Abstract
Inflammatory arthritis is associated with the release of a network of key cytokines. In T cell receptor transgenic K/BxN mice interleukin (IL)-1 plays a key role in joint swelling and destruction, as suggested by the ability of anti–IL-1receptor (IL-1R) antibody treatment to delay the onset and slow the progression of this disease. This mechanism is dependent on the signaling pathway intermediary myeloid differentiation factor 88 (MyD88), such that neither IL-1R nor MyD88-deficient mice developed visually detectable synovitis after transfer of arthritogenic sera. The Toll-like receptors (TLRs) share the same signaling pathway through MyD88 as the IL-1R. The administration of a TLR-4 ligand, lipopolysaccharide, concomitant with arthritogenic serum in IL-1 receptor–deficient mice resulted in acute paw swelling, but not in MyD88-deficient mice. Also, serum transferred arthritis was not sustained in TLR-4 mutant mice compared with controls. These results suggest that innate immune functions via TLR-4 might perpetuate inflammatory mechanisms and bypass the need for IL-1 in chronic joint inflammation.
Keywords: animal model, lipopolysaccharide, rheumatoid arthritis, autoantibody, Toll-like receptor
Introduction
In autoimmune inflammatory joint disease there might be a complex interplay between genetics, immune stimulation and environmental factors. The role of the environment might come into play through the interaction of the innate immune system and pattern-recognition receptors (PRRs). Recently, a family of Toll-like receptors (TLRs) has been identified as PRRs in mammals that serve to recognize pathogen-associated molecular patterns (PAMPs; references 1 and 2). Microbial products such as LPS or peptidoglycan (PG) function as ligands for TLR-4 (3) and –2 (1), respectively. Recognition of these ligands by the innate immune system leads to a cascade of events including the release of cytokines and activation of antigen-presenting cells. Hence, TLR-signaling might potentiate the priming milieu of the adaptive immune system and perpetuate inflammation in a T cell–independent manner.
Innate immune mechanisms have been implicated in establishing and propagating synovitis (for a review, see reference 4). In mice the ability of TLR signaling to lead to destructive arthritis has been demonstrated by direct intraarticular injection of TLR ligands (TLR-Ls; references 5 and 6). In addition subclinical arthritis has been aggravated by systemic injection of LPS or IL-1 (7). Although the TLRs differ in their extracellular domain structure from the cytokine receptors for IL-1 and IL-18, similar cytoplasmic domains allow TLRs to use the same signaling molecules. This pathway includes myeloid differentiation factor 88 (MyD88), which is an adaptor protein that links the IL-1 receptor to IL-1R–associated protein kinase (IRAK), a serine-threonine kinase (8). Upon ligand binding to the IL-1R, IRAK is phosphorylated, subsequently dissociates from the receptor complex, and associates with TNF receptor-associated factor (TRAF)6 (9). This process results in the activation of two different pathways that involve the c-Jun NH2-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) family and the Rel family transcription factor nuclear factor (NF)-κB. Consequently MyD88−/− mice respond poorly if at all to IL-1, IL-18, LPS, or other microbial cell wall components (8, 10).
IL-1 is a critical cytokine in erosive arthritis (11–13). Given that the IL-1R and the TLR signaling pathways converge on MyD88 we hypothesized that MyD88 activation could occur through either IL-1R or TLR stimulation leading to bone erosions and joint inflammation. To test this hypothesis we used the transgenic K/BxN model of spontaneous arthritis or the passive transfer of arthritis, using serum from K/BxN mice. Disruption of IL-1R signaling in transgenic K/BxN mice by anti–IL-1R antibody treatment diminished the extent of spontaneous disease. Concordantly, IL-1R−/− and MyD88−/− mice did not develop arthritis after serum transfer. However, coadministration of the TLR-4 ligand, LPS, and K/BxN serum did induce paw swelling in IL-1R−/−, but not MyD88−/− mice. Furthermore, mice that are mutant in TLR-4 had a shortened course of paw swelling after serum transfer. These data suggest that TLR signaling might additionally activate MyD88 and perpetuate joint inflammation.
Materials and Methods
Mice.
