Abstract
CD4+8+ double positive (DP) thymocytes differentiate into CD4+ and CD8+ mature T cells in response to TCR signals. However, TCR signals that are initiated in DP thymocytes are unlikely to persist throughout all subsequent differentiation steps, suggesting that other signals must sustain thymocyte differentiation after TCR signaling has ceased. Using an in vitro experimental system, we now demonstrate that cytokine receptor signals, such as those transduced by IL-7 receptors, are required for differentiation of signaled DP thymocytes into functionally mature CD8+ T cells as they: (a) up-regulate Bcl-2 expression to maintain thymocyte viability; (b) enhance CD4 gene silencing; (c) promote functional maturation;and (d) up-regulate surface expression of glucose transporter molecules, which improve nutrient uptake and increase metabolic activity. IL-7Rs appear to be unique among cytokine receptors in maintaining the viability of newly generated CD4−8+ thymocytes, whereas several different cytokine receptors can provide the trophic/differentiative signals for subsequent CD8+ thymocyte differentiation and maturation. Thus, cytokine receptors provide both survival and trophic/differentiative signals with varying degrees of redundancy that are required for differentiation of signaled DP thymocytes into functionally mature CD8+ T cells.
Keywords: coreceptor reversal, kinetic signaling, positive selection, IL-7
Introduction
T cell differentiation in the thymus is driven by signals transduced by surface TCR components (1). Differentiation of immature CD4+8+ double positive (DP)* thymocytes into mature CD4+ and CD8+ single positive (SP) T cells is signaled by αβTCR complexes and is referred to as “positive selection,” which has at least three components: cell survival, requiring up-regulation of antiapoptotic proteins such as Bcl-2 (2, 3); CD4/CD8 lineage choice, because positively selected DP thymocytes must differentiate into either CD4SP or CD8SP T cells (4), and functional maturation, because mature T cells must be able to initiate lineage-specific effector programs in response to TCR stimulation (5). It seems unlikely that TCR signals that initiate positive selection of DP thymocytes in the thymic cortex can persist throughout their differentiation into mature T cells which is completed in the thymic medulla. In particular, the TCR signals that drive developing thymocytes to differentiate into CD8+ T cells are thought to be transient and of short duration (6–8).
Cytokine receptors are potential candidates for transducing signals that maintain thymocyte differentiation after TCR signaling has ceased (9–11), although most of what is known about cytokine receptor signaling in thymocyte differentiation involves events before positive selection (12–17). Cytokine receptor expression on CD4−8− double negative thymocytes is extinguished on their differentiation into DP cells (18, 19), which do not express functional cytokine receptors, although they do express the common cytokine receptor γ chain (γc) that, by itself, is unable to transduce intracellular signals (20, 21). In response to TCR-mediated positive selection signals, DP thymocytes differentiate into SP T cells and reexpress IL-7Rα chains, which can associate with preexisting γc to form functional IL-7Rs (6, 10, 18, 22). As a result, IL-7Rs may participate in the differentiation and maturation of positively selected thymocytes. Indeed, IL-7Rs provide survival signals to positively selected thymocytes during differentiation into mature T cells, but it has been argued that cell survival is their only role (22).
Recently, we reported that signaled DP thymocytes first terminate CD8 gene expression to convert into CD4+8− intermediate thymocytes, regardless of their ultimate lineage fate, and that, in the presence of IL-7, intermediate CD4+8− thymocytes subsequently undergo “coreceptor reversal” (i.e., reinitiation of CD8 gene expression and extinction of CD4 gene expression) to differentiate into mature CD8SP T cells (6). These observations have been synthesized into a model of positive selection and lineage commitment called “kinetic signaling,” which posits that CD4/CD8 lineage direction is determined at the intermediate CD4+8− stage of differentiation by whether or not intermediate thymocytes undergo coreceptor reversal (6, 8, 23). Because coreceptor reversal appears to be a key component of the CD4/CD8 lineage decision, we thought it important to identify the role performed by IL-7R signals in coreceptor reversal and in subsequent differentiation of immature thymocytes into mature CD8SP T cells.
The present paper was undertaken to determine whether IL-7R signals might do more than promote cell survival during differentiation of thymocytes into CD8SP T cells. In fact, here we demonstrate that, in addition to up-regulating Bcl-2 expression, IL-7R signals affect CD4 gene silencing during coreceptor reversal, support the acquisition of functional competence in developing CD8SP thymocytes, and up-regulate surface expression of the glucose transporter molecule-1 (glut-1), whose expression is correlated with improved nutrient uptake and increased metabolic activity (24). Interestingly, some IL-7R functions in post-selection thymocytes are unique to IL-7Rs, whereas other functions are redundant. IL-7Rs appear to be unique among cytokine receptors in signaling Bcl-2 up-regulation and promoting thymocyte survival, but IL-7Rs are not unique among cytokine receptors in providing signals that support differentiation of post-selection thymocytes into CD8SP T cells. Thus, the present paper demonstrates that cytokine receptors provide more than survival signals to post-selection thymocytes during differentiation into functionally mature CD8SP T cells.
Materials and Methods
Animals.
C57BL/6 (B6) mice were obtained from the Jackson Laboratory. B6 mice expressing the human Bcl-2 transgene (Bcl-2 Tg; reference 25) were made deficient in β2-microglobulin and are referred to as Bcl-2Tg (β2mo). We refer to mice deficient in both MHC class II and β2m as MHC°, and to mice deficient in murine Bcl-2 as Bcl-2° (26).
Antibodies and Reagents.
Fluorochrome-conjugated antibodies with the following specificities were used for direct immunofluorescence: CD4 (GK1.5); CD8α (53–6.7); mBcl-2 (3F11); hBcl-2 (6C8); TCRβ (H57–597); CD24 (M1–69); IFNγ (XMG1.2); IgG isotype control (all from BD Biosciences); and hCD2 (G11, Caltag). Purified antibodies directed against the following specificities were used for indirect immunofluorescence: IL-7Rα (A7R34, a gift from D. Allman, University of Pennsylvania, Philadelphia, PA; reference 18); γc (4G3 + 3E12; BD Biosciences); CD3 (2C11); and glut-1 (N-20; Santa Cruz Biotechnology, Inc.). The following recombinant cytokines were used: murine IL-7, human IL-15, mouse IL-4 (R&D Systems); human IL-2 (Biological Resources Branch, NCI); mouse IFNγ and mouse TNFα (BD Biosciences). The following reagents were also used: PMA, ionomycin (both from Calbiochem), and carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes).
In Vitro Suspension Culture.
DP thymocytes were purified to >95% by panning with anti-CD8α mAb (83-12-5) and placed in suspension cultures (6, 27). DP thymocytes were transiently stimulated with 0.3 ng/ml PMA and 0.3 μg/ml ionomycin for 14–20 h (signaling culture), harvested, washed, and cultured in medium alone or medium with 6 ng/ml IL-7 for another 16–20 h (recovery culture) or longer. Where indicated, signaled cells were also cultured in IL-2, IL-4, IL-15, IFNγ, and TNFα (all at 100 U/ml).
Where indicated, thymocytes were pronase-treated to remove preexisting surface CD4 and CD8 proteins (28). In brief, cells (5 × 106/ml) were treated with 0.01% pronase (Calbiochem) for 15 min at 37°C and cultured at 37°C overnight, during which time they reexpressed the coreceptor molecules they were actively synthesizing (28).
Fetal Thymic Organ Culture (FTOC).
Embryonic 17.5-d thymus lobes from B6 or Bcl-2Tg mice were placed in an organ culture with 50 μg/ml rat anti-murine IL-7Rα and 50 μg/ml anti-γc mAbs or isotype-matched control IgG. On culture day 3, the lobes were harvested and the thymocytes were subjected to the coreceptor reexpression assay by pronase treatment followed by placement in single cell suspension cultures for an additional day (28).
Flow Cytometry and Electronic Cell Sorting.
Cells were harvested, stained with fluorochrome-conjugated antibodies, and analyzed on a multi-laser FACSVantage® SE (Becton Dickinson). Dead cells were excluded by forward light scatter gating and propidium iodide staining. Analysis was performed using software developed at the National Institutes of Health.
Anti-TCR Stimulation.
24-well plates were coated with 1 μg/ml anti-CD3 and 10 μg/ml anti-CD28 at 4°C overnight. Cells were plated at 0.5–1.0 × 106/ml and cultured for the indicated time.
Intracellular IFNγ Staining.
Purified CD4−8+ T cells from differentiation cultures were stimulated with 1 μg/ml plate-bound anti-CD3 and 10 μg/ml anti-CD28 for 3 d and stimulated with 0.3 μg/ml PMA and 0.2 μg/ml ionomycin in the presence of monensin (BD Biosciences) for the final 4 h. Intracellular staining for IFNγ was performed using Cytofix/Cytoperm Kit® (BD Biosciences) together with PE–anti-IFNγ or PE-IgG isotype control.
CFSE Labeling.
CFSE labeling was performed as described previously (29). Cells were washed in PBS, incubated at 107/ml with 0.25 μM CFSE for 8 min at room temperature and washed in medium with serum before further culture.
Glucose Uptake Assay.
106 cultured thymocytes were starved in PBS at room temperature for 30 min and incubated at 37°C for 10 min in PBS containing 12 μM 2-[14C(U)]-deoxy-d-glucose (PerkinElmer). Cells were washed with PBS, lysed in 1 M KOH, neutralized with 1 M HCl, and counted for 14C-glucose content.
RT-PCR.
Total RNA was isolated and reverse-transcribed as described previously (6). The cDNA was subject to PCR amplification for 35 cycles, each cycle lasting 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The PCR products were hybridized with 32P-labeled internal oligonucleotide probes, and the membranes were analyzed on a PhosphorImager.
