Abstract
A functional hybrid receptor associating the common γ chain (γc) with the granulocyte/macrophage colony-stimulating factor receptor β (GM-CSFRβ) chain is found in mobilized human peripheral blood (MPB) CD34+ hematopoietic progenitors, SCF/Flt3-L primed cord blood (CB) precursors (CBPr CD34+/CD56−), and CD34+ myeloid cell lines, but not in normal natural killer (NK) cells, the cytolytic NK-L cell line or nonhematopoietic cells. We demonstrated, using CD34+ TF1β cells, which express an interleukin (IL)-15Rα/β/γc receptor, that within the hybrid receptor, the GM-CSFRβ chain inhibits the IL-15–triggered γc/JAK3-specific signaling controlling TF1β cell proliferation. However, the γc chain is part of a functional GM-CSFR, activating GM-CSF–dependent STAT5 nuclear translocation and the proliferation of TF1β cells. The hybrid receptor is functional in normal hematopoietic progenitors in which both subunits control STAT5 activation. Finally, the parental TF1 cell line, which lacks the IL-15Rβ chain, nevertheless expresses both a functional hybrid receptor that controls JAK3 phosphorylation and a novel IL-15α/γc/TRAF2 complex that triggers nuclear factor κB activation. The lineage-dependent distribution and function of these receptors suggest that they are involved in hematopoiesis because they modify transduction pathways that play a major role in the differentiation of hematopoietic progenitors.
Keywords: IL-15, GM-CSF, IL-15R, signal transduction, CD34+ cells
Introduction
The development of hematopoietic cells is controlled by hematopoietic growth factors, cytokines and chemokines produced in the bone marrow (BM)* by accessory cells and T lymphocytes (1, 2), and by several other mechanisms (3). Hematopoietic factors constitute a complex network, in which each cytokine may act at various stages: for example, myeloid growth factors such as G-CSF and GM-CSF may also affect the lymphoid compartment (4, 5). GM-CSF inhibits the expansion of the NK cell progenitor population from unprimed BM CD34+ cells, and the generation and functioning of NK cells both in vivo and in vitro (5, 6). IL-15, the most powerful factor inducing the differentiation of CD34+ hematopoietic progenitor cells into CD56+/CD3− NK cells in vivo (7), may also cause a large increase in the number of long-term culture-initiating cells (LTC-IC) when added to the IL-3/SCF/Flt3-L combination (8). Transgenic mice carrying a mutation in the cytoplasmic domain of the common gamma chain (γc), or lacking the signaling kinase JAK3, both of which are necessary for IL-15–mediated signaling, have very low lymphocyte counts and display dysregulated myelopoiesis (9, 10). Moreover, the erythroid burst-forming cells of these mice have a larger than normal fraction of hematopoietic precursors with the CD34+/γc− phenotype (11). These data suggest that there is cross talk between myeloid growth factors and IL-15, which may regulate the balance between the myeloid and lymphoid lineages during hematopoietic differentiation. This regulation may operate at the level of signal transduction because IL-15 and GM-CSF share signaling pathways (JAK2/STAT5 and NF-κB) heavily involved in the control of hematopoiesis (12–16). Alternatively, the cross talk between these two cytokines may involve direct interactions between their receptor subunits, as suggested by the fact that GM-CSF almost totally inhibits IL-2 binding to IL-2/IL-15Rβ/γc in the M07sb CD34+ hematopoietic cell line (17). We investigated whether this cross talk resulted from a physical interaction between the two receptors, possibly involving the γc chain and the GM-CSFRβ chain. Indeed, these two subunits identify two families of cytokines, one with the γc chain (IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, and IL-21; references 7 and 18) and the other, with the GM-CSFRβ chain (IL-3, IL-5, and GM-CSF; reference 19).
In this study, we demonstrate that normal CD34+ cells, but not nonhematopoietic cells, express a novel functional hybrid receptor composed of the γc and GM-CSFRβ chains, in which the GM-CSFRβ chain may inhibit γc/JAK3 signaling. This complex is maintained in CD34+ myeloid and cord blood (CB)Pr CD34+/CD56− cells, but not in mature NK cells, suggesting that it is involved in controlling hematopoietic differentiation.
Materials and Methods
Purification of CD34+ Cells.
Mononuclear cells from CB or adult G-CSF mobilized peripheral blood (MPB) were isolated by centrifugation on a Ficoll gradient (Lymphoprep Nicomed Pharma SA). Adherent cells were isolated by adhesion to plastic Petri dishes for 2 h at 37°C. CD34+ cells were selected by an immunomagnetic method (Miltenyi Biotec), on nonadherent mononuclear cells labeled with a mAb specific for the QBEND10 epitope of the CD34 antigen, achieving a purity >97%, as previously described (20). CB CD34+ cells were used unprimed or after treatment with SCF/Flt3-L to expand the population of CD34+/CD56− primed progenitors (CBPr) in which the frequency of NK cell precursors may be 65 to 235 times higher than that in freshly prepared CD34+ HPCs (7).
Isolation and Culture of Normal Polyclonal NK Cells.
Non-adherent PBMCs from healthy volunteers were isolated by centrifugation on a Ficoll gradient. CD3−/CD4−/CD8− cells were purified by negative depletion and cultured with 100 U/ml rIL-2 (Proleukin; Chiron Corp.) in the presence of 105 irradiated allogenic PBMCs/well and 104 221 lymphoblastoid cells/well. By day 15, almost all the proliferating cells expressed CD16 and CD56 antigens.
Cell Lines.
Human TF1β (IL-15Rα/β/γc) and M07sb (IL-15Rβ/γc) are two IL-15–dependent leukemic cell lines, whereas TF1 (IL-15Rα/γc) is a GM-CSF–dependent cell line. TF1 and TF1β cells have the potential to display proerythroid differentiation whereas M07Sb cells display promegakaryocytic differentiation. NK-L is a functional human cytolytic NK cell line that is dependent on rIL-2. Raji is a B cell line (IL-15Rα/γc) that does not depend on growth factors for its proliferation. The growth characteristics and phenotypes of these cells have been reported elsewhere (21–25). Cells were maintained in RPMI medium (Eurobio) supplemented with 10% FCS (ATGC Biotechnologie), 2 mM glutamine, 1% antibiotics (GIBCO BRL), 10 ng/ml rIL-15, or rGM-CSF (R&D Systems) at 37°C, under an atmosphere of 5% CO2/95% air. Cells were subcultured twice per week by seeding fresh culture medium with 105 cells/ml. The human stromal fibroblast MS9 strain, isolated in culture from the spleen of a patient with a myeloproliferative syndrome, was used as a feeder layer for hematopoietic cells. MS9 fibroblasts express a bioactive membrane-bound IL-15 that induces the NK cell differentiation of CD34+ cells; they also secrete GM-CSF (26, 27). MS9 cells between passages 5 and 10 were cultured for 72 h at a density of 2 × 105 cells/ml in RPMI medium supplemented with 10% FCS in 6-well plates before being brought into contact with hematopoietic cells (5 × 105 cells/well). TF1β and NK-L cells were kindly provided by Drs. Paul Sondel (Dept. of Human Oncology, University of Wisconsin, Madison, WI) and Alessandro Moretta (Department of Experimental Medicine, University of Genoa, Genoa, Italy), respectively.
Proliferation Assays.
TF1β cells and Raji cells (3 × 105 cells/ml) were cultured for 4 d in complete growth medium supplemented with rIL-15 or rGM-CSF (10 ng/ml). Sister cultures were treated with neutralizing anti–IL-15Rα M162 (developed by Immunex Corp., provided by Genmab A/S, Copenhagen, Denmark), rat anti-γc (TUGh4), and anti-GM-CSFRβ (BD Biosciences/Becton Dickinson) mAbs.
TF1β cells (3 × 105 cells/ml) were also cultured for 4 d with MS9 stromal cells as the feeder layer. Sister cultures were treated with anti–IL-15 (R&D Systems), anti–IL-15Rα M162, anti–IL-15Rβ (CF-1; a gift from Dr. Y. Jacques, U463 INSERM, Nantes, France), anti-γc (TUGh4), anti-GM-CSFRβ, or anti-GM-CSFRα (S-20; Santa Cruz Biotechnology, Inc.) mAbs. In some inhibition experiments, we used low concentrations of anti–IL-15Rβ and anti-γc mAbs, because a combination of 1 μg/ml of each mAb is more efficient than a single dose of 10 μg/ml of each mAb at inhibiting IL-15 effects (23). Cells were counted in triplicate. The statistical significance of differences was determined using Student's t test, with P ≤ 0.05 considered significant.
Analysis of IL-15 Signal Transduction in the Human Hematopoietic TF1β Cell Line and in CBPr CD34+/CD56− Precursors.
