Abstract
Activation of CD4+ T cells is governed by interplay between stimulatory and inhibitory receptors; predominance of stimulatory signals favors autoimmune reactions. In patients with rheumatoid arthritis, expression of the critical costimulatory molecule, CD28, is frequently lost. Instead, CD4+CD28null T cells express killer immunoglobulin-like receptors (KIRs) with a preferential expression of the stimulatory receptor, CD158j. The frequency of CD4+CD28null T cells in rheumatoid arthritis (RA) correlates with the risk for more severe disease. Moreover, the KIR2DS2 gene, which encodes for CD158j, is a genetic risk factor for rheumatoid vasculitis. CD158j signals through the adaptor molecule, KARAP/DAP12, to positively regulate cytotoxic activity in NK cells. However, the majority of CD4+CD28null T cell clones lacked the expression of KARAP/DAP12. Despite the absence of KARAP/DAP12, CD158j was functional and augmented interferon-γ production after T cell receptor stimulation. Cross-linking of CD158j resulted in selective phosphorylation of c-Jun NH2-terminal protein kinase (JNK) and its upstream kinase, MKK4 that led to the expression of ATF-2 and c-Jun, all in the absence of extracellular signal–regulated kinase (ERK)1/2 phosphorylation. Mutation of the lysine residue within the transmembrane domain of CD158j abolished JNK activation, suggesting that an alternate adaptor molecule was being used. CD4+CD28null T cells expressed DAP10 and inhibition of phosphatidylinositol 3-kinase, which acts downstream of DAP10, inhibited JNK activation; however, no interaction of DAP10 with CD158j could be detected. Our data suggest that CD158j in T cells functions as a costimulatory molecule through the JNK pathway independent of KARAP/DAP12 and DAP10. Costimulation by CD158j may contribute to the autoreactivity of CD4+CD28null T cells in RA.
Keywords: autoimmunity, pathogenesis, rheumatoid arthritis, costimulation, killer immunoglobulin-like
Introduction
The killer immunoglobulin-like receptors (KIRs)* comprise a multigene family of receptors that are primarily expressed on NK cells. In humans, the KIR family is composed of 12 receptors, each of which recognizes specific MHC class I alleles. Functionally, the members of the KIR family can be either inhibitory or stimulatory. Because both inhibitory and stimulatory KIRs share similar extracellular domains, it is believed that both are capable of binding the same MHC class I alleles, although with differing affinities (1). This led to the hypothesis that the balance between these two classes of receptors regulates NK cell function.
Inhibitory KIRs possess long cytoplasmic tails that contain immunoreceptor tyrosine inhibitory motifs (ITIMs). Upon binding the appropriate MHC class I molecule, the inhibitory receptors activate the phosphatase, SHP-1 (2, 3). This phosphatase dephosphorylates important molecules in the signaling cascade of stimulatory receptors, such as CD16, resulting in inhibition of NK cell cytotoxicity (3, 4). This observation supports the missing-self hypothesis, which suggests that the function of inhibitory KIRs is to recognize self-MHC class I molecules and prevent self-directed NK cell cytotoxicity (5).
In contrast to inhibitory KIRs, stimulatory receptors do not possess a cytoplasmic tail that contains ITIMs, making these receptors unable to activate protein tyrosine phosphatases. In fact, the cytoplasmic tail of stimulatory KIRs does not contain any known signaling motifs and requires an adaptor molecule, KARAP/DAP12, to initiate the signaling cascade (6–8). Signaling through KARAP/DAP12 leads to activation of members of the Syk family of protein tyrosine kinases resulting in extracellular signal–regulated kinase (ERK) activation, calcium mobilization, and NK cell cytotoxicity.
In addition to NK cells, T cell subsets express members of the KIR family. In healthy individuals, the majority of KIR expression on T cells is limited to CD8+ T cells. CD8+ T cells that express KIRs are oligoclonally expanded and possess the memory phenotype, CD44+CD57+ CD45RO+CD28null. It has been proposed that KIR expression on T cells occurs after chronic, antigen-driven stimulation (9).
In contrast to healthy individuals, patients with rheumatoid arthritis (RA) or acute coronary syndromes (ACS) often possess subpopulations of CD4+ T cells that express KIRs (10, 11). CD4+KIR+ T cells are clonally expanded and have lost expression of CD28, suggesting that these cells have undergone chronic stimulation (12). The expansion of CD4+CD28null T cells is associated with increased disease severity in RA (13) and plaque instability in patients with ACS (14). Of the known members of the KIR family, the KIR2DS2 gene, which encodes the CD158j molecule, is the most frequently expressed KIR on CD4+CD28null T cells from patients with RA. Individuals carrying the KIR2DS2 gene are at greater risk of developing RA-associated vasculitis, suggesting that CD158j plays a role in the disease pathogenesis (15).
Because CD158j is a stimulatory receptor on NK cells, we hypothesized that this molecule functions as a stimulatory molecule on CD4+ T cells that have lost CD28 expression. However, most CD4+ T cells do not express KARAP/DAP12 and CD158j stimulation in T cells does not induce activation of the signaling cascade seen in NK cells. Here, we demonstrate that CD158j selectively activates the c-Jun NH2-terminal kinase (JNK) signaling pathway independent of the adaptor molecule, KARAP/DAP12. Activation of this pathway up-regulates IFN-γ expression after suboptimal TCR stimulation. We conclude that CD158j induces inflammation in RA and ACS by regulating the effector functions of CD4+CD28null T cells through the JNK pathway.
Materials and Methods
Cloning of CD4+CD28null T Cells.
The protocol was approved by the Mayo Clinic Institutional Review Board and all patients gave written, informed consent. Peripheral blood mononuclear cells (PBMCs) from patients with RA were isolated using Ficoll-Hypaque (Amersham Biosciences) and were sorted for CD4+CD28null T cells by FACS® using anti-CD4FITC and anti-CD28PE mAbs (Becton Dickinson). Sorted cells were cloned using limiting dilution. Clones were maintained on 1.0 × 106 irradiated heterologous PBMCs/ml, 2.0 × 105 irradiated Epstein Barr virus–transformed B cells/ml, 30 ng/ml anti-CD3 mAb (OKT3, CRL 8001; American Type Culture Collection), and 50 U/ml recombinant human IL-2 (Proleukin; Chiron Corp.). Established T cell clones were characterized for the expression of CD28 and CD158b/j by flow cytometry using anti-CD28FITC (Becton Dickinson) and anti-CD158b/jPE mAbs (GL183; Beckman Coulter).
Reverse Transcriptase PCR.
For all reverse transcriptase (RT)-PCR experiments, total RNA was extracted using TRIzol reagent (Invitrogen/Life Technologies). cDNA was synthesized using an oligo-dT primer and AMV reverse transcriptase (Roche Molecular Diagnostics). CD4+CD28nullCD158b/j+ clones were analyzed for the expression of KIR2DS2 (CD158j), KIR2DL2 (CD158b1), and KIR2DL3 (CD158b2) transcripts using receptor-specific PCR primers described by Uhrberg (16). The CD158 nomenclature for the cell surface molecules and KIR nomenclature for the corresponding genes were used as suggested by the 7th Human Leukocyte Differentiation Antigens Workshop (17). CD4+CD28nullCD158j+ T cell clones were characterized for the expression of KARAP/DAP12 transcript using the primers 5′-CAGTTGCTCTACGGTGAGC-3′ and 5′-TGTGTGTTGAGGTCGCTG-3′. For each RT-PCR analysis, β-actin transcript, amplified by the primers 5′-ATGGCCACGGCTGCTTCCAGC-3′ and 5′-CAGGAGGAGCAATGATCTTGAT-3′, was used as a control.
IFN-γ Analysis.
IFN-γ expression was analyzed by real-time, quantitative PCR and ELISA. For the real-time PCR analysis, resting CD4+CD28nullCD158b/j+ T cell clones were stimulated with immobilized mouse IgG (ICN Biomedicals), anti-CD3 mAb (OKT3), anti-CD158b/j mAb (GL183), anti-CD3 and anti-CD158b/j mAbs, or anti-CD3 and anti-MHC class I (β2-microglobulin; L368, HB149; American Type Culture Collection) mAbs. After 4 h, the cells were harvested, total RNA was extracted, and cDNA was synthesized. The number of IFN-γ transcripts was determined by real-time, quantitative PCR (LightCycler; Roche Molecular Diagnostics). Primers used for the amplification were 5′-ACCTTAAGAAATATTTTAATGC-3′ and 5′-ACCGAATAATTAGTCAGCTT-3′. For quantification of protein production, cell supernatants were harvested after 20 h and IFN-γ was determined by ELISA (Biosource International) according to the manufacturer's instructions.
PathwayFinder cDNA Array.
A resting CD4+CD28null CD158b/j+ T cell clone was treated with either mouse IgG or anti-CD158b/j mAb and cross-linked with rabbit anti–mouse IgG polyclonal Ab (ICN Biomedicals). After 4 h, total RNA was harvested. cDNA was synthesized and used to probe the PathwayFinder cDNA Array (SuperArray) according to the manufacturer's instructions.
Vaccinia Virus Infection.
Jurkat T cells were infected for 18 h at a ratio of 10:1 (viral PFU:T cell ratio) with either wild-type (WR) vaccinia virus or recombinant vaccinia virus containing KIR2DS2 (CD158j) cDNA (both provided by Dr. Paul Leibson, Mayo Clinic). Cell surface expression of CD158j was confirmed by flow cytometry.
Subcloning, Site-directed Mutagenesis, and Transient Transfection.
CDNA for CD158j was amplified using the primers 5′-TTACGGCGGCCGCATGTCGCTCATGGTCGTC-3′ and 5′-CCGGCTCTAGATTATGCGTATGACACCTCCTG-3′. The amplified product was digested with Not and XbaI and cloned into the mammalian expression vector pcDNA3.1. A mutant of CD158j was constructed in which lysine 233 was changed to isoleucine (K233I) according to a previously published protocol (18). Briefly, cDNA for CD158j was amplified in two pieces using the primers 5′-TTACGGCGGCCGCATGTCGCTCATGGTCGTC-3′/5′-GAAAGGGATGATGAC-CACTGAG-3′ and 5′-CTCAGTGGTCATCATCCCTTTC-3′/5′-CCGGCTCTAGATTATGCGTATGACACCTCCTG-3′. After purification, the two PCR products were mixed and amplified as the full-length cDNA for CD158jK233I. This cDNA was digested with NotI and XbaI and cloned into the mammalian expression vector pcDNA3.1. The mutation was confirmed by sequencing.
