1 could provide this function and should thus enhance in trans colonization by protease-defective bacteria that present FHA-related polypeptides at their surface. If, on the other hand, it is the release of FHA that contributes to colonization, the addition of exogenously added purified FHA should enhance the colonization by protease-deficient bacteria.
Mice were therefore intranasally coinfected with SphB1-producing, FHA-deficient BPDR and with SphB1-deficient BPLC9, carrying a gentamycin-resistance marker to distinguish between the two coadministered strains. To detect a potential enhancing effect of SphB1 in trans, it was necessary to increase the persistence of BPDR by inoculating larger numbers. Under these conditions, at least 104 bacteria remained in the respiratory tracts of mice after several days, even though they did not multiply (Fig. 4 , striped bars). The colonization profile of BPLC9 observed in coinfected mice was similar to that observed when BPLC9 was administered alone, as evidenced by the absence of a multiplication peak at day 3 and significant clearance by day 7. The SphB1-producing strain BPDR did thus not enable the protease-deficient bacteria BPLC9 to colonize and multiply in mouse lungs, arguing against SphB1 having a major impact on colonization through its proteolytic activity on host tissue in vivo. Thus, SphB1 may instead have a role in the maturation and release of FHA in vivo.
Figure 4.
Lung colonization by B. pertussis BPLC9 in coinfection. Balb/c mice were infected intranasally with ∼104 CFU of B. pertussis BPLC9 (SphB1−; black bars), or coinfected with 104 CFU of B. pertussis BPLC9 and 106 CFU of B. pertussis BPDR (FHA− SphB1+). The white bars represent the numbers of CFU of BPLC9 (as distinguished by their resistance to gentamycin) in the lungs of coinfected mice, and the striped bars represent the numbers of BPDR in the coinfected mice.
To confirm the hypothesis that shed FHA may play an important role in colonization, mice were treated with 5 μg of purified FHA instilled intranasally and then immediately infected with BPLC6 (SphB1−). As a control, the same bacterial suspension was used to infect mice after an instillation of the same buffer without FHA. 3 d after infection, the numbers of bacteria recovered from the lungs of mice pretreated with FHA were 10-fold higher than those recovered from the lungs of nontreated mice (Fig. 5) . These results indicate that free FHA added exogenously enhanced the colonization of the mouse respiratory tract by B. pertussis strains that are unable to release their surface-associated FHA.
Figure 5.
Effect of an intranasal treatment with FHA on colonization by BPLC6. Balb/c mice were treated by an intranasal instillation of either 20 μl of PBS containing 0.5 M NaCl (PBS/NaCl) and 5 μg of purified FHA or 20 μl of PBS/NaCl, and then immediately infected intranasally with ∼5 × 104 CFU of B. pertussis BPLC6 (SphB1−). At the indicated time points, mice were killed, and the viable bacteria present in the lungs were counted. Black bars, numbers of BPLC6 after mock treatment, white bars, numbers of BPLC6 after the FHA treatment. *P < 0.05 relative to the numbers of BPLC6 CFU administered without FHA.