Results
IL-7R Signals Are Required for Generation of Newly Arising CD4−8+ Thymocytes and Can Be Replaced by Bcl-2.
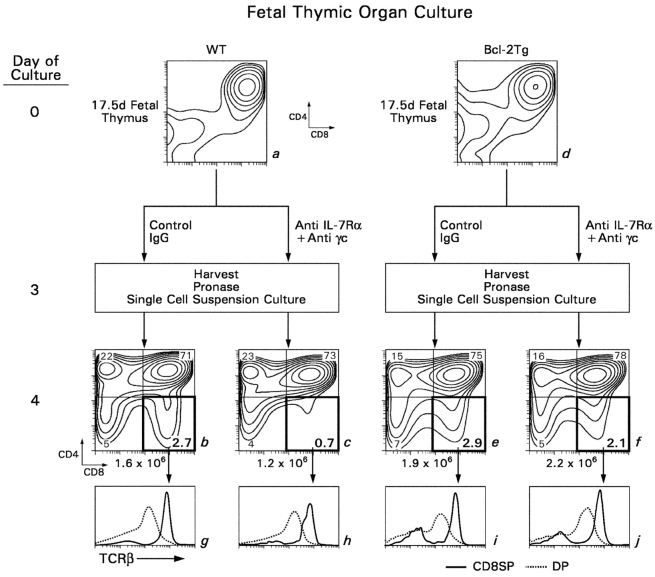
To assess the role of IL-7R signaling in positively selected DP thymocytes, we first examined the effect of anti–IL-7R antibody blockade on intrathymic differentiation in FTOC. We wanted to block IL-7R signaling in positively selected DP thymocytes while minimizing effects of anti–IL-7R blockade on early thymocyte development. Consequently, we cultured embryonic day 17.5 thymic lobes, which contain DP thymocytes that have not yet differentiated into SP T cells, in FTOC in the presence or absence of blocking anti–IL-7R mAbs for only 3 d. After 3 d, developing thymocytes were harvested and subjected to the coreceptor reexpression assay (28); i.e., cells were treated with extracellular pronase to remove preexisting CD4 and CD8 coreceptor molecules from the cell surface and cultured overnight so that they could reexpress the coreceptor molecules they were actively synthesizing (Fig. 1) . Addition to wild-type (WT) FTOC of mAbs specific for the two chains of the IL-7R (IL-7Rα and γc) selectively abrogated the appearance of TCRhi thymocytes that were CD4−8+ in the coreceptor reexpression assay, but did not affect appearance of thymocytes that were CD4+8− (Fig. 1, left). The ability of anti–IL-7R antibodies to block the appearance of newly generated CD4−8+ thymocytes was circumvented by Bcl-2 transgene expression (Fig. 1, right). These results indicate that intrathymic IL-7R signals are required for survival of newly generated CD4−8+ TCRhi thymocytes but not newly generated CD4+8− thymocytes.
Figure 1.
Effect of antibody-mediated blockade of IL-7R signaling on intrathymic T cell development. Fetal thymic lobes from B6 and Bcl-2Tg (β2mο) mice (embryonic day 17.5) were placed in organ cultures to which anti–IL-7R or control rat antibodies were added. On day 3 of culture, the thymocytes were harvested into single cell suspensions, treated with extracellular pronase to strip preexisting CD4/CD8 coreceptor proteins from cell surfaces, and cultured overnight so that they would reexpress the CD4/CD8 coreceptor molecules they were actively synthesizing. Panels a–f depict CD4 versus CD8 expression, whereas panels g–j depict TCRβ expression on DP (dotted lines) and CD8SP (solid lines) thymocytes. Note that most CD8SP thymocytes at this time point were mature as their surface expression of TCRβ was high relative to that of DP thymocytes. Although not shown, similar results were obtained with anti–IL-7Rα and anti-γc individual mAbs as with both together.
Coreceptor Reversal during CD8SP T Cell Differentiation and the Role of IL7R Signals.
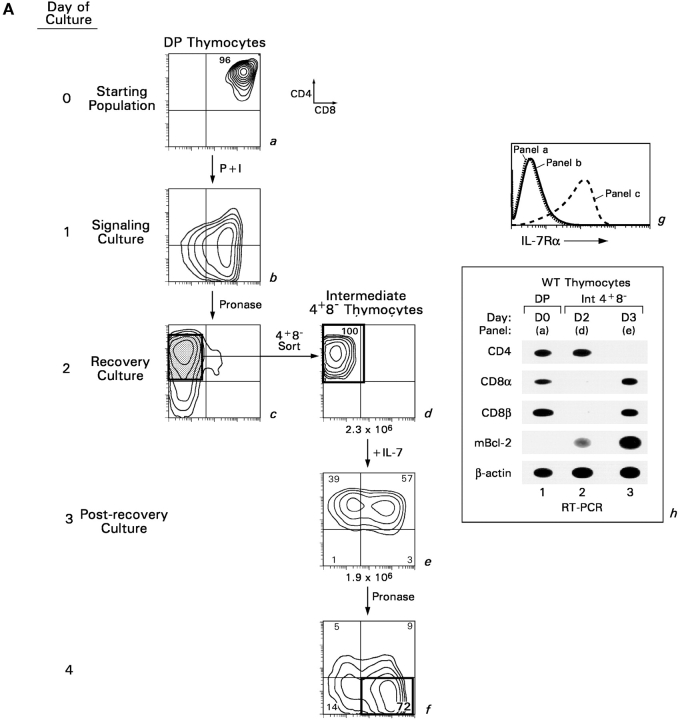
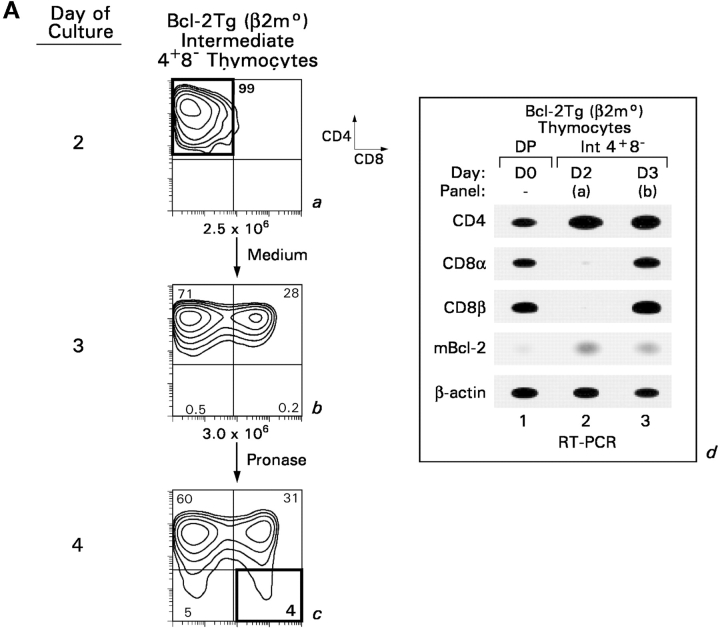
To determine if IL-7R signaling might do more than promote thymocyte survival during CD8SP T cell generation, we used an in vitro experimental model in which DP thymocyte differentiation into CD8SP T cells could be analyzed in individual steps (6, 27). Single cell suspensions of DP thymocytes from MHCo mice were transiently signaled with PMA and ionomycin (Fig. 2 A, P + I, signaling culture), treated with extracellular pronase to remove preexisting surface coreceptor molecules, and placed in nonstimulatory “recovery cultures” (Fig. 2 A, a–c). Note that the apparent conversion of DP thymocytes into CD4−8+ cells in the signaling culture (Fig. 2 A, b) is simply due to selective internalization of surface CD4 protein by PMA stimulation (30). However, cessation of signaling by placement of thymocytes into recovery culture results in termination of CD8 gene expression (Fig. 2 A, c and d, and Fig. 2 A, h, lane 2), up-regulated IL-7Rα expression (Fig. 2 A, g), and differentiation into “intermediate” stage CD4+8− thymocytes.
Figure 2.