KRN T cell receptor (TCR) transgenic mice were a gift from Drs. D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and Institut de Génétique et de Biologie Moléculaire et Cellulaire (Strasbourg, France; reference 14), and were maintained on a C57Bl/6 background (K/B). Arthritic mice were obtained by crossing K/B with NOD/Lt (N) animals (K/BxN). Progeny bearing the Vβ6 transgenic TCR were identified at 2–3 wk of age by cytofluorometry of peripheral blood lymphocytes using anti-CD4 PE (Caltag) and anti-Vβ6 FITC (BD Biosciences) labeled antibodies. C57Bl/6, IL-1R−/−, C3H/HeJ, C3H/HeOU, and NOD/Lt mice were purchased from The Jackson Laboratory. MyD88−/− mice were a generous gift of Dr. Akira (Osaka University, Osaka, Japan; reference 8). The mice were bred and maintained under standard conditions in the University of California, San Diego Animal Facility that is accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols receive prior approval by the institutional review board.
Antibody Inhibition.
Endotoxin and azide free preparations of anti–IL-1 receptor antibody (clone 35F5; BD Biosciences) and rat IgG (Sigma-Aldrich) were diluted in sterile PBS and 150 μg of antibody was injected intraperitoneally into young K/BxN mice on Monday, Wednesday, and Friday of each week. This dose was selected based on a previous report in which the same antibody clone abrogated collagen induced arthritis at 100 and 200 μg doses (15).
Serum Transfer and Arthritis Scoring.
Arthritic adult K/BxN mice were bled and the sera were pooled. Recipient mice are injected with 100 μl intraperitoneally on days 0 and 2. Some groups of mice also received 50 μg of LPS (Sigma-Aldrich) derived from Escherichia coli serotype O111:B4 in sterile PBS intraperitoneally. For each swollen paw 1 point was given, resulting in a maximum score of 4 per mouse. Ankle thickness was measured with a caliper (Manostat) in mm.
Histology.
Whole knee joints and hind paws were fixed in 10% formalin, decalcified, trimmed, and embedded. Sections were prepared from the tissue blocks and stained with hematoxylin and eosin (H&E) (Comparative Biosciences). Histopathological scoring was performed as described below. Knees of arthritic mice were given scores of 0–5 for inflammation, according to the following criteria: 0 = normal; 1 = minimal infiltration of inflammatory cells in periarticular area; 2 = mild infiltration; 3 = moderate infiltration; 4 = marked infiltration; and 5 = severe infiltration. Knees of arthritic mice were given scores of 0–5 for bone resorption, according to the following criteria: 0 = normal; 1 = minimal (small areas of resorption, not readily apparent on low magnification); 2 = mild (more numerous areas of resorption, not readily apparent on low magnification, in trabecular or cortical bone); 3 = moderate (obvious resorption of trabecular and cortical bone, without full-thickness defects in the cortex; loss of some trabeculae; lesions apparent on low magnification); 4 = marked (full-thickness defects in the cortical bone and marked trabecular bone loss, without distortion of the profile of the remaining cortical surface); and 5 = severe (full-thickness defects in the cortical bone and marked trabecular bone loss, with distortion of the profile of the remaining cortical surface). Each slide was scored by two independent observers and the average score was used.
ELISA.
Lapine glucose-6-phosphate isomerase (G6PI) type IV and XI (Sigma-Aldrich) were coated on high affinity 96-well ELISA plates (Costar) at 10 μg/ml in PBS. Plates were then blocked with PBS 1% BSA. Anti–glucose-6-phosphate isomerase antibodies in sera were detected with alkaline phosphatase labeled goat anti–mouse IgG (Southern Biotechnology Associates, Inc.) followed by incubation with p-nitrophenyl phosphate substrate (Sigma-Fast; Boehringer). Absorption was measured at 405 nm. The pool of injected sera was used as standards, arbitrarily set at 106 units of IgG. Data were analyzed using DeltaSOFT II v. 3.66 (Biometallics).
Results
IL-1 Signaling Is Critical to Development of Serum Transferred Arthritis.