Both plasmid constructs were purified using the EndoFree Plasmid Giga Kit (QIAGEN) and ethanol precipitated. To induce expression, Jurkat T cells were transiently transfected with these constructs by electroporation. Cell-surface expression was confirmed by flow cytometry. Cells were used for subsequent experiments 48 h after transfection.
Cell Stimulation.
Resting CD4+CD28nullCD158b/j+ T cell clones or transfected Jurkat T cells were treated with mouse IgG, anti-CD3, anti-CD158b/j, or anti-CD3 and anti-CD158b/j mAbs and then cross-linked with rabbit anti–mouse IgG Ab. For the experiments in which protein phosphorylation was examined, cells were stimulated for 20 min; for the experiments in which protein expression was examined, cells were stimulated for 2 h. For inhibition experiments, cells were treated with 2.0 μM wortmannin for 1 h before stimulation. Cells were harvested immediately after stimulation and were lysed.
Western Blots.
Between 5 × 106 and 10 × 106 cells/sample were harvested for protein extraction. Samples were incubated on ice in 50 mM TrisCl (pH 7.6), 150 mM NaCl, 1.0% Triton X-100, 0.5 mM PMSF, 5.0 μg/ml aprotinin, 10.0 μg/ml leupeptin, 1.0 mM orthovanadate. Clarified cell lysates were collected after centrifugation at 10,000 g for 10 min. The amount of total protein in each sample was quantified with the Protein Assay Kit II (BioRad Laboratories). Lysates were analyzed by SDS-PAGE and transferred onto a nitrocellulose membrane. For the characterization of KARAP/DAP12 expression in CD4+ CD28null T cell clones, membranes were blotted in series with specific Abs against KARAP/DAP12 (provided by Dr. Paul Leibson, Mayo Clinic) and β-actin (Sigma-Aldrich). For clones that were stimulated before lysis, the membranes were blotted in series with specific Abs against phospho-JNK and JNK (both Cell Signaling Technology) or β-actin (Sigma-Aldrich), phospho-ERK1/2 and ERK1/2, phospho-MKK4 and MKK4, or c-Jun, ATF-2 (all Cell Signaling Technology), and β-actin (Sigma-Aldrich). Blots were developed using horseradish peroxidase-conjugated goat anti–rabbit IgG or horseradish peroxidase–conjugated rabbit anti–mouse IgG Abs (both Cell Signaling Technology) and the SuperSignal West Pico Chemiluminescent Detection System (Pierce Chemical Co.).
Immunoprecipitation.
RBL cells, CD158j+ RBL cells, or CD158j+KARAP/DAP12+ RBL cells (19) were washed with 1× PBS, 1.0 mM MgCl2, and 1.0 mM CaCl2 and then biotinylated using 1.0 mg/ml sulfo-NHS-LC-biotin (Pierce Chemical Co.) for 10 min at room temperature. Cells were washed with 1× PBS, 0.1 M Tris, and 0.1 M glycine; 25 × 106 cells/ml were lysed in 1.0% digitonin, 0.12% Triton X-100, 150 mM NaCl, and 20 mM triethanolamine, pH 7.8. Lysates were precleared with protein G-Sepharose beads (Amersham Biosciences), followed by addition of anti-DAP10 or isotype control Abs conjugated to protein G-Sepharose beads. Samples were incubated overnight at 4°C. Immunoprecipitated samples were separated by SDS-PAGE (15%) and transferred to nitrocellulose membranes. Blots were developed using horseradish peroxidase–conjugated streptavidin and the ECL Plus (Amersham Biosciences).
Jurkat T cells were lysed in 50 mM Tris buffer (pH 8.0), 150 mM NaCl, 1.0% NP-40, 1.0 mM EDTA, 1.0 mM PMSF, 10.0 μg/ml aprotinin, and 10.0 μg/ml leupeptin. Lysates were precleared with protein G-Sepharose beads, followed by addition of anti-DAP10 or anti-KARAP/DAP12 Abs conjugated to protein G-Sepharose beads. Samples were incubated overnight at 4°C. Immunoprecipitated samples were separated by SDS-PAGE (15%) and transferred to nitrocellulose membranes. Blots were developed using horseradish peroxidase-conjugated streptavidin and the ECL Plus.
Results
Characterization of CD4+CD28nullCD158b/j+ T Cell Clones.
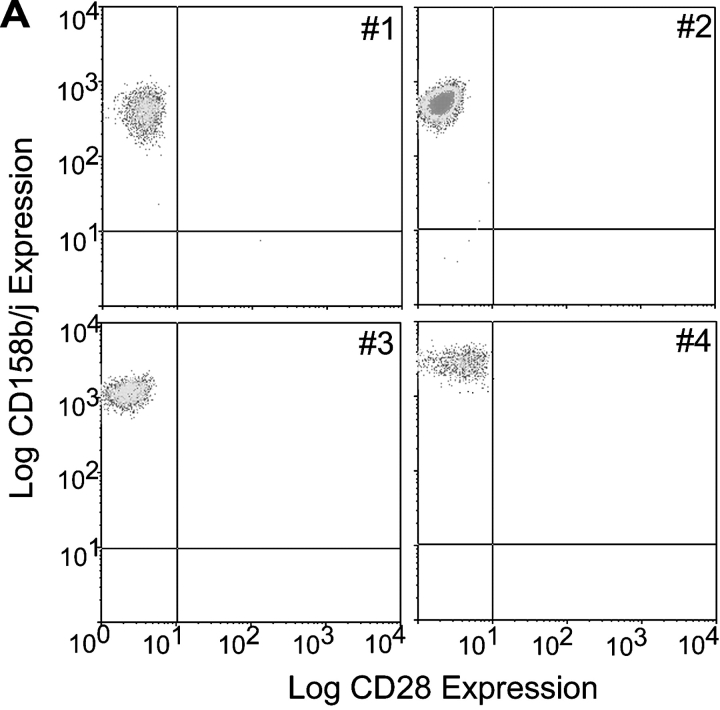
PBMCs were obtained from patients with RA, and CD4+CD28null T cells were sorted by FACS®. After limiting dilution cloning, individual clones were characterized for the cell surface expression of CD158b/j. Within a panel of randomly selected clones, ∼35% of the clones expressed CD158b/j on the cell surface. Representative clones are shown in Fig. 1 A. Each of these clones expressed CD158b/j at levels similar to those previously observed for NK cells.
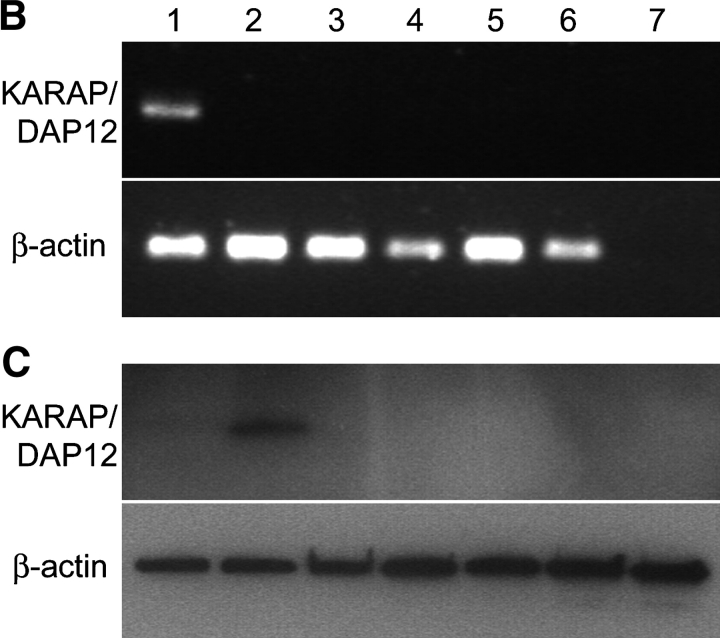
Figure 1.
CD4+CD28null T cell clones express CD158b/j, but do not express KARAP/DAP12. CD4+CD28null T cells were sorted from patients with RA, and clones were established by limiting dilution. Clones were analyzed by flow cytometry for expression of CD28 and CD158b/j. Four representative clones (#1 through #4) are shown. All clones expressed CD4 (unpublished data; A). RT-PCR was used to amplify transcripts for KARAP/DAP12 and β-actin from PBMCs (lane 1), Jurkat T cells (lane 2), and CD4+CD28null T cell clones #1–#4 (lanes 3–6, respectively). cDNA was omitted for the negative control (lane 7) (B). Western blotting was used to detect KARAP/DAP12 and β-actin protein (bottom panels) in Jurkat T cells (lane 1), Jurkat T cells transfected with KARAP/DAP12+ vaccinia virus (lane 2), and CD4+CD28null T cell clones (lanes 3–7) (C).
Within the KIR family there are three receptors that, due to similarities in the extracellular domains, are recognized by the antibody, GL183. These receptors are the stimulatory CD158j (KIR2DS2) and the inhibitory CD158b1 (KIR2DL2) and CD158b2 (KIR2DL3; reference 17). Therefore, it was not possible to determine by flow cytometry if a particular clone expressed one receptor or a mixture of receptors. CD4+CD28nullCD158b/j+ T cell clones were analyzed by RT-PCR with KIR-specific primers. The majority of the selected clones, including those shown in Fig. 1 A, expressed transcripts for CD158j and CD158b1; mRNA for CD158b2 was not detected (unpublished data).
In NK cells, the stimulatory CD158j uses the adaptor molecule, KARAP/DAP12, to activate members of the Syk family of protein tyrosine kinases. However, the majority of CD4+ T cells, including Jurkat T cells, do not express KARAP/DAP12 (8). CD4+CD28nullCD158b/j+ T cell clones were analyzed for expression of the KARAP/DAP12 transcript and protein (Fig. 1, B and C). Neither KARAP/DAP12 transcript nor protein could be detected in any of the clones included in this study or in Jurkat T cells.
CD158b/j Augments IFN-γ Expression Induced by Suboptimal TCR Stimulation.