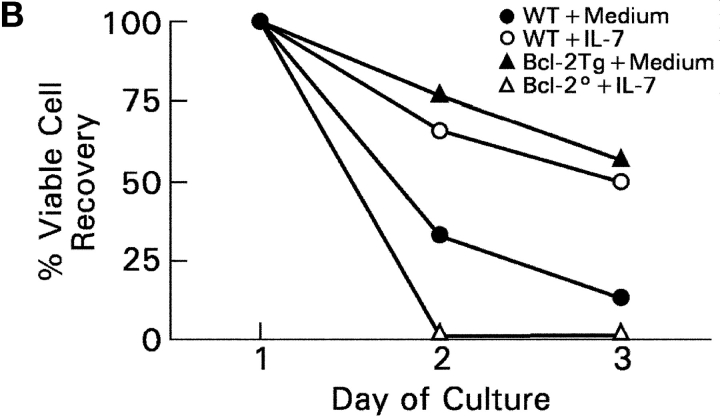
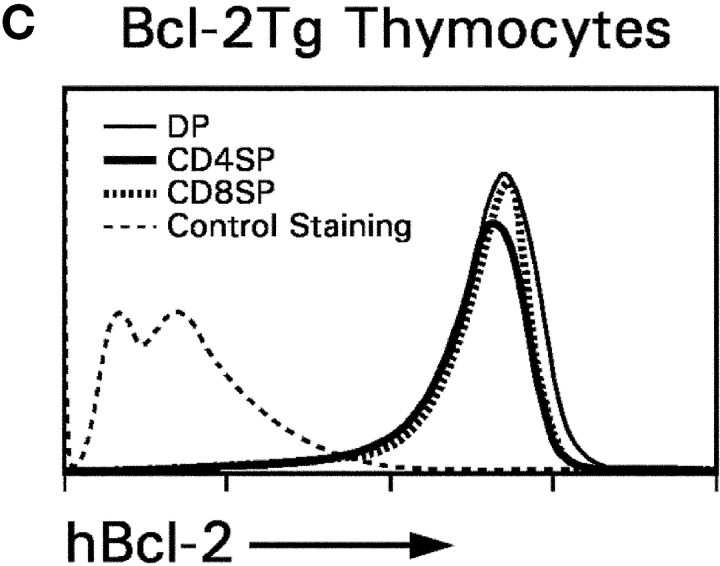
In vitro differentiation of DP thymocytes into CD4−8+ cells: coreceptor reversal and requirement for Bcl-2 expression. (A) Stimulated DP thymocytes differentiate in vitro into intermediate CD4+8− cells and subsequently into CD4−8+ cells. Purified DP thymocytes from MHC° mice (a) were stimulated with PMA + ionomycin (P + I) for 16 h (b, signaling culture) and then allowed to recover in nonstimulatory cultures containing only medium (c, recovery culture). The cells were pronased before placement in recovery culture so that only newly synthesized coreceptor molecules would be expressed on their cell surfaces. In this way, nearly all the cells in recovery culture were CD4+8−, which were purified to homogeneity by electronic cell sorting (d). In vitro–generated CD4+8− cells were referred to as “intermediate thymocytes” because they continued to differentiate. After 1 d of culture in 6 ng/ml murine IL-7 (e), the cells were pronased to identify the coreceptor molecules being actively synthesized, revealing that over 70% were now CD4−8+ (f). IL-7Rα surface expression was examined by flow cytometry (g), and coreceptor gene expression was examined by RT-PCR (h). (B) Survival requirements of signaled DP thymocytes in vitro. DP thymocytes from WT, Bcl-2°, and Bcl-2Tg mice were transiently signaled with P + I and cultured in medium or IL-7. Cell viability for each group was set as 100% at the beginning of recovery culture on day 1, and measured on days 2 and 3. The survival curves of Bcl-2° thymocytes cultured in medium or IL-7 were identical. (C) Bcl-2Tg expression in thymocyte subpopulations. Thymocytes from Bcl-2Tg mice were stained for intracellular human Bcl-2Tg and surface CD4 and CD8. The anti–human Bcl-2 antibody only detects transgenic Bcl-2 protein and does not detect endogenous mouse Bcl-2 protein.
The addition of IL-7 to intermediate CD4+8− thymocytes resulted in a series of events referred to as “coreceptor reversal” (6). By molecular analyses, intermediate CD4+8− thymocytes terminated CD4 RNA expression and reinitiated CD8α and CD8β RNA expression, converting into CD4−8+ T cells (Fig. 2 A, h, lane 3). Assessment of surface coreceptor protein expression revealed that intermediate CD4+8− thymocytes reexpressed surface CD8 coreceptor molecules, appearing to revert into CD4+8+ cells (Fig. 2 A, e); but the reverted CD4+8+ cells were actually CD4−8+ cells because they only reexpressed CD8 coreceptor proteins after extracellular pronase treatment (Fig. 2 A f). Thus, in cultures with IL-7, intermediate CD4+8− thymocytes underwent coreceptor reversal and differentiated into CD4−8+ thymocytes.
To understand the contribution of IL-7R signaling to coreceptor reversal and CD8SP thymocyte generation, we first analyzed the role of IL-7R signals in promoting thymocyte survival. DP thymocytes do not express Bcl-2 RNA transcripts until they have been signaled (Fig. 2 A, h, lanes 1 and 2), after which their expression of Bcl-2 RNA is further up-regulated by IL-7 (Fig. 2 A, h, lane 3). Consistent with its effect on Bcl-2 RNA expression, IL-7 promoted the survival of intermediate thymocytes that otherwise failed to survive in vitro (Fig. 2 B, compare open with closed circles). IL-7's ability to promote thymocyte survival required endogenous Bcl-2 gene expression, as intermediate thymocytes from Bcl-2° mice failed to survive even in the presence of IL-7 (Fig. 2 B, open triangles). And, as observed in FTOC, the survival requirement for IL-7 could be replaced by Bcl-2 transgene expression (Fig. 2 B, closed triangles). Thus, IL-7R signals up-regulate endogenous Bcl-2 expression and can be replaced in intermediate thymocytes by Bcl-2 transgene expression. Importantly, Bcl-2 transgene expression persisted throughout thymocyte differentiation as Bcl-2 transgenic protein was expressed at equally high levels in immature DP and mature SP thymocytes (Fig. 2 C).
Assessment of IL-7R Signaling on CD4/CD8 Gene Expression during Coreceptor Reversal.
The ability of Bcl-2 transgene expression to maintain thymocyte viability in the absence of IL-7 permitted us to examine the differentiation of Bcl-2 transgenic DP thymocytes into CD8SP T cells without IL-7. Notably, we used Bcl-2Tg thymocytes derived from fetal or neonatal mice that were less than 7 d old (to avoid abnormally aged DP thymocytes present in adult Bcl-2Tg mice) and that were also deficient in β2-microglobulin (β2mo) and so contained few CD8SP T cells.
We stimulated Bcl-2 Tg DP thymocytes to generate intermediate CD4+8− thymocytes as in Fig. 2 A, but continued them in culture without IL-7 (Fig. 3 A). Molecular and cellular analyses revealed that intermediate CD4+8− thymocytes were devoid of CD8 RNA transcripts (Fig. 3 A, a and Fig. 3 A, d, lane 2), but that they spontaneously reinitiated CD8 gene expression during further culture in medium (Fig. 3 A, b and Fig. 3 A, d, lane 3). However, few Bcl-2Tg thymocytes terminated CD4 gene expression because most thymocytes still contained CD4 RNA transcripts (Fig. 3 A, d, lane 3) and still reexpressed surface CD4 proteins after pronase treatment (Fig. 3 A, c). Thus, in the absence of IL-7R signaling, Bcl-2Tg intermediate CD4+8− thymocytes reinitiated CD8 gene expression, but most failed to extinguish CD4 gene expression.
Figure 3.
In vitro differentiation of intermediate thymocytes from Bcl-2 transgenic mice in the presence and absence of IL-7. (A) Differentiation of intermediate thymocytes in the absence of IL-7. DP thymocytes were obtained from embryonic day 18 Bcl-2Tg (β2mo) mice, stimulated with P + I for 16 h, pronased, and electronically sorted as in Fig. 2 A to obtain purified intermediate CD4+8− thymocytes (a). 1-d culture in medium alone was sufficient for many of these intermediate thymocytes to reexpress surface CD8 (b). These cells were pronased to reveal the coreceptors being actively synthesized (c). Gene expression was assessed by RT-PCR (d). (B) IL-7R signaling increases CD4−8+ T cell number without cell proliferation. DP thymocytes from BCl-2Tg (β2mo) mice (a) were transiently signaled with P + I and labeled with CFSE on day 1 before placement in recovery cultures with either IL-7 or medium. On day 3, the cells were pronased and on day 4 assessed for CD4/CD8 expression (b, c) and CFSE fluorescence intensity (d). CFSE intensity of thymocytes cultured in medium (solid line) was compared with that of thymocytes cultured in IL-7 (dotted line). As a positive control, B6 lymph node T cells were labeled with CFSE in parallel on day 1 and stimulated by 1 μg/ml anti-CD3 mAb for 3 d (dashed line).
To determine if IL-7R signals were required for extinguishing CD4 gene expression during coreceptor reversal, DP thymocytes from Bcl-2Tg (β2mo) mice were transiently stimulated in vitro, cultured in medium or IL-7, treated with pronase on day 3, and allowed to reexpress the surface coreceptor molecules they were actively synthesizing (Fig. 3 B). CD4−8+ thymocytes were generated in both medium and IL-7, but the frequency of CD4−8+ thymocytes was two- to threefold higher in IL-7 cultures (Fig. 3 B, b and c), despite the fact that Bcl-2 transgene expression appeared to interfere with CD4 extinction (Fig. 3 B, b and c). The higher frequency of CD4−8+ thymocytes in IL-7 cultures was not the result of clonal expansion because CFSE fluorescence revealed no significant proliferation in either culture (Fig. 3 B, d). Thus, changes in coreceptor gene expression during coreceptor reversal can occur without IL-7R signaling, but IL-7R signaling increased by two- to threefold the frequency of thymocytes in which CD4 gene expression is extinguished.
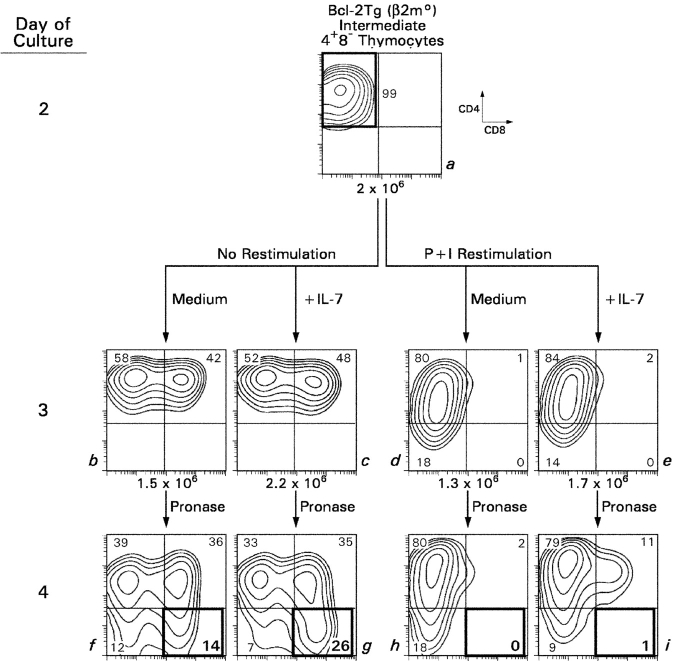
Regulation of Coreceptor Reversal.
