Abstract
Regulatory CD4 T cells (Treg) control inflammatory reactions to commensal bacteria and opportunist pathogens. Activation of Treg functions during these processes might be mediated by host-derived proinflammatory molecules or directly by bacterial products. We tested the hypothesis that engagement of germline-encoded receptors expressed by Treg participate in the triggering of their function. We report that the subset of CD4 cells known to exert regulatory functions in vivo (CD45RBlow CD25+) selectively express Toll-like receptors (TLR)-4, -5, -7, and -8. Exposure of CD4+ CD25+ cells to the TLR-4 ligand lipopolysaccharide (LPS) induces up-regulation of several activation markers and enhances their survival/proliferation. This proliferative response does not require antigen-presenting cells and is augmented by T cell receptor triggering and interleukin 2 stimulation. Most importantly, LPS treatment increases CD4+ CD25+ cell suppressor efficiency by 10-fold and reveals suppressive activity in the CD4+ CD45RBlow CD25− subset that when tested ex-vivo, scores negative. Moreover, LPS-activated Treg efficiently control naive CD4 T cell–dependent wasting disease. These findings provide the first evidence that Treg respond directly to proinflammatory bacterial products, a mechanism that likely contributes to the control of inflammatory responses.
Keywords: inflammation, tolerance, lymphocytes, regulation, innate immunity
Introduction
It is established that a subpopulation of CD4-αβ T cells controls inflammatory responses to commensal bacteria and pathogens (1–4). These cells are encompassed in the naturally activated lymphocytes (CD45RBlow) and enriched in the CD25-expressing subset. In the absence of regulatory T cells (Treg),* alymphoid animals reconstituted with CD4 cells isolated from normal mice develop severe inflammatory bowel disease if colonized by enteric bacteria (1), or lethal pneumonia if infected by Pneumocystis carinii (2). Cotransfers of Treg protect from disease by inhibiting both the protective (2) and inflammatory responses (1, 2), leading to the notion of “quality control” of the immune response (for review see reference 5).
These activities of Treg suggest their engagement at early stages of infection/inflammation. Accordingly, evidence exists for rapid Treg migration to inflammatory sites, likely as a result of the constitutive expression of chemokine receptors (6) and high sensitivity to inflammatory chemokines (7). Once at the site of infection, however, it remains unclear whether activation of Treg function is triggered by components of the host inflammatory response or by direct recognition of microbial products. In either case, engagement of germline-encoded receptors is an attractive possibility. Toll-like receptors (TLRs) ensure vertebrates with the means to recognize a vast range of microbial products and produce immediate, “innate” responses. Thus, TLRs expressed in B lymphocytes trigger effector functions such as antibody production, providing a direct link between innate and adaptive immunity (8, 9). Furthermore, endogenous molecules such as heat shock proteins (HSP; references 10–13) or oligosaccharides of hyaluronan (14) have recently been shown to functionally ligate TLR-4 in macrophages or dendritic cells, and may participate in inflammatory reactions. On the other hand, although TLR-4 expression in murine CD3+ lymphocytes (15) and more specifically in particular subsets of γδ T cells (16) has been reported, expression of TLR genes in CD4-αβ T cell subpopulations has not been directly assessed.
In these experiments, we directly tested whether proinflammatory microbial products activate CD4 cells involved in the control of inflammatory reactions. We first monitored the expression profile of all nine murine TLR genes thus far identified in various subsets of CD4 T cells. The results show that TLR-4, -5, -7, and -8 are selectively expressed in CD4 T cell subsets that contain Treg. We then established that CD4+ CD25+ cells, unresponsive to TCR triggering, are activated and proliferate when treated with the TLR-4 ligand LPS. This proliferative response does not require APC, is augmented by TCR triggering, and synergizes with IL-2 stimulation. Finally, we show that exposure to LPS markedly increases Treg activity as measured in suppression assays in vitro and maintains their regulatory function in vivo.
Materials and Methods
Mice
All animals were bred and maintained under specific pathogen-free conditions in our animal facilities. C57BL/6 and C57BL/6-Thy1.1 mice were originally purchased from The Jackson Laboratory. C57BL/10ScCr mice (B10ScCr) were obtained from CDTA. C3H/HeJ and C3H/HeN were purchased from The Jackson Laboratory. C57BL/6-H-2 u and RAG-1−/− animals were provided by S. Tonegawa, MIT, Boston, MA. C57Bl-H-2 u Thy1a mice have been previously described (17). All animals used in this study were 6–8 wk old.
Cell Purification
Erythrocyte-depleted splenocytes and LN cells were prepared as previously described (17). For flow cytometry purification of CD4+ cell subsets, pooled LN cells were stained with CyChrome-conjugated CD4 mAb (clone RMA-5; BD Biosciences), Alexa Fluor™ 488-CD25 (PC61; produced in the laboratory), and CD45RB-PE (clone 16A; BD Biosciences). In some experiments, the following mAbs were pooled and used in addition to exclude nonconventional CD4 cells: B220-PE (RA3-6B2), CD11c-PE (clone HL3), PanNK-PE (DX-5), Mac I biotin (clone M1/70), and MHC-II biotin (clone M5-114; all from BD Biosciences). Biotinylated Abs were revealed with PE-labeled Streptavidin (BD Biosciences). The reference B cell population was sort-purified after staining with CD19-FITC (clone 1D3; BD Biosciences). Cell sorting was performed on a MoFlo® high speed cellsorter (DakoCytomation). The purity of each cell preparation was >97%. CD4− contaminants in the CD4+ CD25+ preparations represented routinely 0.5%. CD4+ CD25− cells used in the suppression assays were prepared by magnetic purification. Total LN and erythrocyte-lysed splenocytes were first depleted of CD25+ cells by treatment with 7D4 mAb (produced in the laboratory) and complement (low-tox rabbit complement; Cedarlane). Cells were then stained with anti-CD4 (L3T4) microbeads and positively separated on LS+ columns (both from Miltenyi Biotec). Erythrocyte-depleted splenocytes, treated with J1J anti-Thy1 mAb (produced in the laboratory) and complement, were irradiated at 30 Gy and used as a source of APC. Additional antibodies used for FACS® analyses were: class I (H2Kb AF6-88.5) and B.7 (16-10A1), CD69 (H1.2F3), CD44 (IM7), CD38 (90), TCRβ (H57-597), Thy1.1 (OX-7), and Thy1.2 (53-2.1; all from BD Biosciences). FACS® analyses were performed on a FACSCalibur® instrument run with the CellQuest™ program (both from Becton Dickinson). Dead cells were gated out after propidium iodide staining.