For signal transduction analysis, cells were incubated overnight with a low concentration of rIL-15 (0.5 ng/ml) and were then deprived of growth factors for 3 h at 37°C. Cells were then stimulated by incubation with 10 ng/ml rIL-15 for 5 to 15 min. In some experiments, cells were initially treated for 1 h with either 10 μg/ml neutralizing antibody against IL-15, IL-15Rβ, γc, or GM-CSFRβ chains or with the JAK3-specific inhibitor, WHI-P131 (Calbiochem), which has no effect on JAK1 and JAK2.
Immunoblotting: Western Blotting.
Experiments were performed as described previously (12, 14, 28, 29). Briefly, cultures were serum-starved to reduce basal phosphorylation levels. Cells were then washed twice and suspended in lysis buffer supplemented with 0.5% NP-40. For immunoprecipitation, lysates were incubated with anti-γc (TUGh4), anti-JAK3 (Upstate Biotechnology) or polyclonal anti-TRAF2 (Santa Cruz Biotechnology, Inc.) antibody and immune complexes were captured by incubation with protein G-Sepharose beads (Amersham Biosciences) overnight at 4°C. The captured immune complexes were washed twice with lysis buffer. Complexes or lysates (for Western blotting) were then dissolved in Laemmli buffer, boiled, and separated by SDS-PAGE (7.5% or 12% polyacrylamide gels). The protein bands were transferred to PVDF membranes (NEN Life Science Products). Membranes were blocked by incubation with 5% BSA and probed with the following antibodies: anti-JAK1, anti-JAK2, and 4G10 anti-phosphotyrosine (Upstate Biotechnology/USA Euromodex), anti-pJAK1 (Tyr 1022/1023), anti-pJAK2 (Tyr 1007/Tyr 1008), anti-STAT3, anti-pSTAT3 (Tyr705), and anti-STAT6 (Santa Cruz Biotechnology, Inc.), anti-STAT5 (Transduction Laboratories/Becton Dickinson), anti-pSTAT5 (Tyr694), anti-pSTAT6 (Tyr641; Cell Signaling/New England Biolabs, Inc.) and anti-pIκBα (Calbiochem).
Primary antibody binding to the membrane was detected by incubation with peroxidase-conjugated secondary antibodies, followed by the enhanced chemiluminescence (ECL) system (Amersham Biosciences). The membrane was then subjected to densitometry, including correction for background, with analysis using NIH Image software. To correct for possible variations in the amount of protein loaded, values are expressed as pSTAT/STAT ratios. Results are expressed as increases (e.g., three times) with respect to the results obtained for untreated cells.
Confocal Microscopy.
For the double staining of γc and GM-CSFRβ chains, human MPB and CB CD34+ cells, the leukemic cell lines (TF1, TF1β, and M07sb), and MS9 cells were washed and permeabilized by incubation with ORTHOpermeafix (Ortho Diagnostic Systems Inc.) for 45 min at room temperature. Cells were then stained with anti-γc (TUGh4) mAb and with the biotinylated anti–GM-CSFRβ secondary mAb, and were then incubated at room temperature with Alexa Fluor594-GAR and streptavidin-Alexa Fluor594 (Molecular Probes). The extent of association between the two chains was assessed using the colocalization option of Methamorphe Software (Universal Imagine). We also used confocal microscopy to evaluate production of the activated transcription factor, pSTAT5, in TF1β and M07sb cells. Cells were starved of growth factors overnight and were then stimulated with rGM-CSF, as described above. Some samples were pretreated with anti–IL-15β/γc or anti–GM-CSFRβ mAbs. Cells were then permeabilized and indirect immunofluorescence assessed by means of antibodies recognizing the phosphorylated form of the transcription factor STAT5 (pSTAT5). Samples were washed and incubated with Alexa Fluor488-GARa antibody. Nuclei were stained with 2 μg/ml propidium iodide (PI, red staining). All antibodies were dissolved in PBS supplemented with 10 mg/ml BSA to block nonspecific binding. The stained cells were washed with PBS, centrifuged in a Cytospin 3 (Shandon) onto glass slides, and mounted under a coverslip in Prolong Antifade (Molecular Probes) mounting medium. The slides were analyzed by laser scanning confocal microscopy, using a Leica TCS Confocal System.
Results
Human Hematopoietic CD34+ Cells Express a Hybrid γc/GM-CSFRβ Receptor.
We investigated the possible interactions between IL-15R and GM-CSFR complexes in human hematopoietic and nonhematopoietic cells by Western blotting (Fig. 1 A), coimmunoprecipitation (Fig. 1 B), and confocal microscopy (Fig. 1 C). The Western blotting of total lysates (Fig. 1 A) showed that the cells analyzed (TF1β, M07Sb, and MPB CD34+) expressed both the γc chain (a single band of 64 kD) and the GM-CSFRβ chain (a major band of 130 kD, corresponding to the mature protein, and a second band of ∼95 kD, corresponding to the nonglycosylated precursor protein; reference 30).
Figure 1.
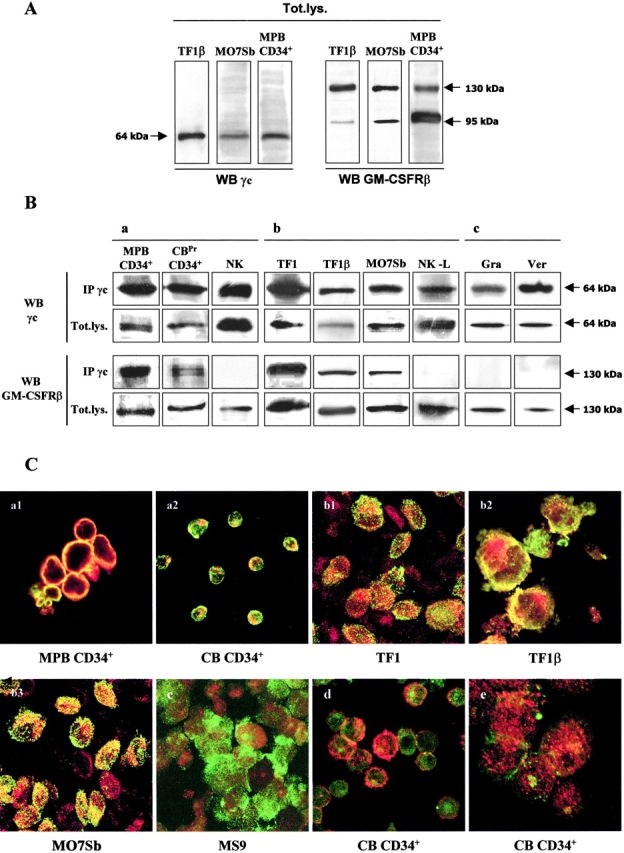
γc/GM-CSFRβ interaction in human hematopoietic and nonhematopoietic cells. (A) Western blots of total lysates. MPB CD34+ cells, TF1, and M07Sb promyeloid CD34+ cell lines were analyzed for the expression for γc and GM-CSFRβ chains. Briefly, cell lysates were subjected to electrophoresis and the protein bands were transferred to PVDF membranes. The total lysate membranes (Tot.lys) were then probed with anti-γc or anti–GM-CSFRβ mAbs. The data shown are representative of three independent experiments. (B) Coimmunoprecipitation MPB CD34+ cells, CBPr CD34+/CD56− and normal polyclonal NK cells (panel a), TF1, TF1β, MO7sb promyeloid CD34+and NK-L cell lines (panel b) and human skin myofibroblasts (panel c) were analyzed for γc/GM-CSFRβ interactions. Briefly, total lysates were subjected to immunoprecipitation (IP) with an anti-γc antibody. Immunoprecipitates were subjected to electrophoresis and the protein bands were transferred to PVDF membranes. Membranes corresponding to the γc immunoprecipitate and to total lysates (Tot.lys) were probed with anti-γc or anti-GM-CSFRβ mAb. The data presented are representative of three independent experiments. (C) Confocal microscopy. Analysis by confocal microscopy of the interactions between the γc chain (green staining) and GM-CSFRβ (red staining) in MPB CD34+ cells (panel a1), CBPr CD34+ cells (panel a2), TF1 cells (panel b1), TF1β cells (panel b2), M07Sb (panel b3), and MS9 spleen myofibroblasts (panel c). As specificity controls, we analyzed the interactions between the γc chain (green staining) and IL-6R gp130 (red staining, panel d) or between the γc chain (green staining) and β1 integrin (red staining, panel e) in CB CD34+ cells. The images presented are compacted overlay pictures from serial optical sections, 1 μm thick, from the outside to the inner compartments of the cell. The yellow staining indicates colocalization of the various molecules. The data presented are representative of three independent experiments.
