It is a key issue in immunology to understand how the immune system discriminates between self- and nonself, inhibiting autoimmune responses but allowing the host to mount immune responses against invading microbes (1–3). This is due to the fact that once antigen-specific lymphocytes are activated in adaptive immune responses, they exhibit essentially the same effector activity whether they respond to a microbe or to a self-constituent. That is, once aberrant immune responses (e.g., autoimmune, immunopathologic, or allergic responses) are triggered, serious damage to the host may ensue because of the power of potent effector activity, high antigen specificity, and immunologic memory of adaptive immunity. There is now accumulating evidence in humans and animals that the normal immune system harbors naturally arising CD4+ regulatory T (TR) cells, which play key roles in controlling such aberrant immune responses (4, 5).
The majority, if not all, of naturally occurring CD4+ TR cells constitutively express CD25 (IL-2 receptor α chain) in the physiologic state (4, 5). At least some of them are produced by the normal thymus as a functionally mature T cell subpopulation with a broad TCR repertoire recognizing self- and nonself antigens (4). They are engaged in the maintenance of immunologic self-tolerance by actively suppressing the activation and expansion of self-reactive lymphocytes that may cause autoimmune disease. This is illustrated by the finding that the removal of CD25+ CD4+ T cells, which constitute 5–10% of peripheral CD4+ T cells in mice, leads to spontaneous development of various autoimmune diseases in otherwise normal mice. Reconstitution of the depleted population prevents the autoimmunity (6). The removal of this population also triggers excessive or misdirected immune responses to microbial antigens, causing immunopathology. For example, depletion of TR cells elicits inflammatory bowel disease in mice due to hyperreaction of the remaining T cells to commensal bacteria in the intestine (7). Their depletion similarly precipitates severe pneumonitis in mice that are opportunistically infected with Pneumocystis carinii (8). In contrast, such treatments can enhance protective immune responses against invading microbes including bacteria, viruses, fungi, and intracellular parasites, leading to their eradication from the host (9–13). Thus, naturally occurring CD25+ CD4+ TR cells not only inhibit immune responses against self-antigens but also hamper or suppress those against microbes invading or cohabiting with the host. This raises a critical question: how do CD25+ CD4+ TR cells manage to suppress autoimmune responses or excessive antimicrobial immune responses causing immunopathology, but allow protective responses against invading pathogenic microbes?
Expression of Toll-like Receptors (TLRs) on CD25+ CD4+ TR Cells.
A report by Caramalho et al. (14) in this issue provides some clues to the above question. It shows that naturally arising CD25+ CD4+ TR cells in normal mice selectively express several members of the TLR family and that stimulation of CD25+ CD4+ TR cells through TLRs can expand them and strengthen their suppressive activity. TLRs, homologues of Toll receptor in Drosophila, are germline-encoded receptors that recognize pathogen-associated molecular patterns shared by large groups of microbes or certain endogenous molecules released during inflammation (15, 16). The ten members of TLR (TLRs 1–10) that have been so far reported in humans and mice respond to different ligands. For example, TLR4 recognizes LPS, a major component of gram-negative bacteria, and is also implicated in the recognition of heat shock protein (HSP) 60/70 and extracellular matrix breakdown products derived from necrotic tissue in inflammation (15, 16). Different classes of TLRs are differentially expressed on immune cells such as dendritic cells (DCs), macrophages, B cells, and certain γδ T cells (15–17). Caramalho et al. (14) show that CD25+ CD4+ T cells in normal naive mice selectively express TLR4, 5, 7, and 8 (TLR1, 2, and 6 appear to be more broadly expressed on CD4+ T cells, and not confined to CD25+ CD4+ T cells). In vitro stimulation of CD25+ CD4+ T cells with LPS elicits their proliferation, prolongs their survival, and augments their in vitro suppressive activity even in the absence of APCs, indicating that LPS