Abstract
Indirect evidence suggests that type-I interferons (IFN-α/β) play a significant role in the pathogenesis of lupus. To directly examine the contribution of these pleiotropic molecules, we created congenic NZB mice lacking the α-chain of IFN-α/βR, the common receptor for the multiple IFN-α/β species. Compared with littermate controls, homozygous IFN-α/βR-deleted NZB mice had significantly reduced anti-erythrocyte autoantibodies, erythroblastosis, hemolytic anemia, anti-DNA autoantibodies, kidney disease, and mortality. These reductions were intermediate in the heterozygous-deleted mice. The disease-ameliorating effects were accompanied by reductions in splenomegaly and in several immune cell subsets, including B-1 cells, the major producers of anti-erythrocyte autoantibodies. Decreases of B and T cell proliferation in vitro and in vivo, and of dendritic cell maturation and T cell stimulatory activity in vitro were also detected. Absence of signaling through the IFN-α/βR, however, did not affect increased basal levels of the IFN-responsive p202 phosphoprotein, encoded by a polymorphic variant of the Ifi202 gene associated with the Nba2 predisposing locus in NZB mice. The data indicate that type-I IFNs are important mediators in the pathogenesis of murine lupus, and that reducing their activity in the human counterpart may be beneficial.
Keywords: autoimmunity, hemolytic anemia, ifi202, B-1 cells, dendritic cells
Introduction
Type-I and -II IFNs, initially identified on the basis of their anti-viral properties, are now known to have multiple effects on the immune system, affecting differentiation, proliferation, and survival of B, T, and NK cells, macrophages, and dendritic cells (DCs),* and the production of, and signaling by, other cytokines (for reviews, see references 1 and 2). These molecules have, therefore, received considerable attention with regard to their roles in autoimmune diseases, including systemic lupus erythematosus (SLE).
The participation of IFN-γ, the only known type-II IFN and the main cytokine produced by Th1 cells, in lupus pathogenesis has been inferred in humans and unequivocally demonstrated in mice (for a review, see reference 3). The most significant findings are disease inhibition in (NZBxNZW)F1 and MRL-Fas lpr mice lacking the IFN-γ or IFN-γR genes (4–7), and in MRL-Fas lpr mice treated with either soluble recombinant IFN-γR (8) or an IFN-γR/Fc-encoding plasmid (9).
The role of type-I IFNs (IFN-α/β) in lupus has also been investigated, but these studies have been limited by the complexity of this family of molecules (∼15 IFN-α, 1 IFN-β) and the lack of broadly reacting Abs. Nonetheless, increased serum IFN-α levels in SLE patients correlating with disease activity has long been known (10), and IFN-α treatment of individuals with viral infections or tumors frequently resulted in SLE-like manifestations (11). The observation that Ab–DNA complexes stimulate production of IFN-α by plasmacytoid DCs, a cell population often found in SLE skin lesions and lymph nodes, is also potentially relevant (12). More importantly, a recent study (13) provided strong evidence that IFN-α in the serum of lupus patients is responsible for monocyte differentiation into antigen-presenting DCs, suggesting a major initiating mechanism of the autoimmune process. Experiments using mouse models of lupus that could further support the role of type-I IFNs in this disease are also limited. Early studies showed that the IFN-α/β inducers poly (I:C) accelerated autoimmunity in (NZBxNZW)F1 mice (14), and renal disease induced in normal mice by viral infection was inhibited with anti-IFN Abs (15). Of interest, IFN-α/β genes are located on chromosome 4 within the boundaries of several NZ lupus susceptibility loci, including Sle2, Nba1/Lbw2, Spm-1, and Aem-1 (for a review, see reference 16).
In this study, we directly evaluated the role of IFN-α/β in the lupus-like disease of NZB mice by creating congenics lacking IFNAR-1, the α-chain of the common receptor for type-I IFNs. Homozygous and even heterozygous IFN-α/βR–deleted mice had significantly reduced serologic, cellular, and histologic disease characteristics, conclusively demonstrating that type-I IFN are important mediators in this disease.
Materials and Methods
Mice.
Female New Zealand Black (NZB) and BALB/c mice were obtained from the Scripps Research Institute breeding colony (La Jolla, CA). Breeding of NZB mice lacking the α-chain of the IFN-α/βR (IFNAR-1, encoded by the Ifnar1 gene) was initiated at Genentech, Inc. by backcrossing with the previously described Ifnar1-deleted 129Sv mouse (17), and further backcrossed with NZB mice at Scripps. The Ifnar1 gene is located on the distal segment of chromosome 16, where no NZB disease-predisposing locus has been identified by genome-wide studies (for a review, see reference 16). Marker assisted selection was used to obtain mice homozygous for all the NZB predisposing Lbw loci on chromosomes 1, 4, 5, 7, 17, and 19 by the N4 generation. The studies presented herein used homozygous IFN-α/βR gene KO, heterozygous (HT), and WT littermate controls generated by intercrossing mice of at least the N6 generation of backcrosses.
Direct Coomb's Test.
Mice were bled bimonthly from 6 to 12 mo of age. RBCs were diluted 1/1,000 in PBS-1.5%BSA and 100 μl incubated in U-bottom plates with 10 μl of goat anti–mouse IgG (Southern Biotechnology Associates, Inc.) at a final concentration of 0, 1, and 10 μg/ml. After 2 h at 37°C, plates were centrifuged and wells scored for agglutination.
Serologic Analysis.
Serum levels of IFN-α before and after intraperitoneal injection of 100 μg poly (I:C; Sigma-Aldrich) were determined using an ELISA kit with a sensitivity of ∼5 pg/ml (PBL Biomedical Laboratories). Serum Igs and autoantibodies (anti-dsDNA and anti-ssDNA) were assessed by ELISA, as described previously (18). Microtiter plates were coated either with 5 μg/ml Fc-specific F(ab′)2 goat anti-mouse IgG (Jackson ImmunoResearch Laboratories), 5 μg/ml anti–mouse IgM (Southern Biotechnology Associates, Inc.), 25 μg/ml calf thymus dsDNA, or 25 μg/ml ssDNA. Total bound IgM or IgG, and IgG subclasses were measured by alkaline phosphatase-labeled goat anti–mouse Abs (Caltag Laboratories) and compared with a standard serum (Bethyl Laboratories).
ELISPOT.
The frequency of B cells secreting total IgM, total IgG, and anti-DNA Abs was determined as described (19).
Histology.
Autopsies and histologic examinations were performed at 12 mo of age, as detailed previously (20). Tissue sections were fixed in zinc formalin and stained with periodic acid-Schiff (PAS) or snap-frozen in OCT for immunofluorescence. Severity of glomerulonephritis (GN) was determined by analyzing >50 representative glomeruli graded blindly on a 0 to 4 scale: 0 = no pathology, 1 = minimal mesangial thickening, 2 = noticeable increases in both mesangium and glomerular cellularity, 3 = the preceding features plus inflammatory exudates and/or capsular adhesion, and 4 = obliteration of glomerular architecture involving >70% of glomeruli; a score ≥ 2.0 was considered pathologic. For immunohistochemistry, kidney sections were air-dried, fixed in ice-cold acetone, blocked sequentially with 10% horse or goat serum and an avidin/biotin blocking kit (Vector Laboratories), and incubated either with anti-IgG-FITC (Vector Laboratories), anti-CD3-biotin, anti-B220-biotin, anti-I-Ad-biotin, anti–ICAM-1 (all from BD Biosciences), anti-F4/80-biotin (Caltag Laboratories), anti-MCP-1 (Santa Cruz Biotechnology, Inc.) and, when required, with biotinylated secondary Abs (Jackson ImmunoResearch Laboratories). Sections were then incubated with streptavidin horseradish peroxidase (Vector Laboratories), developed with a peroxidase substrate AEC kit (Vector Laboratories), and counterstained with Mayer's hematoxylin.