We demonstrated previously in WT thymocytes that coreceptor reversal was dependent on IL-7 and was blocked by TCR signaling (6). Consequently, we wished to determine if P + I signals blocked coreceptor reversal by interrupting IL-7R signaling or by directly affecting coreceptor gene expression. We transiently stimulated Bcl-2Tg DP thymocytes with P + I to generate intermediate CD4+8− thymocytes, which we restimulated and assessed for coreceptor reversal (Fig. 4) . Without restimulation, intermediate CD4+8− thymocytes reinitiated CD8 expression and extinguished CD4 expression whether IL-7 was present or not (Fig. 4, left). In contrast, restimulation of intermediate CD4+8− thymocytes effectively blocked these cells from reinitiating CD8 expression and extinguishing CD4 expression, and the blockade was equally profound in medium and IL-7 (Fig. 4, right). Thus, P + I signals in intermediate thymocytes regulate coreceptor reversal by directly blocking CD8 gene reexpression and CD4 gene extinction, and they do so independently of IL-7R signals.
Figure 4.
Restimulation of intermediate thymocytes blocks coreceptor reversal, even in the absence of IL-7. Intermediate CD4+8− thymocytes (a) were generated from P + I-signaled Bcl-2Tg (β2mo) DP thymocytes as in Figs. 2 and 3. In the absence of restimulation (left), over 40% of these intermediate thymocytes reexpressed CD8 (b and c) with many also terminating CD4 expression to become CD4−8+ T cells (f and g, left). However, in the presence of P + I restimulation (right), intermediate thymocytes remained CD4+8− and neither reexpressed CD8 (d and e) nor terminated CD4 (h and i), regardless of the presence or absence of IL-7.
IL-7R Signals Support the Differentiation of Intermediate Thymocytes into Functionally Competent CD8SP T Cells.
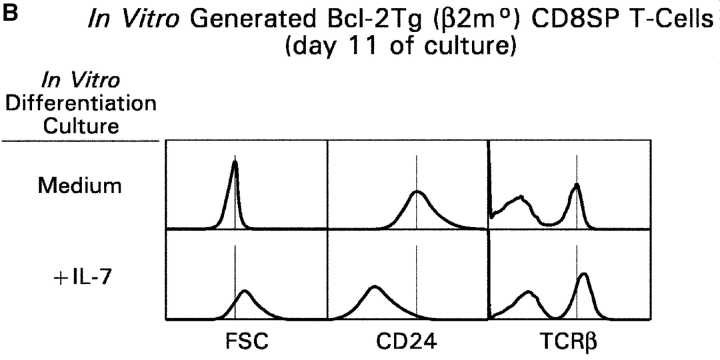
To determine if IL-7R signals support thymocyte differentiation into CD8SP T cells, we transiently signaled Bcl-2Tg (β2mo) thymocytes and placed them in longer term cultures for 11 d (Fig. 5) , because intermediate thymocytes differentiate in longer term cultures into bona fide CD8SP T cells that are CD4− without pronase treatment (6). We observed that intermediate thymocytes phenotypically differentiated into CD8SP T cells more rapidly and in greater frequency with IL-7 than without IL-7 (Fig. 5 A), despite the absence of cell proliferation (6). Importantly, CD8SP T cells generated with IL-7 were significantly more mature than those generated with medium, as revealed by greater cell size, lower surface CD24 expression, and higher surface TCR expression on TCR+ cells (Fig. 5 B). Note that in vitro–generated CD8SP T cells can be either TCR+ or TCR− as all DP thymocytes were pharmacologically signaled to differentiate into SP T cells, even if they failed to productively rearrange their TCRα locus (6).
Figure 5.
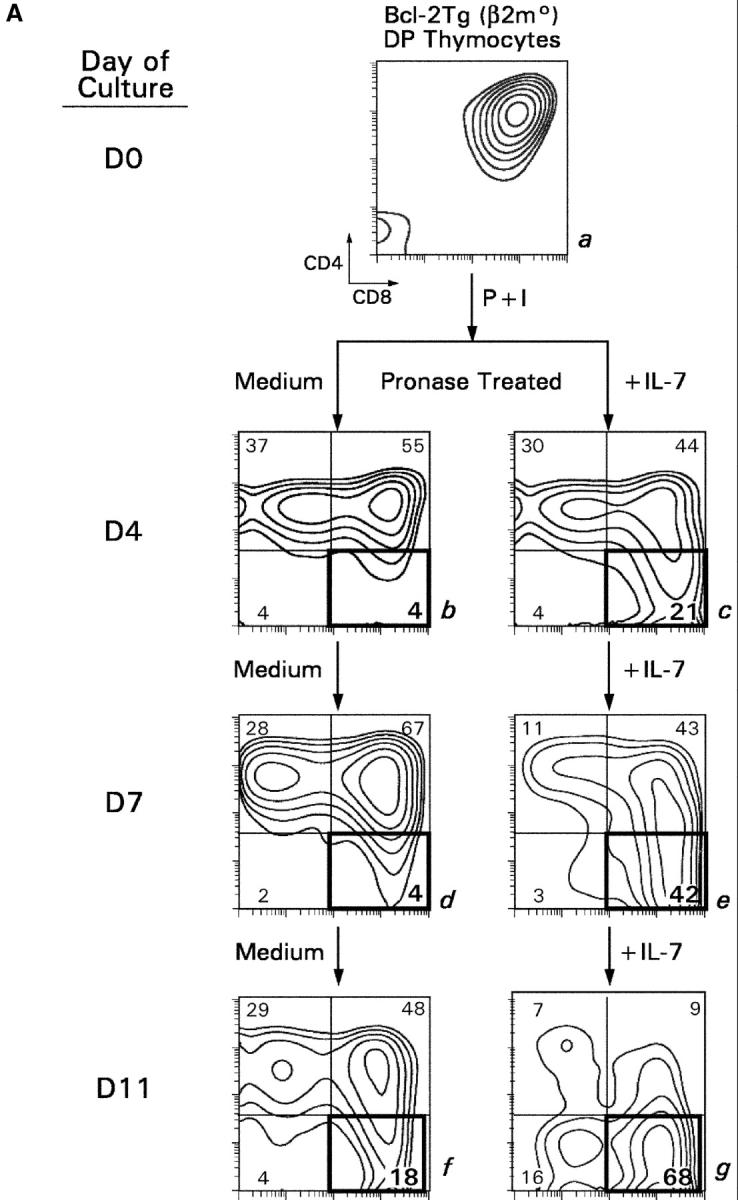
In vitro differentiation of signaled DP thymocytes in longer term cultures. (A) DP thymocytes from Bcl-2Tg (β2mo) mice (a) were signaled with P + I and placed in longer term cultures with either medium (b, d, and f) or IL-7 (c, e, and g). Cells were assessed for CD4/CD8 expression on the days indicated. (B) Effect of IL-7 on phenotypic maturation. Using day 11 cells from A, we determined their forward light scatter (FSC), which is a reflection of cell size, as well as their surface expression of CD24 and TCRβ.
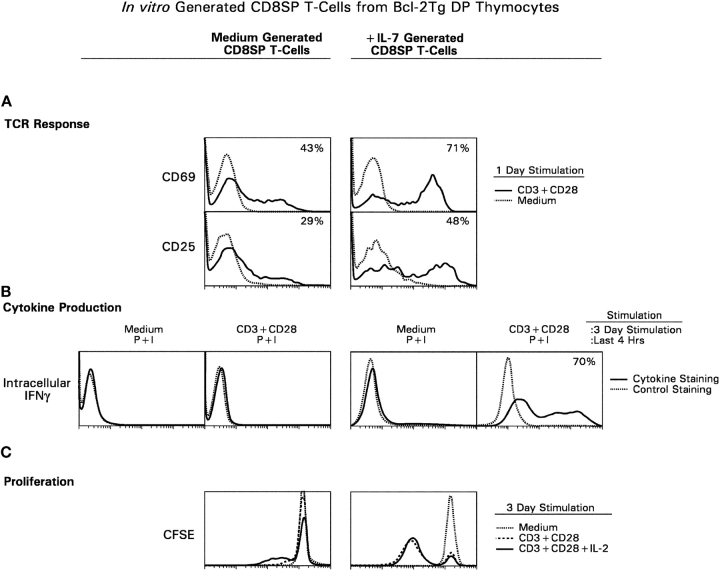
Next, we assessed the functional competence of TCR+ CD8SP T cells (Fig. 6) . In these functional experiments, responder CD8SP thymocytes were derived from Bcl-2Tg (β2mo) intermediate thymocytes, which had been cultured either with or without IL-7, treated with pronase, and allowed to reexpress coreceptor molecules so that we could identify the CD8SP T cells. The ability of these CD8SP T cells to respond to TCR stimulation was assessed in three different assays. First, we assessed the expression of activation markers in response to TCR signals. After 1 d of stimulation, IL-7–generated CD8SP T cells up-regulated both CD69 and CD25 to a significantly greater extent than medium-generated CD8SP T cells (Fig. 6 A). Second, we assessed IFNγ production in response to TCR signals. After 3 d of stimulation, 70% of IL-7–generated CD8SP T cells produced IFNγ as detected by intracellular cytokine staining, whereas medium-generated CD8SP T cells failed to produce IFNγ (Fig. 6 B). And third, we assessed the ability to proliferate in response to TCR signals. After 3 d of stimulation, IL-7–generated CD8SP T cells had undergone significant proliferation as indicated by decreased CFSE fluorescence, whereas medium-generated CD8SP T cells failed to proliferate, even in the presence of exogenously added IL-2 (Fig. 6 C). These results demonstrate that, despite persistent Bcl-2 transgene expression, IL-7R signals are still necessary to support the differentiation of intermediate thymocytes into functionally mature CD8SP T cells.