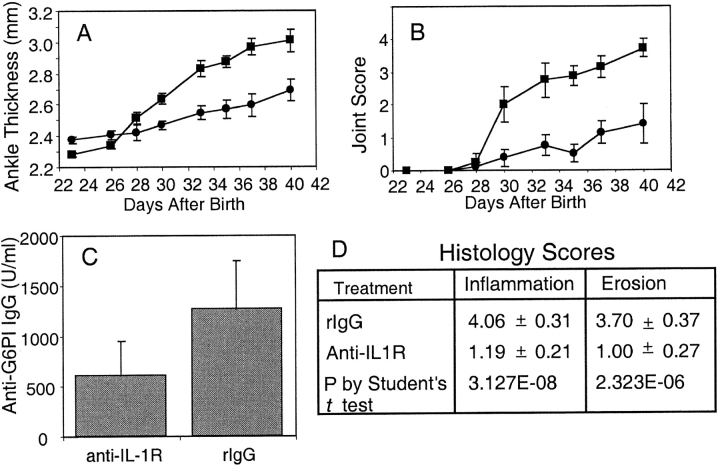
Mice that express both the KRN TCR and the IAg7 MHC class II allele (K/BxN) develop chronic symmetric joint disease with pannus formation, and destructive bone and cartilage erosion of predominantly the distal joints uniformly after 5 wk of life (14). To test the role of IL-1 in the development of arthritis in the K/BxN mouse model, transgenic mice were treated with anti–IL-1 receptor antibody or control rat antibody starting at the time of weaning. Within 5 wk the group that received the control antibody had all developed severe arthritis. In the same time interval only half of the anti–IL-1R–treated mice developed mild clinical disease (Fig. 1) .
Figure 1.
Anti–IL-1R antibody treatment delays the onset of arthritis in K/BxN mice. At ∼3 wk of age transgenic K/BxN mice were started on a course of either 150 μg anti-IL-1R antibody (n = 8) or isotype control antibody (n = 8) intraperitoneally three times a week. Ankle thickness was measured with a caliper (A) and the arthritis was clinically scored (B) for the isotype control group (squares) and the anti–IL-1R antibody group (circles), respectively. (C) At the end of the study the mice were bled and the sera assayed for anti-G6PI (P = 0.29 by Student's t test). (D) The hind limbs of all mice were removed and one knee from each mouse was prepared for histologic scoring. Shown are the average inflammation and erosion scores ± SEM.
The arthritis in these mice usually develops in parallel with the production of anti-G6PI antibodies. At the end of the study the mice were bled and the anti-G6PI antibody titers were measured (Fig. 1 C). Although it appeared that there was a slight trend toward higher titers in the rat IgG–treated control group, there was no statistical difference (P = 0.29). The chief influence of IL-1 in other murine models of inflammatory arthritis appears to be in the latter or destructive phase, rather than in acute synovitis. All of the mice in this study had their knees examined for histologic evidence of synovial inflammation and bone erosion. The anti–IL-1R treatment group had only occasional pockets of inflammatory cell infiltration and fewer areas of bone destruction (Fig. 1 D).
Transfer of Arthritis by K/BxN Sera Is IL-1R and MyD88 Dependent.
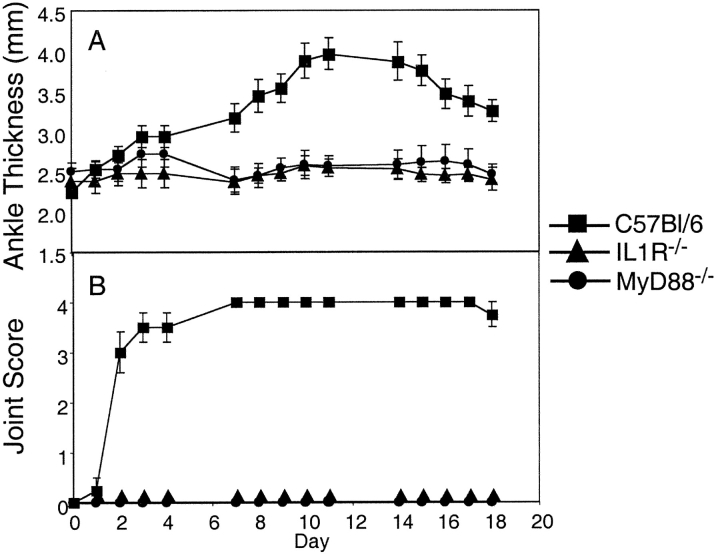
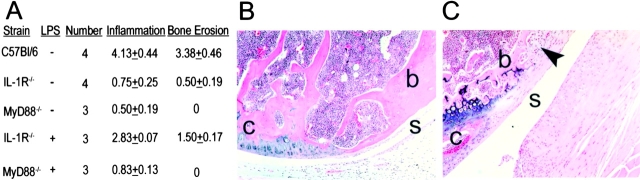
The anti–IL-1–treated K/BxN transgenic mice had a trend toward decreased anti-G6PI antibody production, which might explain the decreased severity of arthritis. Therefore, the role of IL-1 was further tested in a serum transfer model, where a fixed dose of autoantibody-containing serum is administered. The IL-1 receptor–deficient mice that received K/BxN serum failed to develop arthritis (Fig. 2 A). As the IL-1 signaling pathway includes MyD88, these genetically mutated mice were evaluated. Similar to the IL-1R−/− mice, MyD88−/− mice did not develop disease after arthritogenic serum transfer (Fig. 2 B). The knees of sera injected wild-type control, IL-1R−/−, and MyD88−/− mice were examined for the evidence of subclinical inflammation and erosions by histology. There were minimal findings in the IL-1R−/− and MyD88−/− mice compared with the severe erosive destruction seen in the C57Bl/6 control group (see Fig. 5 A).