Antibodies and FACS® Analysis.
mAbs to B220, Gr-1, CD43 (S7), IgD, CD21/35, CD4, CD8, CD5, CD23, CD44, CD69, CD25, CD11b, CD11c, CD40, CD80 (B7.1), CD86 (B7.2), I-Ad, H-2Kd, erythrocytes and precursors (TER-119), IFN-γ, and annexin-V (AV) were purchased from BD Biosciences. Abs to IgM and macrophages (F4/80) were from Caltag Laboratories. Cy5-conjugated streptavidin was from Jackson ImmunoResearch Laboratories, and biotin-conjugated anti-IgG from ICN Biomedicals. For cell surface staining, cells were incubated with various combinations of mAbs and analyzed on a FACSCalibur™ (Becton Dickinson). Calibration and color compensation were performed using fluorescent beads and single color controls, per standard procedures (Becton Dickinson). For intracellular staining of IFN-γ, spleen cells were first stimulated with PMA and ionomycin and then incubated with Brefeldin A to inhibit intracellular protein transport, as described (9). These cells were then stained with mAb to CD4 and CD8, fixed in formaldehyde, resuspended in Saponin buffer containing 1 μg anti-IFN-γ Ab, and analyzed by FACS®.
In Vivo and In Vitro Proliferations.
For in vivo assessments, mice received autoclaved drinking water containing 0.8 mg/ml BrdU (Sigma-Aldrich) prepared fresh every 2 d for 9 d, and spleen cells were stained for various surface-markers and then for BrdU incorporation (BrdU Flow Kit; BD Biosciences). For in vitro proliferation, spleen, or peritoneal cells were incubated for 72 h in complete medium containing either 5 μg/ml LPS (Sigma-Aldrich), 10 μg/ml anti-CD40 plus 1,000 U/ml IL-4 (both from BD Biosciences), 5 μg/ml ConA (Sigma-Aldrich), or 5 μg/ml anti-CD3 (BD Biosciences), and 3H-Thymidine incorporation was measured.
T Cell Homeostatic Proliferation.
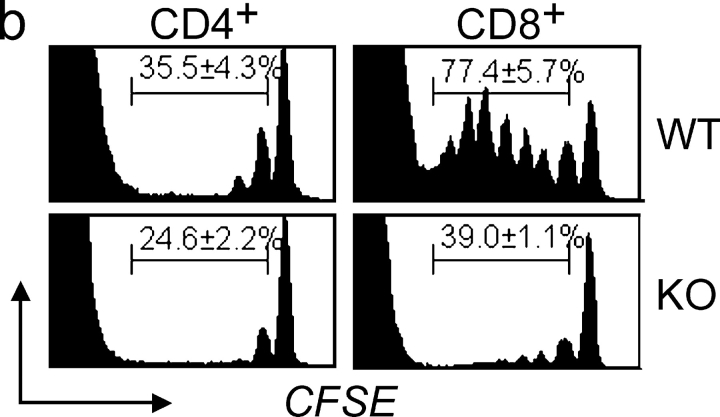
Carboxyfluorescein-diacetate-succinimidyl-ester (CFSE)-stained whole LN cells (5 × 106) from 2–3 mo-old mice were infused into age-matched NZB mice rendered lymphopenic by sublethal irradiation (600 rads) 1 d before (21). On days 3, 5, 8, and 14 after transfer, LN cells were stained with anti-CD4 and anti-CD8 Abs, and CFSE intensity profiles were defined by FACS®.
B Cell Apoptosis.
Spleen cells (106/ml) from 2-mo-old WT and KO mice were incubated with 10 μg/ml of purified F(ab′)2 goat anti–mouse IgM (Jackson ImmunoResearch Laboratories) for 18 h, and preapoptotic B220+ cells were detected by annexin-V staining (22).
DC Differentiation and Allogenic T Cell Proliferation.
A modified version of the technique described by Gallucci et al. (23) was used to derive DCs from bone marrow progenitors. Briefly, RBC-lysed bone marrow cell suspensions were incubated with biotin-conjugated mAbs to B220, CD4, and CD8 for 30 min at 4°C, washed in PBS-0.1%BSA, and negatively depleted by adding streptavidin-coated beads (Dynal). The purity of non-B, non-T cells was ≥98%. For in vitro differentiation of DCs, 5 × 106 cells were incubated in 5 ml RPMI-10% FCS medium supplemented with 3 ng/ml GM-CSF and 5 ng/ml IL-4 (both from BD Biosciences) for 6 d. On days 3 and 6, half of the supernatant was replaced with fresh media containing GM-CSF and IL-4. To induce final maturation, 1,000 U/ml IFN-α (Calbiochem) or 1 μg/ml LPS (Sigma-Aldrich) were added for another 24 h. Washed cells were stained with Abs to CD11c, CD40, CD80, CD86, I-Ad, and H-2Kd, and analyzed by FACS®. For allogenic mixed lymphocyte reaction, CD3+ T cells were purified from LN and spleen of CBA/J (H-2k) mice by FACS®. Triplicates of 105 T cells were then incubated with 104 or 3 × 104 bone marrow (BM) derived, IFN-α– or LPS-induced DCs from WT and KO NZB (H-2d) or normal control BALB/c (H-2d) mice for 3 d at 37°C, and 3H-Thymidine incorporation was measured.
Multiprobe RNase Protection Assay.
Riboprobes of defined length specific for cyclins (D1, D2, D3, E, A, B), cyclin-dependent kinases (CDK4, cdc2), cyclin-dependent kinase inhibitors (CDKI p16, p18, p19, p21), and a housekeeping gene (L32) were prepared and labeled with α-(32P)UTP (Riboprobe system; Promega) as we have described (24). Purified probes were hybridized to 2.5–5 μg total cellular RNA (RPA Kit I; Torrey Pines Biolabs), protected products run on 6% polyacrylamide sequencing gels, and intensity of bands measured by overnight exposure in a PhosphorImager (Amersham Biosciences). Results were expressed as percentage of the housekeeping L32 gene.
IFN-inducible 202 (p202) Protein Levels.
Immunoblotting for the Ifi202 gene-encoded p202 protein was performed as described (25), quantitated by densitometry, and results expressed as percent of actin protein levels.
Statistics.
Unpaired comparisons between groups were analyzed by the Student's t test, except for survival rates and serum (auto)Ab levels, respectively analyzed with the Kaplan-Maier log-rank test and the Mann-Whitney U test. P ≤ 0.05 was considered significant.
Results
Production of Type-I IFN.
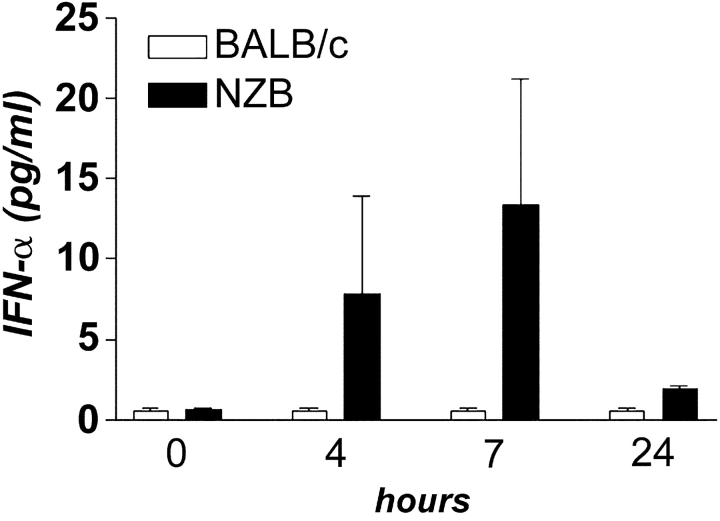
To begin evaluating the role of IFN-α/β in systemic autoimmunity, the frequency of splenic IFN-α/β–producing DCs and serum levels of IFN-α were determined in NZB and control BALB/c mice. The frequency of CD11c+CD8+CD4− DCs and CD11c+CD11b−B220+Gr-1+ DCs, thought to be the murine counterparts of human IFN-α/β-producing plasmacytoid DC (26, 27), was equivalent, and serum IFN-α was undetectable in both autoimmune and normal mice. However, while serum IFN-α remained undetectable in BALB/c mice after poly (I:C) injection, there was variable, but significant, induction in NZB mice (Fig. 1) . This result suggests that NZB mice may mount faster and/or stronger responses to microbial and other stimuli, resulting in higher type-I IFN production.
