Abstract
MHC class I–restricted tumor antigens can be presented to CD8+ T cells by two distinct pathways: via direct and indirect presentation. The relative contribution of these two pathways toward the initial activation of tumor antigen–specific CD8+ T cells and their subsequent tumor rejection is still vigorously debated. Using a tumor model able to dissect the relative contributions of direct and indirect presentation, we show unequivocally the inefficiency of direct presentation and the essential requirement of indirect presentation for the priming of naive tumor antigen–specific T cells leading to tumor rejection. Moreover, we characterize the essential environment under which indirect presentation occurs, and find efficient cross-priming of tumor-specific CD8+ T cells in the complete absence of secondary lymphoid tissues. The independence of this process from local lymph nodes is compromised, however, in the absence of CD4+ T cell help. Therefore, our paper demonstrates that effective immune protection against tumors requires the cross-priming of CD8+ T cells under conditions that require either CD4+ T cell help, or draining lymph nodes.
Keywords: CTL, antigen presentation, cellular proliferation, lymphotoxin α, cell trafficking
Introduction
The failure to prime tumor antigen–specific CD8+ T cells may permit unchecked progression of immunogenic tumors. How to enhance the priming of tumor-specific CD8+ T cells leading to the eradication of an established solid tumor has been a challenging issue. MHC class I–restricted tumor antigens can be presented to CD8+ T cells by two distinct mechanisms: (a) direct priming of CD8+ T cells involves their engagement by cognate peptide–MHC-I complexes of cells for which these presented antigens are of their own production; and (b) cross-priming, or indirect priming, on the other hand, is mediated by host professional APCs that take up and process antigens originally synthesized by other cells. The contributions of these two pathways to priming tumor-specific CD8+ T cells and their pathophysiological relevance have been vigorously debated. There is convincing evidence that exogenous proteins can be a source of class I–restricted antigens (1–4). Seung et al. have shown, for example, that MHC-deficient tumors can stimulate the proliferation of naive tumor-specific CD8+ T cells, with the implication that cytolytic T cells can be generated without direct antigen presentation (5). Furthermore, Huang et al. have shown that bone marrow (BM)*–derived cells are required for the priming of cytolytic T cells against MHC-deficient tumors (6). In contrast, other studies have reported that tumor cells expressing an antigenic peptide from a glycoprotein of lymphocytic choriomenigitis virus could generate cytolytic T lymphocytes (CTL) via direct pathway but failed to induce cross-priming (7–9). Moreover, using genetic models that allow only one mode of antigen presentation, recent studies have shown that both cross-presentation of the tumor antigen by the host APCs and direct antigen presentation by the tumor cells are sufficient to initiate rapid T cell clonal expansion in the lymphoid organs (10). Understanding how tumor antigen–specific CD8+ T cells are activated, by direct or indirect presentation, has an important impact on our understanding of how antigenic tumors escape recognition by CD8+ T cells, and may shape design of therapeutic regiments toward invigorating the cytolytic CD8+ T cell response.
Efficient priming of CD8+ T cells requires the intricate yet robust orchestration of spatial and temporal interaction among immune cells and microenvironments. Host professional APCs are capable of transporting antigens from peripheral tissues to secondary lymphoid organs, where they meet rare antigen-specific lymphocytes. These compartmentalized secondary lymphoid organs, such as spleen and draining lymph nodes (DLNs), provide the microenvironment for optimal priming of naive lymphocytes (4, 11, 12). It has been suggested that draining lymph nodes are key sites for the direct priming of tumor antigen–specific CD8+ T cells by tumor cells, and thus absolutely required for anti-tumor immunity (7–9). CD8+ T cell responses can be divided into CD4+ T cell–independent and CD4+ T cell–dependent responses. CD4+ T cell–dependent type of CD8+ T cell response revealed an important role for CD4+ T cells in the activation of APCs and the generation of memory CD8+ cells (13–16).