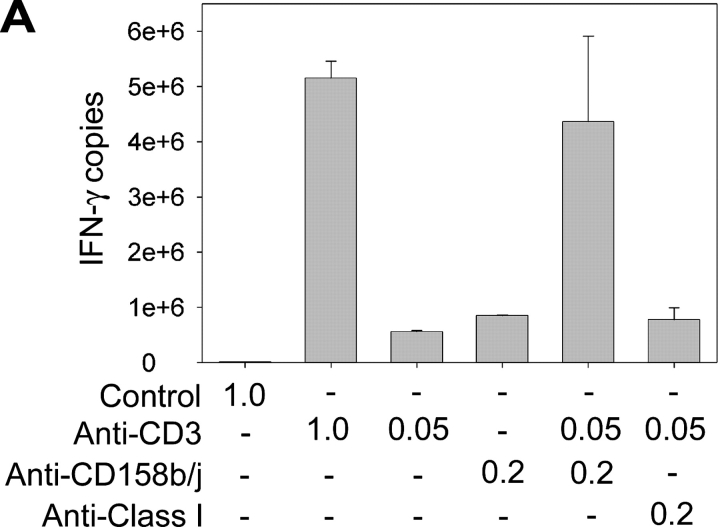
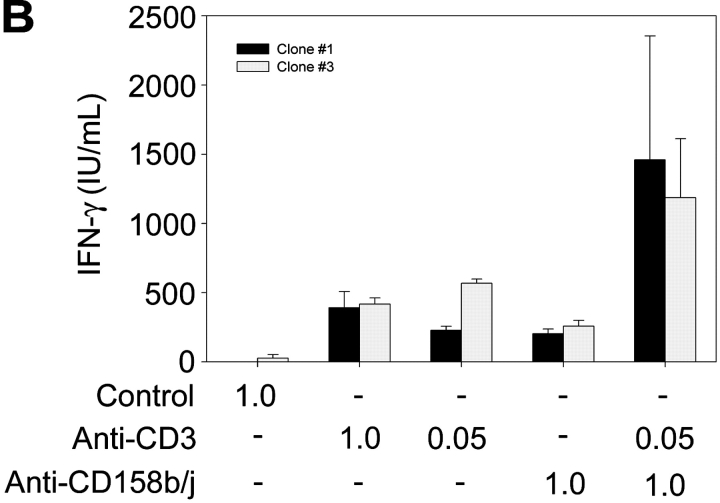
To determine if CD158j could function as a costimulatory molecule despite the lack of KARAP/DAP12, we stimulated CD4+CD28nullCD158b/j+ T cell clones with anti-CD3 and anti-CD158b/j mAbs and analyzed the expression of IFN-γ. A representative experiment using real-time PCR to quantify IFN-γ transcripts is shown in Fig. 2 A. Stimulation with high doses of anti-CD3 mAb resulted in a large increase in IFN-γ transcript production when compared with the control mAb. This level of induction was not observed if the cells were stimulated with either suboptimal concentrations of anti-CD3 mAb or optimal concentrations of anti-CD158b/j mAb. However, when the cells were costimulated with suboptimal concentrations of anti-CD3 and optimal concentrations of anti-CD158b/j mAbs, the increase in IFN-γ production was essentially equivalent to the increase observed with the optimal dose of anti-CD3 mAb alone. Stimulation of the cells with anti-CD3 mAb and a control mAb, anti-MHC class I, did not enhance IFN-γ expression, indicating that the costimulation was a specific function of CD158j. In Fig. 2 B, IFN-γ production was analyzed by ELISA after stimulation of CD4+CD28nullCD158b/j+ T cell clones. As in Fig. 2 A, stimulation through either the TCR or CD158b/j alone resulted in modest increases in IFN-γ expression. However, costimulation through both receptors resulted in approximately a threefold increase in IFN-γ expression over either individual receptor stimulation. These data demonstrate that, in CD4+CD28null T cells, CD158b/j functions as a costimulatory receptor in the absence of KARAP/DAP12.
Figure 2.
CD158b/j costimulates expression of IFN-γ in CD4+ CD28null T cells. CD4+CD28nullCD158b/j+ T cell clones were stimulated using immobilized mouse IgG and anti-CD3 mAb alone or in combination with anti-CD158b/j mAb (shown as μg/ml). The number of IFN-γ transcripts was quantified using real-time PCR. Anti-MHC mAb is included as a negative control (A). Cells were stimulated as above and IFN-γ cytokine production was quantified by ELISA (B).
CD158j Stimulation Results in Increased Transcription of ATF-2.
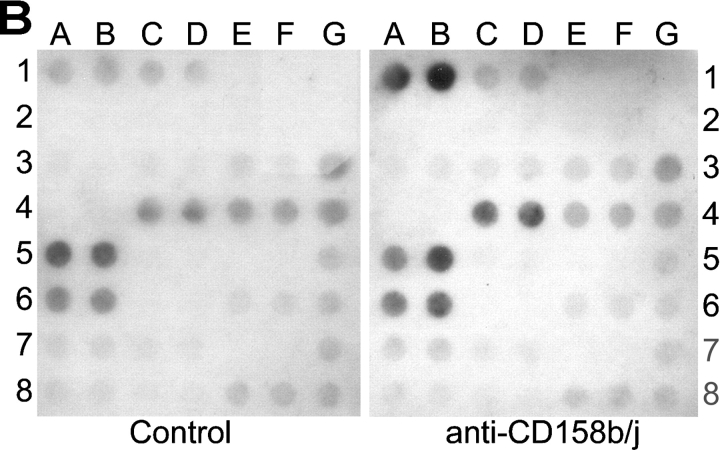
To identify the signaling pathway used by CD158b/j in CD4+CD28null T cells, we used cDNA array technology. The PathwayFinder cDNA Array is a membrane that has been spotted with 23 different cDNAs, each of which is known to be up-regulated by a specific signaling pathway (Fig. 3 A). After stimulation of a CD4+ CD28nullCD158b/j+ T cell clone with either mouse IgG or anti-CD158b/j mAb, total RNA was harvested and used to probe the PathwayFinder cDNA Array. In comparison with the control mAb, stimulation through CD158b/j led to an increase in transcripts for ATF-2 (positions 1A and 1B) and HSP27 (positions 4C and 4D; Fig. 3 B). The β-actin and GAPDH controls (positions 3G through 8G, respectively) indicated that the amount of RNA used for each membrane was equivalent. The transcription of ATF-2 and HSP27 are regulated by the JNK signaling cascade (20, 21). Other pathways represented on the cDNA array, including the NF-κB, ERK, and p53 pathways, were not up-regulated after CD158b/j stimulation, suggesting that the activation was specific for the JNK pathway.
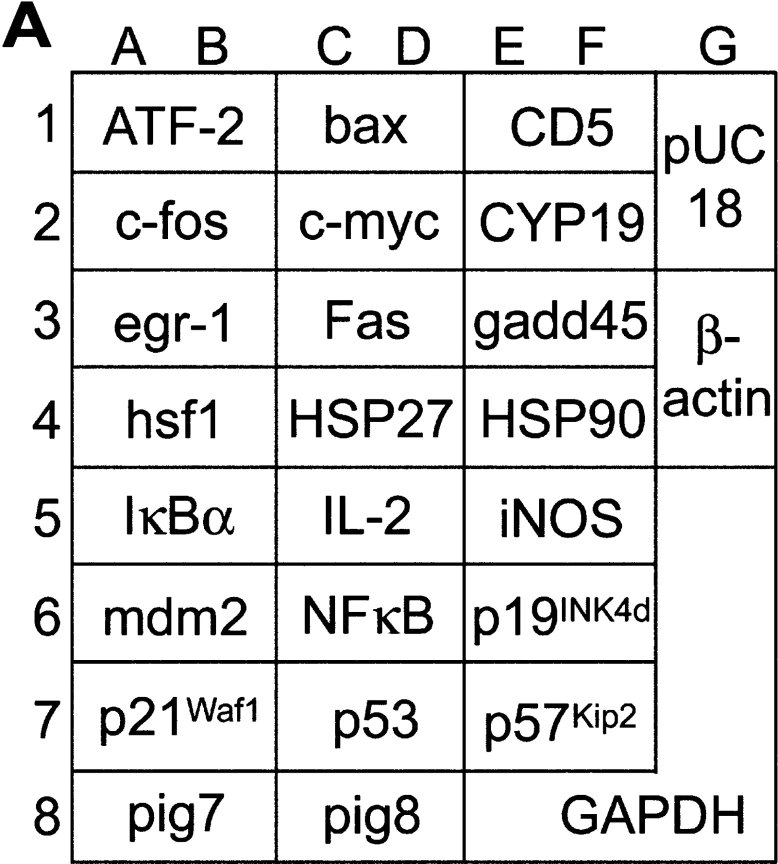
Figure 3.
Stimulation through CD158b/j results in an up-regulation of ATF-2 and HSP27 transcripts. The PathwayFinder cDNA Array is spotted in duplicate with 23 cDNAs. Represented on the membrane are the ERK (egr-1 and c-fos), JNK (ATF-2, hsf1, HSP27, and HSP90), NF-κB (iNos, NF-κB, and IκBα), NFAT (IL-2, Fas, and CD5), TGF-β (p16, p21, and p57Kip2), Wnt (c-myc), p53 (p21, gadd45, pig7, pig8, mdm2, and bax), and CREB pathways (egr-1, CYP19, and c-fos). The membrane also included a negative control (pUC18) and two positive controls (β-actin and GAPDH) (A). A CD4+CD28nullCD158b/j+ T cell clone was stimulated with control mouse IgG or anti-CD158j mAb and cross-linked with rabbit anti–mouse IgG Ab. Total RNA was harvested and used to probe the PathwayFinder cDNA Array (B).
Activation of CD158b/j Leads to Phosphorylation of JNK but Not ERK.