RT-PCR Reactions
Total RNA was extracted from 106 cells using Trizol reagent, treated with DNase I and reverse transcribed using Superscript II RT and oligo(dT)12–18 primer (all four reagents from Life Technologies). The amount of cDNA in each sample was first normalized after nonsaturating PCR for HPRT transcripts. 25 μl reaction mixture contained 1.5 mM MgCl2, 0.2 mM dNTP, 25 pmol sense and antisense primer (5′-GTAATGATCGTCAACGGGGGAC and 5′-CCAGCAAGCTTGCAACCTTAACCA), and 1 U Taq DNA polymerase (Life Technologies) in the manufacturer's buffer. PCR consisted of 5 min at 94°C followed by 26 cycles of 30 s at 94°C, 55°C, and 72°C, terminated by 10 min at 72°C. The specific TLR primer sequences and their respective annealing temperatures are shown in Table I. Other conditions were as described above except for 35 cycles. Reactions were not saturated as controlled by increasing the amount of template (not depicted). All PCRs were performed on a PTC-100™ programmable thermal controller (MJ Research Inc.). After separation on 2% agarose gels containing ethidium bromide (Sigma-Aldrich), the specific bands were quantified with an Eagle Eye® II still video system (Stratagene).
Table I.
Primer Sequences for Mouse TLR RT-PCR Used in This Study
| Nucleic sequencesa | Tpb | |
|---|---|---|
| TLR-1 | TCTCTGAAGGCTTTGTCGATACA | 56 |
| GACAGAGCCTGTAAGCATATTCG | ||
| TLR-2 | TCTAAAGTCGATCCGCGACAT | 58 |
| TACCCAGCTCGCTCACTACGT | ||
| TLR-3 | TTGTCTTCTGCACGAACCTG | 58 |
| CGCAACGCAAGGATTTTATT | ||
| TLR-4 | CAAGAACATAGATCTGAGCTTCAACCC | 62 |
| GCTGTCCAATAGGGAAGCTTTCTAGAG | ||
| TLR-5 | ACTGAATTCCTTAAGCGACGTA | 56 |
| AGAAGATAAAGCCGTGCGAAA | ||
| TLR-6 | AACAGGATACGGAGCCTTGA | 58 |
| CCAGGAAAGTCAGCTTCGTC | ||
| TLR-7 | TTCCGATACGATGAATATGCACG | 56 |
| TGAGTTTGTCCAGAAGCCGTAAT | ||
| TLR-8 | GGCACAACTCCCTTGTGATT | 58 |
| CATTTGGGTGCTGTTGTTTG | ||
| TLR-9c | CCGCAAGACTCTATTTGTGCTGG | 62 |
| TGTCCCTAGTCAGGGCTGTACTCAG |
Sequences are written 5′ to 3′, sense and antisense primers successively.
Annealing temperature in °C.
Reference 48.
Cell Cultures and Proliferation Assays
All cultures were set in RPMI-1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamycin, 50 μM 2β-ME, 10 mM Hepes, and 1 mM sodium pyruvate (all from Life Technologies).
For PCR controls, sort-purified CD4+ CD25− CD45RBhigh (106/well in 24-well plates) were stimulated for 3 d with 1 μg/ml plate-bound anti-CD3 mAb (145.2C11; produced in the laboratory) and 1 μg/ml soluble anti-CD28 mAb (clone 37.51; BD Biosciences).
LPS-induced Proliferation.
Cultures were set in triplicates in 96-well plates maintained for 3 d at 37°C, 5% CO2. Each well contained 2.5 × 104 purified CD4+ cells with or without 5 × 104 APC, 0.5 ug/ml anti-CD3 mAb, IL-2 from X63-IL2 cell supernatant (∼10 U/ml) diluted at 1/500, and 10 μg/ml LPS from Salmonella typhymurium (Sigma-Aldrich). In experiments comparing cells purified from WT, B10ScCr, and C3H/HeJ animals, LPS from Escherichia coli EH100 was HPLC purified (provided by C. Galanos, Max-Planck Institute for Immunobiology, Freiburg, Germany).
IL-2 Production.
Primary cultures were set in triplicate in 96-well plates containing 100 μl medium. After 48 (CD4+ CD25− cells) or 72 (CD4+ CD25+ cells) h, 50 μl of the supernatant was transferred to a new well containing 1,000 CTLL-2 cells and 50 μl fresh medium. Amplification of the response was achieved 48 h later by adding saturating IL-2 (as described above) for 24 h.
LPS Pretreatments.
Sort-purified CD4+ subpopulations (1.5–2 × 106/well) were seeded in 24-well plates for 3 d in the presence of 10 μg/ml LPS (Sigma-Aldrich) with or without 1 μg/ml soluble or plate-bound anti-CD3 mAb. In some experiments, after washing in media, cells were cultured for an additional 3 d in the presence of IL-2 and 1 μg/ml soluble anti-CD3 mAb. To assess the induction of surface molecules, erythrocyte-lysed splenocytes were plated at 1.5 × 106/ml in medium alone or supplemented with 10 μg/ml LPS (Sigma-Aldrich) for 18 h.
Suppression Assay.
CD4+ CD25− cells (target cells) were plated at 2.5 × 104/well in U-shape 96-well plates together with 105 APC and 0.5 μg/ml anti-CD3 mAb and variable numbers of the suppressor populations under test. Each dilution was set in triplicate and culture was maintained for 3 d. All proliferations were monitored by addition of [3H]thymidine (1 μCi/well; Amersham Biosciences) for the last 6 h of culture.
Adoptive Transfer
Pooled LN from C57BL-H-2 u Thy1.2 and C57BL-H-2 u Thy1.1 mice were used to sort purify CD4+ CD25− and CD4+ CD25+ cells, respectively. C57BL-H-2 u RAG-1−/− recipients were injected intravenously at 8 wk of age. Weight and general health status was monitored every 3 d for 21 d.
Results
Selective Expression of TLR-4, -5, -7, and -8 by Treg.