We have developed a tumor model that allows us to dissect direct and indirect presentation pathways to elucidate their relative contributions to CD8+ T cell priming and subsequent tumor rejection. We transfected a fibrosarcoma cell line MC57G originally derived from MC57BL/6 (B6) mice (H-2b) with either Ld or an antigenic peptide SIYRYYGL (SIY). In the B6 hosts, adoptively transferred 2C TCR transgenic T cells recognize Ld only through the direct pathway, but can recognize the SIY peptide via both direct and indirect pathways (17, 18). In our experiments, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled 2C T cells were adoptively transferred into B6 mice that were inoculated with either fibrosarcoma MC57G transfected with Ld (MC57-Ld) or SIY (MC57-SIY). Our approach directly dissects early T cell priming and each step of expansion, maturation, and ultimate tumor rejection to address this critical question. By visualizing the early proliferation and maturation of antigen-specific CD8+ T cells, our data clearly shows that MC57-SIY, but not MC57-Ld, efficiently primes naive 2C T cells in draining lymphoid tissue leading to tumor rejection. Furthermore, our paper reveals that in the absence of DLN, CD8+ T cells become dependent on CD4 help. These results suggest a complementary role of DLN and CD4 help in the priming CD8+ T cells.
Materials and Methods
Mice, Cell Lines, and Reagents.
Female C57BL/6 (B6) and C57BL/6 Ly5.1 (B6/Ly5.1) congenic mice were purchased from the National Cancer Institute, Frederick Cancer Research Facility. C57BL/6-Cd8αtm1Mak (CD8−/−) mice were purchased from the Jackson Laboratory. 2C TCR transgenic mice on RAG-1–deficient background bred into B6 background for 10 generations (2C TCR transgenic mice) were provided by J. Chen (Massachusetts Institute of Technology, Boston, MA). OT-1 TCR transgenic mice were provided by A. Ma (The University of Chicago). Lymphotoxin α–deficient (LTα−/−) mice were backcrossed into B6 background for 10 generations. CD8−/−, 2C, OT-1 TCR transgenic, and LTα−/− mice were bred and maintained in the specific pathogen-free facility at the University of Chicago. Animal care and use were in accord with institutional guidelines. For B6/Ly5.1, LTα−/−, or B6/Ly5.1, B6 BM chimeras, LTα−/− or B6 mice were lethally irradiated with 9.5 Gy and were 4-h later reconstituted with 5 × 106 B6/Ly5.1 BM cells. BM chimeric mice were used in experiments after at least 6 wk after reconstitution.
The MC57G fibrosarcoma cell line was provided by P. Ohashi (University of Toronto, Toronto, Canada) and H. Hengartner (University Hospital, Zurich, Switzerland; references 8, 9). All tumor cell lines used in these experiments were maintained in DMEM (Mediatech) supplemented with 10% FCS (Sigma-Aldrich), 100 U/ml penicillin, and 100 µg/ml streptomycin (BioWhittaker). The hybridoma cell lines producing anti-Ld (clone 30–5-7) and anti–2C TCR (1B2) antibodies were obtained from D. Sachs (National Institutes of Health, Bethesda, MD) and T. Gajweski (The University of Chicago), respectively. Monoclonal antibodies produced by hybridomas were purified from the culture supernatant with protein G column by standard procedure. The 1B2 antibody was conjugated to FITC or biotin by the Monoclonal Antibody Facility of The University of Chicago. PE-coupled anti-CD8α antibody, PE-coupled anti-IFNγ antibody, isotype control PE-coupled rat IgG1 antibody, cy-chrome (CyC)-coupled streptavidin, and CyC-coupled anti-CD44 antibody were purchased from BD Biosciences. FITC–conjugated-anti–mouse IgG was purchased from Caltag. PE-coupled streptavidin was purchased from Immunotech. Brefeldin A, ionomycin, PMA, collagenase (type 4), and heparin were purchased from Sigma-Aldrich. CFSE was purchased from Molecular Probes. Ficoll-paque was purchased from Amersham Biosciences.
Cell Isolation from Peripheral Blood and Lung.
Peripheral blood was collected from the retro-orbital plexus into tubes containing 100 µl of 20 U/ml heparin in PBS, and lymphocytes were purified by Ficoll-paque. For lung leukocyte isolation, the mice were first bled to decrease the blood contamination of lung tissue. The lungs were collected, washed in the PBS, cut into pieces, and resuspended in DMEM supplemented with 2% FCS and 1.25 mg/ml collagenase D (collagenase D solution) for 20 min in a 37°C shaking incubator. The single cell suspension was collected after 20 min, and the cell clumps were digested for another 20 min in the collagenase D solution until all lung tissue had resolved into a single cell suspension.