Figure 1.
Serum concentrations of IFN-α induced by poly (I:C). WT NZB (n = 3) and control BALB/c (n = 4) mice were bled before and, at different time points, after intraperitoneal injection of poly (I:C) and IFN-α levels were determined by ELISA.
Immunopathology.
To directly explore the role of IFN-α/β in lupus pathogenesis, we created NZB mice lacking a functional IFN-α/βR. Disease characteristics were dramatically reduced in these mice (Figs. 2 and 3) . First, KO mice had significantly greater survival than WT littermate controls (Fig. 2 a), i.e., by 12 mo, 45% of WT mice had succumbed compared with none of the KO mice (P = 0.015), while the mortality of heterozygous (HT) animals was intermediate (11%).
Figure 2.
Mortality rates and hemolytic anemia. (a) Cumulative survival of IFN-α/βR KO (n = 11), HT (n = 9), and WT (n = 9) NZB mice followed up to 12 mo; P = 0.015 for KO vs. WT; P > 0.05 for HT vs. WT. (b) Cumulative incidence of direct Coomb's test. Mouse groups were the same as in panel a. HT mice were examined at 12 mo only. (c) FACS® analysis of RBC-bound IgG autoantibodies in 10–12-mo-old mice. Each point represents the mean fluorescence intensity unit (FIU) from a single mouse and horizontal lines indicate the mean FIU; P = 0.004 for KO vs. WT; P = 0.04 for HT vs. WT mice. (d) Frequency of erythroblasts in spleens of 12-mo-old mice. RBC-depleted splenocyte suspensions were stained with an anti-erythroblast Ab (TER119) and analyzed by FACS®. Results of a representative mouse from each group are depicted. Numbers indicate percent of erythroblasts (mean ± SE) of 3–4 mice/group; P = 0.02 for KO or HT vs. WT mice; P = 0.05 for KO vs. HT.
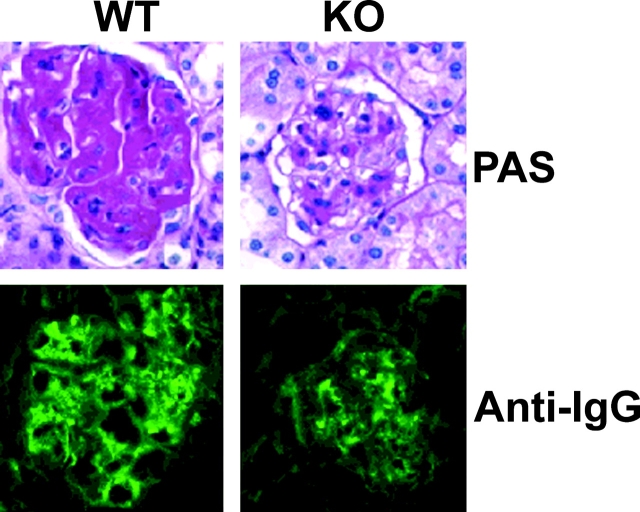
Figure 3.
Kidney immunohistology. Representative sections from 12-mo-old IFN-α/βR WT and KO NZB mice (n = 4 to 10) stained with PAS or FITC-conjugated anti–mouse IgG are shown.
Second, hemolytic anemia, the main pathological manifestation of NZB mice, was remarkably reduced in IFN-α/βR KO and, to a lesser degree, in HT mice, as shown by both incidence and levels of anti-RBC Abs. Thus, 33% of WT mice were positive by direct Coomb's test at 6 mo, 78% at 8 mo, and 100% at 10 mo, while no KO mice were positive at 8 mo, and only 27% at 10 and 12 mo (Fig. 2 b). Furthermore, the amount of IgG autoantibodies bound on the surface of RBCs was significantly lower in KO and HT mice at 10–12 mo (Fig. 2 c). The degree of compensatory extra medullary erythroblastosis, another indication of the severity of hemolytic anemia, was also significantly reduced (Fig. 2 d), i.e., WT mice had marked expansion of splenic erythroblasts (28%), while KO mice had only 5% (P = 0.02) and HT mice 9% at 12 mo.
Third, kidney disease, although generally not very severe in NZB mice, was clearly ameliorated in KO mice. Kidney sections of 12-mo-old KO mice revealed significantly lower glomerular scores (1.8 ± 0.3 vs. 2.5 ± 0.4, P < 0.05) and IgG deposits (Fig. 3) than age-matched WT littermates. Substantially reduced PAS-staining material in glomeruli and reduced mesangial proliferation were also observed. Immunohistochemical analysis indicated absence of B220+ B cells and CD3+ T cells in both types of mice. However, the frequency of infiltrating F4/80+ macrophages, and expression of MCP-1, class-II MHC, and intercellular adhesion molecule (ICAM)-1 were reduced in KO mice (data not depicted).
Polyclonal and Anti-DNA Ig Levels and Ab Forming Cells.
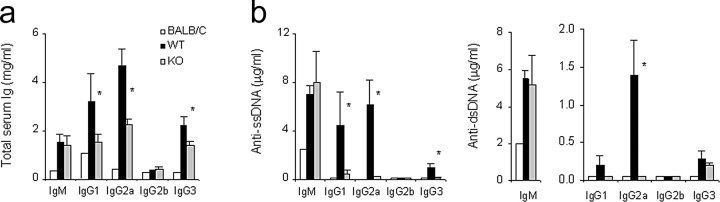
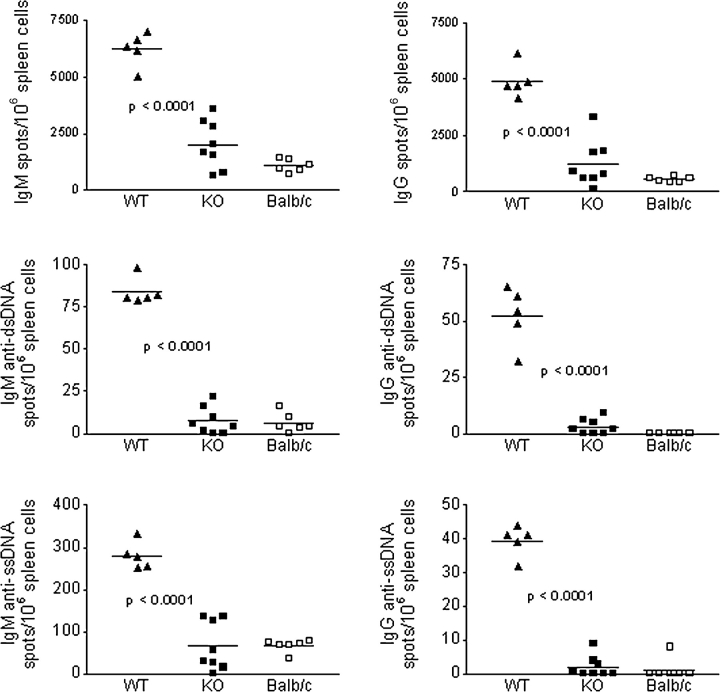
At 8 mo, KO and WT mice had similar serum levels of total IgM and IgM anti-ssDNA and anti-dsDNA Ab (Fig. 4 , a and b), although there were fewer spontaneous IgM-secreting polyclonal and anti-DNA Ab forming cells (AFCs) in the spleen of KO mice (Fig. 5) . The reasons for the nonconcordance between serum IgM levels and frequency of IgM-secreting cells in the KO mice remain to be clarified, but such a dissociation has been previously observed by us (unpublished observations) and others (28, 29), and attributed to independent B cell homeostasis and/or IgM catabolism. Polyclonal IgG subclasses were significantly lower in the serum of KO mice (Fig. 4 a), as were the levels of Abs to ssDNA (several subclasses) and dsDNA (primarily IgG2a; Fig. 4 b). Marked reductions in the frequency of spontaneous spleen AFC secreting total and anti-DNA IgG were also detected (Fig. 5).