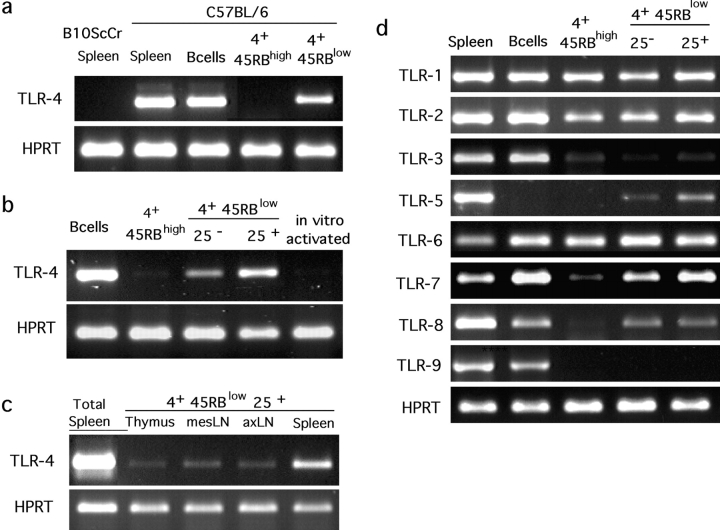
LN lymphocytes were FACS® purified under highly stringent gate definitions according to the CD4 and CD45RB surface markers and TLR-4 expression was assessed in the various subsets by RT-PCR in nonsaturating conditions. Naive CD4+ cells (CD45RBhigh) scored negative whereas the samples of activated/memory CD45RBlow cells prepared in parallel displayed a clear signal (Fig. 1 a). Additional fractionation of the CD4+ CD45RBlow subset according to the expression of the CD25 molecule revealed the heterogeneity of this population. The RT-PCR signal was reproducibly three- to fourfold higher in the CD25+ compared with the CD25− subset (Fig. 1 b). The PCR product obtained in either CD45RBlow population was cloned and sequenced to confirm its identity with the published TLR-4 sequence (not depicted). Expression of TLR-4 by CD4 cells is not merely the result of activation because naive CD4+ cells (CD45RBhigh CD25−) activated in culture for 3 d using plate-bound anti-CD3 together with anti-CD28 acquire a CD45RBlow CD25+ phenotype (not depicted), but continue to score negative for TLR-4 expression in the RT-PCR assay (Fig. 1 b). Finally, analyses of CD4 cells in various lymphoid tissues (thymus, mesenteric LN, axillary LN, and spleen) revealed preferential expression of TLR-4 in CD4+ CD25+ cells independently of their location, although more marked in cells purified from the spleen (Fig. 1 c). Therefore, TLR-4 expression in CD4 T cell subsets is restricted to subpopulations with known in vivo regulatory functions and may represent a specific marker for Treg differentiation.
Figure 1.
Specific expression of TLR genes by Treg. RNA from sort-purified CD19+ (B cells) and various subpopulations of CD4 cells (4+) from B6 mice, either pooled LNs (a, b, and d) or specific lymphoid organs (c) were submitted to RT-PCR. CD4+ CD45RBlow or CD4+ CD45RBhigh (4+ 45RBlow or 4+ 45RBhigh) were sorted as negative for CD11c, CD11b, B220, pan-NK, and MHC II in addition to positive for CD4 and CD45RB (a) or simply according to CD4, CD45RB, and CD25 expression (b, c, and d). Erythrocyte-lysed splenocytes from B6 or TLR-4–deficient B10ScCr animals and in vitro–activated B6 CD4+ CD45RBhigh served as controls. All RT-PCR were performed at least twice on independent samples.
These results prompted us to monitor the expression of all identified murine TLR genes in the same CD4 T cell subsets. As shown in Fig. 1 c, three additional TLRs (TLR-5, -7, and -8) were found preferentially expressed by CD4+ CD45RBlow cells. TLR-5 expression was undetectable in B and naive CD4 T cells and, similarly to TLR-4, markedly increased in the CD25+ subset when compared with CD45RBlow CD25− cells (fourfold). TLR-7 transcripts were detectable in naive CD4 cells albeit to a lower level than in the naturally activated subset of CD4+ cells (fourfold and twofold, respectively, when compared with CD45RBlow CD25+ and CD25−). Finally, TLR-8 appeared specifically expressed in the CD4+ CD45RBlow compartment independently of its CD25 phenotype. Similarly to TLR-4, expression of these genes was not induced by in vitro activation of purified naive CD4+ T cells (not depicted). Less relevant for this study but noteworthy in the interplay of innate and adaptive immunity, expression of TLR-1, -2, and -6 was readily detectable in all CD4+ cell populations analyzed. Finally, expression of both TLR-3 and TLR-9 was clearly detectable in total splenocytes but not significantly in any of the CD4 T cell subsets.
LPS Treatment Induces the Expression of Several Activation Markers on CD4+ CD25+ T Cells.
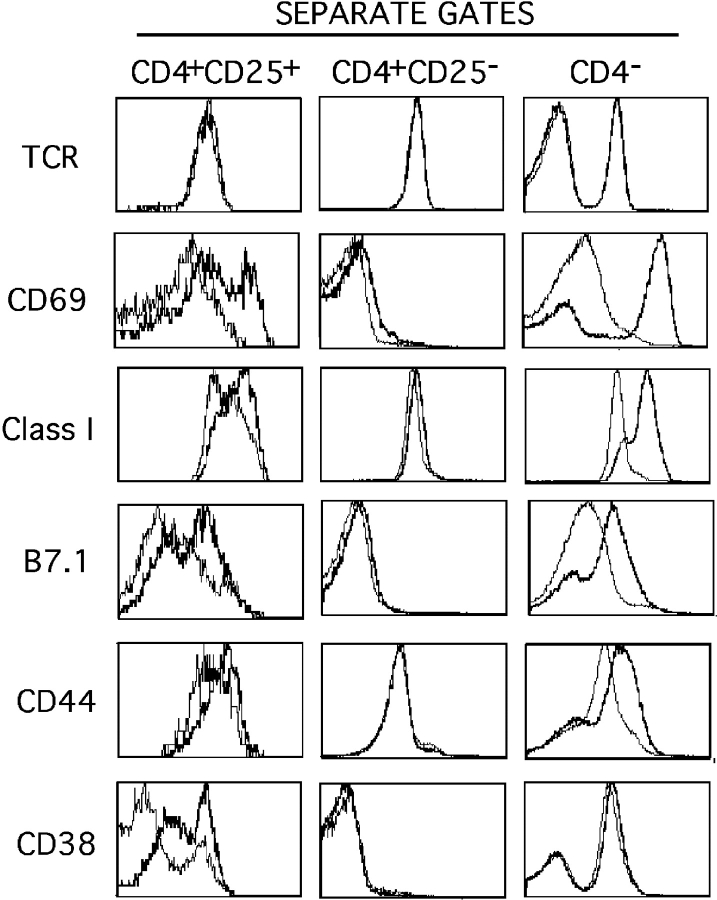
Next, we investigated whether the expression of TLR-4 by CD4+ CD45RBlow CD25+ cells was functionally relevant, i.e., if such cells responded to LPS, the classical TLR-4 ligand for B cells and macrophages (18). In a first step, we performed FACS® analyses of splenocytes treated for 18 h with LPS in vitro. As shown in Fig. 2 , CD4+ CD25+ cells, similarly to macrophages and B lymphocytes, up-regulate the expression of Class I, CD69, and B7.1 surface molecules. In addition, expression of other T cell–specific activation markers (CD44 and CD38) were also significantly enhanced whereas the levels of TCR expression were not affected. In contrast, expression of CTLA-4, cytoplasmic IL-10, and TNF-α remained unaltered upon exposure to LPS (not depicted). Finally, CD4 +CD25− cells failed to up-regulate any of these molecules when exposed to LPS in the same conditions. Up-regulation of CD69 expression by T cells upon exposure to LPS in vitro has been previously described (19) and is a response we attribute to CD4+ CD25+ cells. A previous report also described the induction of B7 expression on CD4+ CD25+ cells upon TCR triggering (20), a finding that we now extend to LPS activation.