Figure 6.
IL-7R signals promote differentiation of thymocytes into functional CD8+ T cells that can respond to TCR signals. Intermediate thymocytes were generated by P + I stimulation of Bcl-2Tg (β2mo) DP thymocytes, placed in short term cultures with either medium or IL-7, and pronased on day 3 to confirm the presence of CD4−8+ T cells. On day 4, cells that had been cultured in either medium (left) or IL-7 (right) were stimulated with immobilized anti-CD3 + anti-CD28 mAbs and assessed for TCR responses (A), intracellular cytokine production (B), and proliferation (C). (A) TCR responses. Cultured cells were stimulated with anti-CD3 + anti-CD28 mAbs for 1 d and assessed for surface expression of CD25 and CD69. Shown are CD25 and CD69 surface expression on gated CD4−8+ T cells (solid line, anti-CD3 + anti-CD28 stimulation; dotted line, medium). (B) Cytokine production. CD4−8+ T cells were purified from in vitro differentiation cultures and stimulated for 3 d with immobilized anti-CD3 + anti-CD28. 4 h before harvest, they were restimulated with P + I in the presence of monensin and assessed for intracellular IFNγ (solid line, intracellular IFNγ staining; dotted line, control IgG staining). (C) Proliferation. Cultured cells were labeled with CFSE and stimulated with anti-CD3 + anti-CD28 for 3 d either with or without additional hIL-2. CFSE intensity on gated CD4−8+ cells was assessed by three color flow cytometry (dotted line, no stimulation; dashed line, stimulation with anti-CD3 + anti-CD28; solid line, stimulation with anti-CD3 + anti-CD28 in the presence of 50 U/ml hIL-2). Comparable results were obtained with P + I stimulation instead of antibody stimulation (not depicted).
IL-7R Signals Up-regulate Expression of glut-1 and Increase Nutrient Uptake by Developing CD8SP Thymocytes.
What might IL-7R signals provide to developing thymocytes beyond expression of antiapoptotic Bcl-2 proteins? In mature lymphocytes, transgenic expression of Bcl-2 family members blocks cell death but does not block cellular atrophy (24). Rather, signals transmitted through either TCR or cytokine receptors prevent cellular atrophy by promoting nutrient uptake and utilization, which are functions correlated with increased surface expression of glut-1 (24). Consequently, we considered that IL-7R signals might similarly function in developing CD8SP thymocytes to up-regulate surface expression of glut-1.
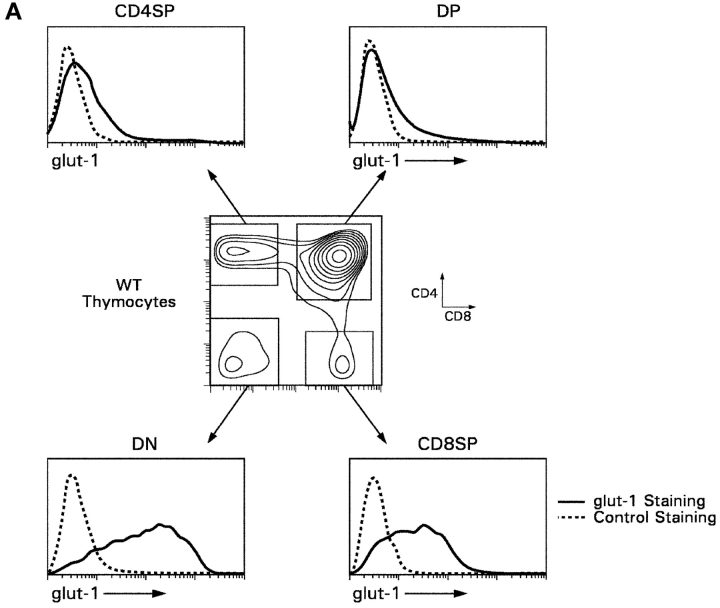
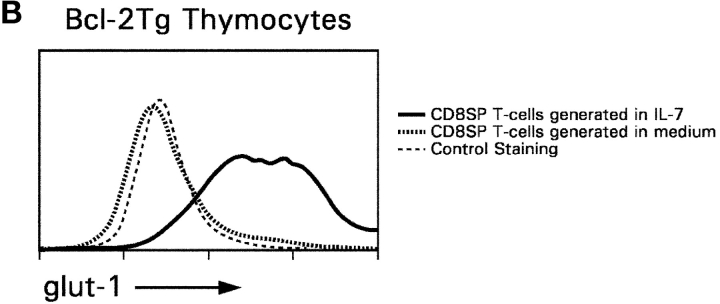
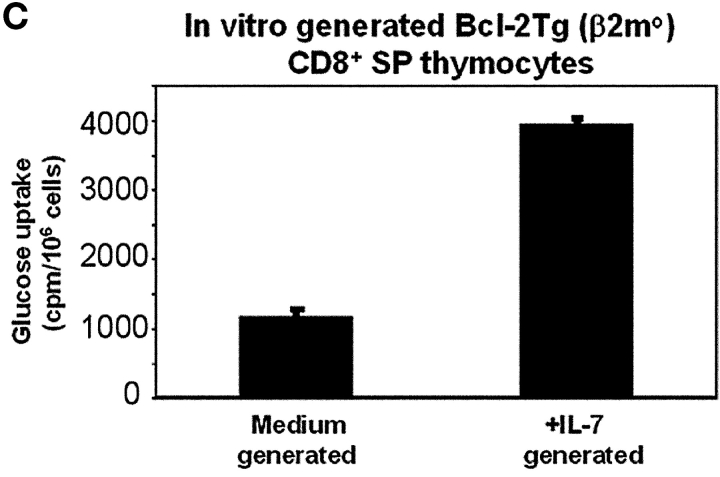
First, we examined glut-1 surface expression on adult thymocytes from normal mice. Glut-1 was highly expressed on double negative and CD8SP thymocytes but was expressed in barely detectable amounts on DP and CD4SP thymocytes (Fig. 7 A). Next, we examined if IL-7R signals up-regulated glut-1 expression on in vitro–generated CD8SP T cells (Fig. 7 B). We found that IL-7R signals were required for glut-1 expression on in vitro–generated CD8SP thymocytes, as CD8SP thymocytes generated in medium without IL-7 failed to express glut-1 (Fig. 7 B). In parallel with their up-regulation of glut-1 expression, IL-7R signals also increased glucose uptake by in vitro–generated CD8SP thymocytes by about fourfold (Fig. 7 C). Thus, IL-7R signals up-regulate glut-1 expression and promote glucose uptake by developing CD8SP thymocytes.
Figure 7.
glut-1 surface expression and glucose uptake by developing CD8SP thymocytes. (A) glut-1 surface expression on thymocyte subpopulations from WT mice. Thymocytes from WT mice were stained for surface glut-1, CD4, and CD8 and analyzed by multicolor flow cytometry. Staining for glut-1 was performed by incubating thymocytes with goat anti–mouse glut-1 antibody, followed by incubation with FITC-conjugated sheep anti–goat IgG. The cells were incubated with purified rat IgG to saturate any free-binding sites on the sheep IgG before incubation with anti-CD4 and anti-CD8 mAbs. Surface expression of glut-1 is indicated relative to control staining with the secondary sheep anti–goat IgG in the absence of primary antibody. (B) IL-7R signals induce glut-1 surface expression on in vitro–generated CD8SP thymocytes. CD8SP thymocytes were generated in vitro by stimulation of Bcl-2Tg (β2mo) DP thymocytes followed by culture in either medium or IL-7. On day 7, cells were assessed for surface glut-1 expression by immuno-fluorescence and multicolor flow cytometry. (C) IL-7R signals promote glucose take. CD8SP thymocytes were generated in vitro by stimulation of Bcl-2Tg (β2mo) DP thymocytes, followed by culture in either medium or IL-7. On day 7, the cells were assayed for glucose uptake with 2-[14C(U)]-deoxy-d-glucose.
Assessment of Redundant Cytokine Receptor Functions in Developing CD8SP Thymocytes.