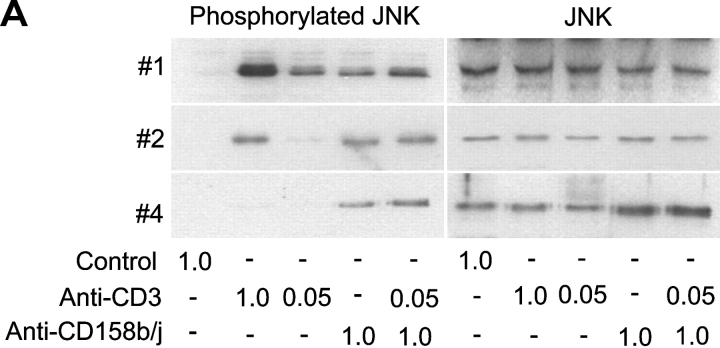
Activation of the JNK signaling cascade involves phosphorylation of several tyrosine kinases, which in turn phosphorylate MKK4 on serine and threonine residues. These kinases phosphorylate JNK on tyrosine, serine, and threonine residues. To confirm the results obtained with the PathwayFinder cDNA Array, we analyzed the induction of JNK phosphorylation after CD158b/j stimulation. CD4+CD28nullCD158b/j+ T cell clones were stimulated with mouse IgG, anti-CD3 mAb, anti-CD158b/j mAb, or a combination of anti-CD3 and anti-CD158b/j mAbs. The cell lysates were then analyzed by Western blot for phosphorylated and total cellular JNK (Fig. 4 A). In all of the clones analyzed, stimulation with anti-CD158b/j mAb, either alone or in combination with suboptimal concentrations of anti-CD3 mAb, resulted in phosphorylation of JNK, even in clone #4 that did not phosphorylate JNK upon CD3 stimulation. Thus, in the CD4+CD28null T cells, stimulation through CD158b/j led to phosphorylation of JNK and activation of the JNK pathway.
Figure 4.
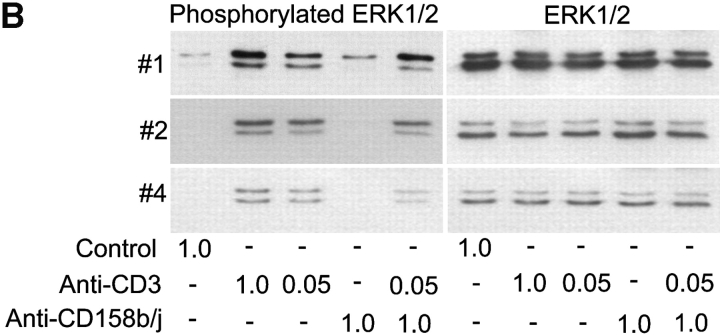
Stimulation of CD158b/j in CD4+CD28null T cells leads to JNK, but not ERK, phosphorylation. CD4+CD28nullCD158j+ T cell clones shown in Figure 1 (clones #1, #2, and #4) were stimulated with anti-CD3 and/or anti-CD158b/j mAbs and cross-linked with rabbit anti–mouse IgG Ab. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of JNK (left panels), after which the blots were stripped and reprobed with Abs against total JNK (right panels) (A). After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of ERK (left panels), after which the blots were stripped and re-probed with Abs against total ERK (right panels) (B).
Results from the PathwayFinder cDNA array suggested that the JNK pathway was specifically activated after CD158b/j stimulation and that there was no activation of the mitogenic ERK pathway. In contrast, CD158j stimulation in NK cells, signaling through KARAP/DAP12, leads to ERK phosphorylation. Three CD4+CD28nullCD158b/j+ T cell clones were stimulated as previously described and then analyzed for ERK phosphorylation (Fig. 4 B). Each of the three clones induced ERK phosphorylation after either optimal or suboptimal TCR stimulation. In contrast, stimulation through CD158b/j in two of the three clones did not result in ERK phosphorylation. A third clone did display some ERK phosphorylation after CD158b/j activation, although it was minimal in comparison with the level of ERK phosphorylation detected after TCR stimulation. In summary, this data suggests that, in the absence of KARAP/DAP12, CD158b/j specifically uses the JNK cascade as its primary signaling pathway.
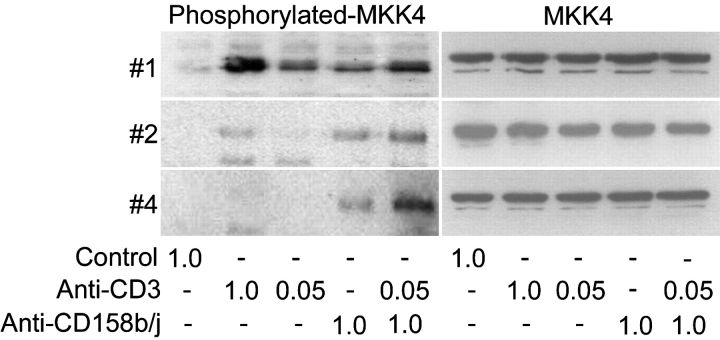
Activation of CD158b/j Leads to Phosphorylation of MKK4.
To further characterize the signaling pathway used by CD158b/j in CD4+CD28null T cells, we analyzed the activation of the kinase MKK4 directly upstream of JNK. Several CD4+CD28nullCD158b/j+ T cell clones were stimulated as described previously. The cell lysates were then analyzed by Western blot for the phosphorylation of MKK4 (Fig. 5) . In two of the three clones analyzed, high concentrations of anti-CD3 mAb induced phosphorylation of MKK4. This phosphorylation was decreased or absent when the cells were stimulated with suboptimal doses of anti-CD3 mAb. All of the clones, when stimulated with anti-CD158b/j mAb alone or in combination with suboptimal anti-CD3 mAb, induced phosphorylation of MKK4. This confirmed the initial findings that stimulation through CD158b/j activates the JNK pathway in CD4+CD28null T cells.
Figure 5.
Stimulation of CD158b/j in CD4+CD28null T cells leads to MKK4 phosphorylation. CD4+CD28nullCD158j+ T cell clones (clones #1, #2, and #4) were stimulated with anti-CD3 and/or anti-CD158b/j mAbs and cross-linked with rabbit anti–mouse IgG Ab. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of MKK4 (left panels). The blots were stripped and reprobed with Ab against total MKK4 (right panels).
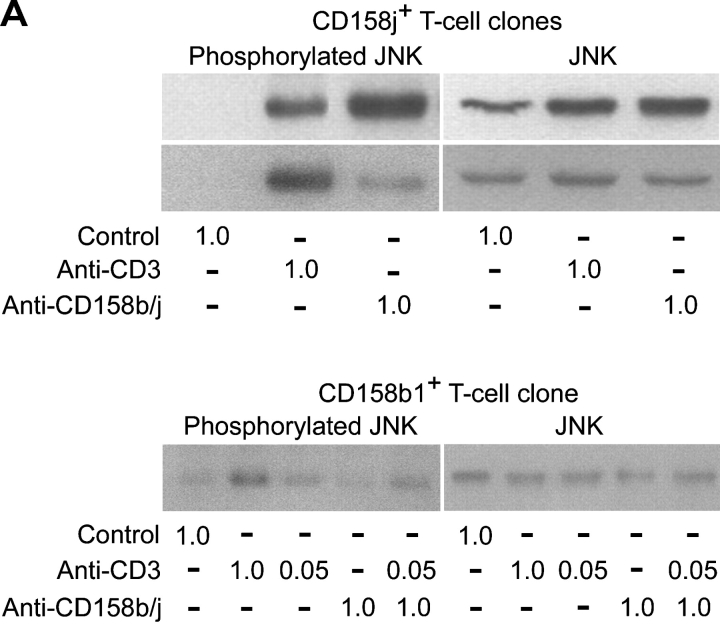
CD158j, and Not CD158b, Induces Activation of the JNK Pathway.
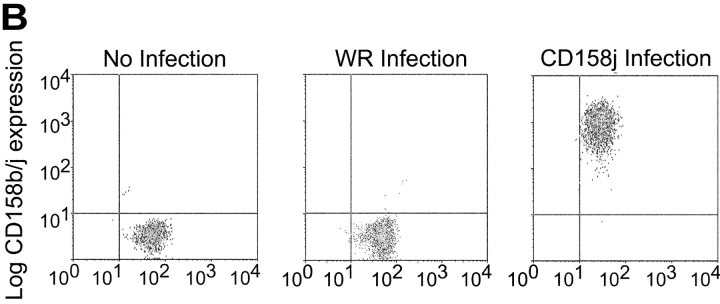
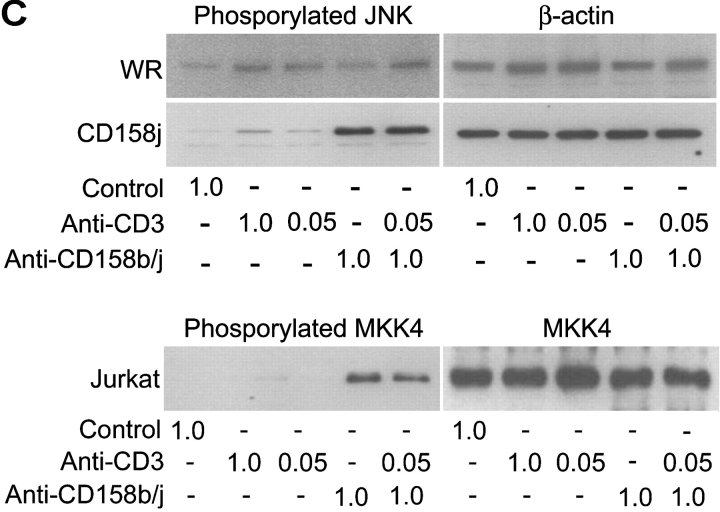
As previously stated, the mAb used to stimulate CD158b/j recognizes three related gene products, CD158b1/b2 and CD158j. CD4+CD28nullCD158b/j+ T cell clones used so far for the signaling studies expressed transcript for both CD158b1 and CD158j. CD158b1 is known to activate protein phosphatases and to inhibit other activating receptors. However, it has also been demonstrated to bind to and activate phosphatidylinositol 3-kinase (PI3K; reference 22). To experimentally address the question of which receptor, CD158j or CD158b1, activates the JNK pathway, we identified three CD4+CD28null DAP12null T cell clones, two of which only expressed CD158j and the other of which expressed only CD158b1. For the two CD158j+ clones, stimulation through either the TCR or CD158j resulted in phosphorylation of JNK (Fig. 6 A, top panels). In contrast, in the CD158b1+ clone, only stimulation through the TCR activated the JNK pathway (Fig. 6 A, bottom panel). To confirm that CD158j was specifically responsible for the activation of this pathway in CD4+CD28null T cells, we infected Jurkat T cells with recombinant vaccinia virus containing the CD158j cDNA. This allowed for the expression of CD158j in the absence of any other receptors recognized by the GL183 mAb. After infection, the expression of CD158j was confirmed by flow cytometry (Fig. 6 B). Prior to infection or after infection with wild-type vaccinia virus, the Jurkat T cells did not express any receptors recognized by the anti-CD158b/j mAb. However, after infection with vaccinia virus containing the cDNA for CD158j, essentially all of the cells expressed CD158j at levels equivalent to the endogenous expression observed on CD4+CD28null T cells. Infected Jurkat T cells were stimulated with a control mAb, anti-CD3 mAb, anti-CD158b/j mAb, or a combination of anti-CD3 and anti-CD158b/j mAbs and then analyzed for JNK phosphorylation (Fig. 6 C). Although anti-CD3 mAb induced limited phosphorylation of JNK, stimulation through CD158j activated the signaling cascade that resulted in significant JNK phosphorylation. This activation after CD158j stimulation was not observed in Jurkat cells infected with wild-type virus. In addition, stimulation through CD158j also induced MKK4 phosphorylation in transfected CD158j+ Jurkats (Fig. 6 C). These results confirmed that, in the CD4+CD28nullCD158b/j+ T cells, CD158j is responsible for the activation of the JNK signaling pathway. They also demonstrate that the CD158j-mediated JNK activation is not limited to CD4+CD28null T cells but principally is possible in other KARAP/DAP12-negative CD4+ T cells.