Figure 2.
Activation of CD4+ CD25+ cells upon LPS treatment of splenocytes in vitro. Erythrocyte-depleted splenocytes were cultured for 18 h in the presence of LPS (bold) or medium alone (plain) and stained for CD4, CD25, and various other surface molecules. Histograms correspond to FACS® analyses for the indicated molecules inside the three independent gates. Representative analysis out of four independent assessments is shown. A minimum of 5,000 events was acquired in the CD4+ CD25+ gate.
LPS Directly Induces CD4+ CD25+ Cell Survival/Proliferation.
Previous studies indicate that LPS stimulation of B cells in vitro enhances their survival (21, 22). Similarly, survival of purified CD4+ CD25+ cells maintained in culture was greatly enhanced by the addition of LPS. Thus, although the number of cells recovered after a 3-d culture in medium supplemented or not with anti-CD3 antibody represented routinely 1–2% of the initial number of seeded cells, these values reached ∼15% in cultures containing anti-CD3 antibody and LPS or IL-2 and ∼30% when media was supplemented with both LPS and IL-2. In 6-d cultures containing anti-CD3 and IL-2, the addition of LPS for the first 3 d enhanced the cell recoveries from 32 to 62% (not depicted). These observations confirm that exocrine IL-2 acts as a survival/growth factor for CD4+ CD25+ cells (23) and demonstrates similar effects for LPS.
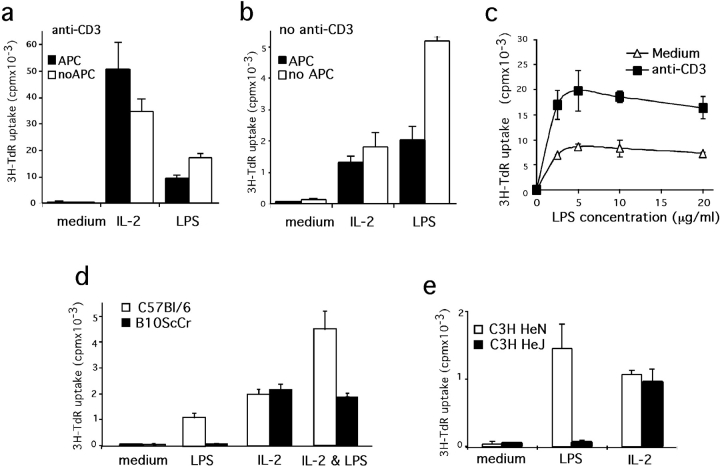
Because LPS is a potent B lymphocyte mitogen, we also tested its effect on CD4+ CD25+ proliferation. As reported (24, 25), highly purified CD4+ CD25+ cells do not proliferate in APC-supplemented cultures in response to TCR ligation whereas they do expand in the presence of IL-2. The addition of LPS to otherwise unresponsive cultures containing anti-CD3 antibodies and APC induced a readily detectable incorporation of [3H]thymidine that was consistently 20-fold higher than in controls, although 5-fold lower than IL-2–induced responses (Fig. 3 a). The removal of APC from these cultures resulted in higher [3H]thymidine incorporation in response to LPS while reducing the IL-2–mediated response. The responses of CD4+ CD25+ cells to LPS are also detectable in the absence of TCR triggering, albeit to a much lower level (Fig. 3 b, note the different scale). Interestingly, in the absence of both APC and anti-CD3 antibodies, responses to LPS scored higher than those to IL-2. Assessing the dose response of CD4+ CD25+ to LPS treatment (Fig. 3 c) reveals a peak of proliferation at 10 μg/ml, a result similar to what is routinely obtained when testing B lymphocytes (not depicted). This dose is much higher than that required to induce maximal responses in components of the innate immune system like dendritic cells and macrophages (in the order of nanograms). Contrary with B cell responses to LPS and anti-BCR (26), proliferation of CD4+ CD25+ is increased by the addition of anti-CD3 independently of the dose of LPS. These results indicate that LPS treatment induces TCR triggering sensitivity whereas TCR engagement does not affect the sensitivity to LPS.
Figure 3.
LPS directly induces CD4+ CD25+ proliferation through TLR-4. (a and b) Proliferative responses of CD4+ CD25+ cells to LPS are APC independent. Sort-purified CD4+ CD25+ from B6 animals were maintained in culture for 3 d in the presence of anti-CD3 (a) or not (b) and supplemented or not with APC (solid or open bars). For each culture condition, proliferative responses induced by either LPS or IL-2 are compared. (c) Purified CD4+ CD25+ cells were cultured for 3 d in the absence of APC in medium supplemented with various doses of LPS. (d and e) TLR-4–deficient CD4+ CD25+ cells do not proliferate in response to LPS. CD4+ CD25+ were sort purified from TLR-4–competent animals B6 (d) and C3H/HeN (e; open bars) or TLR-4–deficient B10ScCr (d) and C3H/HeJ (e) mice (solid bars) and maintained for 3 d in culture without APC nor anti-CD3. The medium was supplemented with LPS, IL-2, or both, as indicated. Each assay has been performed at least twice on independent cell samples and with similar results.
To directly assess whether LPS induces proliferation of CD4+ CD25+ cells through engagement of the TLR-4 receptor, we tested cells from LPS nonresponder and responder animals (27). In similar cultures to those described above, CD4+ CD25+ cells purified from TLR-4–deficient B10ScCr or C3H/HeJ (28, 29) mice did not respond to LPS (Fig. 3, d and e) regardless of the presence of APC or anti-CD3 (not depicted), but they did respond to IL-2. Furthermore, the LPS and IL-2 effects are additive in B6 animals but not in the B10ScCr T cells. Finally, using either B6 or B10ScCr APC altered neither the LPS response of B6 CD4+ CD25+ cells nor the unresponsiveness of TLR-4–deficient CD4+ CD25+ cells (not depicted), confirming that such T cell responses are not mediated by LPS activation of APC.
Taken together, these results demonstrate that LPS directly activates survival/proliferation of CD4+ CD25+ T cells through the TLR-4 receptor molecule.
TCR Triggering of LPS-activated CD4+ CD25+ Cells Induces IL-2 Production.