Human MPB CD34+ cells, CBPr CD34+/CD56− progenitors and normal polyclonal NK cells were immunoprecipitated with anti-γc mAb, and the γc membranes were reprobed with mAbs recognizing the γc and GM-CSFRβ chains. Reprobing with the anti-γc mAb confirmed the presence of a specific 64 kD band, whereas reprobing with anti–GM-CSFRβ mAb resulted in detection of the specific 130 kD band in MPB CD34+ and CBPr CD34+/CD56− cells, but not in polyclonal NK cells from normal donors (Fig. 1 B, panel a). Thus, normal human CD34+ cells express a γc/GM-CSFRβ complex, which is not detectable in mature NK cells.
The γc/GM-CSFRβ association was also detected both in the proerythroid TF1 and TF1β cell lines, which expressed IL-15Rα/γc and IL-15Rα/β/γc, respectively, and in the promegakaryocytic (M07sb) cells expressing IL-15Rβ/γc, whereas it was not detected in the cytolytic NK-L cell line (Fig. 1 B, panel b). In addition, the γc/GM-CSFRβ complex was not found in human skin myofibroblasts despite the presence of the γc and GM-CSFRβ chains in these cells (Fig. 1 B, panel c). Reprobing the γc membrane with anti-GM-CSFRα or anti-gp130 mAbs did not give a specific signal (unpublished data).
We demonstrated, by confocal microscopy, the colocalization of the γc (green staining) and GM-CSFRβ (red staining) chains in MPB and CB CD34+ progenitors, as well as in the TF1, TF1β and M07sb cell lines (Fig. 1 C). The images presented are overlay-compacted images from confocal analysis of 1 μm serial optical sections from the cell surface to the inner compartment.
In MPB CD34+ and unprimed CB CD34+ cells (Fig. 1 C, panels a1 and a2), all cells displayed strong colocalization of the two molecules (yellow staining), restricted to the membrane/submembrane compartment. In TF1 and TF1β cells (Fig. 1 C, panels b1 and b2), we observed similar yellow staining of the membrane/submembrane compartment and spotted yellow staining in the cytoplasm, suggesting that the hybrid receptor was taken up by some of the cells. This punctate distribution of the γc/GM-CSFRβ complex in the cytoplasms was the predominant staining pattern in M07sb cells (Fig. 1 C, panel b3). No colocalization was observed in human MS9 myofibroblasts (Fig. 1 C, panel c). The specificity of γc and GM-CSFRβ chain colocalization was further confirmed by the lack of colocalization of the γc chain and the IL-6R subunit gp130 (Fig. 1 C, panel d) or the β1 integrin (Fig. 1 C, panel e) in normal CB CD34+ cells. Computerized quantification of the extent to which the γc chain and the GM-CSFRβ chain were colocalized in normal and leukemic CD34+ cells showed that 74.9% (MPB CD34+), 66.3% (TF1β), 50.2% (TF1), and 58.3% (M07sb) of the GM-CSFRβ chain colocalized with the γc chain, whereas 94.1% (MPB CD34+), 99.6% (TF1β), 82.1% (TF1), and 93.6% (M07sb) of the γc chain colocalized with the GM-CSFRβ chain. Our results indicate that the γc chain is almost completely associated with the GM-CSFRβ chain, with ∼30–40% of the GM-CSFRβ chain left free.
The expression of this hybrid receptor is constitutive and does not seem to be influenced by cytokine stimulation, as it is observed in CB hematopoietic progenitors, whether unprimed or treated with SCF/Flt3-L, in MPB CD34+ cells, and in CD34+ myeloid cell lines, which are dependent on GM-CSF (TF1) or IL-15 (TF1β, M07sb) for growth.
IL-15– and GM-CSF–dependent Proliferation in TF1β Cells Is Modified by Anti–GM-CSFRβ and Anti-γc mAbs, Respectively.
The presence of the hybrid receptor on CD34+ cells dependent on IL-15 or GM-CSF led us to investigate the role of this receptor in cell proliferation. As illustrated in Fig. 2 A, TF1β cells proliferated in the presence of rIL-15 (+200%) or rGM-CSF (+300%). IL-15–dependent proliferation was inhibited by anti–IL-15Rα, but not by anti-γc mAbs. Anti-GM-CSFRβ mAb slightly, but significantly increased (+20–25%, P < 0.001) the rate of cell proliferation.
Figure 2.
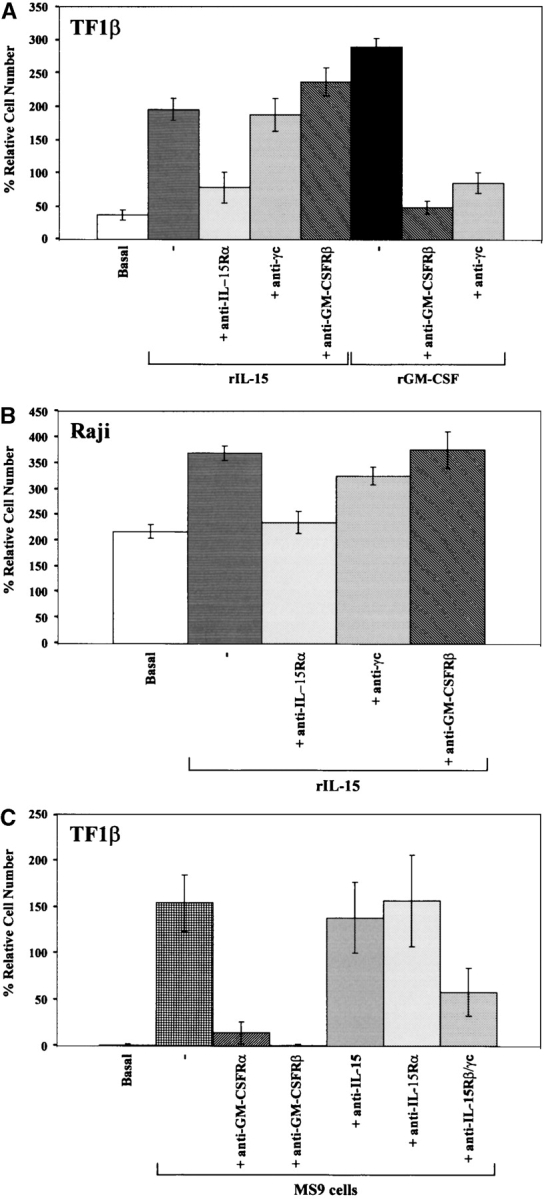
Proliferation induced by rIL-15 and rGM-CSF: IL-15R/GM-CSFR cross talk in TF1β cells. (A) Proliferative response of TF1β cells to recombinant IL-15 and GM-CSF. TF1β (IL-15Rα/β/γc) CD34+ cells were cultured for 4 d with rIL-15 or rGM-CSF (10 ng/ml) and their proliferation potential was then analyzed. Sister cultures were continuously incubated with neutralizing mAbs recognizing the IL-15Rα, γc, and GM-CSFRβ chains. In TF1β cells cultured with rIL-15, the proliferation rates of samples treated with an anti–GM-CSFRβ mAb were significantly higher (+25%; P < 0.001) than those of samples incubated with rIL-15 alone or with rIL-15 plus an anti-γc mAb. (B) Proliferative response of Raji cells to rIL-15. Raji cells (IL-15Rα/γc) were cultured for 4 d with rIL-15 (10 ng/ml) and analyzed for proliferation. Sister cultures were continuously incubated with neutralizing mAbs recognizing the IL-15Rα, γc, and GM-CSFRβ chains. (C) Proliferative response of TF1β cells to MS9 cells. TF1β cells were cocultured for 4 d with MS9 cells and analyzed for proliferation. Sister cultures were continuously incubated with neutralizing mAbs recognizing the GM-CSFRα, GM-CSFRβ, IL-15Rβ/γc, IL-15Rα chains, and IL-15. Cells were counted in an electronic Coulter counter and the data are expressed as % difference in proliferative potential with respect to control untreated samples. The data presented are representative of three independent experiments.
In contrast, GM-CSF–dependent proliferation was totally inhibited not only by anti–GM-CSFRβ mAb, but also by anti-γc mAb, further suggesting that there is cross talk between these two cytokine receptors in these cells. As a control, we used the Raji cell line, the growth of which is not dependent on IL-15, and which expresses IL-15Rα and γc, but not IL-15Rβ chains (reference 30; Fig. 2 B). After 4 d in culture, Raji cells fed with DMEM supplemented with 10% FCS proliferated, resulting in a 200% increase in cell density. The addition of 10 ng/ml of rIL-15 increased the proliferation of these cells (+30%). This effect was inhibited by anti-IL-15Rα mAb, but not by anti-γc or anti-GM-CSFRβ mAbs. Anti–IL-15 and anti–IL-15R mAbs have been shown to have similar effects on Raji cells (25).