directly acts on TLR4 molecules expressed by TR cells. On the other hand, it is known that LPS stimulation of DCs triggers their maturation, leading to enhanced antigen processing, increased MHC expression, migration to the lymph nodes, production of proinflammatory cytokines and chemokines, and most importantly, induction of costimulatory molecules such as CD80 and CD86 (18). Thus, stimulation of TLRs have different effects on immune responses, i.e., one is to trigger DC maturation and augment T cell–mediated adaptive immunity and another is to activate endogenous CD25+ CD4+ TR cells and thereby down-regulate immune responses. Although these effects are apparently conflicting, it is of note that the concentration of LPS required for evoking the proliferation of TR cells and augmenting their suppressive activity in vitro is several orders of magnitude higher than the concentration required for in vitro activation of DCs (14, 18). It is therefore likely that upon gram-negative bacterial infection, LPS stimulation of TLRs on DCs will trigger maturation of DCs, thereby evoking the activation, expansion, and differentiation of microbe-specific naive T cells to effector T cells. If so large an amount of LPS is produced, TR cells activated by LPS through TLRs may augment their suppressive activity and thereby prevent local or systemic immunopathology (such as septic shock) due to the production of large amounts of proinflammatory cytokines (such as TNFα and IL-1) by macrophages. IL-10 produced by activated TR cells may suppress activation of macrophages and their cytokine production (12). Furthermore, IL-2 in the local milieu and TCR stimulation by microbial antigens may synergistically augment the suppressive activity of TLR-stimulated TR cells (14). The TR cells thus activated may also suppress the activation and expansion of self-reactive T cells that are recruited to the infection site where APCs present self-peptides derived from damaged tissues.
Tuning of the Intensity of T Cell–mediated Immunoregulation.
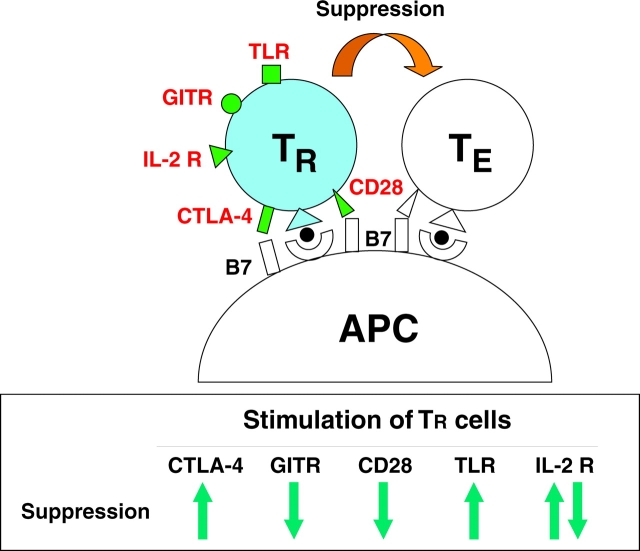
In addition to TLRs, other molecules expressed by endogenous CD25+ CD4+ TR cells transduce signals to TR cells upon interacting with particular ligands and thereby contribute to tuning the degree of their expansion and suppressive activity. Accessory molecules on TR cells, for example, interact with costimulatory molecules on APCs, DCs in particular, and thereby confer signals augmenting or attenuating the activation state of TR cells with consequences for their suppressive activity. Unlike other T cells, CD25+ CD4+ TR cells constitutively express CTLA-4 (CD152) and this appears to be required for their activation because specific blockade of the interaction between CTLA-4 on CD25+ CD4+ TR cells and CD80/CD86 on APCs abrogates suppression (4). In contrast, active signaling through CD28, along with TCR stimulation, neutralizes suppression (4). CTLA-4 has much higher (∼100-fold) affinity for CD80/CD86 molecules than CD28 (19). Based on these findings, the following scenario can be envisaged for the roles of TR cells in controlling immune responses in the normal state or upon infection (Fig. 1) . In the absence of infection, APCs express basal levels of CD80/CD86 and a low level of class II MHC binding self-peptides. CD25+ CD4+ TR cells with self-specificity might be activated by recognizing self-peptides (e.g., derived from apoptotic cells) and receiving a signal through CTLA-4, but not through CD28, and thereby suppress autoreactive T cells. Upon infection, APCs are activated and increase their expression of CD80/CD86 and class II MHC molecules bound to microbe-derived peptides. CD25+ CD4+ TR cells recognizing the peptides will receive a signal through CD28 in addition to CTLA-4, lessen their suppressive activity, and allow the activation/expansion of effector T cells capable of eliminating microbes. IL-2 from responding effector T cells and a signal through glucocorticoid-induced TNF receptor–related gene (GITR) also act to attenuate the suppressive activity of TR cells in synergy with the signal through CD28 (Fig. 1 and see below; reference 20). With the elimination of microbes, together with a reduction of the APCs presenting the microbial peptides and a decrease of CD80/CD86 expression, TR cells revert to their original state and reacquire the basal regulatory activity.