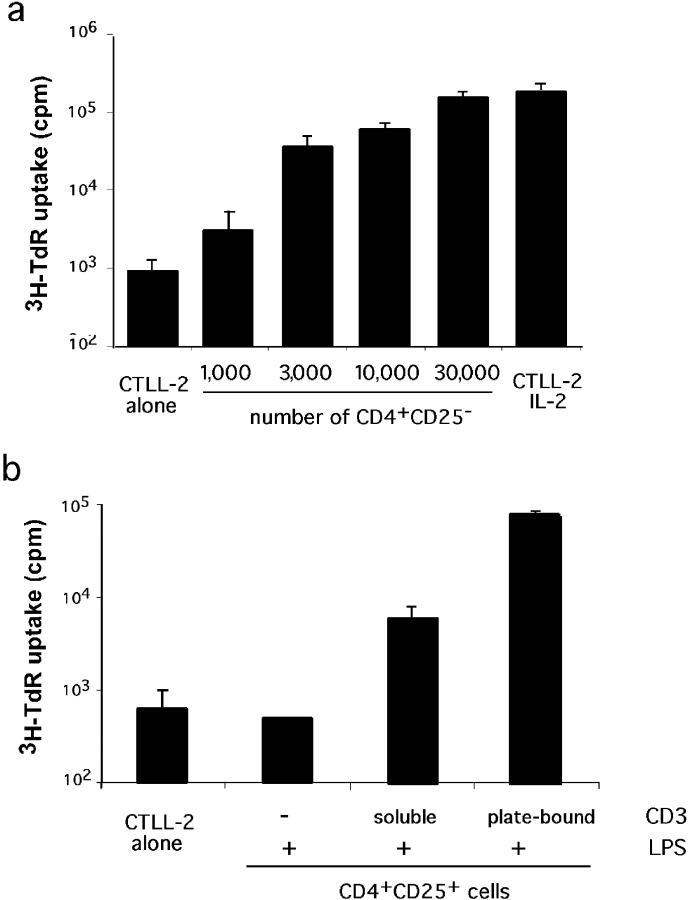
The inability of CD4+ CD25+ cells to engage in cycle upon TCR triggering has been attributed to their incapacity to produce IL-2 (24, 25). We described above that upon LPS stimulation, CD4+ CD25+ cells respond to TCR triggering by increased proliferation. This result may indicate that LPS induces endogenous IL-2 production. To directly address this point, we developed a highly sensitive assay to detect IL-2 produced by cells maintained in culture. As shown in Fig. 4 a, supernatants from 1,000 naive CD4 cells stimulated for 48 h in presence of APC and anti-CD3 induce readily detectable proliferation of the IL-2–dependent CTLL-2 cell line. This proliferation is proportional to the number of CD4 cells seeded. When testing the supernatants from 2.105 CD4+ CD25+ cells maintained in culture for 3 d in the presence of LPS, IL-2 production was undetectable (Fig. 4 b). However, similar cultures supplemented with anti-CD3 provided enough IL-2 to induce detectable proliferation of the CTLL-2 cells. Moreover, production of IL-2 was markedly enhanced when anti-CD3 was provided plate bound instead of soluble.
Figure 4.
IL-2 production by CD4+ CD25+ cells upon LPS stimulation. (a) Sensitivity of the assay. Supernatant of various numbers of CD4+ CD25− cells cultured for 48 h in the presence of APC and anti-CD3 were used as a source of IL-2 to support CTLL-2 cell proliferation. Proliferation of CTLL-2 cells in the absence of supernatant (alone) or when they were provided with saturating amount of IL-2 (IL-2) are shown. (b) CTLL-2 proliferation induced by supernatants of 2.105 CD4+ CD25+ cells maintained in culture for 3 d in the presence of LPS and anti-CD3 as indicated. Background proliferation is shown on the left. Each assay has been performed twice, each in triplicates.
These results indicate that LPS per se does not induce endogenous IL-2 production. However, it seems to confer sensitivity to TCR triggering, a signal that in turn leads to a low level of IL-2 synthesis.
Exposure of CD45RBlow CD25+ or CD45RBlow CD25− T Cells to LPS Markedly Enhances Their Suppressive Functions.
Next, we investigated whether LPS affects the effector functions of CD4+ CD25+ cells. Currently, the ability of CD4+ CD25+ cells to suppress the proliferative response of naive CD4 T cells to TCR triggering in the presence of APC is used as an in vitro correlate of their regulatory function in vivo (24, 25). According to the observations described above on LPS-induced survival/proliferation, we tested cells that were first differentially exposed to LPS for 3 d and then provided with IL-2 for an additional 3-d period. The inhibition of CD4+ CD25− proliferation is plotted in Fig. 5 as a function of the ratio of “regulatory” to target cells. The ratio corresponding to a 50% inhibition (I50%) can serve as an index of suppression efficiency. The I50% of freshly isolated CD4+ CD25+ cells was ∼0.25 (i.e., one regulatory to four target cells) in several independent experiments. As shown in Fig. 5 a, culturing this cell population for 6 d with IL-2 (and anti-CD3) improved the I50% to 0.07 (1 to 14), a result consistent with previous findings (30). In the same conditions, however, exposure to LPS for the first 3 d resulted in even higher levels of suppressive activity, as indicated by an I50% lower than 0.025, the last point in our titrations (1 to 40). We also tested CD4+ CD25+ cells that were pretreated with LPS in the absence or presence of plate-bound anti-CD3 antibodies. Independently of the presence of anti-CD3, the I50% was consistently fourfold higher when cells had been exposed successively to LPS and IL-2, as compared with IL-2 alone (not depicted). These results demonstrate LPS-dependent activation of effector functions in CD4+ CD25+ cells.
Figure 5.
LPS treatment enhances the suppressor function of Treg. (a) Suppressor efficiency of CD4+ CD25+ is greatly enhanced upon exposure to LPS. Sort-purified CD4+ CD25+ T cells were maintained in culture in medium supplemented with anti-CD3 and either LPS for 3 d followed by 3 d with IL-2 (LPS/IL-2) or with IL-2 for 6 d (IL-2). The same cell population not submitted to culture was used as reference control (fresh). Proliferation of CD4+ CD25− stimulated with anti-CD3 and APC in the presence of increasing numbers of these CD4+ CD25+ cells was monitored on day 3. The percent of inhibition ([cpm in control − cpm in experiment]/cpm in control) is plotted versus the ratio of CD4+ CD25+/CD4+CD25− cell number at the origin of the culture. (b and c) LPS treatment of CD4+ CD45RBlow CD25− cells reveals their suppressor functions. Sort-purified CD4+ CD45RBlow CD25− cells were tested in suppression assays after 6 d culture (b) or after only 3 d exposure to LPS and anti-CD3. Suppression by CD4+ CD25+ treated similarly is also shown. (c) Freshly isolated CD4+ CD25+ and CD4+ CD45RBlow CD25− served as control. Nomenclatures are as described in a. Each measurement has been performed at least twice on independent cell samples and resulted in similar curves.