We investigated whether the growth of TF1β cells could be stimulated by contact with human stromal MS9 cells, which have been shown to secrete several hematopoietic growth factors (26), and whether the hybrid receptor was involved in these interactions. TF1β cells proliferated when cultured in contact with MS9 cells, doubling in number after 4 d (Fig. 2 C). The proliferation induced by MS9 cells was totally inhibited by neutralizing mAbs against the GM-CSFRα and GM-CSFRβ chains. Neutralizing mAbs directed against IL-15 or the IL-15Rα chain had no effect whereas anti–IL-15Rβ/γc mAbs inhibited the proliferation of TF1β cells by 60%. Thus, TF1β cell proliferation is completely dependent on GM-CSF produced by MS9 cells (26) and can be efficiently inhibited by anti–IL-15Rβ/γc mAbs, consistent with the existence of cross talk between the two cytokine receptors.
IL-15–induced JAK/STAT Signaling Is Inhibited by Anti–GM-CSFRβ mAb.
The inability of the anti-γc mAb to block IL-15–dependent proliferation in TF1β cells suggests that the γc chain cannot mediate the effects of IL-15 in these cells. We investigated the signal transduction triggered by IL-15 and the involvement of the various subunits of the IL-15R, in TF1β cells.
Densitometric analysis revealed that rIL-15 (10 ng/ml) caused the levels of phosphorylation of JAK1 and STAT3 to increase by a factor of seven within 15 min (Fig. 3 A). Neutralizing anti–IL-15Rβ mAb totally inhibited the phosphorylation of JAK1 and inhibited that of STAT3 by 50%. In contrast, anti-γc mAb had no effect on either pathway. These data confirm the results of the proliferation assays, demonstrating the incapacity of the γc chain to mediate IL-15–dependent functions in TF1β cells.
Figure 3.
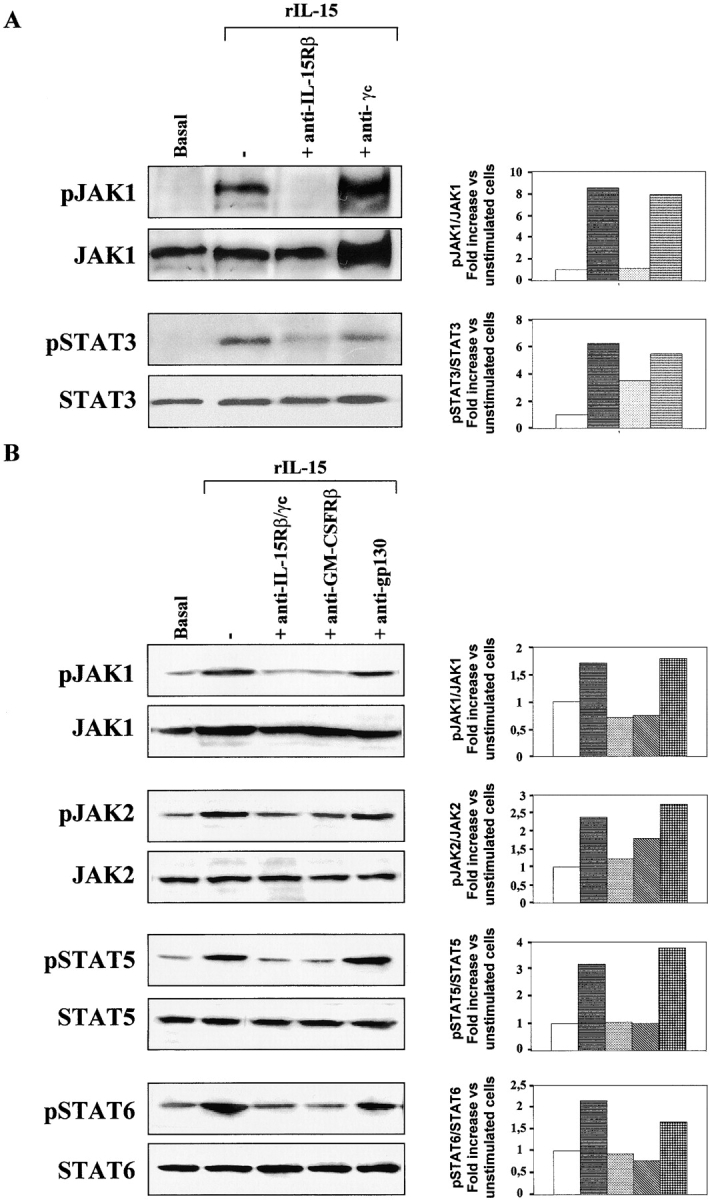
IL-15 signal transduction: IL-15R/GM-CSFR cross talk in TF1β cells. Analysis of JAK/STAT signal transduction by Western blotting. TF1β cells were incubated with 10 ng/ml rIL-15 for 15 min at 37°C. Sister cultures were pretreated for 1 h with neutralizing anti–IL-15Rβ/γc, anti-GM-CSFRβ, or anti-IL-6R gp130 mAbs. Cell extracts were analyzed by Western blotting using anti-phospho-JAK1 (pJAK1), anti-phospho-JAK2 (pJAK2), anti-phospho-TYK2 (pTYK2), anti-phospho-STAT5 (pSTAT5), and anti-phospho-STAT6 (pSTAT6) antibodies. Membranes were then reprobed with antibodies recognizing the native proteins. To correct for possible variations in the amount of protein loaded, values are expressed as pJAK/JAK or pSTAT/STAT ratios. pJAK/JAK and pSTAT/STAT levels were determined by densitometry including correction for background (NIH Image software). Results are expressed as increases (e.g., two times) with respect to untreated cells. The data presented are representative of three independent experiments.
We then investigated whether the GM-CSFRβ chain interfered with γc chain activity, as suggested by the results of the proliferation assays. Constitutive levels of JAK1, JAK2, STAT5, and STAT6 phosphorylation were low in TF1β cells (Fig. 3 B). Densitometric analysis showed that rIL-15 (10 ng/ml) induced an increase by a factor of at least two in the level of phosphorylation of all of these molecules, within 15 min. The phosphorylation of JAK1, STAT5, and STAT6 was totally inhibited not only by the synergistic combination of anti–IL-15Rβ and anti-γc mAbs, but also by neutralizing anti–GM-CSFRβ mAb.
In contrast, JAK2 phosphorylation was totally inhibited by anti–IL-15Rβ/γc mAb but was only inhibited by 30% with the anti-GM-CSFRβ mAb. The use of an isotype-matched anti-gp130 mAb had no significant effect on the level of phosphorylation of the various molecules. In contrast to what was observed in TF1β cells, anti–GM-CSFRβ mAb did not affect IL-15 signaling in CD34+ promegakaryocytic M07sb cells expressing a low-affinity IL-15Rβ/γc receptor (unpublished data).
GM-CSF–induced pSTAT5 Nuclear Translocation Is Inhibited by Anti–IL-15Rβ/γc mAbs in TF1β Cells.
We analyzed the effects of anti–IL-15Rβ/γc mAbs on the signal transduction activated by rGM-CSF (Fig. 4 A). Densitometric analysis revealed that rGM-CSF (at a concentration of 10 ng/ml) doubled the phosphorylation of JAK2 and quadrupled that of STAT5 in TF1β cells in a 15-min period. In experiments with anti–IL-15Rβ/γc mAbs, the specific JAK3 inhibitor WHI-P31 or the irrelevant anti-gp130 mAb, we observed no significant inhibition of either pathway, which demonstrated that neither of the two chains of the IL-15R affected these steps of GM-CSF signaling. The effects of rGM-CSF and IL-15Rβ/γc mAbs on the localization of pSTAT5 were analyzed by confocal microscopy (Fig. 4 B). Cells were stained for pSTAT5 (green staining) and with propidium iodide (specific for nuclei; red staining); superimposition of the two stains gave a yellow coloration. In basal culture conditions, all cells displayed green staining of the cytoplasm, indicating the presence of pSTAT5 protein in this compartment. The intensity of the green staining increased after GM-CSF stimulation suggesting that the level of STAT5 phosphorylation increased. No inhibition was observed in the presence of neutralizing anti-IL-15Rβ/γc mAbs, or of the specific JAK3 inhibitor WHI-P31 (unpublished data), confirming the results obtained by Western blotting.
Figure 4.