Figure 2.
Transfer of arthritis by K/BxN sera is IL-1R and MyD88 dependent. Adult K/BxN mice were bled and the sera pooled. Adult C57Bl/6 (square), IL-1R−/− (triangle), and MyD88−/− (circle) mice were injected on days 0 and 2 with 100 μl of pooled sera intraperitoneally. Arthritis was clinically scored (B) and ankle thickness was measured with a caliper (A). The means of 3–4 mice per group ± SEM are shown.
Figure 5.
Inflammatory infiltration and bone erosion in IL-1R−/− mice treated with LPS and K/BxN serum. (A) One knee from each of the mice above was prepared for histologic scoring. Shown are the average inflammation and erosion scores ± SEM. The inflammation and erosion scores were significantly greater in the C57BL/6 than the MyD88−/− or IL-1R−/− groups (P < 0.005 by Student's t test). (B) Shown is an example of a serum treated IL-1R−/− mouse knee at the site where the cartilage meets the bone. The cartilage is smooth and the synovium is a single cell layer. (C) In IL-1R−/− mice that were treated with LPS and K/BxN sera bone erosions and remodeling were noted. The scalloped edges of the synovium invading the bone can be seen (arrow). B and C are 100× original magnification. For orientation the s overlies synovium, b designates bone, and c indicates cartilage.
TLR-4 Signaling Modulates Arthritis.
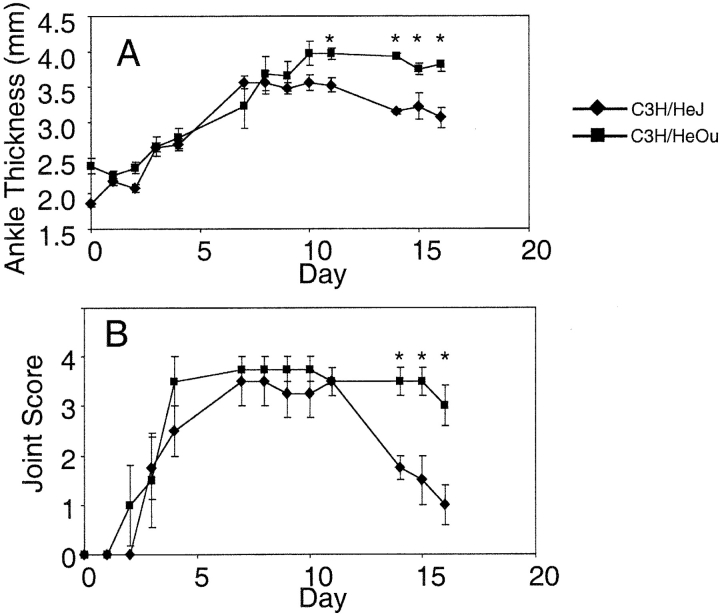
The lack of arthritis in the MyD88-deficient mice suggested that other receptors, which use this signaling mediator, like TLR-4, might have an effect on the transfer of arthritis. C3H/HeJ mice have a missense mutation in the third exon of the TLR-4 gene, predicted to substitute proline with histidine at position 712 of the polypeptide chain (3). K/BxN serum given to C3H/HeJ mice resulted in acute paw swelling. However, the paw swelling subsided more rapidly than in C3H/HeOU control mice, which do not share the TLR-4 defect (Fig. 3) .
Figure 3.