Figure 4.
Serum concentrations of (a) polyclonal Ig and (b) anti-ss and dsDNA autoantibodies. BALB/c mice (a pool of 3 individuals) and IFN-α/βR WT (n = 8), or KO (n = 7) NZB mice were analyzed at 10–12 mo. Bars indicate mean ± SE; *P < 0.05.
Figure 5.
Frequency of splenic polyclonal Ig and anti-DNA AFCs. ELISPOT results for total IgM, total IgG, and corresponding anti-dsDNA or anti-ssDNA AFC in 12-mo-old BALB/c and IFN-α/βR WT or KO NZB mice are depicted. Data result from two separate experiments and each dot represents a single mouse. In all measurements, P < 0.0001 for WT vs. KO or BALB/c mice.
Spleen, BM, and Peritoneal Cell Populations.
IFN-α/βR deficiency was associated with a 2–3-fold decrease in the number of splenic T cells, B cells, and macrophages, consistent with the reduction of average spleen weights in 12 mo-old KO mice to about half that of WT animals (P < 0.002, Table I). The overall proportions of the various cell subsets were, however, unaffected, with a slight increase in the frequency of splenic F4/80+ macrophages being attributed to the reduction of erythroblasts. Also, there was no difference in the frequency of regulatory CD4+CD25+ cells, or activated/memory (CD44hi) splenic CD4+ and CD8+ T cells. Significantly, however, the frequency of activated splenic B cells (CD19+CD69+) in KO mice was reduced ∼2-fold compared with WT mice (14.5 ± 1.2% vs. 26.2 ± 5.0%, P = 0.002), consistent with decreases in both polyclonal and autoantibody IgG. At 2–3 mo of age, WT and KO mice were similar in phenotypic characteristics and percentages of marginal zone (CD23lowCD21high, 23 ± 1.9%) and follicular (CD23highCD21int, 42 ± 7.8%) B cells, i.e., KO mice retained the reduced CD23 expression and the expanded marginal zone B cell population typical of NZB mice (30). Similarly, BM analysis showed that B cell development was unaffected in IFN-α/βR KO mice, with no difference in the proportions of pro- (B220+ CD43+IgM−), pre- (B220+CD43−IgM−), or immature (B220+IgM+IgD−) B cells compared with WT mice (data not depicted). In contrast, splenic and, particularly, peritoneal B-1 cells, considered a major source of autoantibodies to RBC (31, 32), were significantly reduced in KO mice to levels similar to nonautoimmune BALB/c mice (Table I and Fig. 6) .
Table I.
Frequency and Number of Splenic Cell Subsets in BALB/c, IFN-α/βR WT, and KO NZB Mice
| Spleen
|
T cell subsets
|
B cell subsets
|
Macrophages
|
|||
|---|---|---|---|---|---|---|
| Mice | Weight | CD4+ | CD8+ | B-2 (CD5−) | B-1 (CD5+) | (F4/80+) |
| WT | 1,006 ± 203 | 185.9 ± 68.0 | 64.1 ± 19.2 | 144.8 ± 48.9 | 34.7 ± 8.7 | 62.3 ± 18.4 |
| (25.6 ± 4.3) | (9.1 ± 1.4) | (20.0 ± 2.3) | (5.5 ± 1.6) | 8.9 ± 0.9 | ||
| KO | 483 ± 34a | 58.1 ± 14.0a | 25.8 ± 6.7a | 40.7 ± 10.8a | 17.1 ± 4.0a | 26.9 ± 5.6a |
| (26.9 ± 1.5) | (11.7 ± 0.7) | (20.4 ± 4.6) | (8.7 ± 2.1) | (13.8 ± 1.8a) | ||
| BALB/c | 288 ± 33 | 78.2 ± 12.8 | 26.0 ± 4.8 | 112.4 ± 18.8 | 13.3 ± 3.5 | 14.6 ± 1.3 |
| (32.4 ± 3.3) | (10.5 ± 0.8) | (46.2 ± 4.2) | ( 5.0 ± 0.9) | (6.3 ± 0.5) | ||
Mean ± SE of spleen wet weight (mg), total number ×10−6 and percentage (in parentheses) of splenocyte subset. Data from 11–12-mo-old females are shown (n = 3–6 mice/group). Cells were stained with Abs to CD4, CD8, B220, CD5, and F4/80, then analyzed by flow cytometry (see Materials and Methods).
P < 0.05 for WT vs. KO.
Figure 6.
Frequency of peritoneal B-1 cells. Representative density plots of total peritoneal lymphocytes are shown, with numbers indicating percentages (mean ± SE) of gated B220+CD5+ cells in 12-mo-old IFN-α/βR WT and KO NZB mice (n = 3–6 mice/group); P = 0.01.
In Vitro and In Vivo Proliferation of Spleen and Peritoneal Cells.
In vitro proliferation of splenic B cells (mostly B-2) after LPS stimulation, and of peritoneal B cells (mostly B-1) after stimulation with either LPS or anti-CD40 plus IL-4, was significantly reduced in KO mice (Table II). Proliferation of splenic T cells stimulated with ConA or anti-CD3 was, however, equivalent in KO and WT mice. In vivo turnover (proliferation), assessed at 12 mo of age by long-term BrdU administration, was significantly reduced in KO mice for B-1, B-2, and CD8+ (but not CD4+) T cells (Fig. 7 a). Homeostatic T cell proliferation, mediated by recognition of self-MHC/peptide ligands (33) was also assessed by adoptive transfer of CFSE-stained LN cells from WT or KO mice into sublethally-irradiated (lymphopenic) WT NZB hosts. Marked reductions in the percentage of KO CD8+ T cells and, to a lesser extent, CD4+ T cells, that had undergone one or more divisions compared with WT T cell subsets, were observed (Fig. 7 b).
Table II.
In Vitro Proliferation of Splenocytes and Peritoneal Cells from IFN-α/βR WT and KO NZB Mice
| Splenocytes
|
Peritoneal cells
|
||||
|---|---|---|---|---|---|
| Mice | LPS | Con A | Anti-CD3 | LPS | Anti-CD40/IL-4 |
| WT | 35.6 ± 1.4 | 22.9 ± 11.2 | 122.4 ± 6.7 | 23.1 ± 2.4 | 95.8 ± 5.1 |
| KO | 23.2 ± 3.7a | 21.6 ± 8.8 | 104.7 ± 17.7 | 9.8 ± 4.6a | 52.1 ± 5.5a |
Mean ± SE of cpm ×10−3 are shown for three 10-wk-old mice/group.
P < 0.05 for WT vs. KO.
Figure 7.
In vivo lymphocyte proliferation. (a) Spontaneous proliferation of splenic B and T cell subsets in 12 mo-old BALB/c, IFN-α/βR WT, and KO mice (n = 2–3/group) defined by BrdU-incorporation. Representative histograms and percentage of BrdUhi cells (mean ± SE) are shown. (b) Lymphopenia-induced homeostatic T cell proliferation. LN cells from 2–3 mo-old WT and KO mice were labeled with CFSE and infused into sublethally-irradiated WT NZB recipients (n = 3/group). Representative histograms at 8 d post-transfer and percentage (mean ± SE) of cells that had undergone 1 to 7 divisions are shown, with peaks of decreasing CFSE-intensity identifying sequential cellular divisions. Reduced in vivo proliferation of KO T cells was also observed 5 and 14 d after transfer (data not depicted).