Figure 1.
Signaling to CD25+ CD4+ TR cells via several cell surface molecules including TCR, CD28, CTLA-4, GITR, IL-2 R (CD25), and TLRs results in augmentation or attenuation of their suppressive activity. During the proliferation of TR cells in the presence of high dose IL-2 along with TCR stimulation, they lose suppressive activity whereas they show augmented suppressive activity upon withdrawal of IL-2 after expansion (references 22 and 23). TLR stimulation may also show a similar sequence of events, i.e., loss of suppressive activity during proliferation and augmented suppression after expansion.
CD25+ CD4+ TR cells constitutively express GlTR (20, 21). Active signaling through GITR to CD25+ CD4+ TR cells attenuates their suppressive activity, leading to enhanced immune responses to self- and nonself antigens (20). Mere blockade of GITR on TR cells fails to abrogate the suppression (20). GITR-ligand (L) is induced on DCs upon maturation (unpublished data). Thus, GITR–GITR-L interaction may also contribute to the tuning of CD25+ CD4+ TR cell–mediated immunoregulation.
The expression patterns of other accessory molecules on CD25+ CD4+ TR cells are CD45RBlow, CD44high, CD5high, CD54 (intercellular adhesion molecule 1)high, CD11a/CD18 (LFA-1)high, partly CD62Llow (4). This pattern suggests that they might be in a “primed,” “activated,” “effector,” or “memory” state probably due to constant stimulation by self-peptides/MHCs on APCs in the normal internal milieu (4). Upon infection, they can be more swiftly recruited to the infection site than other T cells as recently demonstrated by Belkaid et al. (12).
Besides accessory molecules on CD25+ CD4+ TR cells, cytokines also play key roles in tuning their expansion and activation. High dose IL-2 along with TCR stimulation, for example, triggers their proliferation and neutralizes the suppressive activity during their proliferation (22). Such expanded TR cells show more potent suppressive activity than before expansion (23). TLR4 stimulation with LPS in the presence of IL-2 also results in their acquisition of more potent suppressive activity than LPS stimulation alone (14). It is thus likely that T cell immune responses to microbes may locally produce a high IL-2 milieu, contributing to the expansion of microbe-specific TR cells at the site of infection. Other cytokines may also participate in the activation or expansion of endogenous TR cells (7, 12).
Collectively, these findings indicate that various signals contribute to the activation and expansion of endogenous CD25+ CD4+ TR cells and thereby to the fine tuning of the suppressive activity of TR cells, hence the magnitude of immune responses. Strong suppression may make the host succumb to the infection whereas weak suppression may lead to complete eradication of the microbes but may lose concomitant immunity because of insufficient maintenance of memory T cells due to lack of microbe persistence (12). It is therefore required to elucidate how various signals, some of which are apparently in opposition (e.g., signals through CTLA-4 vs. CD28), are integrated in TR cells to determine the degree of their expansion and suppression.
Adaptive Nature of CD25+ CD4+ TR Cells and Their Roles in Immunological Diseases.