TLR-4 signaling modulates antibody-mediated arthritis. Adult K/BxN mice were bled and the sera pooled. C3H/HeJ (TLR-4 mutant; diamond) and C3H/HeOu (square) mice were injected on days 0 and 2 with 100 μl of pooled sera intraperitoneally. Arthritis was clinically scored (B) and ankle thickness was measured with a caliper (A). The means of four mice per group ± SEM are shown. Days when C3H/HOu mice have significantly greater paw swelling than are noted (*), (P < 0.05 by Student's t test).
TLR-4 Ligand Stimulation Circumvents a Requirement for IL-1R Signaling.
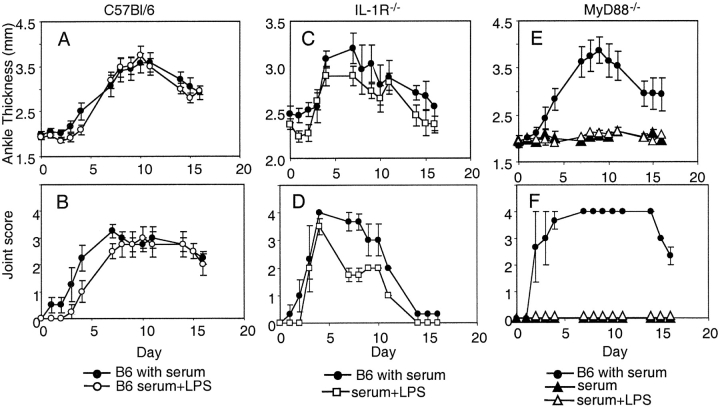
As the TLR-4 mutant mice resolved their paw swelling more rapidly after serum transfer, a complementary approach was used to evaluate the effect of TLR-4 signaling in the serum transfer model. Mice were given a systemic TLR-4 ligand, LPS, on day 0 concurrently with K/BxN sera. In C57Bl/6 mice this regimen did not result in accelerated arthritis. However, the administration of LPS to IL-1 receptor–deficient mice resulted in arthritis similar to wild-type controls (Fig. 4) . The lack of arthritis induction in the MyD88-deficient mice suggested that the LPS signaled through this pathway bypassing the requirement for IL-1R signaling (Fig. 4). Concordantly there was histologic evidence of increased inflammatory infiltration and bone erosion in IL-1R−/− mice that received LPS, which was not appreciated in the MyD88−/− mice (Fig. 5, A–C) .
Figure 4.
TLR-4 ligand stimulation circumvents requirement for IL-1R signaling. Adult K/BxN mice were bled and the sera pooled. Adult were injected with 100 μl of pooled sera intraperitoneally on days 0 and 2. In addition, some groups also received an injection of 50 μg LPS intraperitoneally on day 0. C57Bl/6 mice treated with sera are presented as a positive control in each experiment. (A and B) C57Bl/6 treated with sera (closed circle), and LPS and sera (open circle); (C and D) C57Bl/6 treated with sera (closed circle) and IL-1R−/− mice treated with sera and LPS (open square); and (E and F) C57Bl/6 treated with sera (closed circle), MyD88−/− mice treated with LPS and sera (open triangle) and sera alone (closed triangle). Arthritis was clinically scored and ankle thickness was measured with a caliper. The means of 3–4 mice per group ± SEM are shown.
Discussion
Much interest has been raised by the KRN model of spontaneously occurring erosive arthritis. In this model a single autoreactive T cell receptor escapes negative selection in mice bearing a specific MHC class II allele, IAg7 (14). In the periphery these T cells are able to promote a breach in B cell tolerance and high levels of anti–glucose-6-phosphate isomerase are secreted (16). The accumulation of these autoantibodies in the serum then results in a destructive and erosive arthritis similar to that seen in human rheumatoid arthritis (RA). The adoptive transfer of the serum from these mice results in the generation of arthritis in most recipient strains (14, 17). In previous reports key factors involved in developing arthritis after serum transfer included the alternate complement pathway, Fcγ receptors, and subsets of Fcγ receptor bearing cells (17–22). In the K/BxN serum transfer the IL-1R was also found to play a critical role in paw swelling (20).