IFN-γ–producing T Cells.
Signaling via the IFN-α/βR is thought to promote induction of IFN-γ (1). Therefore, we investigated whether deletion of this receptor led to decreases in IFN-γ-producing T cells. Indeed, KO mice had a significant reduction in IFN-γ–expressing CD4+ (10.6 ± 2.8% vs. 20.4 ± 1.7%, P = 0.01) and CD8+ (18.1 ± 2.1% vs. 36.0 ± 4.7%, P = 0.003) T cells compared with WT mice.
Anti-IgM–mediated Apoptosis.
Previous studies indicated that NZB B cells are defective in BCR-mediated apoptosis (22). Because IFN-α/β were shown to inhibit B cell apoptosis (34–36), we investigated whether this apoptosis defect was corrected in the IFN-α/βR KO mice. Susceptibility to apoptosis induced by IgM-cross-linking was, however, equivalent for KO and WT B cells.
DC Maturation and Function.
IFN-α in human lupus sera was recently shown to accelerate differentiation and maturation of monocytic cells into antigen-presenting DCs, an effect proposed to play a major role in this disease (13). We examined the effect of IFN-α on NZB DC maturation and, as expected, WT, but not KO, BM-derived DCs cultured with recombinant IFN-α up-regulated significantly B7.1, B7.2, and class-I MHC, marginally class-II MHC (Fig. 8a) , and not CD40. Consequently, the IFN-α treated WT DCs induced stronger allogenic T cell responses than similarly-treated KO DCs (Fig. 8 b). It is notable, however, that even without exogenous IFN-α, WT DCs were more efficient in this assay than KO DCs, suggesting that sufficient maturation occurred in response to endogenous IFN-α/β, produced at basal levels and/or secondarily induced during the alloresponse. Interestingly, differences between WT and KO DCs were also evident after in vitro stimulation with LPS, further suggesting the importance of IFN-α/βR-signaling in DC activity (Fig. 8 b). IFN-α- or LPS-treated BALB/c DC showed equivalent expression of costimulatory molecules (data not depicted) and allostimulation (Fig. 8 b) to those of WT NZB mice.
Figure 8.
Maturation and function of DCs. (a) Effect of IFN-α on DC maturation. BM cells from IFN-α/βR WT and KO NZB mice were incubated with GM-CSF and IL-4 for 6 d, then with or without IFN-α for another 24 h to induce final maturation. The frequency of cells that had up-regulated MHC and B7 costimulatory molecules was determined by FACS® on gated live, CD11c+ cells. Basal levels (horizontal lines) for MHC class II, B7.1, and B7.2 were set on the basis of parallel stainings in the absence of the specific antibodies, and for MHC class I on the basis of constitutive expression defined with specific antibody. Percentages are the mean ± SE of three separate experiments with pooled cells from 3 mo-old mice (n = 2–4/pool); *P = 0.05. (b) DCs (3 × 104) from IFN-α/βR WT, KO, and BALB/c mice, derived as described in panel a using either medium, IFN-α or LPS, were incubated with allogenic T cells (105) for 3 d and proliferative responses determined by H3-thymidine incorporation. Patterns of allostimulation remained similar when lower numbers (104) of DCs were used (data not depicted). Results are the mean ± SE of three separate experiments; *P < 0.05.
Cell Cycle–related Genes.
Previous reports indicated that IFN-α/β can regulate cell-cycle protein expression and mediate G1 arrest by inducing p21 and p15 genes (for a review, see reference 37). Therefore, the effect of IFN-α/βR deficiency on the expression of several cell cycle-related genes was assessed by a multiprobe RNase protection assay. With the exception of a significant reduction of the CDKI p21 in KO mice (10.4 ± 2.1% vs. 17.4 ± 1.1%, P = 0.02), transcript levels for all other tested genes were equivalent in WT and KO mice.
Interferon-inducible p202 Expession.
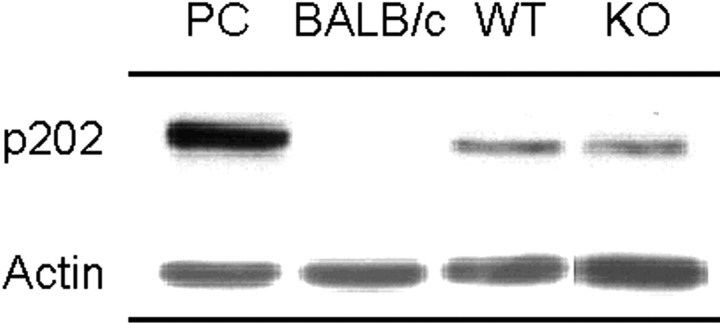
An allelic polymorphism in the promoter region of the IFN-inducible gene, Ifi202, which results in high RNA and protein levels of p202 in B cells (and other non-B, non-T cells), has recently been implicated as a candidate lupus susceptibility gene for NZB mice (25). Although spleen cells from WT NZB mice indeed showed higher p202 levels than nonautoimmune BALB/c mice, these high levels were retained in IFN-α/βR KO NZB mice (Fig. 9) , thereby indicating that signaling through this receptor is not required for high expression of the Ifi202. Of note, BALB/c mice carry the same Ifi202 promoter polymorphism as NZB mice (25), and have been reported to show increased transcription of this gene after stimulation with IFN-inducers (38). Our results, however, showed low basal p202 protein expression in BALB/c mice, thereby supporting a posttranslational regulation of this gene (39).
Figure 9.
Expression of Ifi202-encoded p202. Protein extracts from spleens of BALB/c, IFN-α/βR WT, and KO NZB mice (n = 3) were analyzed by immunoblotting using an anti-p202 polyclonal antiserum. Representative results are shown. Expression levels (mean ± SE) of p202, calculated as percent of actin expression, were 13.6 ± 3.4% for WT mice, 12.2 ± 1.7% for KO mice, and 0.7 ± 0.2% for BALB/c mice. PC, positive control (recombinant p202).
Discussion
The present study with NZB mice lacking a functional IFN-α/βR directly demonstrates the long-suspected role of IFN-α/β as major effectors in the pathogenesis of systemic autoimmunity. IFN-α/βR–deficient NZB mice had significantly reduced serologic and histologic disease characteristics, including anti-erythrocyte autoantibodies and hemolytic anemia, as well as anti-DNA autoantibodies, kidney disease, and mortality. Reflecting the pleiotropic functions of IFN-α/β, these protective effects were associated with multiple cellular modifications, including reduced splenomegaly and numbers of B-1 and B-2 cells, T cells, macrophages, autoantibody-secreting B cells, and IFN-γ–secreting T cells. Proliferative in vitro and in vivo responses of B and T cells were also decreased, as were the in vitro maturation and T cell stimulatory capacities of DCs.
The major clinical characteristic of NZB disease is hemolytic anemia (40). Anti-erythrocyte autoantibody titers and erythroblastosis were significantly reduced in the KO mice and, to a lesser degree, in HT mice, suggesting that even partial ablation of IFN-α/β signaling is beneficial. Previous studies have shown that the B-1 subset is the primary cell type for the production of anti-erythrocyte autoantibodies (31, 32). As reaffirmed here, WT NZB mice have increased B-1 cells in both the peritoneal cavity and spleen, but these cells were significantly decreased in KO mice, especially in the peritoneal cavity. Correspondingly, in vivo proliferation of splenic B-1 cells and in vitro proliferation of peritoneal cells stimulated with either LPS or anti-CD40 plus IL-4, were reduced. Thus, IFN-α/β signaling appears to significantly affect the B-1 subset. Whether this signaling is acting directly or indirectly through the induction of other cytokines/chemokines in the generation, self-renewal, or survival of the B-1 subset, remains to be determined. Several cytokines and chemokines have been shown to affect B-1 cell numbers, homing and differentiation, including IFN-γ, IL-5, IL-10, IL-12, B lymphocyte chemoattractant (CXCL13), and stromal cell-derived factor-1 (CXCL12; references 41–45). Some of these mediators, particularly IL-12 and IFN-γ, as well as IL-5 and IL-10, can be induced, and their signaling magnified, by IFN-α/β (1, 2, 46, 47).