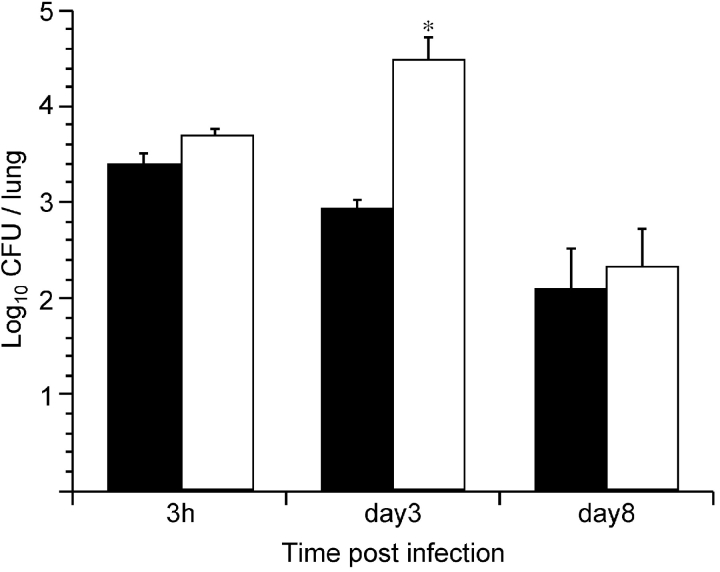
To support the role of free FHA in enhancing colonization by SphB1-deficient B. pertussis, mice were coinfected with BPRA (FHA+ SphB1+) and BPLC9 (FHA+ SphB1−), carrying a gentamycin-resistance marker. In this experimental setting, physiological amounts of FHA could be released by BPRA to help BPLC9 colonize. The coinfection with BPRA resulted in significantly enhanced colonization by BPLC9 compared with that observed in the absence of BPRA (Fig. 6) . This enhancement was conspicuous 3 d after infection, when the coinfecting parental strain has multiplied and secreted FHA. It is likely that the release of FHA by BPRA during the first days of infection leads to the dispersal of the bacteria from mixed microcolonies containing both BPRA and BPLC9. This in turn may facilitate the spread of the mutant bacteria and hence their persistence, as was observed at day 3. These data confirm that the maturation and release of FHA substantially contribute to colonization.
Figure 6.
Lung colonization by B. pertussis BPLC9 in coinfection. Balb/c mice were infected intranasally with ∼104 CFU of B. pertussis BPLC9 (SphB1−, black bars), or coinfected with 104 CFU of B. pertussis BPLC9 and 105 CFU of B. pertussis BPRA (FHA+ SphB1+). The white bars represent the numbers of CFU of BPLC9 (as distinguished by their resistance to gentamycin) in the lungs of coinfected mice. *P < 0.05 relative to the number of CFU of BPLC9 administered alone.
Discussion
Bacterial adhesins promote colonization at the initial stages of an infection by mediating attachment to host tissues, thus avoiding nonspecific host defenses such as mechanical clearance and allowing bacterial multiplication to occur within the host (1). To exert these functions, adhesins need to be presented at the surface of the bacterium. Like typical adhesins, B. pertussis' FHA attaches the bacterium to receptors in the respiratory tract (17–21, 28). However, in addition to being surface-associated, large amounts of FHA are also released into the extracellular milieu (15). This has so far only been observed in vitro. In this work, we show for the first time that FHA is likely to also be released in vivo, and that its secretion is necessary for efficient colonization in a mouse model of infection. Our results support the paradigm that the secreted form of bacterial adhesins may participate in pathogenesis.
The use of B. pertussis strains deficient in FHA release but presenting FHA polypeptides at their surface has shown that the maturation and release of FHA are of paramount importance in vivo. Bacteria that do not release FHA are defective in their ability to multiply and persist in the lungs of mice, despite their increased adhesiveness in vitro. This defect was apparent 3 d after infection, which corresponds to the peak of multiplication of wild-type bacteria under the same conditions. These observations indicate that the lack of FHA maturation and release enhances the clearance of the bacteria. Surface-associated FHA has been shown to cause auto-agglutination of B. pertussis (22). The exacerbation of this aggregative phenotype in SphB1-deficient bacteria as observed in vitro might be detrimental to colonization. Similarly, the highly adherent phenotype of these bacteria to respiratory cells and their inability to release FHA may be counter-productive for colonization. Therefore, a first role of adhesin release may be to facilitate the dispersal of bacteria from microcolonies and their detachment from epithelial surfaces.
The addition of free FHA or the coadministration of B. pertussis proficient in FHA release transiently enhanced the in vivo multiplication and persistence of bacteria that are unable to release their surface-bound FHA. Their enhanced persistence at day 3 is compatible with the notion of complementation in trans by exogenously added FHA or after coadministered bacteria had multiplied and secreted FHA. In the first experiment, exogenously added FHA may have contributed to the spread of the protease-deficient bacteria by facilitating their binding to the respiratory tract via homotypic FHA–FHA interactions, while limiting their aggregation. In the coinfection experiments, the persistence of the SphB1-deficient bacteria was transiently enhanced most likely because their dispersal in the respiratory tract was facilitated by the presence of wild type bacteria. This is consistent with the fact that in vitro the aggregation of SphB1-deficient bacteria generally observed in the late exponential phase of liquid cultures was markedly reduced in cocultures containing bacteria proficient for FHA release. In a similar manner, it is possible that mixed bacterial aggregates formed during the course of infection in vivo were dissociated by the effect of FHA release by wild-type bacteria. In a dynamic process such as colonization, the flexibility afforded by the partitioning of adhesins between the bacterial surface and the external milieu is an asset to facilitate the dispersal of bacteria and the spread of the infection throughout the respiratory tract.