Figure 6.
Phosphorylation of JNK is initiated by stimulation specifically through CD158j. Two CD4+CD28nullCD158j+ T cell clones (top panels) and a CD4+CD28nullCD158b1+ T cell clone (bottom panels) were stimulated with anti-CD3 and/or anti-CD158b/j mAbs and cross-linked with rabbit anti–mouse IgG Ab. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of JNK (left panels). The blots were then stripped and reprobed with Abs against total JNK (right panels; A). Jurkat T cells were infected with either wild-type vaccinia virus (WR) or vaccinia virus containing CD158j cDNA and were analyzed for expression of CD158j by flow cytometry (B). Jurkat T cells infected with WR vaccinia virus or CD158j+ vaccinia virus were stimulated with anti-CD3 and/or anti-CD158b/j mAbs and cross-linked with rabbit anti–mouse IgG Ab. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of JNK (top left panels) and MKK4 (bottom left panel). The blots were stripped and reprobed with Abs against β-actin (top right panels) or MKK4 (bottom right panel) (C).
Activation of CD158j Leads to Expression of ATF-2 and c-Jun.
Activation of the JNK signaling pathway leads to the phosphorylation and activation of specific transcription factors, including ATF-2 and c-Jun (20, 21). These two transcription factors form a heterodimer and bind to modified AP-1 sites, one of which is located within the promoter region of the IFN-γ gene (23). Stimulation through CD158j in CD4+CD28null T cells led to increased IFN-γ expression (Fig. 2), possibly mediated through JNK-induced activation of ATF-2 and c-Jun. For all CD4+CD28nullCD158b/j+ T cell clones tested, we observed that stimulation through either the TCR or CD158j led to phosphorylation of both ATF-2 and c-Jun (data not shown). In addition to regulating the expression of IFN-γ, activation of the JNK pathway leads to increased expression of c-Jun (20, 21). As indicated by the PathwayFinder cDNA Array, transcription of the ATF-2 gene is also regulated by the JNK pathway. To determine whether CD158j stimulation regulates the expression of these transcription factors, CD4+CD28null CD158b/j+ T cells and Jurkat T cells infected with CD158j+ vaccinia virus were stimulated and then analyzed for expression of ATF-2 and c-Jun (Fig. 7) . In two of the clones, stimulation with optimal, but not suboptimal, concentrations of anti-CD3 mAb led to increased expression of both ATF-2 and c-Jun. In the remaining clone, clone #4 and in the vaccinia virus-infected Jurkat T cells, stimulation through the TCR did not induce the expression of either transcription factor. This is consistent with the failure of anti-CD3 to induce MKK4 and JNK phosphorylation in these cells (Figs. 4 A, 5, and 6). In contrast, for each of the clones analyzed, stimulation through CD158j alone or in combination with low-dose anti-CD3 mAb resulted in expression of both c-Jun and ATF-2. This was also observed for Jurkat T cells induced to express CD158j after infection with vaccinia virus. This suggests that CD158j, in conjunction with the TCR, costimulates IFN-γ production in CD4+CD28null T cells through the expression and activation of ATF-2 and c-Jun after induction of the JNK signaling pathway.
Figure 7.
Stimulation of CD158j in CD4+CD28null T cells leads to expression of ATF-2 and c-Jun. CD4+CD28nullCD158j+ T cell clones (clones #2, #3, #4), and Jurkat T cells expressing CD158j were stimulated with anti-CD3 and/or anti-CD158b/j mAbs and cross-linked with rabbit anti–mouse IgG Ab. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for expression of ATF-2 and c-Jun. The membranes were stripped and reprobed with Ab against β-actin.
The Adaptor Binding Motif in the Transmembrane Region Is Required for CD158j Function.
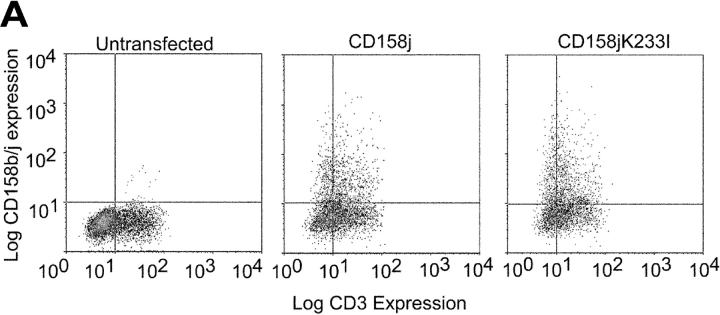
All known stimulatory members of the KIR family possess a dramatically shortened cytoplasmic tail in comparison with the inhibitory receptors and a positively charged residue within the transmembrane domain. The cytoplasmic domains of these receptors are not capable of directly initiating signaling events and must rely on adaptor molecules, such as KARAP/DAP12. Because each of the known adaptor molecules contains a negatively charged residue in their transmembrane domains, it is hypothesized that the interaction between a stimulatory receptor and its corresponding adaptor molecule is partially modulated through an electrostatic interaction. We have demonstrated that in CD4+ CD28null T cells, CD158j is capable of activating the JNK signaling pathway and IFN-γ expression in the absence of KARAP/DAP12. To explore whether CD158j is using an alternate adaptor molecule to activate the JNK pathway that also interacts through the lysine residue within the transmembrane domain, we constructed a mutant of CD158j in which this lysine residue was changed to a hydrophobic residue (CD158jK233I). After transfection into Jurkat T cells, both CD158j and CD158jK233I were expressed on the cell surface (Fig. 8 A) at similar levels. Although stimulation through CD158j resulted in phosphorylation of JNK, stimulation through CD158jK233I did not (Fig. 8 B). This suggests that mutation of the transmembrane lysine residue disrupted the interaction between the receptor and its unknown adaptor molecule, preventing activation of the JNK signaling pathway.
Figure 8.
Mutation of transmembrane lysine residue in CD158j abolishes ability to induce JNK phosphorylation. Jurkat T cells were transiently transfected with constructs containing the CD158j cDNA or the CD158j233I cDNA. Cell-surface expression was confirmed by flow cytometry (A). Jurkat T cells transfected with either CD158j or CD158jK233I were stimulated with anti-CD3 or anti-CD158b/j mAb and cross-linked with rabbit anti–mouse IgG Ab. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of JNK (left panels). The blots were then stripped and reprobed with Abs against total JNK (right panels) (B).
Lack of Association between DAP10 and CD158j.
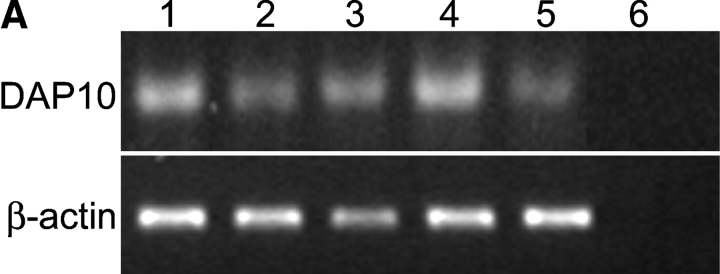
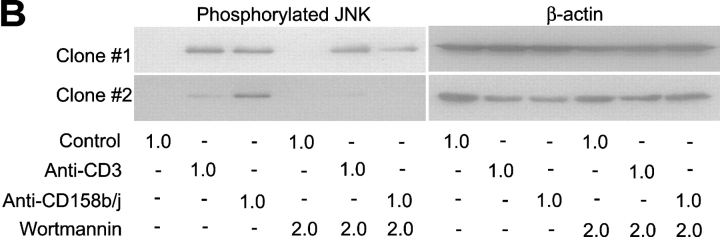
As previously stated, stimulatory receptors within the KIR family require an adaptor molecule to activate signaling cascades. Stimulatory receptors from other families, such as the C-type lectin family, also display this requirement. Some adaptor molecules, such as the common FcRγ chain and KARAP/DAP12, associate with a number of different receptors (24). We hypothesized that, in CD4+CD28null T cells, CD158j is associating with another known adaptor molecule previously characterized for its interaction with a different stimulatory receptor. One such candidate molecule is DAP10. DAP10 associates with NKG2D and is found in most NK cells and CD8+ T cells. Whereas KARAP/DAP12 contains ITAMs and activates the tyrosine kinases Syk and ZAP-70, DAP10 contains PI3K binding domains within its cytoplasmic domain. This made it a potential candidate for association with CD158j in CD4+CD28null T cells because PI3K is capable of activating the JNK pathway. In addition, although NKG2D can induce cytotoxicity in NK cells, it functions solely as a costimulatory receptor in CD8+ T cells (25). This is similar to the functional activity displayed by CD158j in CD4+CD28null T cells. We analyzed several CD4+ CD28null T cell clones and found expression of DAP10 transcript (Fig. 9 A). If CD158j is signaling through DAP10 and PI3K, blocking experiments with the PI3K inhibitor wortmannin should inhibit JNK activation induced by CD158j. CD4+CD28nullCD158j+ T cell clones were stimulated with anti-CD3 or anti-CD158j mAb in the presence or absence of wortmannin, and were then analyzed for JNK phosphorylation (Fig. 9 B). Although wortmannin had a limited inhibitory effect on TCR-mediated JNK phosphorylation, it led to significant inhibition of JNK activation mediated by CD158j.