Generation of SIY and Ld Expression Vectors and Clones (19).
In brief, to generate pcDNA3.1-Ld, the LK444 plasmid containing Ld cDNA (provided by A. Sant, The University of Chicago) was digested with EcoRI and ligated to an EcoRI-digested, calf intestinal phosphatase–treated pcDNA 3.1 (Invitrogen). To generate iSIY–LEGFP, an SlY minigene was made from two dsDNA oligonucleotides (SIY-5′ and SIY-3′) annealed, phosphorylated, and ligated together. SIY-5′ was made by annealing 5′-TCGACGCCACCATGGTGTCTATTTACAGGTACTACGGCCT-GGCTGCTTACTCTATTTACA-3′ with 5′-TACCTGTAAATAGAGTAAGCAGCCAGGCCGTAGTACCTGTAAATA-GACACCATGGTGGCG-3′. SIY-3′ was made by annealing 5′-GGTACTACGGCCTGGCTGCTTACTCTATTTACAGGTACTACGGCCTGGCTGCTTACGGG-3′ with 5′-GATCCCCGTAAGCAGCCAGGCCGTAGTACCTGTAAATAG-AGTAAGCAGCCAGGCCGTAG-3′. The boldface indicates SalI and BamHI overhangs for SIY-5′ and SIY-3′, respectively; the underlined base pairs indicate the overhang of SIY-5′, which is complementary with the overhang of SIY-3′. The SIY minigene was ligated to a SalI–BamHI fragment of pLEGFP (CLONTECH Laboratories, Inc.), generating SIY-LEGFP. To generate pcDNA-SIY, SIY-LEGFP was digested with EcoRI–BamHI, and the fragment containing the SIY peptide was ligated to an EcoRI–BamHI fragment of pcDNA3.1. The EGFP gene was generated by PCR with the primers 5′-GGGGATCCAGGCGGCGGCATGGTGAGCAAG-3′ and 5′-CCAAGCTTCATTGATGAGTTTGGACAAACC-3′. This EGFP PCR product was digested with BamHI and HindIII and ligated to a BamHI–HindIII fragment of pcDNA3.1-SIY to generate the vector pcDNA3.1-SIY-EGFP.
To generate MC57-Ld, MC57-SIY, and MC57-EGFP, MC57G cells were transfected with pcDNA3.1-Ld, pcDNA3.1-SIY-EGFP, or pcDNA3.1-EGFP using Superfect (QIAGEN). 48 h after transfection, the cell lines were treated with 1 mg/ml G418 and cloned by limiting dilution.
Analysis of Cells by FACS®.
For analysis of Ld expression, tumor cells were incubated with the anti-Ld antibody, washed, and incubated with FITC-coupled anti–mouse IgG antibody. For detection of proliferation of CFSE-labeled 2C T cells, isolated lymph node (LN) cells, splenocytes, and cells from peripheral blood and lung were stained with biotinylated 1B2 antibody, washed, and stained with CyC-coupled streptavidin and PE-coupled anti-CD8α. For analysis of CFSE-labeled 2C T cells and CD44 expression, isolated LN cells or splenocytes were stained with biotinylated 1B2 antibody, washed, and stained with a mixture of PE-coupled streptavidin and CyC-coupled anti-CD44. For analysis of IFN-γ production, isolated LN cells or splenocytes were incubated with 50 ng/ml PMA, 500 ng/ml ionomycin, and 10 μg/ml Brefeldin A for 4 h in complete RPMI 1640 (Mediatech) at 37°C. The cells were washed and stained with biotinylated 1B2, washed, and stained with CyC-coupled streptavidin. The cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% saponin in PBS with 1% BSA and 0.1% azide. The cells were stained with PE-coupled anti-IFNγ or the isotype control. Samples were analyzed on a FACScan™ and data was analyzed with CELLQuest™ software.
Adoptive Transfer of 2C T Cells.