Although the mouse model reproduces certain features of human whooping cough, such as lymphocytosis, and has been extensively used in many laboratories to tackle the pathophysiological issues of pertussis (37), it differs from the human model by the fact that in mice the infection occurs largely in the lungs, while in humans it is essentially confined to the upper airways. In spite of this difference, it is nevertheless possible that the release of FHA by B. pertussis may also promote colonization of the human respiratory tract by facilitating the dispersal of the bacteria. Unfortunately, this can only be addressed conclusively by human infection experiments using the FHA release mutants.
The kinetics of adhesin synthesis and the ability to shed adhesins are likely to both be important during infection. FHA is among the first adhesins participating in the colonization by B. pertussis (38). Accordingly, fhaB is among the first genes to be activated upon a shift from nonvirulent to virulent growth conditions in vitro (39, 40), and artificially delayed expression of fhaB by promoter replacement affects colonization (41). Similarly, it has been suggested that the time-dependent maturation of FHA may be important (6). Here, we extend these dynamics to the importance of FHA release for optimal colonization. Maximal FHA-mediated adherence may occur when the adhesin is cell-associated, which might be followed by its subsequent release into the external milieu to facilitate the detachment of bacteria from host cells it initially bound to and the spread to the surroundings. This may also be the case for other adhesins, as has been suggested for Hap of Haemophilus influenzae, a protein which also mediates both adherence to eukaryotic epithelial cells and bacterial aggregation during the initial stages of colonization. This adhesin undergoes auto-catalyzed maturation and release (7), which might facilitate H. influenzae spreading in the respiratory tract, although this could only be tested in vitro so far. A variation on this theme is exemplified by the type IV pili of Neisseria meningitidis and enteropathogenic Escherichia coli, which are responsible for the initial steps of colonization (42, 43). In both cases, the progression of the infection requires the loss of piliation, which is mediated by pilus retraction (42, 43).
In addition to mediating the attachment of bacteria to host tissues, adhesins, and in particular free adhesins, may have additional functions in the development of the infection. They may be biological effectors in vivo and thus influence the outcome of the host–pathogen interaction (44). In fact, FHA has recently been shown to affect the inflammatory and apoptotic responses (45, 46). Therefore, the release of certain adhesins by bacterial pathogens may contribute to infection through activities other than their strict adhesive functions, the discovery of which may open the way for new and effective interventions against infectious diseases.
Acknowledgments
We thank Jean Dubuisson for his critical reading of the manuscript.
L. Coutte is supported by the Ministère de l'Education Nationale, de la Recherche et de la Technologie, and F. Jacob-Dubuisson is a Researcher of the Centre National de la Recherche Scientifique. This work was supported in part by Institut National de la Sante et de la Recherche Medicale, the Institut Pasteur de Lille, and the Région Nord-Pas-de Calais.
L. Coutte and S. Alonso contributed equally to this work.
S. Alonso's present address is Microbiology and Immunology, 5173 Veterinary Medical Center, Cornell University, Ithaca, NY 14853.
Footnotes
Abbreviations used in this paper: BG, Bordet-Gengou; FHA, filamentous hemagglutinin; PTX, pertussis toxin, PBSG, PBS with 5% glycerol.