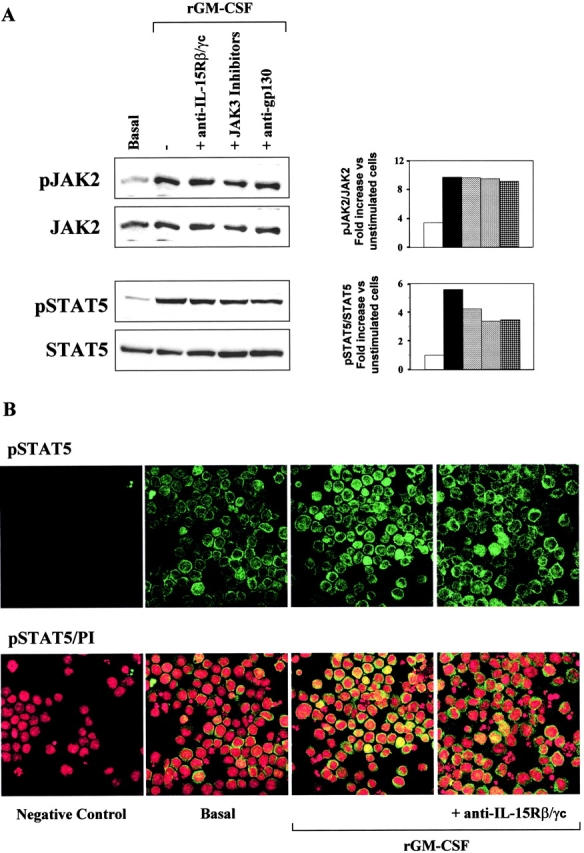
GM-CSF signal transduction: IL-15R/GM-CSFR cross talk in TF1β cells. (A) Analysis of JAK2/STAT5 signal transduction by Western blotting. TF1β cells were incubated with 10 ng/ml rGM-CSF for 15 min at 37°C. Sister cultures were pretreated for 1 h with neutralizing anti–IL-15Rβ/γc mAbs or specific JAK3 inhibitors. Cell extracts were analyzed by Western blotting with anti-phospho-JAK2 (pJAK2) and anti-phospho-STAT5 (pSTAT5) antibodies. Membranes were then reprobed with antibodies recognizing the native proteins. To correct for possible variations in the amount of protein loaded, values are expressed as pJAK/JAK or pSTAT/STAT ratios. pJAK/JAK and pSTAT/STAT levels were determined by densitometry, including correction for background (NIH Image software). Results are expressed as an increase (e.g., two times) with respect to untreated cells. The data presented are representative of three independent experiments. (B) Analysis of pSTAT5 nuclear localization by confocal microscopy. TF1β cells were incubated with 10 ng/ml rGM-CSF for 15 min at 37°C. Sister cultures were pretreated for 1 h with neutralizing anti-IL-15Rβ/γc mAbs. Control (basal) and treated cultures were analyzed by confocal microscopy for pSTAT5 distribution in the cell, focusing particularly on whether this protein was present in the nucleus. Propidium iodide stains nuclei red whereas pSTAT5 is stained green. Yellow staining indicates the presence of pSTAT5 in the nucleus.
In basal culture conditions, only a few cells displayed yellow staining indicating the presence of pSTAT5 in the nucleus. Control cultures displayed only red nuclear staining when treated with PI and the second reagent. Short-term stimulation with rGM-CSF (15 min) induced the massive nuclear translocation of pSTAT5 in 30% of the cells, as demonstrated by intense yellow staining of the nucleus. Finally, anti–IL-15Rβ/γc mAbs efficiently inhibited the effects of rGM-CSF on pSTAT5 nuclear translocation, as shown by a loss of the yellow staining, whereas the isotype-matched anti-gp130 mAb had no effect (unpublished data). The effects on STAT5 of anti-IL-15Rβ/γc mAbs and of the specific JAK3 inhibitor WHI-P31 were also observed in M07Sb cells (unpublished data).
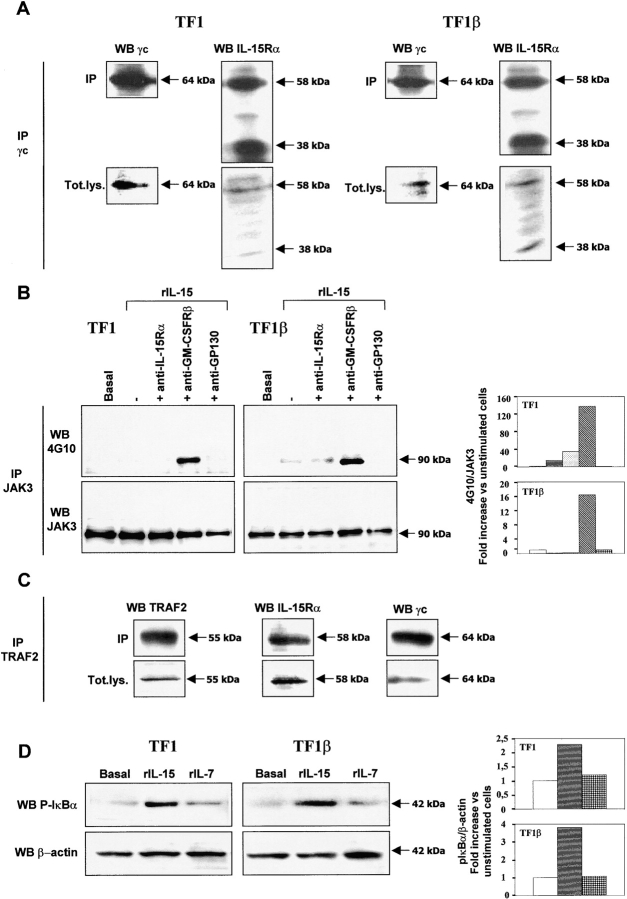
TF1 Cells Express Functional IL-15Rα/γc Complex, the Formation of which Is Controlled by the Hybrid γc/GM-CSFRβ Receptor.
The absence of proliferation in IL-15Rα/γc-positive TF1 cells in response to IL-15 has been attributed to the lack of the IL-15Rβ subunit (23), which is indispensable for IL-15 binding and γc chain signaling (7). However, it has recently been reported that the IL-15Rα chain may transduce a signal in the absence of links with any other receptor subunit (12), leading to NF-κB activation via the cytoplasmic interaction of this molecule with the signaling molecule TRAF2. We therefore investigated the possible presence of a functional IL-15Rα/γc receptor in TF1 cells. Analysis of total lysates showed that the TF1 and TF1β cell lines expressed the γc chain (64 kD) and at least two IL-15Rα isoforms, 58 and 38 kD in size (Fig. 5 A). The two cell lines were then incubated with an anti-γc mAb for immunoprecipitation. The γc membrane was reprobed with the anti-γc mAb. A strong specific signal was identified at 64 kD. In contrast, two major bands, 58 and 38 kD in size, were identified if the membrane was reprobed with anti–IL-15Rα mAb. Thus, in both TF1 and TF1β cells, the γc chain is physically associated with the two IL-15Rα isoforms.
Figure 5.
IL-15Rα/γc interaction in TF1 and TF1β cells. (A) Coimmunoprecipitation. TF1 and TF1β cells were analyzed for IL-15Rα/γc interactions. Briefly, lysates were subjected to immunoprecipitation (IP) with an anti-γc antibody. Immunoprecipitates were subjected to electrophoresis and the protein bands were transferred to PVDF membranes. Membranes corresponding to immunoprecipitated γc and total lysates (Tot.lys.) were probed with anti-γc or anti–IL-15Rα mAbs. The data presented are representative of three independent experiments. (B) Analysis of JAK3 phosphorylation by Western blotting. TF1 and TF1β cells were incubated with 10 ng/ml rIL-15 for 15 min at 37°C. Sister cultures were pretreated for 1 h with neutralizing anti–IL-15Rα, anti–GM-CSFRβ, and anti-gp130 mAbs. Cell extracts were subjected to immunoprecipitation with an anti-JAK3 mAb. JAK3 membranes were then re-probed with anti-phosphotyrosine 4G10 or anti-JAK3 mAbs. To correct for possible variations in the amount of protein loaded, values are expressed as pJAK3/JAK3 ratios. pJAK3/JAK3 levels were determined by densitometry, including correction for background (NIH Image software). Results are expressed as increases (e.g., two times) with respect to untreated cells. The data presented are representative of three independent experiments. (C) Coimmunoprecipitation. TF1 cells were analyzed for interactions between TRAF2, IL-15Rα, and γc. Briefly, lysates were subjected to immunoprecipitation (IP) with an anti-TRAF2 antibody. Immunoprecipitates were subjected to electrophoresis and the protein bands were transferred to PVDF membranes. Membranes corresponding to immunoprecipitated TRAF2 and total lysates (Tot.lys.) were probed with anti-TRAF2, anti-γc, or anti-IL-15Rα mAbs. The data presented are representative of three independent experiments. (D) Analysis of IκBα phosphorylation by Western blotting. TF1 and TF1β cells were incubated with 10 ng/ml rIL-15 or rIL-7 for 15 min at 37°C. Cell extracts were analyzed by Western blotting with anti-phospho-IκBα (pIκBα) antibody. Membranes were then reprobed with an antibody recognizing β-actin. To correct for possible variations in the amount of protein loaded, values are expressed as pIκBα/β actin ratios. pIκBα/β actin levels were determined by densitometry, including correction for background (NIH Image software). Results are expressed as increases with respect to untreated cells. The data presented are representative of three independent experiments.