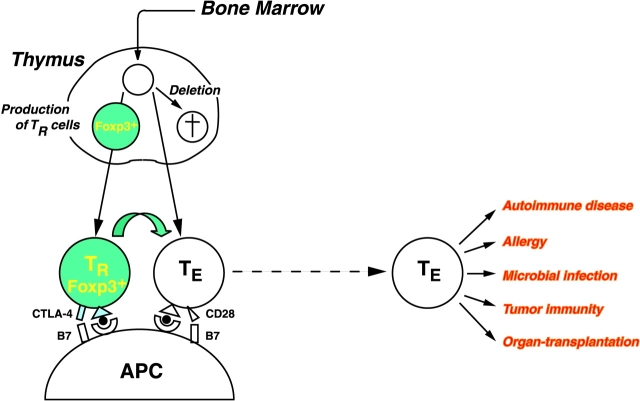
There is now evidence not only for the presence of CD25+ CD4+ TR cells in humans but also for their essential role in controlling autoimmunity, immunopathology, and allergy in human diseases. For example, an X-linked recessive autoimmune/inflammatory syndrome called IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) develops autoimmune diseases (such as type 1 diabetes), inflammatory bowel disease, and allergy similar to those produced in mice by depletion of CD25+ CD4+ TR cells (24). The causative gene, Foxp3, which encodes a transcription factor, is specifically expressed in CD25+ CD4+ T cells in the thymus and periphery. Forced expression of the Foxp3 gene in naive T cells converts them to TR cells phenotypically and functionally similar to naturally arising CD25+ CD4+ TR cells (25). The results indicate that a genetically determined abnormality in the generation or function of CD25+ CD4+ TR cells can be a cause of immunological disease in humans (Fig. 2) . It remains to be determined how Foxp3, a master regulatory gene for the development and/or function of CD25+ CD4+ TR cells, controls the expression and functions of TR-associated molecules such as CD25, CTLA-4, GITR, and TLRs. It is likely that other genetic abnormalities or environmental insults may also affect CD25+ CD4+ TR cells and thereby cause autoimmune and other immunological diseases (26). Furthermore, defects or polymorphism of other genes encoding those molecules (including TLRs) that contribute to the control of TR cells (see above) may determine the susceptibility or resistance to autoimmunity, allergy, and immunopathology.
Figure 2.
At least some of CD25+ CD4+ TR cells are produced by the normal thymus as a functionally mature T cell subpopulation. They specifically express the Foxp3 gene. Reduction of CD25+ CD4+ TR cells or attenuation of their suppressive activity by manipulating various molecules shown in Fig. 1 may elicit autoimmunity or enhance autoimmunity, tumor immunity, microbial immunity, or allergy. In contrast, an increase of the number of TR cells or augmentation of their suppressive activity can delay the rejection of organ grafts and induce transplantation tolerance.
As discussed above, one important feature of naturally arising CD25+ CD4+ TR cells is that they are immunologically adaptive, i.e., they can expand and augment their suppressive activity when stimulated through TCR along with high dose IL-2 or through TLRs (Fig. 1). This means that repeated microbial infections may not only increase T cell immunity by generating memory T cells but also strengthen the suppressive activity of endogenous CD25+ CD4+ TR cells. Another level of equilibrium set between the strengthened TR cells and the memory T cells may ensure effective secondary responses without damage to the host upon reinfection. Furthermore, considering that CD25+ CD4+ TR cells activated by a specific antigen exert antigen-nonspecific suppression, TR cells stimulated by microbial antigens may suppress self-reactive T cells as well in a bystander manner (22, 23). For example, inoculation of complete Freund's adjuvant, which contains killed tubercle bacilli, inhibits the development of autoimmune disease in animal models (27, 28). It is likely in this finding that HSP of tubercle bacilli may activate endogenous TR cells through stimulating their TLRs, thereby suppressing self-reactive T cells (29). Other TLR ligands including endogenous HSPs may also be able to strengthen the regulatory activity of endogenous TR cells and prevent autoimmune disease.
Furthermore, assuming that endogenous TR cells play key roles in preventing autoimmune diseases and allergy in humans as exemplified in IPEX syndrome, the recent increase of the incidence of autoimmune disease and allergy in developed countries could be attributed to insufficient adaptive expansion or strengthening of endogenous TR cells due to less frequent opportunities for microbial infections in hygienic societies (26, 30).
Future Perspective.
In addition to the control of T cell activation or nonactivation at the level of APCs that discriminate between “infectious nonself” and “noninfectious self” or sensing “danger” signals (2, 3), TR cells mediate another mode of control of the activation and expansion of aberrant T cells. As shown by Caramalho et al. (14), CD25+ CD4+ TR cells are an evolutionarily unique T cell subpopulation bearing two kinds of the receptor: TCRs that recognize a broad repertoire of various self- and nonself antigens and TLRs that sense conserved molecular patterns shared by microbial components or certain endogenous molecules (such as HSPs) released in inflammation. This dual signaling source for TR cells, together with other signals via accessory molecules, may enable CD25+ CD4+ TR cells to efficiently and finely tune their activity to control adaptive immune responses against self- and nonself antigens (Fig. 2; reference 4). Additional elucidation of the mechanisms by which endogenous TR cells are generated, activated, or expanded and those by which they control other T cells will contribute to our better control of physiologic and pathologic adaptive immune responses.