References
- 1.Beachey, E.H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachement of bacteria to mucosal surfaces. J. Infect. Dis. 143:325–345. [DOI] [PubMed] [Google Scholar]
- 2.Isberg, R.R., and G. Tran Van Nhieu. 1994. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 2:10–14. [DOI] [PubMed] [Google Scholar]
- 3.Rostand, K.S., and J.D. Esko. 1997. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadström, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223–233. [DOI] [PubMed] [Google Scholar]
- 5.Menozzi, F.D., K. Pethe, P. Bifani, F. Soncin, M.J. Brennan, and C. Locht. 2002. Enhanced bacterial virulence through exploitation of host glycosaminoglycans. Mol. Microbiol. 43:1379–1386. [DOI] [PubMed] [Google Scholar]
- 6.Arico, B., S. Nuti, V. Scarlato, and R. Rappuoli. 1993. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proc. Natl. Acad. Sci. USA. 90:9204–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink, D.L., L.D. Cope, E.J. Hansen, and J.W. St Geme. 2001. The Haemophilus influenzae Hap autotransporter is a chymotryspin clan protease and undergoes autoproteolysis via an intermolecular mechanism. J. Biol. Chem. 276:39492–39500. [DOI] [PubMed] [Google Scholar]
- 8.Finn, T.M., and L.A. Stevens. 1995. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol. Microbiol. 16:625–634. [DOI] [PubMed] [Google Scholar]
- 9.Stathopoulos, C., D.L. Provence, and R. Curtiss. 1999. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward, C.K., S.R. Lumbley, J.L. Latimer, L.D. Cope, and E.J. Hansen. 1998. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J. Bacteriol. 180:6013–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St. Geme, J.W., III, and S. Grass. 1998. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol. Microbiol. 27:617–630. [DOI] [PubMed] [Google Scholar]
- 12.Hewlett, E.L. 1997. Pertussis: current concepts of pathogenesis and prevention. Pediatr. Infect. Dis. J. 16:S78–84. [DOI] [PubMed] [Google Scholar]
- 13.Locht, C. 1999. Molecular aspects of Bordetella pertussis pathogenesis. Int. Microbiol. 2:137–144. [PubMed] [Google Scholar]
- 14.Domenighini, M., D. Relman, C. Capiau, S. Falkow, A. Prugnola, V. Scarlato, and R. Rappuoli. 1990. Genetic characterization of Bordetella pertussis filamentous hemagglutinin: a protein processed from an unusually large precursor. Mol. Microbiol. 4:787–800. [DOI] [PubMed] [Google Scholar]
- 15.Locht, C., P. Bertin, F.D. Menozzi, and G. Renauld. 1993. The filamentous haemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol. Microbiol. 9:653–660. [DOI] [PubMed] [Google Scholar]
- 16.Renauld-Mongénie, G., J. Cornette, N. Mielcarek, F.D. Menozzi, and C. Locht. 1996. Distinct roles of the N-terminal and the C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J. Bacteriol. 178:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomanen, E., H. Towbin, G. Rosenfelder, D. Braun, G. Larson, G.C. Hansson, and R. Hill. 1988. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J. Exp. Med. 168:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad, S.M., Y. Yin, E. Rodzinski, E.I. Tuomamen, and H.R. Masure. 1993. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect. Immun. 61:2780–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannah, J.H., F.D. Menozzi, G. Renauld, C. Locht, and M.J. Brennan. 1994. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect. Immun. 62:5010–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relman, D., E. Tuomanen, S. Falkow, D.T. Golenbock, K. Saukkonen, and S.D. Wright. 1990. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 61:1375–1382. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi, Y., S. Claus, and D.A. Relman. 1994. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18). J. Exp. Med. 180:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menozzi, F.D., P.E. Boucher, G. Riveau, C. Gantiez, and C. Locht. 1994. Surface-associated filamentous hemagglutinin induces autoagglutination of Bordetella pertussis. Infect. Immun. 62:4261–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutte, L., R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J. 20:5040–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siezen, R.J., and J.A.M. Leunissen. 1997. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6:501–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoine, R., S. Alonzo, D. Raze, L. Coutte, S. Lesjean, E. Willery, C. Locht, and F. Jacob-Dubuisson. 2000. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol. 182:5902–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locht, C., R. Antoine, and F. Jacob-Dubuisson. 2001. Bordetella pertussis, molecular pathogenesis under multiple aspects. Curr. Opin. Microbiol. 4:82–89. [DOI] [PubMed] [Google Scholar]