Conventional B-2 cells and Ig secreting cells were also decreased in the spleen of KO mice, consistent with reductions in polyclonal and autoantibody Ig, as well as kidney immune deposits and pathology. These findings are compatible with the reported ability of type-I IFNs to potently enhance humoral immunity and to promote isotype switching by DC stimulation in vivo (48). Decreases in B-2 cells could be attributed to defective lymphogenesis, enhanced apoptosis, and/or decreased proliferation. Defective lymphogenesis appears unlikely since KO mice had similar numbers of B cell precursors as WT mice, and development of pro-B cells and their progeny was previously shown to be reduced at high, rather than low, IFN-α/β levels (49). Increased B cell apoptosis was also considered, as IFN-α/β inhibit this process, presumably by up-regulating Bcl-2 and Bcl-XL and/or activating NF-κB (34–36). This possibility also appears unlikely, because the resistance of WT NZB B cells to anti-IgM–induced apoptosis was not corrected in the KO mice. Therefore, the most likely possibility is that the decline in B cell numbers is caused by reduced antigen receptor-mediated proliferative responses, known to be enhanced by IFN-α/β (36).
Significant decreases in splenic T cells, especially in the CD8+ subset, were also observed in KO mice. Although CD4+ T cells are thought to be the primary subset involved in lupus autoimmune responses, studies in β2-microglobulin-deleted NZB mice suggested that CD8+ T cells may also contribute, particularly with regard to the anti-erythrocyte response (50). The decline of CD8+ cells in the KO mice may be due to defects in their development, decreased proliferation, and/or increased apoptosis. Impaired development of CD8+ cells may result from reduced expression of the IFN regulatory transcription factor-1 (IRF-1), as mice deleted of this gene showed defective CD8+ cell maturation (51, 52). It is of interest that deletion of IRF-1 confers resistance to CD8+ cell-mediated autoimmune diseases (53, 54) while, conversely, deletion of IRF-2, an attenuator of IRF-1 and IFN-α/β signaling, leads to development of an inflammatory skin disease (55). Although type-I IFNs are known to exert anti-proliferative effects (1), in vivo proliferation of CD8+ T cells in the KO mice was also decreased, as measured by both BrdU incorporation and lymphopenia-induced homeostatic expansion. It should be noted, however, that the frequency of proliferating cells defined by these assessments is a reflection of the balance between dividing and apoptosing cells. Recent studies have shown that IFN-α/β directly inhibit apoptosis of activated CD8+ (and CD4+) T cells without the need of other cytokines nor by raising the levels of Bcl-2 or Bcl-XL (56). Moreover, it has been reported that survival and proliferation of activated/memory CD8+ cells in vivo is significantly enhanced by type-I IFNs or their inducers (LPS, CpG DNA, poly I:C), an effect attributed to production of IL-15 by APCs (57, 58). In addition, efficient activation of naive CD8+ T cells was shown to be dependent on IFN-α/β–induced CXCR3 chemokine signalling (59). In this context, the decline in p21 in T cells of the KO mice may also promote apoptosis (24, 37). Indeed, we have recently shown that p21-deleted lupus-predisposed BXSB mice have reduced accumulation of replication- and apoptosis-resistant activated T cells, accompanied by disease amelioration (unpublished data).
The absolute number of macrophages was also reduced in the KO mice, which might lead to less efficient antigen presentation and, more importantly, decreased participation of these cells in end-organ inflammatory processes. The mechanism causing reduced numbers of macrophages in the KO mice is unclear but, again, a likely possibility is decreased expression of certain IRFs, such as IRF-1 and IRF-8, known to exert a major influence on macrophage maturation and function as well as production of IL-12 and iNOS (60).
Promoting DC maturation is a major mechanism by which IFN-α/β may contribute to lupus pathogenesis (13). As expected, incubation of immature, bone marrow–derived DCs from WT, but not KO, NZB mice with recombinant IFN-α significantly enhanced expression of the costimulatory molecules CD80 and CD86, and of class I MHC. Correspondingly, WT DCs induced stronger in vitro T cell alloresponses than KO DCs. It is of interest, however, that the stimulatory capacity of WT DCs was equivalent irrespective of whether they had been cultured with or without exogenous IFN-α, presumably because the levels of endogenous IFN-α/β were sufficient to induce appropriate expression of costimulatory and MHC molecules. Interestingly, even after stimulation with LPS, the alloresponses induced by WT DCs were more efficient than those by KO DCs. This finding suggests that induction of IFN-α/β by stimuli of the innate immune system may promote autoimmune responses through enhanced self-antigen presentation and engagement of nontolerant, low affinity, autoreactive T cells.
Several predisposing loci have been identified for NZB and other lupus strains but, with the exception of a few, the exact nature of the associated genes is unknown (16, 61, 62). The Nba2/Lbw7 locus on chromosome 1 of NZB mice, predisposing to autoantibody production, splenomegaly, and GN, has recently been linked to a polymorphism in the promoter region of the IFN-inducible Ifi202 gene, leading to increased p202 expression in B cells and other non-B, non-T cells (25). The Ifi202 is a family of two genes, Ifi202a and Ifi202b, and both appear to contribute to the high p202 levels in NZB mice (unpublished data). The p202 phosphoprotein is thought to affect cell-signaling pathways by binding several transcription factors and inhibiting cell proliferation and apoptosis (39), but the exact mechanism by which it predisposes to systemic autoimmunity remains to be elucidated. Although the Ifi202 polymorphism is not confined to the NZB strain, backcross studies have suggested that increased p202 levels contribute to disease in NZB and (NZBxNZW)F1 mice (25). We confirmed the high expression of p202 in NZB splenocytes, but found that high expression was maintained in the absence of IFN-α/βR, suggesting that signaling through this receptor is not required for Ifi202 activation. It is conceivable, however, that the two Ifi202 genes may be affected differently by IFN-α/βR signaling and studies are in progress to address this question. In addition, previous investigations have suggested that IFN-γ and other factors can also induce p202 (39). Nonetheless, the findings indicate that the adverse effects mediated by the Nba2-associated Ifi202 allele (and other predisposing loci) are attenuated in the absence of IFN-α/βR signaling.
The present results clearly indicate that absence of IFN-α/βR significantly reduces systemic autoimmunity. Although this effect may be a direct consequence of ablating the IFN-α/β activities, it is conceivable that absence of this receptor may also reduce the adverse effects of IFN-γ in this disease. This possibility stems from the findings that IFN-α/β induce IFN-γ production (1), and that a week IFN-α/βR stimulation by spontaneously produced IFN-α/β is necessary for efficient IFN-γ signaling (2). The latter effect appears to be mediated by a physical association between tyrosine-phosphorylated IFNAR-1 and IFNGR-2 receptor subunits at the caveolar membrane domains, thereby providing docking sites for efficient STAT-1 dimerization and IFN-stimulated gene factor 3 (ISGF3) formation (63). The present finding of decreased frequency of IFN-γ–producing T cells and of IgG2a autoantibody levels in the IFN-α/βR KO mice are compatible with this concept, and suggests that therapeutic interventions to inhibit signaling through the IFN-α/βR may decrease the pathogenic effects of both type-I and -II IFNs in lupus.