CD4+ CD45RBlow CD25− cells exert regulatory functions in vivo (31), but do not show suppressor activity in vitro when freshly isolated (Fig. 5 b and reference 24). Strikingly, however, CD4+ CD45RBlow CD25− cells manifested a remarkable I50% of ∼0.1 (1 to 10) after in vitro exposure to LPS followed by IL-2 (Fig. 5 b). As titration curves for cells exposed sequentially to LPS and IL-2 or to IL-2 alone were similar, we also tested CD4+ CD45RBlow CD25− cells immediately after 3 d of LPS activation. As can be seen in Fig. 5 c, LPS clearly promotes suppressive activity in this cell population. CD4+ CD25+ similarly exposed for 3 d to LPS without subsequent exposure to IL-2 showed lower suppressor efficiency than untreated cells. This result is best explained by the poor survival of CD4+ CD25+ cells in absence of exogenous IL-2 (as described above), preventing a proper evaluation of suppressor efficiency on a per cell basis. This argument is supported by our results above, which show that when subsequently provided with IL-2, the same cell population displays maximal suppressor efficiency. Finally, as expected, CD4+ CD45RBhigh cells stimulated for 6 d in cultures supplemented with IL-2 and soluble anti-CD3 did not show any suppressor activity, regardless of their differential exposure to LPS for the first 3 d of culture (not depicted).
We conclude that LPS markedly enhances the suppressive activity of naturally activated CD45RBlow T cells, whether these are CD25+ or CD25−.
LPS-activated CD4+ CD25+ T Cells Prevent Wasting Disease Induced by Naive CD4 T Cells in Alymphoid Recipients.
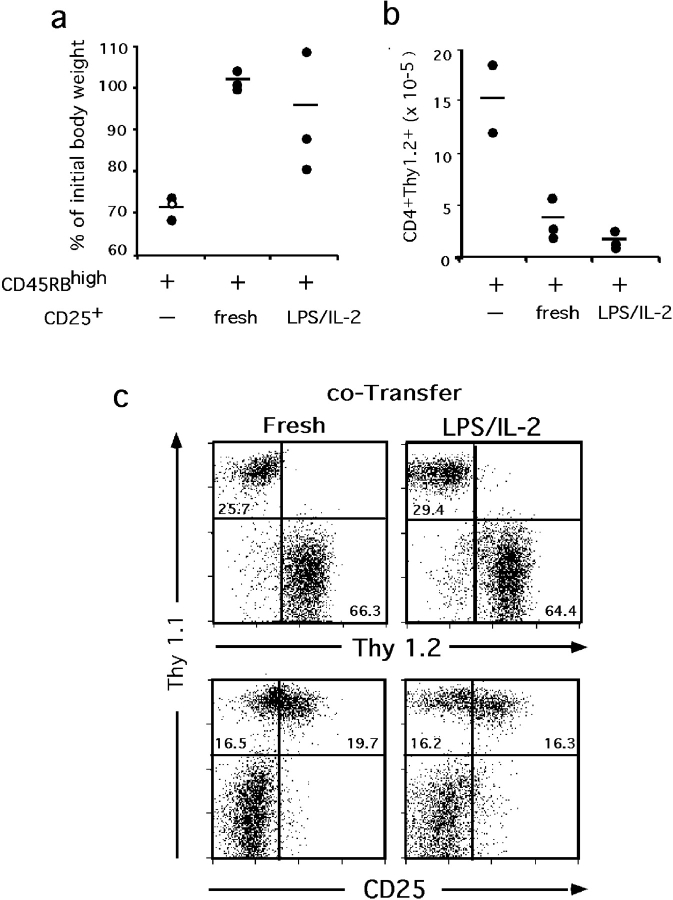
To assess whether the suppressive function we monitored in vitro correlates with in vivo regulatory functions, we tested the ability of LPS-treated CD4+ CD25+ cells to control the wasting disease that is induced in RAG-deficient animals upon transfer of naive CD4 cells (1). C57BL/6-H-2 u RAG-1−/− recipients of CD4+ CD45RBhigh (Thy1.2+) cells developed a lethal wasting disease in less than 3 wk after adoptive transfer. This extremely fast development associated neither with pathological intestinal inflammation nor pneumonia, contrarily to what is observed in other genetic backgrounds (1, 2). Animals that received CD4+ CD25+ (Thy1.1), either freshly isolated or exposed to LPS followed by IL-2, remained healthy (Fig. 6 a). In addition, cotransfer with either population of CD4+ CD25+ cells reduced the number of transferred naive Thy1.2+ lymphocytes recovered from mesenteric LNs (Fig. 6 b), spleen and pooled axillary and cervical LNs (not depicted) to a similar extent. Finally, the numbers of transferred CD4+ CD25+ cells recovered in recipients of the cotransfers were similar in the two groups of animals as were their respective levels of CD25 expression (exemplified in Fig. 6 c).
Figure 6.
LPS-treated CD4+ CD25+ are efficient regulatory cells in vivo. RAG-1–deficient animals (three per experimental group) received 105 CD4+ CD45RBhigh Thy1.2 cells alone or together with the same number of CD4+ CD25+ Thy1.1 cells, either freshly isolated or maintained in culture sequentially with LPS and IL-2 for a total of 6 d (as described in Fig. 4). Mice were analyzed at 21 d after transfer. (a) Control of wasting disease. The white dot represents an animal found dead on day 17 after transfer. Its weight monitored on day 16 was used. (b) Control of lymphocyte expansion. Numbers of CD4+ Thy1.2+ cells (originally CD45RBhigh) recovered in the mesenteric LNs. (c) Viability and phenotype of the transferred CD4+ CD25+ cells. Representative staining of axillary LNs from recipients of cotransfers.
These results demonstrate efficient control of inflammation and homeostatic expansion by LPS activated CD4+ CD25+ cells in vivo.
Discussion
Here we provide the first evidence for the selective expression of pathogen-associated “pattern recognition receptors” by Treg and the functional relevance of one of them, TLR-4, in the physiology of this cell subpopulation. Our findings that LPS promotes Treg survival/proliferation and enhances their suppressive functions also demonstrate that T cells involved in the control of inflammation directly respond to proinflammatory microbial products. Thus, this analysis provides another link between innate and adaptive immunity and reveals a novel mechanism for the control of immune responses.