Acknowledgments
The author thanks Z. Fehervari and K.J. Wood for critically reading the manuscript.
References
- 1.Cohn, M. 1994. The wisdom of hindsight. Annu. Rev. Immunol. 12:1–62. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov, R., and C.A. Janeway, Jr. 2002. Decoding the patterns of self and nonself by the innate immune system. Science. 296:298–300. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger, P. 2002. The danger model: a renewed sense of self. Science. 296:301–305. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 5.Shevach, E.M. 2001. Certified professionals: CD4+CD25+ suppressor T cells. J. Exp. Med. 193:F41–F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 7.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. [DOI] [PubMed] [Google Scholar]
- 8.Hori, S., T.L. Carvalho, and J. Demengeot. 2002. CD25+ CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291. [DOI] [PubMed] [Google Scholar]
- 9.Aseffa, A., A. Gumy, P. Launois, H.R. MacDonald, J.A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 169:3232–3241. [DOI] [PubMed] [Google Scholar]
- 10.Iwashiro, M., R.J. Messer, K.E. Peterson, I.M. Stromnes, T. Sugie, and K.J. Hasenkrug. 2001. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. USA. 98:9226–9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montagnoli, C., A. Bacci, S. Bozza, R. Gaziano, P. Mosci, A.H. Sharpe, and L. Romani. 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169:6298–6308. [DOI] [PubMed] [Google Scholar]
- 12.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 13.Xu, D., L.H. Liu, M. Komai-Koma, C. Campbell, C. McSharry, J. Alexander, and F.Y. Liew. 2003. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 170:394–399. [DOI] [PubMed] [Google Scholar]
- 14.Caramalho, I., T. Lopes-Carvalho, D. Ostler, S. Zelenay, M. Haury, and J. Demengeot. 2003. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 16.Akira, S., K. Takeda, and K. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 17.Mokuno, Y., T. Matsuguchi, M. Takano, H. Nishimura, J. Washizu, T. Ogawa, O. Takeuchi, S. Akira, Y. Nimura, and Y. Yoshikai. 2000. Expression of toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J. Immunol. 165:931–940. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 19.Salomon, B., and J.A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19:225–252. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142. [DOI] [PubMed] [Google Scholar]
- 21.McHugh, R.S., M.J. Whitters, C.A. Piccirillo, D.A. Young, E.M. Shevach, M. Collins, and M.C. Byrne. 2002. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 23.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 24.Bennett, C.L., and H.D. Ochs. 2001. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr. Opin. Pediatr. 13:533–538. [DOI] [PubMed] [Google Scholar]
- 25.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor FOXP3. Science. In press. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi, S., M. Toda, M. Asano, M. Itoh, S.S. Morse, and N. Sakaguchi. 1996. T cell-mediated maintenance of natural self-tolerance: its breakdown as a possible cause of various autoimmune diseases. J. Autoimmun. 9:211–220. [DOI] [PubMed] [Google Scholar]
- 27.Qin, H.Y., M.W. Sadelain, C. Hitchon, J. Lauzon, and B. Singh. 1993. Complete Freund's adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J. Immunol. 150:2072–2080. [PubMed] [Google Scholar]
- 28.Lehmann, D., and A. Ben-Nun. 1992. Bacterial agents protect against autoimmune disease. I. Mice pre-exposed to Bordetella pertussis or Mycobacterium tuberculosis are highly refractory to induction of experimental autoimmune encephalomyelitis. J. Autoimmun. 5:675–690. [DOI] [PubMed] [Google Scholar]
- 29.Elias, D., D. Markovits, T. Reshef, R. van der Zee, and I.R. Cohen. 1990. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc. Natl. Acad. Sci. USA. 87:1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bach, J.F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347:911–920. [DOI] [PubMed] [Google Scholar]