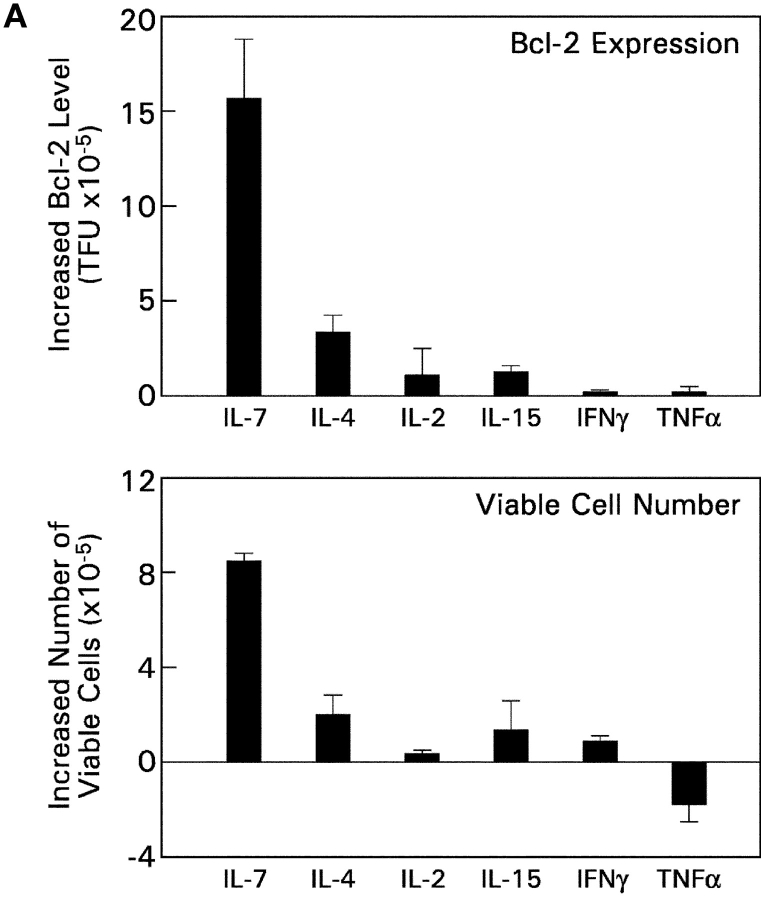
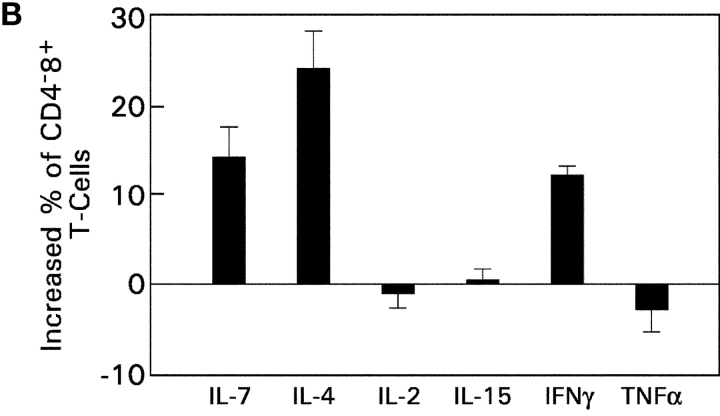
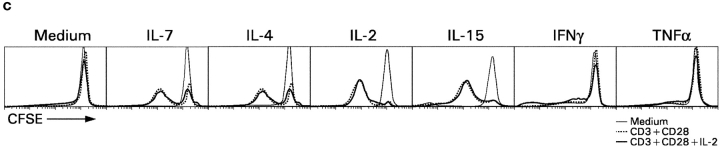
To determine whether IL-7R signals are unique in their ability to promote CD8SP thymocyte differentiation or whether other cytokine receptors have similar effects, we examined the ability of five other cytokines (IL-2, IL-4, IL-15, IFNγ, and TNFα) to promote differentiation of intermediate CD4+8− thymocytes into functionally mature CD8SP T cells (Fig. 8) . Specifically, we examined the ability of these other cytokines to do the following: (a) up-regulate Bcl-2 expression and promote thymocyte survival; (b) support extinction of CD4 gene expression during coreceptor reversal; and (c) promote the acquisition of functional competence in developing CD8SP T cells. With regard to thymocyte survival, IL-7 was superior to other cytokines in up-regulating Bcl-2 and promoting survival of in vitro–generated intermediate CD4+8− thymocytes (Fig. 8 A). With regard to promoting extinction of CD4 gene expression in intermediate CD4+8− thymocytes during coreceptor reversal, IL-4 and IFNγ were as effective as IL-7 in promoting conversion of intermediate CD4+8− thymocytes into CD4−8+ cells (Fig. 8 B). And, with regard to functional maturation, IL-2, -4, and -15 were as effective as IL-7 in promoting functional competence as measured by proliferative responses to CD3 + CD28 stimulation (Fig. 8 C). These results are summarized in Table I and demonstrate that Bcl-2 up-regulation and promotion of thymocyte survival in developing CD8SP T cells are unique to IL-7Rs, whereas the differentiative functions supported by IL-7Rs can also be supported by other cytokine receptors.
Figure 8.
Assessment of cytokine receptor redundancy in developing CD8SP thymocytes. (A) Ability of other cytokines to up-regulate Bcl-2 expression and promote thymocyte survival. In vitro–generated intermediate CD4+8− thymocytes from MHC° mice were cultured overnight in medium or one of the indicated cytokines. (top) Cells were assessed for expression of Bcl-2 by intracellular staining, which was quantitated into total fluorescence units (TFU) and is displayed relative to that of cells cultured in medium alone (TFU of medium cultured cells was 4.6 × 105). (bottom) Total number of viable cells in each culture was determined and is displayed relative to that of cells cultured in medium alone (viable cell number of medium cultured cells was 2.5 × 105). (B) Ability of various cytokines to promote extinction of CD4 gene expression during coreceptor reversal. In vitro–generated intermediate CD4+8− thymocytes from Bcl-2Tg (β2mo) mice were cultured overnight in either medium or one of the indicated cytokines. The next day, cells were pronase-stripped to determine the percentage of CD4−8+ cells that had extinguished CD4 gene expression and undergone coreceptor reversal. The frequency of CD4−8+ cells in each cytokine culture is displayed relative to that of medium alone (frequency of CD4−8+ cells in medium alone was 13.4%). (C) Ability of various cytokines to promote functional maturation of CD8SP thymocytes. In vitro–generated intermediate CD4+8− thymocytes from Bcl-2Tg (β2mo) mice were cultured overnight in either medium or one of the indicated cytokines. The next day, cells were pronase-stripped to identify CD4−8+ cells, labeled with CFSE, and stimulated for 3 d with anti-CD3 + anti-CD28, either with or without additional IL-2 as indicated. Proliferation of CD4−8+ cells was assessed by CFSE intensity.
Table I.
Summary Effects of Different Cytokines on CD8+ Thymocyte Differentiation
| γc-dependent cytokines
|
||||||
|---|---|---|---|---|---|---|
| Function | IL-7 | IL-4 | IL-2 | IL-15 | IFNγ | TNFα |
| Promote survival | + | − | − | − | − | − |
| Promote differentiation into CD4−8+ |
+ | + | − | − | + | − |
| Promote functional maturation |
+ | + | + | + | − | − |
Discussion
Using an in vitro model of CD8+ T cell differentiation, the present paper demonstrates that cytokine receptor signaling is required for terminal differentiation of thymocytes into functionally mature CD8+ T cells. TCR-signaled DP thymocytes initially differentiate into intermediate CD4+8− cells that must undergo coreceptor reversal to transcriptionally become CD4−8+ thymocytes that can differentiate into functional CD8+ T cells. Importantly, IL-7R signals maintain the viability of intermediate CD4+8− thymocytes during coreceptor reversal and can be replaced by Bcl-2 transgene expression. In addition to up-regulating Bcl-2 expression, IL-7R signals also enhance CD4 gene silencing and promote functional competence in developing CD8+ T cells, perhaps by improving nutrient uptake to provide the metabolic energy needed to activate new genetic programs. And finally, IL-7Rs are unique among cytokine receptors in maintaining thymocyte viability during differentiation into CD8+ T cells, but they are not unique among cytokine receptors in supporting the trophic/differentiative functions required for terminal differentiation of mature CD8+ T cells. Thus, cytokine receptors provide both survival and trophic/differentiative signals required for differentiation of developing thymocytes into functionally mature CD8+ T cells (Fig. 9) .
Figure 9.
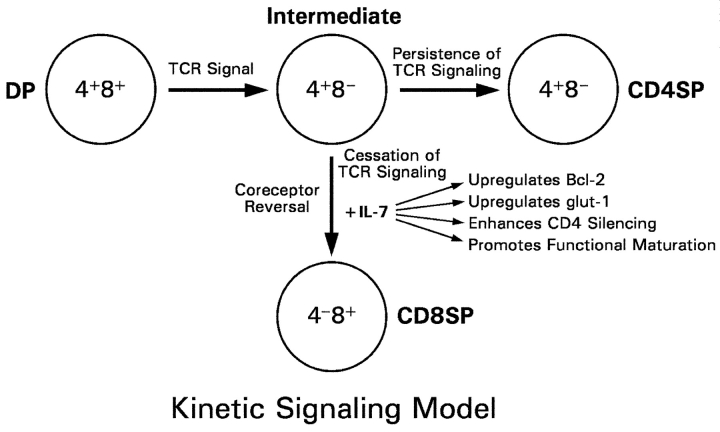
Kinetic signaling model of T cell development: role of IL-7. The kinetic signaling model postulates that TCR signals drive DP thymocytes to first differentiate into intermediate CD4+8− cells, and it is at the intermediate CD4+8− stage that CD4/CD8 lineage direction is determined by whether TCR signals are present or have ceased. Persistence of TCR signals in intermediate thymocytes maintains CD4+8− gene expression and results in differentiation into CD4+ T lineage cells; cessation of TCR signals in intermediate thymocytes results in coreceptor reversal (i.e., reinitiation of CD8 gene expression and silencing of CD4 gene expression) and differentiation into CD8+ T lineage cells. As a consequence of absent TCR signals, IL-7R signals are required to maintain Bcl-2 expression and viability of intermediate thymocytes during coreceptor reversal and differentiation into CD8+ T cells. In addition, cytokine receptor signaling promotes CD8+ T cell differentiation by providing trophic signals that support CD4 gene silencing and functional maturation in developing CD8SP thymocytes.