The rather high proportion of TLR genes, classical components of the innate immune system, selectively expressed in a subset of CD4 cells endowed with the function of regulating adaptive immune responses, may help us delineate the evolutionary scheme that led to the establishment of an adaptive immune system. Among the nine known murine TLR genes analyzed here, four (TLR-4, -5, -7, and -8) are selectively expressed in this particular CD4+ subset. TLR-5 expression appeared the most selective for Treg. In line with the present observations on LPS activation of Treg through TLR-4, it is striking that the TLR-5 receptor binds flagellin (32), another bacterial product. Furthermore, all endogenous ligands identified to date, which in addition to LPS, bind TLR-4 and induce signaling in various cell types, are molecules involved in inflammatory responses. Thus, this is the case for HSP, HSP-60 (10), HSP-70 (12), and GP96 (11), heparan sulfate (33), surfactant protein A (34), and the product of degradation of the extracellular matrix hyaluronic acid (14). Natural ligands for TLR-7 and TLR-8 are not yet identified, but the finding that seven out of nine TLR molecules are expressed by Treg suggests that a rather large universe of inflammation-related endogenous and pathogen-associated molecules might directly modulate their activities.
LPS effects on murine T cells have been generally interpreted as indirectly mediated through activation of accessory cells (19, 35, 36), although sporadic evidence of direct triggering has also been provided (37–39). The frequency of CD4 T cells expressing TLR-4 at the cell surface could not be assessed because the available antibodies that specifically recognize the murine TLR-4–MD2 complex on peritoneal macrophages fails to interact with both B and T cells (40 and unpublished data). Assuming homogeneous expression levels by positive cells, a quantitative interpretation of the present RT-PCR analyses would indicate TLR-4 expression in, at most, some 15% of the total CD4 compartment (10% CD25+ and 1/4 of the 20% CD45RBlow CD25−). In addition, the results of LPS-stimulated cell cultures suggest that only a fraction of CD4+ CD45RBlow CD25+/− cells respond to TLR-4 ligation, thus being compatible with a previous report indicating that 3% total T splenocytes are LPS responders (37). The essential finding here, namely that all CD4 T cells that respond to LPS are encompassed in the Treg subpopulation, provides additional evidence for the evolutionary and functional relationship between innate and adaptive immunity. In turn, our results may suggest that Treg belong to a particular class of “nonclassical” αβ T lymphocytes, along with NK and γδ T cells. Whether the selective TLR expression by Treg described here defines a unique lineage or a particular differentiation stage will be a crucial issue to address in future experiments.
Several models of inflammation induced by pathogens in immunocompromised animals have shown that Treg are necessary to prevent deleterious immune responses (1, 2). The process of Treg activation in these systems had not been addressed until now. Our findings that a bacterial product can directly activate Treg and enhance their effector functions suggest polyclonal activation of these cells during infection. In turn, they provide the first evidence that cells involved in the control of inflammation respond to proinflammatory microbial molecules and add to previous reports demonstrating that Treg are particularly sensitive to inflammatory chemokines (6, 7). The survival/proliferative responses to LPS, TCR ligation, and IL-2 may suggest, however, that during bacterial infection unspecific activation of Treg through TLR is amplified by specific recognition of antigens. Moreover, specific delayed-type hypersensitivity and graft-versus-host reactions, as well as graft rejection and classical immunization, have all been shown to be down modulated or abolished by pretreatment of mice with various doses of LPS (41–43). Activation of Treg by LPS may well be the basis for these observations. In the course of an infection, however, Treg activation does not fully impair the protective response even though it may limit its magnitude (2) and, certainly, its deleterious pathogenic consequences for the host (1, 2). The specific immune response to nonself antigens may take place as the consequence of a natural ratio of regulatory to effector cells heavily biased toward the latter and a Treg TCR repertoire seemingly biased toward the recognition of self-antigens (17, 44–46). Thus, presentation of nonself peptides during acute infection is predominant, resulting in the initial preferential activation of naive responder cells. As infection regresses and pathogens are cleared, presentation of self-ligands to Treg predominates, leading to the control of inflammation and preventing the activation of naive, autoreactive T cells by self-peptides presented in an inflammatory context. Importantly, inflammatory stimuli may lead to detrimental systemic effects and the stimulation of Treg through TLRs, either by microbial or endogenous ligands released during stress or tissue damage, might be required to prevent generalized immunopathologies.
Finally, these findings may contribute novel insights to the cellular basis of chronic infections and to the beneficial effect of various infections on the onset of autoimmune diseases (47, 48).
Acknowledgments
We thank Rosa Maria Santos for antibody production and labeling and Bruce Lenhart and Dolores Bonaparte for mouse production. We are grateful to Alexander Poltorak for his help in designing the TLR-4 RT-PCR protocol. We are most indebted to Werner Haas, Jorge Carneiro, and Antonio Coutinho for continuous support during the development of this work and Miguel Soares and Jan Andersson for critical reading of the manuscript.
This work was supported by the Fundação para a Ciênca e a Tecnologia, Portugal, grant 43063MGI2001, and fellowships PRAXISXXI/BD/21599/99 and 21671/99 to I. Caramalho and T.L. Carvalho, respectively.
Footnotes
Abbreviations used in this paper: HSP, heat shock proteins; I50%, 50% inhibition; TLR, Toll-like receptor; Treg, regulatory T cells.