Using this model we describe the potential for signaling through the TLRs to circumvent the role of a key cyto-kine, namely IL-1. The TLR family members have been shown to trigger responses primarily to microbial components. The specificities of some of the family members have been elucidated. In particular TLR-2 and TLR-4 have been shown to be essential for the recognition of distinct bacterial cell wall components (3, 23). Microbial products, however, are not specifically required for TLR signaling, as mammalian ligands have also been identified for TLR-4. Amongst these ligands heat shock protein (HSP)60 (24), HSP70 (25), and fibronectin (26) are abundantly present in the joint, and thus might be instrumental in perpetuating synovial inflammation.
There is growing evidence that the signaling pathways associated with each TLR are not identical and might, therefore, result in different biological responses. Studies in MyD88−/− macrophages have suggested differences between TLR-2 and TLR-4 signaling (10, 27). MALP activation of NF-κB and MAPK, which is mediated by TLR-2, is completely abolished in both TLR-2−/− and MyD88−/− macrophages. However, LPS activates NF-κB, and JNK and p38 in MyD88−/− macrophages, albeit with delayed kinetics (10). This suggests that a MyD88-independent pathway(s) can mediate NF-κB, JNK, or p38 activation after TLR-4 signaling. In the serum transfer model, however, LPS failed to induce inflammation in the MyD88−/− mice, suggesting that this pathway was the dominant one.
Exposure to environmental pathogens might shape the subsequent adaptive immune response and might also sustain antigen-specific responses by recruiting and triggering the activity of monocytes and macrophages in the area exposed to microorganisms. The innate immune system might “prime” the target organ to be receptive to damage from further insult, as might be the case in adjuvant and collagen induced arthritis where CFA is used. Alternatively, antigen-independent release of IL-1 and TNFα by TLR exposed synovial macrophages could perpetuate fibroblast proliferation and increase synoviocyte secretion of IL-6, GM-CSF, and collagenase. In recent clinical trials biologic blockade of IL-1 or TNFα was not universally effective (28–30). In these patients with established rheumatoid arthritis the redundancy in the innate signaling receptors might make them refractory to treatments targeting one ligand. Disruption of intraarticular TLR signaling might provide an additional therapeutic modality for patients with refractory RA.
Acknowledgments
We are grateful to Drs. Akira, Benoist, and Mathis for their generous gifts of mice, and Drs. N. Zvaifler, G. Firestein, and D. Carson for review of the manuscript. We appreciate the assistance of P. Charos, N. Noon, and J. Uhle. We are thankful to Dr. D. Broide, J.Y. Cho, and J. Feramiglo for assistance with photomicroscopy, and to the Rheumatic Diseases Care Center.
This work was funded by grants from the National Institutes of Health. Abstract submitted to American College of Rheumatology for presentation as a poster at their annual meeting, October 25–28, 2002 in New Orleans, LA.
References
- 1.Brightbill, H.D., D.H. Libraty, S.R. Krutzik, R.B. Yang, J.T. Belisle, J.R. Bleharski, M. Maitland, M.V. Norgard, S.E. Plevy, S.T. Smale, et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 285:732–736. [DOI] [PubMed] [Google Scholar]
- 2.Rock, F.L., G. Hardiman, J.C. Timans, R.A. Kastelein, and J.F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA. 95:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 4.Firestein, G.S., and N.J. Zvaifler. 2002. How important are T cells in chronic rheumatoid synovitis?: II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 46:298–308. [DOI] [PubMed] [Google Scholar]
- 5.Esser, R.E., S.K. Anderle, C. Chetty, S.A. Stimpson, W.J. Cromartie, and J.H. Schwab. 1986. Comparison of inflammatory reactions induced by intraarticular injection of bacterial cell wall polymers. Am. J. Pathol. 122:323–334. [PMC free article] [PubMed] [Google Scholar]
- 6.Deng, G.M., and A. Tarkowski. 2000. The features of arthritis induced by CpG motifs in bacterial DNA. Arthritis Rheum. 43:356–364. [DOI] [PubMed] [Google Scholar]
- 7.Kagari, T., H. Doi, and T. Shimozato. 2002. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL- 6, in the development of monoclonal antibody-induced arthritis. J. Immunol. 169:1459–1466. [DOI] [PubMed] [Google Scholar]