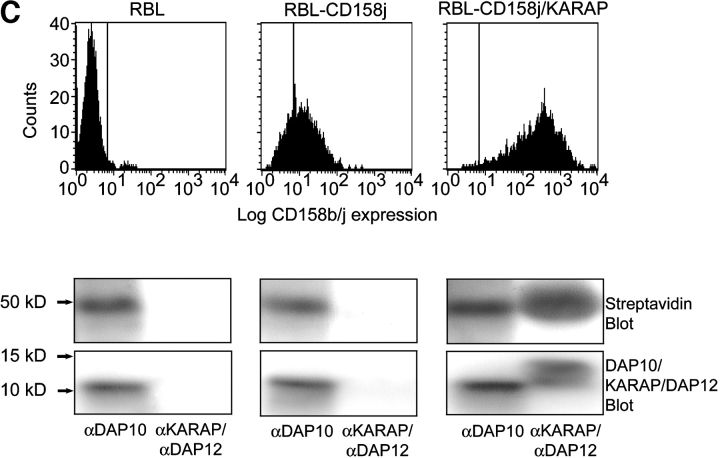
Figure 9.
CD158j and DAP10 do not associate. RT-PCR was used to amplify transcripts for DAP10 from PBMCs (lane 1) and CD4+CD28null T cell clones (lanes 2–5). cDNA was omitted for the negative control (lane 6) (A). CD4+CD28nullCD158b/j+ T cell clones were stimulated with anti-CD3 or anti-CD158b/j in the presence or absence of 2.0 μM wortmannin. After SDS-PAGE and transfer to a nitrocellulose membrane, the cell lysates were analyzed for phosphorylation of JNK (left panels). The blots were then stripped and reprobed with Abs against β-actin (right panels). Results from two T cell clones are shown (B). DAP10-expressing RBL cells (left panel) were stably transfected with CD158j alone (middle panel) or with CD158j and KARAP/DAP12 (right panel). Cell surface expression of CD158j was confirmed by flow cytometry (top panels). DAP10 or KARAP/DAP12 was immunoprecipitated from lysates of biotinylated transfected RBL cells. After SDS-PAGE and transfer to nitrocellulose membranes, coimmunoprecipitated cell-surface proteins were detected by streptavidin-HRP (middle panels). Immunoprecipitation of DAP10 and KARAP/DAP12 was confirmed by immunoblot with anti-DAP10 or anti-KARAP/DAP12 Ab (bottom panels) (C). DAP10 or KARAP/DAP12 was immunoprecipitated from Jurkat T cells (lanes 1–3) or RBL cells (lanes 4–6). After SDS-PAGE and transfer to a nitrocellulose membrane, samples (protein-G preclear, lanes 1 and 4; DAP10 immunoprecipitate, lanes 2 and 5; KARAP/DAP12 immunoprecipitate, lanes 3 and 6) were analyzed by Western blot using DAP10 Ab (D).
CD158j/DAP10 association was difficult to study in T cell clones because of the high cell numbers required. To determine if DAP10 associates with CD158j, we used RBL cells, which expressed endogenous DAP10 but not KARAP/DAP12, and which had been stably transfected with CD158j. As indicated by flow cytometry, CD158j was expressed on the cell surface of RBL cells despite the lack of KARAP/DAP12 (Fig. 9 C, top panel). In CD158jnull RBL cells, DAP10 coimmunoprecipitated with a protein of ∼50 kD, the identity of which was unclear. In CD158j+ RBL cells, the immunoprecipitation pattern was essentially unchanged (Fig. 9 C, bottom panel). Coimmunoprecipitation of KARAP/DAP12 and CD158j from KARAP/DAP12+CD158j+ RBL cells was included as a control. The apparent molecular weight of CD158j is ∼50 kD. However, even if CD158j was included in this 50-kD band the amount would be minuscule. In support of this interpretation, CD158j did not function as a costimulatory molecule in RBL cells (unpublished data). Moreover, DAP10 appeared not to be involved in the CD158j induced activation of the JNK pathway in Jurkat T cells. In these experiments, DAP10 was immunoprecipitated from Jurkat T cells and RBL cells, then analyzed through a Western blot with a DAP10 antibody (Fig. 9 D). Compared with the RBL cells, miniscule amounts of DAP10 were present in Jurkat T cells that could only be detected after overexposure of the Western blot. Thus, despite the detection of DAP10 transcript and inhibition of JNK activation with wortmannin, our immunoprecipitation data do not support the hypothesis that DAP10 is functioning as the adaptor molecule for CD158j in CD4+CD28null T cells.
Discussion
The results presented here demonstrate that stimulation of the MHC class I–recognizing receptor, CD158j, activates the JNK signaling pathway in CD4+ T cells. The activation of this pathway is independent of the adaptor molecule, KARAP/DAP12, which usually associates with CD158j (7, 8). Activation of the JNK pathway by CD158j led to increased expression and phosphorylation of the transcription factors ATF-2 and c-Jun, which regulate the expression of IFN-γ (23). Indeed, triggering of CD158j provided costimulatory signals for IFN-γ production by the responding CD4+ T cell population.
We and others have reported that CD158b/j and other MHC class I–recognizing receptors are expressed on a small subset of CD4+ T cells that shows evidence of an extensive replicative history (10, 26, 27). These T cells have many characteristics of NK-T cells. They express perforin and granzyme B and they have lost the costimulatory molecule, CD28; however, they do not express the canonical TCR structure typically seen on NK-T cells (28). Mandelboim and colleagues (26) were the first to demonstrate that stimulatory MHC class I–recognizing receptors provided costimulatory signals in this subtype of T cells. They showed that these receptors acted as costimulatory molecules for superantigen-induced T cell proliferation. Expression of CD158j and its proposed ligand, HLA-C, are constitutive and are not under regulatory control, in contrast to most costimulatory receptors and ligands. The authors, therefore, proposed that CD158j expression and signaling may be important in breeches of tolerance, leading to autoimmunity. In support of this model, we demonstrated that CD158j is frequently expressed on CD4+ T cells in patients with RA, particularly those who have developed vascular complications (13, 15). We also confirmed that CD158j provides costimulatory signals for proliferation (27). However, in contrast to CD158j on NK cells, CD158j on cytotoxic CD4+ T cells did not induce or augment a cytotoxic T cell response, raising the possibility that the signaling pathways of stimulatory KIRs in T cells and NK cells are different.
CD158j does not possess a substantial cytoplasmic domain. As a result, it is dependent on an adaptor molecule to initiate signaling events. In NK cells, this adaptor molecule is KARAP/DAP12. KARAP/DAP12 has a cytoplasmic ITAM motif and is able to recruit and activate Syk and ZAP-70 (8, 29, 30). Activation of these tyrosine kinases induces ERK activation via the Raf/Mek/ERK cascade (31). It also activates PLC-γ1, leading to intracellular calcium flux. Strikingly, the vast majority of CD4+ T cells, including the CD4+CD28null T cell clones characterized in this paper, do not express KARAP/DAP12 even in the presence of cell-surface CD158j. This would explain why CD158j does not induce any calcium flux and cannot induce cytotoxic activity in CD4+CD28null T cells (27). We also have isolated CD4 T cell clones that express KARAP/DAP12 and CD158j triggering of these T cell clones induces cytotoxicity. Specifically, we have found an increased frequency of KARAP/DAP12 expression in CD4+ CD28null T cells from patients with ACS (32). Such clones are highly infrequent in healthy controls and it is currently unknown how frequent these cells are in patients with RA. However, the majority of CD4+ T cells in RA are clearly KARAP/DAP12 negative. CD158j stimulation activates the JNK pathway and costimulates IFN-γ expression in CD4+CD28null T cells that do not express this adaptor molecule regardless of whether they are derived from RA patients or other sources. These data show that CD158j has as yet undescribed functions in T cells that are distinct from the CD158j/KARAP/DAP12/Syk pathway described in NK cells.
Our mutational analysis demonstrated that the activation of the JNK pathway by CD158j is dependent on a charged residue in the transmembrane domain, suggesting that an adaptor molecule is involved. Certain stimulatory receptors, specifically NKG2D, can bind to and signal through both KARAP/DAP12 and another adaptor molecule DAP10 (33–36). Other receptors related to the stimulatory KIRs, including immunoglobulin-like transcript-1 (ILT-1) and paired-immunoglobulin receptor-A (PIR-A), can also signal through other adaptor molecules that share with KARAP/DAP12 the presence of a negatively charged residue within the transmembrane domain (37, 38). These molecules include the γ chain of the Fc receptor (FcRγ) and the CD3 ζ chain. Although both ILT-1 and PIR-A interact with FcRγ, CD158j does not. CD3 ζ is certainly a potential adaptor molecule because it is expressed in all T cells; however, as is the case with FcRγ, CD3 ζ is unable to interact with CD158j (39). Even if CD3 ζ or FcRγ were capable of binding to CD158j, their signaling properties would not be expected to induce a preferential activation of the JNK pathway. Both of these molecules signal through multiple pathways, including ERK and PLC-γ; however, neither of these is activated after CD158j stimulation, suggesting that CD158j binds to an unidentified adaptor molecule that leads to the activation of the JNK pathway.
We focused on DAP10 as a possible adaptor molecule for CD158j in CD4+CD28null T cells for several reasons. First, the level of sequence homology that exists between KARAP/DAP12 and DAP10 suggests that they may be capable of substituting for one another with regards to receptor association (40). Second, PI3K, which is activated by DAP10, can induce JNK phosphorylation through the intermediate, Vav1. This would correlate with our data demonstrating that activation of the JNK pathway by CD158j in CD4+CD28null T cells can be inhibited by wortmannin. Third, initial experiments indicated that DAP10 is expressed in CD4+CD28null T cell clones. Experiments were undertaken in an attempt to demonstrate a physical association between CD158j and DAP10. However, these experiments failed to show any such association. In addition, CD158j is capable of inducing JNK phosphorylation in Jurkat T cells, which express only limited amounts of DAP10. In summary, our data do not support the hypothesis that DAP10 serves as the adaptor molecule for CD158j in CD4+CD28null T cells. This is in agreement with previously published reports that also failed to demonstrate any interaction between CD158j and DAP10 (39).