Stimulation with rIL-15 induced JAK3 phosphorylation (increase by a factor of 15) only in the presence of anti–GM-CSFRβ mAb, indicating that the IL-15Rα/γc receptor is potentially functional in TF1 cells (Fig. 5 B). A similar result was also obtained with TF1β cells.
Total lysates of the TF1 and TF1β cell lines were subjected to immunoprecipitation with an anti-JAK3 mAb, and the JAK3 membranes were probed with the anti-phosphotyrosine mAb 4G10. A single phosphorylated band was detected for the samples treated with an anti-GM-CSFRβ mAb. Reprobing of the membrane with an anti-JAK3 mAb resulted in the detection of a single protein with migration characteristics identical to those of the phosphorylated band detected on the 4G10 Western blot, indicating that the phosphorylated protein is, indeed, JAK3. These data indicate that the γc subunit of the IL-15Rα/γc receptor is functional in TF1 cells, even in the absence of the IL-15Rβ chain, but that signaling is controlled by GM-CSFRβ. The hybrid receptor is, therefore, functional in these cells.
We analyzed whether the IL-15Rα/γc complex was associated with the signaling molecule TRAF2 (Fig. 5 C). Analysis of total lysates with anti-TRAF2, anti-γc, or anti–IL-15Rα mAbs showed that TF1 cells expressed TRAF2 (55 kD), the 58 kD IL-15Rα isoform and the γc chain (64 kD). After immunoprecipitation with an anti-TRAF2 antibody, reprobing of the TRAF2 membranes with mAbs recognizing the IL-15Rα and γc chains resulted in the detection of specific 58 and 64 kD bands, respectively, indicating that TRAF2 is physically associated with the IL-15Rα 58 kD standard isoform and the γc chain.
We also showed that rIL-15, but not rIL-7, triggered phosphorylation of the p65 NF-κB subunit inhibitor IκBα (42 kD) within 15 min in TF1 and TF1β cells (Fig. 5 D). Reprobing the membranes with an anti–β-actin mAb identified a band of similar intensity at 42 kD in each sample, indicating that the gel had been evenly loaded in terms of amount of protein. We also showed by confocal microscopy that rIL-15, but not rIL-7, induced the translocation to the nucleus of the p65 NF-κB subunit (unpublished data). These data indicate that, in TF1 cells, the IL-15Rα chain is functional and able to activate the NF-κB factor. Moreover, not only does its association with the γc chain not interfere with NF-κB activation, it also probably renders the γc chain functional even in the absence of the IL-15Rβ chain, as shown by the induction of JAK3 phosphorylation by rIL-15.
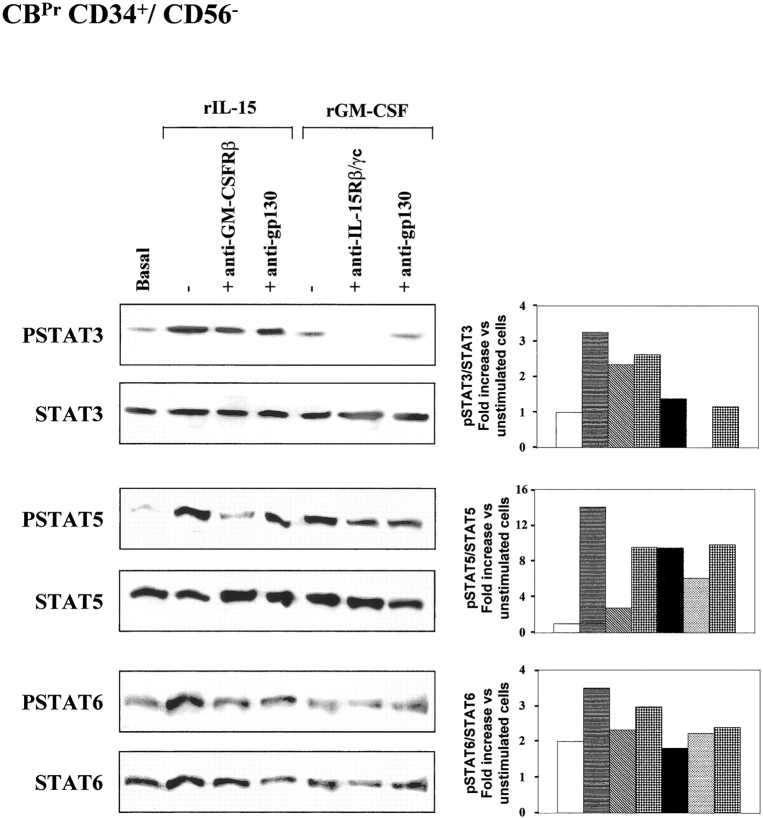
IL-15–induced STAT5 Signaling Is Inhibited by Anti–GM-CSFRβ mAb in Normal CBPr CD34+/CD56− Cells.
Finally, we investigated the functional significance of the hybrid receptor in normal CB hematopoietic progenitors and the properties of this receptor in these cells. We used populations of CB progenitors that had been expanded for 5 d in the presence of SCF and Flt3-L (CBPr CD34+/CD56−). This facilitates proliferation of the hematopoietic progenitors, which maintain a CD34+/CD56− phenotype with no expression of lineage-specific markers (7). We found that rIL-15 quadrupled levels of STAT3 phosphorylation, which was not significantly affected by anti–GM-CSFRβ or isotype-matched control anti-gp130 mAbs (Fig. 6) . As expected, rGM-CSF did not induce STAT3 phosphorylation. In contrast, anti-γc, but not anti-gp130 mAbs inhibited constitutive STAT3 phosphorylation, suggesting interference with an autocrine IL-15–dependent loop. Indeed, unprimed hematopoietic precursors secrete several hematopoietic cytokines that regulate normal hematopoiesis (3) by autocrine/paracrine loops, and IL-15 belongs to this group (20).
Figure 6.
IL-15 signal transduction: IL-15R/GM-CSFR cross talk in CBPr CD34+/CD56− cells. Analysis of STAT signal transduction by Western blotting. CB CD34+ cell populations were expanded by incubation for 5 d in the presence of SCF and Flt3-L. These cells displayed a CD34+/CD56− phenotype with no expression of lineage-specific markers. These CBPr CD34+/CD56− progenitors were incubated with 10 ng/ml rIL-15 or rGM-CSF for 15 min at 37°C. Sister cultures were pretreated for 1 h with neutralizing anti-IL-15Rβ/γc, anti-GM-CSFRβ, or anti-IL-6R gp130 mAbs. Cell extracts were analyzed by Western blotting with anti-phospho-STAT3 (pSTAT3), anti-phospho-STAT5 (pSTAT5), and anti-phospho-STAT6 (pSTAT6) antibodies. Membranes were then reprobed with antibodies recognizing the native proteins. To correct for possible variations in the amount of protein loaded, values are expressed as pSTAT/STAT ratios. pSTAT/STAT levels were determined by densitometry, including correction for background (NIH Image software). Results are expressed as increases with respect to untreated cells. The data presented are representative of two independent experiments.
Both rIL-15 and rGM-CSF induced STAT5 phosphorylation (increases by factors of 10 and 7, respectively). Anti–GM-CSFRβ mAb totally abolished the effect of rIL-15 whereas the action of rGM-CSF action was inhibited by only 35% with an anti-γc mAb. No significant inhibition was observed if an anti-gp130 mAb was used. Finally, CBPr CD34+/CD56− progenitors displayed discrete constitutive STAT6 phosphorylation, with an 80% increase in phosphorylation in response to rIL-15, but no effect of rGM-CSF. The various neutralizing mAbs had no significant effect on STAT6 phosphorylation.