Kinetic Signaling, Coreceptor Reversal, and the CD4/CD8 Lineage Decision
The kinetic signaling model posits that positively selected DP thymocytes are stimulated by TCR signals to initiate a preset program of molecular events characterized by induction of Bcl-2 expression, induction of IL-7Rα expression, and selective termination of CD8 gene expression with the result that signaled DP thymocytes transcriptionally convert into intermediate CD4+8− thymocytes. If TCR signaling persists on conversion of DP into intermediate CD4+8− thymocytes, the intermediate thymocytes differentiate into CD4SP T cells; however, if TCR signaling is disrupted on conversion of DP into intermediate CD4+8− thymocytes, the intermediate thymocytes undergo coreceptor reversal and differentiate into CD8SP T cells. Thus, sustained TCR signaling results in differentiation into CD4+ T cells, whereas transient TCR signaling results in coreceptor reversal and differentiation into CD8+ T cells (6, 8, 23).
From the kinetic signaling perspective, coreceptor reversal in intermediate CD4+8− thymocytes causes positively selected thymocytes to differentiate into CD8+ T cells. Because coreceptor reversal occurs in intermediate CD4+8− thymocytes when TCR signaling has ceased, thymocytes undergoing coreceptor reversal cannot depend on TCR signals to maintain Bcl-2 expression for their survival, but instead must rely on IL-7R signals. A strict requirement for IL-7R signaling to maintain thymocyte viability during coreceptor reversal is consistent with our finding that anti–IL-7R antibodies added to FTOC blocked the ability of WT thymocytes to survive to transcriptionally become CD4−8+ cells, but did not block their ability to survive to transcriptionally become CD4+8− cells (Fig. 1; and reference 6). Thus, our FTOC experiments, which used the coreceptor reexpression assay to focus on the lineage commitment phase of positive selection, demonstrated that IL-7R signals were critical for thymocyte survival during CD8 lineage commitment but not CD4 lineage commitment. However, Akashi et al. observed previously that administration of anti–IL-7R antibody to host mice blocked intrathymically transferred DP thymocytes from differentiating into both CD4+ and CD8+ T cells (22). We think that our results and those of Akashi et al. are both consistent with the concept that IL-7R signals are required for survival of positively selected thymocytes after TCR signaling has ceased; but that TCR signaling ceases before lineage commitment in developing CD8+ thymocytes, and ceases sometime after lineage commitment in developing CD4+ thymocytes.
This paper primarily focused on the role of cytokine receptor signaling in developing CD8+ thymocytes. We replaced the viability-promoting effects of IL-7R signals with Bcl-2 transgene expression and assessed the role of IL-7R signals in coreceptor reversal and subsequent differentiation into functional CD8+ T cells. We observed that, after cessation of TCR signaling in intermediate CD4+8− thymocytes, each subsequent step in CD8+ thymocyte differentiation was more dependent on IL-7R signaling than the previous step. That is, intermediate CD4+8− thymocytes differentiate into mature CD8+ T cells first by reinitiating CD8 expression, which occurred independently of IL-7R signaling; next by extinguishing CD4 expression, which was quantitatively dependent on IL-7R signaling; and finally by acquiring functional competence, which was highly dependent on IL-7R signaling. This hierarchy of IL-7R dependence (CD8 reexpression < CD4 extinction < functional maturation) suggests that, after cessation of TCR signaling, thymocytes deplete intracellular stores and become increasingly dependent on IL-7R signaling for the metabolic energy required for each subsequent developmental step (24, 31).
IL-7R Signaling and Coreceptor Reversal
Here, we demonstrate that IL-7R signaling is strictly required for thymocyte survival during coreceptor reversal, but is not strictly required for either CD8 gene reexpression or CD4 gene silencing. CD8 gene transcription is regulated by stage-specific enhancer elements in that immature CD8 enhancers drive CD8 gene transcription in immature DP thymocytes and mature CD8 enhancers drive CD8 gene transcription in mature SP thymocytes (32, 33). CD8 gene expression may terminate in intermediate CD4+8− thymocytes because these cells represent an inflection point in which immature CD8 enhancers have turned off but mature CD8 enhancers are still in the process of turning on. Consequently, when mature CD8 enhancers do turn on, CD8 gene reexpression appears “spontaneously” and independently of IL-7R signaling.
IL-7R signaling is also not strictly required for CD4 gene silencing during coreceptor reversal as CD4 gene termination occurred in intermediate thymocytes even in the absence of IL-7. However, the number of intermediate thymocytes in which CD4 gene termination occurred, and the rapidity with which it occurred, was significantly increased by IL-7. CD4 gene silencing during thymocyte development is dependent on activation of a negative regulatory element (CD4 silencer; references 34–37). IL-7R signals may contribute to CD4 gene silencing in intermediate thymocytes either directly, by enhancing activation of the CD4 silencer element, or indirectly, by promoting nutrient uptake and the metabolic energy needed to activate the CD4 silencer element (24, 31).
As coreceptor reversal did not require IL-7R signaling, blockade of coreceptor reversal also did not require IL-7R signaling. Coreceptor reversal was shown previously to be blocked by TCR signaling in intermediate thymocytes (6). The present paper demonstrates that P + I signaling, which mimics TCR signaling while having other potential effects, directly interferes with CD8 gene reexpression and CD4 gene silencing during coreceptor reversal. Current knowledge of how CD8 and CD4 genes are regulated provides some insight into how TCR signaling might regulate reciprocal expression of these two gene loci. In developing thymocytes, CD8 gene expression is regulated positively by activation of stage-specific enhancer elements, whereas CD4 gene expression is somewhat more complex. CD4 enhancer elements have been described that are differentially active in mature and immature thymocytes (38), but gene knockout mice have demonstrated that tissue-specific CD4 expression depends on the CD4 silencer element (36, 37). Consequently, if TCR signals blocked activation of CD8 enhancer and CD4 silencer elements, the result would be to maintain CD4 gene expression “on” and to turn CD8 gene expression “off.” Interestingly, CD4+8− is precisely the state of coreceptor gene expression in TCR-signaled DP thymocytes that results in their conversion into intermediate thymocytes, and is precisely the state of coreceptor gene expression that is maintained in intermediate thymocytes by persistent TCR signaling during differentiation into mature CD4+ T cells (Fig. 9).
IL-7R Signaling Requirements for Functional Maturation of CD8+ T Cells
The present paper was performed in an in vitro system so that CD8+ T cell differentiation could be studied in discrete steps during which cytokine exposure was specifically controlled. An important finding is that CD8+ thymocytes require cytokine receptor signals for functional maturation. We found that CD8+ T cells could be inefficiently generated in vitro without IL-7R signaling so long as cell survival was maintained by Bcl-2 transgene expression, but such CD8+ T cells failed to acquire functional competence. In response to TCR stimulation, CD8+ T cells generated in medium failed to fully up-regulate CD69 and CD25, failed to produce cytokines such as IFNγ, and failed to proliferate even in the presence of exogenously added IL-2, whereas CD8+ T cells generated in IL-7 were fully functional. It remains to be determined if IL-7R signals specifically activate a functional gene program in developing CD8+ thymocytes, or if IL-7R signals provide nonspecific trophic effects that support the activation of a functional gene program in developing CD8+ thymocytes (24). Importantly, the present paper demonstrates that, by whatever mechanism, cytokine receptor signals such as those transduced by IL-7Rs are required for acquisition of functional competence in developing CD8+ T cells through a mechanism that is distinct from Bcl-2 up-regulation and promotion of thymocyte survival.
Limited Cytokine Receptor Redundancy
Although this work has focused on IL-7R signaling in developing CD8+ thymocytes, it was also important to assess potential cytokine receptor redundancy. We found that other cytokine receptors (including IL-2, IL-4, IL-15, and IFNγ but not TNFα) are able to provide the differentiative functions performed by IL-7Rs in CD8+ thymocyte differentiation, but we were unable to identify a cytokine receptor other than IL-7R that could efficiently up-regulate Bcl-2 expression and promote thymocyte survival. Notably, we have not directly examined the role of the cytokine TSLP, whose receptor consists of IL-7Rα complexed to a unique heavy chain, but efficient blockade of CD8+ T cell generation in FTOC by anti-γc antibody indicates that TSLP does not play a major role in promoting survival of positively selected CD8+ thymocytes. Consequently, we think that IL-7R signaling is virtually indispensable for survival of WT thymocytes during CD8+ T cell development, but that its differentiative functions can also be signaled by receptors for other intrathymic cytokines. Interestingly, the receptors that affect differentiation of post-selection thymocytes include other γc cytokine receptors (IL-2, -4, -15) and the non-γc cytokine receptor for IFNγ.
Redundancy of cytokine receptor signaling makes it nearly impossible to identify a differentiative role for cytokine receptor signaling in CD8+ thymocyte development, except in an in vitro system. Indeed, in vivo experiments have concluded that IL-7Rs only provide survival signals to positively selected thymocytes (22). However, it was not possible to appreciate in in vivo experiments that other cytokine receptors might have replaced the intrathymic differentiative or trophic signals otherwise provided by IL-7Rs in positively selected Bcl-2 transgenic thymocytes.
Although our use of P + I to stimulate DP thymocytes is clearly artificial, the differentiative events induced in DP thymocytes after transient P + I stimulation, including coreceptor reversal and terminal CD8+ thymocyte differentiation, are identically produced by TCR stimulation in vitro and occur in vivo in response to normal intrathymic ligands (6). Thus, regardless of how DP thymocytes are stimulated, transient stimulation initiates differentiative events in vitro that closely resemble the differentiative events occurring in the thymus during normal CD8+ T cell differentiation.