- 8.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 9.Hacker, H., R.M. Vabulas, O. Takeuchi, K. Hoshino, S. Akira, and H. Wagner. 2000. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J. Exp. Med. 192:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 11.Firestein, G.S., D.L. Boyle, C. Yu, M.M. Paine, T.D. Whisenand, N.J. Zvaifler, and W.P. Arend. 1994. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 37:644–652. [DOI] [PubMed] [Google Scholar]
- 12.Arend, W.P., and J.M. Dayer. 1995. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 38:151–160. [DOI] [PubMed] [Google Scholar]
- 13.Abramson, S.B., and A. Amin. 2002. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford). 41:972–980. [DOI] [PubMed] [Google Scholar]
- 14.Kouskoff, V., A.S. Korganow, V. Duchatelle, C. Degott, C. Benoist, and D. Mathis. 1996. Organ-specific disease provoked by systemic autoimmunity. Cell. 87:811–822. [DOI] [PubMed] [Google Scholar]
- 15.Williams, R.O., L. Marinova-Mutafchieva, M. Feldmann, and R.N. Maini. 2000. Evaluation of TNF-alpha and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-alpha/anti-CD4 therapy. J. Immunol. 165:7240–7245. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto, I., A. Staub, C. Benoist, and D. Mathis. 1999. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 286:1732–1735. [DOI] [PubMed] [Google Scholar]
- 17.Ji, H., D. Gauguier, K. Ohmura, A. Gonzalez, V. Duchatelle, P. Danoy, H.J. Garchon, C. Degott, M. Lathrop, C. Benoist, and D. Mathis. 2001. Genetic influences on the end-stage effector phase of arthritis. J. Exp. Med. 194:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wipke, B.T., and P.M. Allen. 2001. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167:1601–1608. [DOI] [PubMed] [Google Scholar]
- 19.Kyburz, D., D.A. Carson, and M. Corr. 2000. The role of CD40 ligand and tumor necrosis factor alpha signaling in the transgenic K/BxN mouse model of rheumatoid arthritis. Arthritis Rheum. 43:2571–2577. [DOI] [PubMed] [Google Scholar]
- 20.Ji, H., A. Pettit, K. Ohmura, A. Ortiz-Lopez, V. Duchatelle, C. Degott, E. Gravallese, D. Mathis, and C. Benoist. 2002. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J. Exp. Med. 196:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji, H., K. Ohmura, U. Mahmood, D.M. Lee, F.M. Hofhuis, S.A. Boackle, K. Takahashi, V.M. Holers, M. Walport, C. Gerard, et al. 2002. Arthritis critically dependent on innate immune system players. Immunity. 16:157–168. [DOI] [PubMed] [Google Scholar]
- 22.Lee, D.M., D.S. Friend, M.F. Gurish, C. Benoist, D. Mathis, and M.B. Brenner. 2002. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 297:1689–1692. [DOI] [PubMed] [Google Scholar]
- 23.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C.J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406–17409. [DOI] [PubMed] [Google Scholar]
- 24.Vabulas, R.M., P. Ahmad-Nejad, C. da Costa, T. Miethke, C.J. Kirschning, H. Hacker, and H. Wagner. 2001. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 276:31332–31339. [DOI] [PubMed] [Google Scholar]
- 25.Asea, A., M. Rehli, E. Kabingu, J.A. Boch, O. Bare, P.E. Auron, M.A. Stevenson, and S.K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028–15034. [DOI] [PubMed] [Google Scholar]
- 26.Okamura, Y., M. Watari, E.S. Jerud, D.W. Young, S.T. Ishizaka, J. Rose, J.C. Chow, and J.F. Strauss III. 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276:10229–10233. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P.F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554–557. [DOI] [PubMed] [Google Scholar]
- 28.Lipsky, P.E., D.M. van der Heijde, E.W. St Clair, D.E. Furst, F.C. Breedveld, J.R. Kalden, J.S. Smolen, M. Weisman, P. Emery, M. Feldmann, et al. 2000. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N. Engl. J. Med. 343:1594–1602. [DOI] [PubMed] [Google Scholar]
- 29.Weinblatt, M.E., J.M. Kremer, A.D. Bankhurst, K.J. Bulpitt, R.M. Fleischmann, R.I. Fox, C.G. Jackson, M. Lange, and D.J. Burge. 1999. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340:253–259. [DOI] [PubMed] [Google Scholar]
- 30.Cohen, S., E. Hurd, J. Cush, M. Schiff, M.E. Weinblatt, L.W. Moreland, J. Kremer, M.B. Bear, W.J. Rich, and D. McCabe. 2002. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46:614–624. [DOI] [PubMed] [Google Scholar]