References
- 1.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. [DOI] [PubMed] [Google Scholar]
- 2.Hori, S., T.L. Carvalho, and J. Demengeot. 2002. CD25+ CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291. [DOI] [PubMed] [Google Scholar]
- 3.Zuany-Amorim, C., E. Sawicka, C. Manlius, A. Le Moine, L.R. Brunet, D.M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625–629. [DOI] [PubMed] [Google Scholar]
- 4.McGuirk, P., C. McCann, and K.H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho, A., S. Hori, T. Carvalho, I. Caramalho, and J. Demengeot. 2001. Regulatory T cells: the physiology of autoreactivity in dominant tolerance and “quality control” of immune responses. Immunol. Rev. 182:89–98. [DOI] [PubMed] [Google Scholar]
- 6.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+ CD25+ regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bystry, R.S., V. Aluvihare, K.A. Welch, M. Kallikourdis, and A.G. Betz. 2001. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2:1126–1132. [DOI] [PubMed] [Google Scholar]
- 8.Barton, G.M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380–383. [DOI] [PubMed] [Google Scholar]
- 9.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558–561. [DOI] [PubMed] [Google Scholar]
- 11.Vabulas, R.M., S. Braedel, N. Hilf, H. Singh-Jasuja, S. Herter, P. Ahmad-Nejad, C.J. Kirschning, C. Da Costa, H.G. Rammensee, H. Wagner, et al. 2002. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J. Biol. Chem. 277:20847–20853. [DOI] [PubMed] [Google Scholar]
- 12.Vabulas, R.M., P. Ahmad-Nejad, S. Ghose, C.J. Kirschning, R.D. Issels, and H. Wagner. 2002. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277:15107–15112. [DOI] [PubMed] [Google Scholar]
- 13.Asea, A., M. Rehli, E. Kabingu, J.A. Boch, O. Bare, P.E. Auron, M.A. Stevenson, and S.K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028–15034. [DOI] [PubMed] [Google Scholar]
- 14.Termeer, C., F. Benedix, J. Sleeman, C. Fieber, U. Voith, T. Ahrens, K. Miyake, M. Freudenberg, C. Galanos, and J.C. Simon. 2002. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuguchi, T., K. Takagi, T. Musikacharoen, and Y. Yoshikai. 2000. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 95:1378–1385. [PubMed] [Google Scholar]
- 16.Mokuno, Y., T. Matsuguchi, M. Takano, H. Nishimura, J. Washizu, T. Ogawa, O. Takeuchi, S. Akira, Y. Nimura, and Y. Yoshikai. 2000. Expression of toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J. Immunol. 165:931–940. [DOI] [PubMed] [Google Scholar]
- 17.Hori, S., M. Haury, A. Coutinho, and J. Demengeot. 2002. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 99:8213–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752. [PubMed] [Google Scholar]
- 19.Vilanova, M., D. Tavares, P. Ferreira, L. Oliveira, A. Nobrega, R. Appelberg, and M. Arala-Chaves. 1996. Role of monocytes in the up-regulation of the early activation marker CD69 on B and T murine lymphocytes induced by microbial mitogens. Scand. J. Immunol. 43:155–163. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact–dependent immunosuppression by CD4+ CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechsler-Reya, R.J., and J.G. Monroe. 1996. Lipopolysaccharide prevents apoptosis and induces responsiveness to antigen receptor cross-linking in immature B cells. Immunology. 89:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillot, D.A., R. Merino, J.C. Pena, W.C. Fanslow, F.D. Finkelman, C.B. Thompson, and G. Nunez. 1996. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J. Exp. Med. 183:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papiernik, M., M.L. de Moraes, C. Pontoux, F. Vasseur, and C. Penit. 1998. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371–378. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 25.Thornton, A.M., and E.M. Shevach. 1998. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modigliani, Y., J. Demengeot, R. Vasconcellos, J. Andersson, A. Coutinho, and A. Grandien. 1997. Differential sensitivity of B lymphocyte populations to IgM receptor ligation is determined by local factors. Int. Immunol. 9:755–762. [DOI] [PubMed] [Google Scholar]
- 27.Coutinho, A., L. Forni, F. Melchers, and T. Watanabe. 1977. Genetic defect in responsiveness to the B cell mitogen lipopolysaccharide. Eur. J. Immunol. 7:325–328. [DOI] [PubMed] [Google Scholar]
- 28.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi, S.T., L. Lariviere, G. Leveque, S. Clermont, K.J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 31.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T.C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166:3008–3018. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi, F., K.D. Smith, A. Ozinsky, T.R. Hawn, E.C. Yi, D.R. Goodlett, J.K. Eng, S. Akira, D.M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410:1099–1103. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, G.B., G.J. Brunn, Y. Kodaira, and J.L. Platt. 2002. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 168:5233–5239. [DOI] [PubMed] [Google Scholar]
- 34.Guillot, L., V. Balloy, F.X. McCormack, D.T. Golenbock, M. Chignard, and M. Si-Tahar. 2002. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J. Immunol. 168:5989–5992. [DOI] [PubMed] [Google Scholar]
- 35.Castro, A., V. Bemer, A. Nobrega, A. Coutinho, and P. Truffa-Bachi. 1998. Administration to mouse of endotoxin from gram-negative bacteria leads to activation and apoptosis of T lymphocytes. Eur. J. Immunol. 28:488–495. [DOI] [PubMed] [Google Scholar]
- 36.Tough, D.F., S. Sun, and J. Sprent. 1997. T cell stimulation in vivo by lipopolysaccharide (LPS). J. Exp. Med. 185:2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel, S.N., M.L. Hilfiker, and M.J. Caulfield. 1983. Endotoxin-induced T lymphocyte proliferation. J. Immunol. 130:1774–1779. [PubMed] [Google Scholar]
- 38.Watanabe, T., T. Inoue, H. Ochi, M. Terashima, Y. Asano, and T. Nakatani. 1999. Lipid A directly inhibits IL-4 production by murine Th2 cells but does not inhibit IFN-gamma production by Th1 cells. Eur. J. Immunol. 29:413–418. [DOI] [PubMed] [Google Scholar]
- 39.Ismaili, J., J. Rennesson, E. Aksoy, J. Vekemans, B. Vincart, Z. Amraoui, F. Van Laethem, M. Goldman, and P.M. Dubois. 2002. Monophosphoryl lipid A activates both human dendritic cells and T cells. J. Immunol. 168:926–932. [DOI] [PubMed] [Google Scholar]
- 40.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471–3475. [DOI] [PubMed] [Google Scholar]
- 41.Persson, U. 1977. Lipopolysaccharide-induced suppression of the primary immune response to a thymus-dependent antigen. J. Immunol. 118:789–796. [PubMed] [Google Scholar]
- 42.Molendijk, A., H. Bril, L.M. Hussaarts-Odijk, R.J. van Gurp, and R. Benner. 1988. Lipopolysaccharide-induced suppression of DTH-reactivity to histocompatibility antigens. I. Kinetic aspects and specificity. Immunobiology. 176:255–271. [DOI] [PubMed] [Google Scholar]
- 43.Winchurch, R.A., C. Hilberg, W. Birmingham, and A.M. Munster. 1982. Inhibition of graft rejection by LPS: further evidence for effects on T lymphocytes. J. Reticuloendothel. Soc. 31:31–42. [PubMed] [Google Scholar]
- 44.Kawahata, K., Y. Misaki, M. Yamauchi, S. Tsunekawa, K. Setoguchi, J. Miyazaki, and K. Yamamoto. 2002. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol. 168:4399–4405. [DOI] [PubMed] [Google Scholar]
- 45.Romagnoli, P., D. Hudrisier, and J.P. van Meerwijk. 2002. Preferential recognition of self antigens despite normal thymic deletion of CD4(+)CD25(+) regulatory T cells. J. Immunol. 168:1644–1648. [DOI] [PubMed] [Google Scholar]
- 46.Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 47.Serreze, D.V., K. Hamaguchi, and E.H. Leiter. 1989. Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2:759–776. [DOI] [PubMed] [Google Scholar]
- 48.Bach, J.F. 2001. Protective role of infections and vaccinations on autoimmune diseases. J. Autoimmun. 16:347–353. [DOI] [PubMed] [Google Scholar]