This and other studies establish that IFNs are key molecules in the pathogenesis of autoimmune diseases, but their effects may be detrimental or beneficial depending on the specific clinical entity. Thus, IFN-γ promotes lupus (5–9) and myasthenia gravis (64, 65) but inhibits EAE (66, 67), whereas IFN-α/β promotes lupus (reference 13, and present study) and IDDM (68) but inhibits myasthenia gravis (69, 70), EAE, and multiple sclerosis (71). Understanding the differences in the autoimmune disease-modifying activities of IFNs and other cytokines will certainly have a considerable impact in further dissecting the etiologies and pathways of these syndromes and in developing new treatments.
Acknowledgments
The authors would like to thank Kat Occhipinti-Bender for editorial assistance, and Vanessa Clifton, Lynn Simpson, and Wesley Scott for technical support.
This work was supported by National Institutes of Health grants AR39555 and AR31203. This is publication number 15431-IMM from the Department of Immunology, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037.
Footnotes
Abbreviations used in this paper: AFC, Ab forming cell; BM, bone marrow; CFSE, carboxyfluorescein-diacetate-succinimidyl-ester; DC, dendritic cell; IRF-1, IFN regulatory transcription factor-1; PAS, periodic acid-Schiff; SLE, systemic lupus erythematosus.
References
- 1.Biron, C.A. 2001. Interferons alpha and beta as immune regulators–a new look. Immunity. 14:661–664. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi, T., and A. Takaoka. 2001. A weak signal for strong responses: interferon-alpha/beta revisited. Nat. Rev. Mol. Cell Biol. 2:378–386. [DOI] [PubMed] [Google Scholar]
- 3.Theofilopoulos, A.N., S. Koundouris, D.H. Kono, and B.R. Lawson. 2001. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 3:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng, S.L., J. Moslehi, and J. Craft. 1997. Roles of interferon-gamma and interleukin-4 in murine lupus. J. Clin. Invest. 99:1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balomenos, D., R. Rumold, and A.N. Theofilopoulos. 1998. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J. Clin. Invest. 101:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas, C., B. Ryffel, and M. Le Hir. 1998. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB x NZW)F1 mice. J. Immunol. 160:3713–3718. [PubMed] [Google Scholar]
- 7.Schwarting, A., T. Wada, K. Kinoshita, G. Tesch, and V.R. Kelley. 1998. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J. Immunol. 161:494–503. [PubMed] [Google Scholar]
- 8.Ozmen, L., D. Roman, M. Fountoulakis, G. Schmid, B. Ryffel, and G. Garotta. 1995. Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferon-gamma receptor inhibits the onset of glomerulonephritis. Eur. J. Immunol. 25:6–12. [DOI] [PubMed] [Google Scholar]
- 9.Lawson, B.R., G.J. Prud'homme, Y. Chang, H.A. Gardner, J. Kuan, D.H. Kono, and A.N. Theofilopoulos. 2000. Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J. Clin. Invest. 106:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preble, O.T., R.J. Black, R.M. Friedman, J.H. Klippel, and J. Vilcek. 1982. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science. 216:429–431. [DOI] [PubMed] [Google Scholar]
- 11.Ioannou, Y., and D.A. Isenberg. 2000. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 43:1431–1442. [DOI] [PubMed] [Google Scholar]
- 12.Ronnblom, L., and G.V. Alm. 2001. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 22:427–431. [DOI] [PubMed] [Google Scholar]
- 13.Blanco, P., A.K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 294:1540–1543. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter, D.F., A.D. Steinberg, P.H. Schur, and N. Talal. 1970. The pathogenesis of autoimmunity in New Zealand mice. II. Acceleration of glomerulonephritis by polyinosinic-polycytidylic acid. Lab. Invest. 23:628–634. [PubMed] [Google Scholar]
- 15.Gresser, J., L. Morel-Maroger, P. Verroust, Y. Riviere, and J.C. Guillon. 1978. Anti-interferon globulin inhibits the development of glomerulonephritis in mice infected at birth with lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. USA. 75:3413–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kono, D.H., and A.N. Theofilopoulos. 2000. Genetics of systemic autoimmunity in mouse models of lupus. Int. Rev. Immunol. 19:367–387. [DOI] [PubMed] [Google Scholar]
- 17.Muller, U., U. Steinhoff, L.F. Reis, S. Hemmi, J. Pavlovic, R.M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 18.Burlingame, R.W., R.L. Rubin, R.S. Balderas, and A.N. Theofilopoulos. 1993. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self antigen. J. Clin. Invest. 91:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedgwick, J.D., and P.G. Holt. 1983. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J. Immunol. Methods. 57:301–309. [DOI] [PubMed] [Google Scholar]
- 20.Andrews, B.S., R.A. Eisenberg, A.N. Theofilopoulos, S. Izui, C.B. Wilson, P.J. McConahey, E.D. Murphy, J.B. Roths, and F.J. Dixon. 1978. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J. Exp. Med. 148:1198–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst, B., D.S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 22.Kozono, Y., B.L. Kotzin, and V.M. Holers. 1996. Resting B cells from New Zealand black mice demonstrate a defect in apoptosis induction following surface IgM ligation. J. Immunol. 156:4498–4503. [PubMed] [Google Scholar]
- 23.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 24.Sabzevari, H., S. Propp, D.H. Kono, and A.N. Theofilopoulos. 1997. G1 arrest and high expression of cyclin kinase and apoptosis inhibitors in accumulated activated/ memory phenotype CD4+ cells of older lupus mice. Eur. J. Immunol. 27:1901–1910. [DOI] [PubMed] [Google Scholar]
- 25.Rozzo, S.J., J.D. Allard, D. Choubey, T.J. Vyse, S. Izui, G. Peltz, and B.L. Kotzin. 2001. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 15:435–443. [DOI] [PubMed] [Google Scholar]
- 26.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 27.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. Cd11c(+)b220(+)gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agenes, F., and A.A. Freitas. 1999. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 189:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klinman, D.M., and A.D. Steinberg. 1987. Novel ELISA and ELISA-spot assays used to quantitate B cells and serum antibodies specific for T cell and bromelated mouse red blood cell autoantigens. J. Immunol. Methods. 102:157–164. [DOI] [PubMed] [Google Scholar]
- 30.Wither, J.E., A.D. Paterson, and B. Vukusic. 2000. Genetic dissection of B cell traits in New Zealand black mice. The expanded population of B cells expressing up-regulated costimulatory molecules shows linkage to Nba2. Eur. J. Immunol. 30:356–365. [DOI] [PubMed] [Google Scholar]
- 31.Murakami, M., T. Tsubata, M. Okamoto, A. Shimizu, S. Kumagai, H. Imura, and T. Honjo. 1992. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 357:77–80. [DOI] [PubMed] [Google Scholar]
- 32.Murakami, M., H. Yoshioka, T. Shirai, T. Tsubata, and T. Honjo. 1995. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int. Immunol. 7:877–882. [DOI] [PubMed] [Google Scholar]
- 33.Jameson, S.C. 2002. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2:547–556. [DOI] [PubMed] [Google Scholar]
- 34.Su, L., and M. David. 1999. Inhibition of B cell receptor-mediated apoptosis by IFN. J. Immunol. 162:6317–6321. [PubMed] [Google Scholar]