Activation of the JNK pathway has been postulated to be a hallmark of costimulatory signals. CD28 is the principal costimulatory molecule in CD4+ T cells and predominantly facilitates the production of IL-2. Several downstream events of CD28 signaling have been described (41, 42). Although controversial, there are several lines of experimental evidence that suggest activation of the JNK pathway is central to CD28 responses (43–45). JNK-induced phosphorylation is critical in the activation of c-Jun, a component of the transcription factor AP-1, which is known to bind to the IL-2 promoter as well as to the IFN-γ promoter (23, 46, 47). Activation of the JNK pathway in Jurkat T cells requires cotriggering of CD3 and CD28 (48). Furthermore, JNK activation is blunted in anergic Th1 clones (49).
It is interesting to note that costimulatory signals that can, in part, substitute for CD28 have also been shown to signal through the JNK pathway. Ligand engagement of the 4–1BB (CD137) molecule, which is expressed on activated T cells, costimulates IL-2 production and proliferation independent of CD28 ligation (50). CD137 signals through TRAF2, which leads to the downstream activation of the JNK pathway (47). Ox-40 (CD134), also a costimulatory molecule selectively expressed on activated T cells, associates with TRAF2 and probably also activates the JNK pathway (51, 52). Cannons and colleagues (50) found evidence for the hypothesis that activation of the JNK pathway by other inducers of stress-activated kinases, such as hyperosmotic shock, provides costimulatory signals to T cells. It is, therefore, of particular interest that CD158j triggering in T cells, in the absence of KARAP/DAP12, selectively targets the activation of the JNK pathway.
The receptors CD158b1, CD158b2, and CD158j share virtually identical extracellular domains. As a result, it was hypothesized that each would recognize the same ligand, specifically HLA-C. The inhibitory receptors CD158b1/b2 clearly recognize specific alleles of HLA-C. Target cells expressing HLA-Cw1, 3, 7, or 8 are protected from lysis by CD158b1/b2+ NK cells. In addition, in vitro experiments demonstrate direct binding between CD158b1/b2 and appropriate HLA-C alleles (1, 53). However, despite the high degree of sequence similarity between the extracellular domains of the inhibitory and stimulatory receptors, no binding between soluble CD158j and HLA-C has been demonstrated. This lack of interaction is attributed to the sequence difference between CD158j and CD158b1/b2 at position 45. In CD158b1/b2, the residue at this position is a phenylalanine while in CD158j, the residue is a tyrosine. Mutation of this residue in CD158j to phenylalanine partially restored the interaction with HLA-C, indicating the importance of this residue (1). Currently, the identity of the ligand for CD158j is unconfirmed. It is possible that, in vivo, CD158j is capable of interacting with particular HLA-C molecules. The interaction between CD158b1/b2 and HLA-C is dependent on the sequence of the peptide bound to the class I molecule (1, 54), which may also be the case for CD158j. Further experimentation is required before the in vivo ligand for CD158j can be confirmed.
Unopposed costimulatory function could occur if CD158j is expressed in the absence of any inhibitory receptors. In this context, it is of interest that CD4+CD28null T cells that express members of the KIR family usually do not express CD94 or any inhibitory members of the C-type lectin receptors (27). Moreover, we (55) and others have demonstrated that KIR expression is acquired during clonal expansion of T cells (56, 57). T cells expressing identical TCRs express different repertoires of KIRs, clearly indicating that KIR expression is initiated after TCR rearrangement. Because KIR expression appears to be a stochastic event, it can be envisioned that T cell clones are generated that express stimulatory KIRs in the absence of inhibitory receptors. Indeed, we have shown that this is the case in patients with RA. Patients with RA frequently possess expanded populations of CD4+CD28null T cells that express various members of the KIR family. This population of CD4+CD28null T cells results from the expansion of a limited number of autoreactive clones (12, 58). CD158j is most frequently expressed, often in the absence of CD158b1/b2 (27). Genotyping studies have shown that the CD158j gene is a risk factor for extraarticular manifestations of RA, such as rheumatoid vasculitis (15). Because CD158j costimulates proliferation, this receptor may play a role in the expansion of these autoreactive T cells. In addition, CD158j may also be an important regulatory molecule for effector functions. It has been shown that the JNK pathway is most important for regulating T cell effector function and not T cell activation (59). The activation of CD158j, in conjunction with TCR, leads to production of the inflammatory cytokine, IFN-γ. Coactivation of the JNK pathway by CD158j, therefore, regulates several important cell functions as a costimulatory molecule. Expression of CD158j on T cells may essentially undermine tolerance and amplify autoimmune mechanisms.
In summary, we have demonstrated that CD158j, depending upon its coupling to adaptor molecules, can have various functions. Whereas CD158j in association with KARAP/DAP12 acts as a primary recognition structure in NK cells, CD158j, presumably associated with an as of yet unidentified adaptor molecule, functions as a costimulatory complex in concert with the TCR in KARAP/DAP12null T cells. This CD28-independent costimulatory activity is mediated through the JNK signaling pathway. The activation of the JNK pathway has important implications for the stimulatory activity of CD158j. We hypothesize that this could have dramatic effects on the function of the CD4+CD28null T cells that express CD158j and on their pathogenic role in autoimmunity.
Acknowledgments
We thank Dr. Paul J. Leibson for the CD158j and KARAP/DAP12 vaccinia constructs, Sophie Guia for excellent technical assistance, James W. Fulbright for aiding with figure preparation and editorial assistance, and Linda H. Arneson for secretarial support.
This work was supported by National Institutes of Health grants (RO1 AR41974, RO1 HL63919, and RO1 AR42527). M.R. Snyder is supported by a National Arthritis Foundation Fellowship (AF21). This research was supported in part by institutional grants from INSERM, CNRS, Ministère de l'Enseignement Supérieur et de la Recherche, and specific grants from Ligue Nationale contre le Cancer (to M. Lucas and ‘Equipe labellisée La Ligue’ to E. Vivier).
Footnotes
Abbreviations used in this paper: ACS, acute coronary syndromes; ERK, extracellular signal–regulated kinase; JNK, c-Jun NH2-terminal kinase; KIR, killer immunoglobulin-like receptor; RA, rheumatoid arthritis.
References
- 1.Vales-Gomez, M., H.T. Reyburn, R.A. Erskine, and J. Strominger. 1998. Differential binding to HLA-C of p50-activating and p58-inhibitory natural killer cell receptors. Proc. Natl. Acad. Sci. USA. 95:14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burshtyn, D.N., A.M. Scharenberg, N. Wagtmann, S. Rajagopalan, K. Berrada, T. Yi, J.P. Kinet, and E.O. Long. 1996. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 4:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olcese, L., P. Lang, F. Vely, A. Cambiaggi, D. Marguet, M. Blery, K.L. Hippen, R. Biassoni, A. Moretta, L. Moretta, et al. 1996. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J. Immunol. 156:4531–4534. [PubMed] [Google Scholar]
- 4.Binstadt, B.A., K.M. Brumbaugh, C.J. Dick, A.M. Scharenberg, B.L. Williams, M. Colonna, L.L. Lanier, J.P. Kinet, R.T. Abraham, and P.J. Leibson. 1996. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 5:629–638. [DOI] [PubMed] [Google Scholar]
- 5.Ljunggren, H.G., and K. Karre. 1990. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today. 11:237–244. [DOI] [PubMed] [Google Scholar]
- 6.Tomasello, E., L. Olcese, F. Vely, C. Geourgeon, M. Blery, A. Moqrich, D. Gautheret, M. Djabali, M.G. Mattei, and E. Vivier. 1998. Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP-12. J. Biol. Chem. 273:34115–34119. [DOI] [PubMed] [Google Scholar]
- 7.Olcese, L., A. Cambiaggi, G. Semenzato, C. Bottino, A. Moretta, and E. Vivier. 1997. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 158:5083–5086. [PubMed] [Google Scholar]
- 8.Lanier, L.L., B.C. Corliss, J. Wu, C. Leong, and J.H. Phillips. 1998. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 391:703–707. [DOI] [PubMed] [Google Scholar]
- 9.Mingari, M.C., F. Schiavetti, M. Ponte, C. Vitale, E. Maggi, S. Romagnani, J. Demarest, G. Pantaleo, A.S. Fauci, and L. Moretta. 1996. Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc. Natl. Acad. Sci. USA. 93:12433–12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warrington, K.J., S. Takemura, J.J. Goronzy, and C.M. Weyand. 2001. CD4+,CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 44:13–20. [DOI] [PubMed] [Google Scholar]
- 11.Liuzzo, G., S.L. Kopecky, R.L. Frye, W.M. O'Fallon, A. Maseri, J.J. Goronzy, and C.M. Weyand. 1999. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 100:2135–2139. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt, D., P.B. Martens, C.M. Weyand, and J.J. Goronzy. 1996. The repertoire of CD4+ CD28- T cells in rheumatoid arthritis. Mol. Med. 2:608–618. [PMC free article] [PubMed] [Google Scholar]
- 13.Martens, P.B., J.J. Goronzy, D. Schaid, and C.M. Weyand. 1997. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 40:1106–1114. [DOI] [PubMed] [Google Scholar]
- 14.Liuzzo, G., J.J. Goronzy, H. Yang, S.L. Kopecky, D.R. Holmes, R.L. Frye, and C.M. Weyand. 2000. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 101:2883–2888. [DOI] [PubMed] [Google Scholar]