Discussion
We found that human CD34+ hematopoietic progenitors express a novel hybrid receptor composed of the γc chain of IL-2R and the GM-CSFRβ chain. This receptor was also found in CD34+ hematopoietic cell lines with proerythroid (TF1, TF1β) or promegakaryocytic (M07sb) differentiation potentials and in CBPr CD34+/CD56− precursors, but not in the cytolytic NK-L cell line, normal NK cells or nonhematopoietic human cells, even though all these cells produce both chains. The hybrid receptor is functional and mediates signal transduction by both the IL-15 and GM-CSF pathways. Indeed, the results obtained with an anti-GM-CSFRβ mAb suggested that, in TF1β cells, the GM-CSFRβ chain replaces the γc chain, inhibiting its specific signaling (phosphorylation of JAK3), but that this chain cooperates with the IL-15Rβ chain, facilitating the IL-15–dependent activation of other JAK/STAT pathways (JAK1, JAK2, STAT5, and STAT6) and controlling the proliferation of these cells. In contrast, an anti-γc mAb inhibited the GM-CSF–dependent proliferation of TF1β cells treated with the recombinant cytokine or cocultured with human stromal hematopoietic cells secreting GM-CSF. This suggests that the hybrid receptor may have important effects in vivo, interfering in the interactions between CD34+ cells and stromal cells that play a major role in the control of both normal and pathological hematopoiesis (1, 2). In addition, anti–IL-15Rβ/γc mAbs, and the specific JAK3 inhibitor WHI-P31, inhibited the nuclear translocation of STAT5 induced by rGM-CSF but did not inhibit the phosphorylation of STAT5. These effects on STAT5 were also observed in M07Sb cells. Our observation that the γc subunit of the hybrid receptor controls the GM-CSF–dependent nuclear translocation of pSTAT5 rather than STAT5 phosphorylation is consistent with recent results showing that the tyrosine phosphorylation and nuclear translocation of STAT proteins are regulated independently (31, 32). Indeed, in human keratinocytes, which express a type II IL-4R, IL-4 induces the phosphorylation of STAT3, but not its nuclear translocation (31), whereas the prolactin receptor (PRLR) regulates STAT5 tyrosine phosphorylation and nuclear translocation by means of two independent pathways (32). With PRLR, the number of phosphorylated tyrosines on the receptor itself seems to play a critical role in the activation of a cellular component that controls the number of STAT5 complexes in the cytoplasm, by controlling the entry of these complexes into the nucleus (32). Moreover, STAT5 tyrosine phosphorylation and DNA binding via the IL-2Rβ chain requires receptor phosphotyrosines (33), whereas similar events mediated by the G-CSFR are independent of receptor phosphotyrosines (34). Thus, the structural organization of some receptors and the subsequent activation of particular signaling pathways may affect the translocation of STAT protein to the nucleus.
On the basis of these results, we propose a model for the activation of STAT5 by rGM-CSF via the hybrid receptor γc/GM-CSFRβ. We suggest that STAT5 tyrosine phosphorylation and nuclear translocation are regulated independently and that the translocation pathway is controlled by the γc/JAK3 complex, which, in cells with type I IL-4R, is necessary for the translocation of STAT5 to the nucleus (35).
The hybrid receptor is functional not only in leukemic cell precursors, but also in normal progenitors, although its functions appear more limited in normal cells. Indeed, in normal CBPr CD34+/CD56− progenitors, the hybrid receptor controls the IL-15–dependent phosphorylation of STAT5 but not of STAT3 and STAT6. Moreover, levels of GM-CSF–dependent STAT5 phosphorylation are decreased by anti-γc mAb in CBPr CD34+/CD56− progenitors, but not in TF1β cells. These differences may be due to differences in origin (normal versus pathological) and/or degree of primitiveness of the two types of CD34+ cell. In any case, our results emphasize the involvement of the hybrid receptor in the control of normal and pathological hematopoiesis, with this receptor probably displaying various functions during hematopoietic development.
The absence of the hybrid receptor on nonhematopoietic cells strengthens the evidence for its “specific” involvement in hematopoiesis. The distribution of this receptor in cells of various lineages and functions suggests that it is involved in the development of hematopoiesis, probably affecting engagement in myeloid, rather than lymphoid, lineages. Indeed, the inhibition of γc/JAK3 signaling by the GM-CSFRβ chain should be interpreted in light of the inhibition of lymphocyte maturation and myelopoiesis dysregulation observed in mice and humans bearing γc or JAK3 deletions or mutations (9–11).
In erythrocytic TF1 cells, we describe, for the first time, a novel IL-15Rα/γc/TRAF2 complex that triggers specific IL-15 signaling, even though these cells do not express the IL-15Rβ chain23, a subunit indispensable for the γc chain functions (7). Both subunits of the IL-15Rα/γc heterodimer are functional, although they behave differently from what would be expected based on the results obtained for other cell types. It has been reported that IL-15Rα, associating with the signaling molecule TRAF2, activates the transcription factor NF-κB, in the absence of a link with the γc chain (12, 24). In contrast, in TF1 cells, IL-15Rα and γc are associated but we nevertheless observed the phosphorylation, not only of IκBα, but also of JAK3 factors. However, the phosphorylation of JAK3 factor was only observed in samples treated with rIL-15 and anti–GM-CSFRβ mAb, indicating that the γc/JAK3 pathway is functional in the absence of the IL-15Rβ chain but is controlled by the hybrid receptor.
The association of IL-15Rα/γc/TRAF2 may play a regulatory role at certain stages of hematopoiesis, associating NF-κB activation, which is involved in the control of erythropoiesis (15), with the conditional activation of JAK3-dependent pathways.
Finally, we showed that rIL-15 induced the phosphorylation of STAT6 both in leukemic TF1β proerythroid precursors, and in normal CBPr CD34+/CD56− progenitors. Our data suggest that this unusual IL-15–dependent pathway, the activation of which in mast cells was attributed to the high-affinity p65 IL-15RX receptor (28, 29), is also triggered in other types of CD34+ precursor. However, in TF1β cells, this pathway is controlled by the classical IL-15Rα/β/γc receptor and/or by the γc/GM-CSFRβ hybrid receptor, whereas in normal CBPr, CD34+/CD56− IL-15–dependent STAT6 phosphorylation is not controlled by the hybrid receptor. Overall, these results suggest the possible and complex involvement of STAT6 in the control of hematopoiesis (36) and provide further evidence that IL-15/GM-CSF are involved in cross talk.
Recent data have shown that unprimed hematopoietic progenitors secrete several mediators activating autocrine/paracrine regulatory loops (3). For instance, CD34+ cells may express GM-CSF transcripts (3) and a biologically active IL-15 that controls expression of the γc chain, but is not competent for inducing the spontaneous differentiation of these cells into NK cells (20). Thus, IL-15 and GM-CSF may contribute, by means of the hybrid receptor, to the autocrine/paracrine cross talk that controls the development of hematopoietic cells (3), perhaps by inhibiting the spontaneous engagement of these cells in the lymphoid lineage. In contrast, in leukemic hematopoiesis, the action of the hybrid receptor is probably irreversible and may be involved in the molecular mechanisms responsible for the inhibition of differentiation in certain leukemic clones.
Acknowledgments
This work was supported by grants from the Association pour la Recherche sur le Cancer (ARC, N° 5801), NRB-Vaincre le Cancer, FE.GE.FLUC, Fondation de France (N° 99-003929), Italian Association for Cancer Research (AIRC), and the Italian National Council for Research (CNR).
Footnotes
Abbreviations used in this paper: BM, bone marrow; CB, cord blood; MPB, mobilized peripheral blood.