Trophic Effects of IL-7R Signaling
It has recently been appreciated by Thompson and colleagues that lymphocytes require extrinsic stimulation to induce expression of surface receptors such as glut-1 that promote nutrient uptake and increase metabolic activity (24, 31). TCR and IL-7R signals are among the signals that induce glut-1. The importance of extrinsic stimulation to promote nutrient uptake was originally revealed by the observation that Bcl-2 transgene expression maintains cell survival but does not itself prevent cell atrophy resulting from limited energy supplies due to inadequate nutrient uptake (24, 31). Interestingly, the present paper demonstrates that glut-1 is expressed in high amounts on developing CD8+ thymocytes and that its expression is dependent on IL-7R signaling. In parallel with increased glut-1 expression, the present paper also demonstrates that IL-7R signaling significantly improves nutrient uptake in developing CD8+ thymocytes. Consequently, we think that IL-7R signals are needed by developing CD8+ thymocytes to provide the metabolic energy for initiating and altering gene programs during their differentiation into mature T cells.
In conclusion, the present paper documents that cytokine receptors provide critical survival and trophic/differentiative signals involved in terminal differentiation of post-selection CD8+ thymocytes after TCR signaling has ceased. In addition, the present paper documents that redundancy exists among cytokine receptors for the differentiative functions, but not the survival functions, performed by IL-7Rs in CD8+ T cell development.
Acknowledgments
We thank Larry Granger and Anthony Adams for expert flow cytometry; Drs. Samuel Cushman and Desmond Hunt for help with the glucose uptake assay; and Drs. Remy Bosselut, Ronald Gress, Phil Lucas, and Dinah Singer for their critical readings of the manuscript.
The present address of Avinash Bhandoola is Dept. of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Footnotes
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate succinimidyl ester; DP, double positive; FTOC, fetal thymic organ culture; γc, common cytokine receptor γ chain; glut-1, glucose transporter molecule-1; SP, single positive; WT, wild-type.
References
- 1.Jameson, S.C., K.A. Hogquist, and M.J. Bevan. 1995. Positive selection of thymocytes. Annu. Rev. Immunol. 13:93–126. [DOI] [PubMed] [Google Scholar]
- 2.Linette, G.P., M.J. Grusby, S.M. Hedrick, T.H. Hansen, L.H. Glimcher, and S.J. Korsmeyer. 1994. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1:197–205. [DOI] [PubMed] [Google Scholar]
- 3.Moore, N.C., G. Anderson, G.T. Williams, J.J. Owen, and E.J. Jenkinson. 1994. Developmental regulation of bcl-2 expression in the thymus. Immunology. 81:115–119. [PMC free article] [PubMed] [Google Scholar]
- 4.Singer, A., R. Bosselut, and A. Bhandoola. 1999. Signals involved in CD4/CD8 lineage commitment: current concepts and potential mechanisms. Semin. Immunol. 11:273–281. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg, E.V. 1992. The development of functionally responsive T cells. Adv. Immunol. 51:85–214. [DOI] [PubMed] [Google Scholar]
- 6.Brugnera, E., A. Bhandoola, R. Cibotti, Q. Yu, T.I. Guinter, Y. Yamashita, S.O. Sharrow, and A. Singer. 2000. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 13:59–71. [DOI] [PubMed] [Google Scholar]
- 7.Yasutomo, K., C. Doyle, L. Miele, C. Fuchs, and R.N. Germain. 2000. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 404:506–510. [DOI] [PubMed] [Google Scholar]
- 8.Singer, A. 2002. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr. Opin. Immunol. 14:207–215. [DOI] [PubMed] [Google Scholar]
- 9.Akashi, K., M. Kondo, and I.L. Weissman. 1998. Role of interleukin-7 in T-cell development from hematopoietic stem cells. Immunol. Rev. 165:13–28. [DOI] [PubMed] [Google Scholar]
- 10.Akashi, K., M. Kondo, and I.L. Weissman. 1998. Two distinct pathways of positive selection for thymocytes. Proc. Natl. Acad. Sci. USA. 95:2486–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata, M., T. Kuwata, M. Mukai, Y. Tozawa, and M. Yokoyama. 1996. Differential induction of helper and killer T cells from isolated CD4+CD8+ thymocytes in suspension culture. Eur. J. Immunol. 26:2081–2086. [DOI] [PubMed] [Google Scholar]
- 12.Moore, T.A., U. von Freeden-Jeffry, R. Murray, and A. Zlotnik. 1996. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7−/− mice. J. Immunol. 157:2366–2373. [PubMed] [Google Scholar]
- 13.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, Y.W., and T.R. Malek. 1996. Interleukin-7 receptor α is essential for the development of γ δ + T cells, but not natural killer cells. J. Exp. Med. 184:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao, X., E.W. Shores, J. Hu-Li, M.R. Anver, B.L. Kelsall, S.M. Russell, J. Drago, M. Noguchi, A. Grinberg, E.T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 2:223–238. [DOI] [PubMed] [Google Scholar]
- 17.Schlissel, M.S., S.D. Durum, and K. Muegge. 2000. The interleukin 7 receptor is required for T cell receptor γ locus accessibility to the V(D)J recombinase. J. Exp. Med. 191:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi, H. Yoshida, and S. Nishikawa. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlotnik, A., and T.A. Moore. 1995. Cytokine production and requirements during T-cell development. Curr. Opin. Immunol. 7:206–213. [DOI] [PubMed] [Google Scholar]
- 20.Kondo, M., Y. Ohashi, K. Tada, M. Nakamura, and K. Sugamura. 1994. Expression of the mouse interleukin-2 receptor gamma chain in various cell populations of the thymus and spleen. Eur. J. Immunol. 24:2026–2030. [DOI] [PubMed] [Google Scholar]
- 21.DiSanto, J.P., S. Certain, A. Wilson, H.R. MacDonald, P. Avner, A. Fischer, and G. de Saint Basile. 1994. The murine interleukin-2 receptor gamma chain gene: organization, chromosomal localization and expression in the adult thymus. Eur. J. Immunol. 24:3014–3018. [DOI] [PubMed] [Google Scholar]
- 22.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. [DOI] [PubMed] [Google Scholar]
- 23.Bosselut, R., L. Feigenbaum, S.O. Sharrow, and A. Singer. 2001. Strength of signaling by CD4 and CD8 coreceptor tails determines the number but not the lineage direction of positively selected thymocytes. Immunity. 14:483–494. [DOI] [PubMed] [Google Scholar]
- 24.Rathmell, J.C., M.G. Vander Heiden, M.H. Harris, K.A. Frauwirth, and C.B. Thompson. 2000. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell. 6:683–692. [DOI] [PubMed] [Google Scholar]
- 25.Sentman, C.L., J.R. Shutter, D. Hockenbery, O. Kanagawa, and S.J. Korsmeyer. 1991. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 67:879–888. [DOI] [PubMed] [Google Scholar]
- 26.Veis, D.J., C.M. Sorenson, J.R. Shutter, and S.J. Korsmeyer. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 75:229–240. [DOI] [PubMed] [Google Scholar]
- 27.Ohoka, Y., T. Kuwata, Y. Tozawa, Y. Zhao, M. Mukai, Y. Motegi, R. Suzuki, M. Yokoyama, and M. Iwata. 1996. In vitro differentiation and commitment of CD4+ CD8+ thymocytes to the CD4 lineage, without TCR engagement. Int. Immunol. 8:297–306. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, H., J.A. Punt, L.G. Granger, and A. Singer. 1995. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity. 2:413–425. [DOI] [PubMed] [Google Scholar]
- 29.Bird, J.J., D.R. Brown, A.C. Mullen, N.H. Moskowitz, M.A. Mahowald, J.R. Sider, T.F. Gajewski, C.R. Wang, and S.L. Reiner. 1998. Helper T cell differentiation is controlled by the cell cycle. Immunity. 9:229–237. [DOI] [PubMed] [Google Scholar]
- 30.Kaldjian, E., S.A. McCarthy, S.O. Sharrow, D.R. Littman, R.D. Klausner, and A. Singer. 1988. Nonequivalent effects of PKC activation by PMA on murine CD4 and CD8 cell-surface expression. FASEB J. 2:2801–2806. [DOI] [PubMed] [Google Scholar]
- 31.Rathmell, J.C., E.A. Farkash, W. Gao, and C.B. Thompson. 2001. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167:6869–6876. [DOI] [PubMed] [Google Scholar]
- 32.Ellmeier, W., S. Sawada, and D.R. Littman. 1999. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu. Rev. Immunol. 17:523–554. [DOI] [PubMed] [Google Scholar]
- 33.Hostert, A., M. Tolaini, R. Festenstein, L. McNeill, B. Malissen, O. Williams, R. Zamoyska, and D. Kioussis. 1997. A CD8 genomic fragment that directs subset-specific expression of CD8 in transgenic mice. J. Immunol. 158:4270–4281. [PubMed] [Google Scholar]
- 34.Sawada, S., J.D. Scarborough, N. Killeen, and D.R. Littman. 1994. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 77:917–929. [DOI] [PubMed] [Google Scholar]
- 35.Siu, G., A.L. Wurster, D.D. Duncan, T.M. Soliman, and S.M. Hedrick. 1994. A transcriptional silencer controls the developmental expression of the CD4 gene. EMBO J. 13:3570–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung, R.K., K. Thomson, A. Gallimore, E. Jones, M. Van den Broek, S. Sierro, A.R. Alsheikhly, A. McMichael, and A. Rahemtulla. 2001. Deletion of the CD4 silencer element supports a stochastic mechanism of thymocyte lineage commitment. Nat. Immunol. 2:1167–1173. [DOI] [PubMed] [Google Scholar]
- 37.Zou, Y.R., M.J. Sunshine, I. Taniuchi, F. Hatam, N. Killeen, and D.R. Littman. 2001. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nat. Genet. 29:332–336. [DOI] [PubMed] [Google Scholar]
- 38.Adlam, M., D.D. Duncan, D.K. Ng, and G. Siu. 1997. Positive selection induces CD4 promoter and enhancer function. Int. Immunol. 9:877–887. [DOI] [PubMed] [Google Scholar]