- 27.Alonso, S., K. Pethe, N. Mielcarek, D. Raze, and C. Locht. 2001. Role of ADP-ribosyltransferase activity of pertussis toxin in toxin-adhesin redundancy with filamentous hemagglutinin during Bordetella pertussis infection. Infect. Immun. 69:6038–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menozzi, F.D., R. Mutombo, G. Renauld, C. Gantiez, J.H. Hannah, E. Leininger, M.J. Brennan, and C. Locht. 1994. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect. Immun. 62:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoine, R., and C. Locht. 1990. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect. Immun. 58:1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stibitz, S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 235:458–465. [DOI] [PubMed] [Google Scholar]
- 31.Quandt, J., and M.F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene. 127:15–21. [DOI] [PubMed] [Google Scholar]
- 32.Locht, C., M.-C. Geoffroy, and G. Renauld. 1992. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 11:3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menozzi, F.D., C. Gantiez, and C. Locht. 1991. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol. Lett. 78:59–64. [DOI] [PubMed] [Google Scholar]
- 34.Leininger, E., S. Bowen, G. Renauld-Mongenie, J.H. Rouse, F.D. Menozzi, C. Locht, I. Heron, and M.J. Brennan. 1997. Immunodominant domains present on the Bordetella pertussis vaccine component filamentous hemagglutinin. J. Infect. Dis. 175:1423–1431. [DOI] [PubMed] [Google Scholar]
- 35.Dei-Cas, E., M. Brun-Pascaud, V. Bille-Hansen, A. Allaert, and E.M. Aliouat. 1998. Animal models of pneumocystis. FEMS Immunol. Med. Microbiol. 22:163–168. [DOI] [PubMed] [Google Scholar]
- 36.Travis, J., J. Potempa, and H. Maeda. 1995. Are bacterial proteinases pathogenic factors? Trends Microbiol. 3:405–407. [DOI] [PubMed] [Google Scholar]
- 37.Khelef, N., C.-M. Bachelet, B.B. Vargaftig, and N. Guiso. 1994. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutant deficient in adhesins or toxins. Infect. Immun. 62:2893–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mooi, F.R., W.H. Jansen, H. Brunings, H. Gielen, J.G.J. van der Heide, H.C. Walvoort, and P.A.M. Guinee. 1992. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb. Pathog. 12:127–135. [DOI] [PubMed] [Google Scholar]
- 39.Cotter, P.A., and V.J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519–565. [DOI] [PubMed] [Google Scholar]
- 40.Rappuoli, R. 1994. Pathogenicity mechanisms of Bordetella. Curr. Top. Microbiol. Immunol. 192:319–336. [DOI] [PubMed] [Google Scholar]
- 41.Kinnear, S.M., R.R. Marques, and N.H. Carbonetti. 2001. Differential regulation of Bvg-activated virulence factors plays a role in Bordetella pertussis pathogenicity. Infect. Immun. 69:1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bieber, D., S. Ramer, C.-Y. Wu, W.J. Murray, T. Tobe, R. Fernandez, and G.K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 280:2114–2118. [DOI] [PubMed] [Google Scholar]
- 43.Pujol, C., E. Eugene, M. Marceau, and X. Nassif. 1999. The meningococcal PilT protein is required for induction of intimate attachement to epithelial cells following pilus-mediated adhesion. Proc. Natl. Acad. Sci. USA. 96:4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoepelman, A.I.M., and E.I. Tuomanen. 1992. Consequences of microbial attachment: directing host cell functions with adhesins. Infect. Immun. 60:1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abramson, T., H. Kedem, and D.A. Relman. 2001. Proinflammatory and proapoptotic activites associated with Bordetella pertussis filamentous hemagglutinin. Infect. Immun. 69:2650–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mc Guirk, P., and K.H.G. Mills. 2000. Direct anti-inflammatory virulence factor: IL-10-dependent suppresion of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30:415–422. [DOI] [PubMed] [Google Scholar]