References
- 1.Ratajczak, M.Z., and A.M. Gewirtz. 1995. The biology of hemopoietic stem cells. Semin. Oncol. 22:210–217. [PubMed] [Google Scholar]
- 2.Sharkis, S.J., C. Cremo, M.I. Collector, S.J. Noga, and A.D. Donnenberg. 1986. Thymic regulation of hematopoiesis: isolation of helper and suppressor populations using counterflow centrifugal elutriation. Blood. 68:787–789. [PubMed] [Google Scholar]
- 3.Majka, M., A. Janowska-Wieczorek, J. Ratajczak, K. Ehrenman, Z. Pietrzkowski, M.A. Kowalska, A.M. Gewirtz, S.G. Emerson, and M.Z. Ratajczak. 2001. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 97:3075–3085. [DOI] [PubMed] [Google Scholar]
- 4.Shibuya, A., K. Taguchi, H. Kojima, and T. Abe. 1991. Inhibitory effect of granulocyte-macrophage colony-stimulating factor therapy on the generation of natural killer cells. Blood. 78:3241–324. [PubMed] [Google Scholar]
- 5.Miller, J.S., F. Prosper, and V. McCullar. 1997. Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood. Blood. 90:3098–3105. [PubMed] [Google Scholar]
- 6.Taguchi, K., A. Shibuya, Y. Inazawa, and T. Abe. 1992. Suppressive effect of granulocyte-macrophage colony-stimulating factor on the generation of natural killer cells in vitro. Blood. 79:3227–3232. [PubMed] [Google Scholar]
- 7.Fehniger, T.A., and M.A. Caligiuri. 2001. Interleukin 15: biology and relevance to human disease. Blood. 97:14–32. [DOI] [PubMed] [Google Scholar]
- 8.Pierelli, L., G. Scambia, G. Bonanno, A. Coscarella, R. De Santis, A. Mele, A. Battaglia, A. Fattorossi, V. Romeo, G. Menichella, et al. 1999. Expansion of granulocyte colony-stimulating factor/chemotherapy-mobilized CD34+ hematopoietic progenitors: role of granulocyte-macrophage colony-stimulating factor/erythropoietin hybrid protein (MEN11303) and interleukin-15. Exp. Hematol. 27:416–424. [DOI] [PubMed] [Google Scholar]
- 9.Ohbo, K., T. Suda, M. Hashiyama, A. Mantani, M. Ikebe, K. Miyakawa, M. Moriyama, M. Nakamura, M. Katsuki, K. Takahashi, et al. 1996. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 87:956–967. [PubMed] [Google Scholar]
- 10.Grossman, W.J., J.W. Verbsky, L. Yang, L.J. Berg, L.E. Fields, D.D. Chaplin, and L. Ratner. 1999. Dysregulated myelopoiesis in mice lacking Jak3. Blood. 94:932–939. [PubMed] [Google Scholar]
- 11.Itano, M., S. Tsuchiya, S. Morita, H. Fujie, N. Ishii, T. Yanagisawa, Y. Ohashi, M. Minegishi, K. Sugamura, and T. Konno. 1996. IL-2 receptor gamma chain expression on CD34 positive hematopoietic progenitor cells from bone marrow and cord blood. J. Exp. Med. 78:389–398. [DOI] [PubMed] [Google Scholar]
- 12.Bulfone-Paus, S., E. Bulanova, T. Pohl, V. Budagian, H. Durkop, R. Ruckert, U. Kunzendorf, R. Paus, and H. Krause. 1999. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 13:1575–1585. [DOI] [PubMed] [Google Scholar]
- 13.McDonald, P.P., M.P. Russo, S. Ferrini, and M.A. Cassatella. 1998. Interleukin-15 (IL-15) induces NF-kappaB activation and IL-8 production in human neutrophils. Blood. 92:4828–4835. [PubMed] [Google Scholar]
- 14.Liu, R.Y., C Fan, R. Garcia, R. Jove, and K.S. Zuckerman. 1999. Constitutive activation of the JAK2/STAT5 signal transduction pathway correlates with growth factor independence of megakaryocytic leukemic cell lines. Blood. 9:2369–2379. [PubMed] [Google Scholar]
- 15.Zhang, M.Y., S.C. Sun, L. Bell, and B.A. Miller. 1998. NF-kappaB transcription factors are involved in normal erythropoiesis. Blood. 91:4136–4144. [PubMed] [Google Scholar]
- 16.Ihle, J.N. 2001. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13:211–217. [DOI] [PubMed] [Google Scholar]
- 17.Kanakura, Y., H. Sugahara, H. Mitsui, H. Ikeda, T. Furitsu, H. Yagura, H. Kitayama, Y. Kanayama, and Y. Matsuzawa. 1993. Functional expression of interleukin 2 receptor in a human factor-dependent megakaryoblastic leukemia cell line: evidence that granulocyte-macrophage colony-stimulating factor inhibits interleukin 2 binding to its receptor. Cancer Res. 53:675–680. [PubMed] [Google Scholar]
- 18.Asao, H., C. Okuyama, S. Kumaki, N. Ishii, S. Tsuchiya, D. Foster, and K. Sugamura. 2001. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheim, J.J. 2001. Cytokines: past, present, and future. Int. J. Hematol. 74:3–82. [DOI] [PubMed] [Google Scholar]
- 20.Carayol, G., J. Giron-Michel, B. Azzarone, L. Castagna, N. Cambier, Z. Mishal, J.H. Bourhis, S. Chouaib, and A. Caignard. 2000. Altered natural killer cell differentiation in CD34+ progenitors from chronic myeloid leukemia patients. Oncogene. 19:2758–2766. [DOI] [PubMed] [Google Scholar]
- 21.Avanzi, G.C., P. Lista, B. Giovinazzo, R. Miniero, G. Saglio, G. Benetton, R. Coda, G. Cattoretti, and L. Pegoraro. 1988. Selective growth response to IL-3 of a human leukaemic cell line with megakaryoblastic features. Br. J. Haematol. 69:359–366. [DOI] [PubMed] [Google Scholar]
- 22.Farner, N.L., J. Gan, J.L. de Jong, T.P. Leary, T.S. Fenske, P. Buckley, S. Dunlap, and P.M. Sondel. 1997. Alteration of the CD34+ Tf-1 beta cell line profile in response to long-term exposure to IL-15. Cytokine. 9:316–327. [DOI] [PubMed] [Google Scholar]
- 23.Meazza, R., S. Basso, A. Gaggero, D. Detotero, L. Trentin, R. Pereno, B. Azzarone, and S. Ferrini. 1998. Interleukin (IL)-15 induces survival and proliferation of the growth factor-dependent acute myeloid leukemia M-07e through the IL-2 receptor beta/gamma. Int. J. Cancer. 78:189–195. [DOI] [PubMed] [Google Scholar]
- 24.Robertson, M.J., K.J. Cochran, C. Cameron, J.M. Le, R. Tantravahi, and J. Ritz. 1996. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 24:406–415. [PubMed] [Google Scholar]
- 25.Bulanova, E., V. Budagian, T. Pohl, H. Krause, H. Durkop, R. Paus, and S. Bulfone-Paus. 2001. The IL-15Ralpha chain signals through association with Syk in human B cells. J. Immunol. 167:6292–6302. [DOI] [PubMed] [Google Scholar]
- 26.Brouty-Boye, D., D. Briard, B. Azzarone, M.-C. Le Bousse-Kerdiles, D. Clay, C. Pottin-Clemenceau, and C. Jasmin. 2001. Effects of human fibroblasts from myelometaplasic and non-myelometaplasic hematopoietic tissues on CD34+ stem cells. Int. J. Cancer. 92:484–488. [DOI] [PubMed] [Google Scholar]
- 27.Brouty-Boye, D., D. Briard, B. Azzarone, M.C. Le Bousse-Kerdiles, D. Clay, C. Pottin-Clemenceau, and C. Jasmin. 1998. Phenotypic diversity in human fibroblasts from myelometaplasic and non-myelometaplasic hematopoietic tissues. Int. J. Cancer. 76:767–773. [DOI] [PubMed] [Google Scholar]
- 28.Masuda, A., T. Matsuguchi, K. Yamaki, T. Hayakawa, M. Kubo, W.J. LaRochelle, and Y. Yoshikai. 2000. Interleukin-15 induces rapid tyrosine phosphorylation of STAT6 and the expression of interleukin-4 in mouse mast cells. J. Biol. Chem. 275:29331–29337. [DOI] [PubMed] [Google Scholar]
- 29.Tagaya, Y., J.D. Burton, Y. Miyamoto, and T.A. Waldmann. 1996. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 15:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashida, K., T. Kitamura, D.M. Gorman, K. Arai, T. Yokota, and A. Miyajima. 1990. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc. Natl. Acad. Sci. USA. 87:9655–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wery-Zennaro, S., M. Letourneur, M. David, and J.P. Bertoglio. 1999. Binding of IL-4 to the IL-13Ralpha(1)/IL-4Ralpha receptor complex leads to STAT3 phosphorylation but not to its nuclear translocation. FEBS Lett. 464:91–96. [DOI] [PubMed] [Google Scholar]
- 32.Ali, S., and S. Ali. 1998. Prolactin receptor regulates Stat5 tyrosine phosphorylation and nuclear translocation by two separate pathways. J. Biol. Chem. 273:7709–7716. [DOI] [PubMed] [Google Scholar]
- 33.Gaffen, S.L., S.Y. Lai, M. Ha, X. Liu, L. Hennighausen, W.C. Greene, and M.A. Goldsmith. 1996. Distinct tyrosine residues within the interleukin-2 receptor beta chain drive signal transduction specificity, redundancy, and diversity. J. Biol. Chem. 271:21381–21390. [DOI] [PubMed] [Google Scholar]
- 34.Fujitani, Y., M. Hibi, T. Fukada, M. Takahashi-Tezuka, H. Yoshida, T. Yamaguchi, K. Sugiyama, Y. Yamanaka, and K. Nakajima. 1997. An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene. 14:751A–761A. [DOI] [PubMed] [Google Scholar]
- 35.Lischke, A., R. Moriggl, S. Brandlein, S. Berchtold, W. Kammer, W. Sebald, B. Groner, X. Liu, L. Hennighausen, and K. Friedrich. 1998. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma–chain. J. Biol. Chem. 273:31222–31229. [DOI] [PubMed] [Google Scholar]
- 36.Soon, L., L. Flechner, J.S. Gutkind, L.H. Wang, R. Baserga, J.H. Pierce, and W. Li. 1999. Insulin-like growth factor I synergizes with interleukin-4 for hematopoietic cell proliferation independent of insulin receptor substrate expression. Mol. Cell. Biol. 19:3816–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]