- 15.Yen, J.H., B.E. Moore, T. Nakajima, D. Scholl, D.J. Schaid, C.M. Weyand, and J.J. Goronzy. 2001. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J. Exp. Med. 193:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhrberg, M., N.M. Valiante, B.P. Shum, H.G. Shilling, K. Lienert-Weidenbach, B. Corliss, D. Tyan, L.L. Lanier, and P. Parham. 1997. Human diversity in killer cell inhibitory receptor genes. Immunity. 7:753–763. [DOI] [PubMed] [Google Scholar]
- 17.Andre, P., R. Biassoni, M. Colonna, D. Cosman, L.L. Lanier, E.O. Long, M. Lopez-Botet, A. Moretta, L. Moretta, P. Parham, et al. 2001. New nomenclature for MHC receptors. Nat. Immunol. 2:661. [DOI] [PubMed] [Google Scholar]
- 18.Vallejo, A.N., R.J. Pogulis, and L.R. Pease. 1994. In vitro synthesis of novel genes: mutagenesis and recombination by PCR. PCR Methods Appl. 4:S123–S130. [DOI] [PubMed] [Google Scholar]
- 19.Blery, M., J. Delon, A. Trautmann, A. Cambiaggi, L. Olcese, R. Biassoni, L. Moretta, P. Chavrier, A. Moretta, M. Daeron, and E. Vivier. 1997. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J. Biol. Chem. 272:8989–8996. [DOI] [PubMed] [Google Scholar]
- 20.van Dam, H., D. Wilhelm, I. Herr, A. Steffen, P. Herrlich, and P. Angel. 1995. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 14:1798–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiriyama, M.T., M. Oka, M. Takehana, and S. Kobayashi. 2001. Expression of a small heat shock protein 27 (HSP27) in mouse skin tumors induced by UVB-irradiation. Biol. Pharm. Bull. 24:197–200. [DOI] [PubMed] [Google Scholar]
- 22.Marti, F., C.W. Xu, A. Selvakumar, R. Brent, B. Dupont, and P.D. King. 1998. LCK-phosphorylated human killer cell-inhibitory receptors recruit and activate phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 95:11810–11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penix, L.A., M.T. Sweetser, W.M. Weaver, J.P. Hoeffler, T.K. Kerppola, and C.B. Wilson. 1996. The proximal regulatory element of the interferon-gamma promoter mediates selective expression in T cells. J. Biol. Chem. 271:31964–31972. [DOI] [PubMed] [Google Scholar]
- 24.Tomasello, E., M. Blery, E. Vely, and E. Vivier. 2000. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin. Immunol. 12:139–147. [DOI] [PubMed] [Google Scholar]
- 25.Groh, V., R. Rhinehart, J. Randolph-Habecker, M.S. Topp, S.R. Riddell, and T. Spies. 2001. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2:255–260. [DOI] [PubMed] [Google Scholar]
- 26.Mandelboim, O., D.M. Davis, H.T. Reyburn, M. Vales-Gomez, E.G. Sheu, L. Pazmany, and J.L. Strominger. 1996. Enhancement of class II-restricted T cell responses by costimulatory NK receptors for class I MHC proteins. Science. 274:2097–2100. [DOI] [PubMed] [Google Scholar]
- 27.Namekawa, T., M.R. Snyder, J.H. Yen, B.E. Goehring, P.J. Leibson, C.M. Weyand, and J.J. Goronzy. 2000. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J. Immunol. 165:1138–1145. [DOI] [PubMed] [Google Scholar]
- 28.Elewaut, D., and M. Kronenberg. 2000. Molecular biology of NK T cell specificity and development. Semin. Immunol. 12:561–568. [DOI] [PubMed] [Google Scholar]
- 29.McVicar, D.W., L.S. Taylor, P. Gosselin, J. Willette-Brown, A.I. Mikhael, R.L. Geahlen, M.C. Nakamura, P. Linnemeyer, W.E. Seaman, S.K. Anderson, et al. 1998. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 273:32934–32942. [DOI] [PubMed] [Google Scholar]
- 30.Lanier, L.L., B. Corliss, J. Wu, and J.H. Phillips. 1998. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 8:693–701. [DOI] [PubMed] [Google Scholar]
- 31.Qian, D., and A. Weiss. 1997. T cell antigen receptor signal transduction. Curr. Opin. Cell Biol. 9:205–212. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima, T., S.L. Kopecky, D. Elewaut, R.L. Frye, J.J. Goronzy, and C.M. Weyand. 2002. Cytotoxic CD4 T cells in the unstable athersclerotic plaque: Gene complementation of MHC class I-recognizing receptors and the adapter molecule DAP12. Clin. Immunol. 103:S30 (Abstract). [Google Scholar]
- 33.Chang, C., J. Dietrich, A.G. Harpur, J.A. Lindquist, A. Haude, Y.W. Loke, A. King, M. Colonna, J. Trowsdale, and M.J. Wilson. 1999. Cutting edge: KAP10, a novel transmembrane adapter protein genetically linked to DAP12 but with unique signaling properties. J. Immunol. 163:4651–4654. [PubMed] [Google Scholar]
- 34.Diefenbach, A., E. Tomasello, M. Lucas, A.M. Jamieson, J.K. Hsia, E. Vivier, and D.H. Raulet. 2002. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat. Immunol. 3:1142–1149. [DOI] [PubMed] [Google Scholar]
- 35.Gilfillan, S., E.L. Ho, M. Cella, W.M. Yokoyama, and M. Colonna. 2002. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat. Immunol. 3:1150–1155. [DOI] [PubMed] [Google Scholar]
- 36.Long, E.O. 2002. Versatile signaling through NKG2D. Nat. Immunol. 3:1119–1120. [DOI] [PubMed] [Google Scholar]
- 37.Kubagawa, H., P.D. Burrows, and M.D. Cooper. 1997. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl. Acad. Sci. USA. 94:5261–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima, H., J. Samaridis, L. Angman, and M. Colonna. 1999. Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor gamma-chain. J. Immunol. 162:5–8. [PubMed] [Google Scholar]
- 39.Wu, J., H. Cherwinski, T. Spies, J.H. Phillips, and L.L. Lanier. 2000. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J. Exp. Med. 192:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomasello, E., C. Cant, H.J. Buhring, F. Vely, P. Andre, M. Seiffert, A. Ullrich, and E. Vivier. 2000. Association of signal-regulatory proteins beta with KARAP/DAP-12. Eur. J. Immunol. 30:2147–2156. [DOI] [PubMed] [Google Scholar]
- 41.Kane, L.P., P.G. Andres, K.C. Howland, A.K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2:37–44. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro, V.S., K.E. Truitt, J.B. Imboden, and A. Weiss. 1997. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol. Cell. Biol. 17:4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su, B., E. Jacinto, M. Hibi, T. Kallunki, M. Karin, and Y. Ben-Neriah. 1994. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 77:727–736. [DOI] [PubMed] [Google Scholar]
- 44.Salojin, K.V., J. Zhang, and T.L. Delovitch. 1999. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J. Immunol. 163:844–853. [PubMed] [Google Scholar]
- 45.Rivas, F.V., S. O'Herrin, and T.F. Gajewski. 2001. CD28 is not required for c-Jun N-terminal kinase activation in T cells. J. Immunol. 167:3123–3128. [DOI] [PubMed] [Google Scholar]
- 46.Kaminuma, O., M. Deckert, C. Elly, Y.C. Liu, and A. Altman. 2001. Vav-Rac1-mediated activation of the c-Jun N-terminal kinase/c-Jun/AP-1 pathway plays a major role in stimulation of the distal NFAT site in the interleukin-2 gene promoter. Mol. Cell. Biol. 21:3126–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saoulli, K., S.Y. Lee, J.L. Cannons, W.C. Yeh, A. Santana, M.D. Goldstein, N. Bangia, M.A. DeBenedette, T.W. Mak, Y. Choi, and T.H. Watts. 1998. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 187:1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hehner, S.P., T.G. Hofmann, O. Dienz, W. Droge, and M.L. Schmitz. 2000. Tyrosine-phosphorylated Vav1 as a point of integration for T-cell receptor- and CD28-mediated activation of JNK, p38, and interleukin-2 transcription. J. Biol. Chem. 275:18160–18171. [DOI] [PubMed] [Google Scholar]
- 49.Li, W., C.D. Whaley, A. Mondino, and D.L. Mueller. 1996. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 271:1272–1276. [DOI] [PubMed] [Google Scholar]
- 50.Cannons, J.L., K.P. Hoeflich, J.R. Woodgett, and T.H. Watts. 1999. Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. J. Immunol. 163:2990–2998. [PubMed] [Google Scholar]
- 51.Arch, R.H., and C.B. Thompson. 1998. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol. Cell. Biol. 18:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawamata, S., T. Hori, A. Imura, A. Takaori-Kondo, and T. Uchiyama. 1998. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 273:5808–5814. [DOI] [PubMed] [Google Scholar]
- 53.Vales-Gomez, M., R.A. Erskine, M.P. Deacon, J.L. Strominger, and H.T. Reyburn. 2001. The role of zinc in the binding of killer cell Ig-like receptors to class I MHC proteins. Proc. Natl. Acad. Sci. USA. 98:1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajagopalan, S., and E.O. Long. 1997. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J. Exp. Med. 185:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snyder, M.R., L.O. Muegge, C. Offord, W.M. O'Fallon, Z. Bajzer, C.M. Weyand, and J.J. Goronzy. 2002. Formation of the killer Ig-like receptor repertoire on CD4+CD28null T cells. J. Immunol. 168:3839–3846. [DOI] [PubMed] [Google Scholar]
- 56.Vely, F., M. Peyrat, C. Couedel, J. Morcet, F. Halary, F. Davodeau, F. Romagne, E. Scotet, X. Saulquin, E. Houssaint, et al. 2001. Regulation of inhibitory and activating killer-cell Ig-like receptor expression occurs in T cells after termination of TCR rearrangements. J. Immunol. 166:2487–2494. [DOI] [PubMed] [Google Scholar]
- 57.Uhrberg, M., N.M. Valiante, N.T. Young, L.L. Lanier, J.H. Phillips, and P. Parham. 2001. The repertoire of killer cell Ig-like receptor and CD94:NKG2A receptors in T cells: clones sharing identical alpha beta TCR rearrangement express highly diverse killer cell Ig-like receptor patterns. J. Immunol. 166:3923–3932. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt, D., J.J. Goronzy, and C.M. Weyand. 1996. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 97:2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong, C., R.J. Davis, and R.A. Flavell. 2001. Signaling by the JNK group of MAP kinases. J. Clin. Immunol. 21:253–257. [DOI] [PubMed] [Google Scholar]