- 35.Yang, C.H., A. Murti, S.R. Pfeffer, L. Basu, J.G. Kim, and L.M. Pfeffer. 2000. IFNalpha/beta promotes cell survival by activating NF-kappa B. Proc. Natl. Acad. Sci. USA. 97:13631–13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun, D., I. Caramalho, and J. Demengeot. 2002. IFN-alpha/beta enhances BCR-dependent B cell responses. Int. Immunol. 14:411–419. [DOI] [PubMed] [Google Scholar]
- 37.Johnson, D.G., and C.L. Walker. 1999. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 39:295–312. [DOI] [PubMed] [Google Scholar]
- 38.Gribaudo, G., L. Riera, L. Hertel, and S. Landolfo. 1999. In vitro and in vivo expression analysis of the interferon-inducible 203 gene. J. Interferon Cytokine Res. 19:129–136. [DOI] [PubMed] [Google Scholar]
- 39.Choubey, D., and B.L. Kotzin. 2002. Interferon-inducible p202 in the susceptibility to systemic lupus. Front. Biosci. 7:e252–e262. [DOI] [PubMed] [Google Scholar]
- 40.Theofilopoulos, A.N., and F.J. Dixon. 1985. Murine models of systemic lupus erithematosus. Adv. Immunol. 37:269–390. [DOI] [PubMed] [Google Scholar]
- 41.Nisitani, S., T. Tsubata, M. Murakami, and T. Honjo. 1995. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur. J. Immunol. 25:3047–3052. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe, N., K. Ikuta, S. Nisitani, T. Chiba, and T. Honjo. 2002. Activation and differentiation of autoreactive B-1 cells by interleukin 10 induce autoimmune hemolytic anemia in Fas-deficient antierythrocyte immunoglobulin transgenic mice. J. Exp. Med. 196:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velupillai, P., J. Sypek, and D.A. Harn. 1996. Interleukin-12 and -10 and gamma interferon regulate polyclonal and ligand-specific expansion of murine B-1 cells. Infect. Immun. 64:4557–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansel, K.M., R.B. Harris, and J.G. Cyster. 2002. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 16:67–76. [DOI] [PubMed] [Google Scholar]
- 45.Balabanian, K., A. Foussat, L. Bouchet-Delbos, J. Couderc, R. Krzysiek, A. Amara, F. Baleux, A. Portier, P. Galanaud, and D. Emilie. 2002. Interleukin-10 modulates the sensitivity of peritoneal B lymphocytes to chemokines with opposite effects on stromal cell-derived factor-1 and B-lymphocyte chemoattractant. Blood. 99:427–436. [DOI] [PubMed] [Google Scholar]
- 46.Wiesemann, E., D. Sonmez, F. Heidenreich, and A. Windhagen. 2002. Interferon-beta increases the stimulatory capacity of monocyte-derived dendritic cells to induce IL-13, IL-5 and IL-10 in autologous T-cells. J. Neuroimmunol. 123:160–169. [DOI] [PubMed] [Google Scholar]
- 47.Byrnes, A.A., J.C. McArthur, and C.L. Karp. 2002. Interferon-beta therapy for multiple sclerosis induces reciprocal changes in interleukin-12 and interleukin-10 production. Ann. Neurol. 51:165–174. [DOI] [PubMed] [Google Scholar]
- 48.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D.F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461–470. [DOI] [PubMed] [Google Scholar]
- 49.Lin, Q., C. Dong, and M.D. Cooper. 1998. Impairment of T and B cell development by treatment with a type I interferon. J. Exp. Med. 187:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen, S.Y., Y. Takeoka, L. Pike-Nobile, A.A. Ansari, R. Boyd, and M.E. Gershwin. 1997. Autoantibody production and cytokine profiles of MHC class I (beta2-microglobulin) gene deleted New Zealand black (NZB) mice. Clin. Immunol. Immunopathol. 84:318–327. [DOI] [PubMed] [Google Scholar]
- 51.Penninger, J.M., C. Sirard, H.W. Mittrücker, A. Chidgey, I. Kozieradzki, M. Nghiem, A. Hakem, T. Kimura, E. Timms, R. Boyd, et al. 1997. The interferon regulatory transcription factor IRF-1 controls positive and negative selection of CD8+ thymocytes. Immunity. 7:243–254. [DOI] [PubMed] [Google Scholar]
- 52.Matsuyama, T., T. Kimura, M. Kitagawa, K. Pfeffer, T. Kawakami, N. Watanabe, T.M. Kundig, R. Amakawa, K. Kishihara, and A. Wakeham. 1993. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 75:83–97. [PubMed] [Google Scholar]
- 53.Tada, Y., A. Ho, T. Matsuyama, and T.W. Mak. 1997. Reduced incidence and severity of antigen-induced autoimmune diseases in mice lacking interferon regulatory factor-1. J. Exp. Med. 185:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakazawa, T., J. Satoh, K. Takahashi, Y. Sakata, F. Ikehata, Y. Takizawa, S.I. Bando, T. Housai, Y. Li, C. Chen, et al. 2001. Complete suppression of insulitis and diabetes in NOD mice lacking interferon regulatory factor-1. J. Autoimmun. 17:119–125. [DOI] [PubMed] [Google Scholar]
- 55.Hida, S., K. Ogasawara, K. Sato, M. Abe, H. Takayanagi, T. Yokochi, T. Sato, S. Hirose, T. Shirai, S. Taki, and T. Taniguchi. 2000. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 13:643–655. [DOI] [PubMed] [Google Scholar]
- 56.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tough, D.F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 272:1947–1950. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, X.H., S.Q. Sun, I.K. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 59.Ogasawara, K., S. Hida, Y. Weng, A. Saiura, K. Sato, H. Takayanagi, S. Sakaguchi, T. Yokochi, T. Kodama, M. Naitoh, et al. 2002. Requirement of the IFN-α/β-induced CXCR3 chemokine signalling for CD8(+) T cell activation. Genes Cells. 7:309–320. [DOI] [PubMed] [Google Scholar]
- 60.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623–655. [DOI] [PubMed] [Google Scholar]
- 61.Vyse, T.J., and B.L. Kotzin. 1998. Genetic susceptibility to systemic lupus erythematosus. Annu. Rev. Immunol. 16:261–292. [DOI] [PubMed] [Google Scholar]
- 62.Wakeland, E.K., K. Liu, R.R. Graham, and T.W. Behrens. 2001. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 15:397–408. [DOI] [PubMed] [Google Scholar]
- 63.Takaoka, A., Y. Mitani, H. Suemori, M. Sato, T. Yokochi, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science. 288:2357–2360. [DOI] [PubMed] [Google Scholar]
- 64.Balasa, B., C. Deng, J. Lee, L.M. Bradley, D.K. Dalton, P. Christadoss, and N. Sarvetnick. 1997. Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J. Exp. Med. 186:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, G.X., B.G. Xiao, X.F. Bai, P.H. van der Meide, A. Orn, and H. Link. 1999. Mice with IFN-gamma receptor deficiency are less susceptible to experimental autoimmune myasthenia gravis. J. Immunol. 162:3775–3781. [PubMed] [Google Scholar]
- 66.Chu, C.Q., S. Wittmer, and D.K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahng, A.W., I. Maricic, B. Pedersen, N. Burdin, O. Naidenko, M. Kronenberg, Y. Koezuka, and V. Kumar. 2001. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 194:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stewart, T.A., B. Hultgren, X. Huang, S. Pitts-Meek, J. Hully, and N.J. MacLachlan. 1993. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 260:1942–1946. [DOI] [PubMed] [Google Scholar]
- 69.Deng, C., E. Goluszko, S. Baron, B. Wu, and P. Christadoss. 1996. IFN-alpha therapy is effective in suppressing the clinical experimental myasthenia gravis. J. Immunol. 157:5675–5682. [PubMed] [Google Scholar]
- 70.Shenoy, M., S. Baron, B. Wu, E. Goluszko, and P. Christadoss. 1995. IFN-alpha treatment suppresses the development of experimental autoimmune myasthenia gravis. J. Immunol. 154:6203–6208. [PubMed] [Google Scholar]
- 71.Martin, R., C.S. Sturzebecher, and H.F. McFarland. 2001. Immunotherapy of multiple sclerosis: where are we? Where should we go? Nat. Immunol. 2:785–788. [DOI] [PubMed] [Google Scholar]