LN cells and splenocytes were isolated from 2C mice and CD8+ T cells were negatively selected with a CD8+ T cell enrichment kit (StemCell Technologies, Inc.). When analyzed, >90% of the enriched CD8+ cells expressed the 2C receptor. Approximately 7–9 × 105 2C T cells were transferred into CD8-deficient mice for assays of tumor growth. The same number of 2C T cells were transferred to each mouse in each experiment. To transfer CFSE-labeled T cells, T cells at a concentration of 2 × 107/ml were labeled with 10 µM CFSE in PBS at 37°C for 30 min. The cells were quenched with equal volume of FCS for 1 min and washed three times, and 3 × 106 CFSE-labeled T cells were injected intravenously into the retro-orbital plexus in a 0.2-ml volume. The next day, mice were challenged subcutaneously with tumor cell suspensions ∼0.5 cm above the tail base. Cells were isolated from the inguinal lymph nodes (DLNs), the other lymph nodes (nondraining lymph nodes [NDLN]), peripheral blood, lung, or spleen at the time indicated. Data for 2C T cell responses to challenges of tumor cells are representative of at least three experiments.
51Cr Release Assay.
To compare the cytolytic activity of T cells from MC57-Ld– or MC57-SIY–challenged mice, spleen cells were incubated with mitomycin C–treated MC57-Ld or MC57-SIY tumor cells, respectively, in a mixed lymphocyte-tumor cell culture as described previously (20). The cytotoxicity of mixed lymphocyte-tumor cell culture effector cells was determined in a 4.5-h 51Cr release assay at different effector-to-target ratios as described previously (20). The percent-specific lysis was calculated by the formula: percent lysis = ([experimental release − spontaneous release]/[maximum release − spontaneous release]) × 100. Spontaneous release was ≤15% of maximum. Maximum release was determined by detergent lysis of targets.
LN Ablation Protocol.
To ablate peripheral LN development, pregnant female C57BL/6 mice were injected intravenously with 100 µg of purified murine LTβ receptor-Ig (LTβR-Ig) on gestation day 12. To ablate all LN, including peripheral and mucosal LN, pregnant female C57BL/6 mice were treated with the combination of 100 µg each of TNF receptor-Ig (TNFR55-Ig) and LTβR-Ig before gestation day 11 (21). The presence or absence of mucosal and/or peripheral LN was carefully determined by dissection of representative progeny of each litter, and confirmed in each individual animal at necropsy.
Results
Direct Presentation Was Not Sufficient, Whereas Cross-presentation Was Essential for the Induction of Tumor Antigen–specific CD8+ T Cell Proliferation.
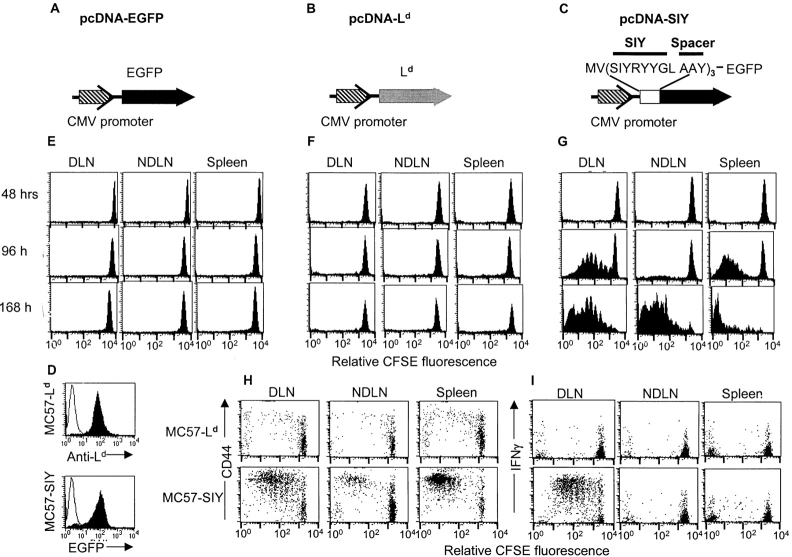
It has been proposed that direct priming of CD8+ T cells in the DLN is essential for the rejection of MC57G, whereas indirect presentation is insignificant (8, 9). To elucidate the importance of direct and indirect presentation in the priming of CD8+ T cells, we developed a tumor model in which we can separate these two distinctive pathways. MC57G cells were transfected with either pcDNA3.1-Ld (MC57-Ld; Fig. 1 B) or pcDNA3.1-SIY-EGFP (MC57-SIY; Fig. 1 C). As the control, MC57G was transfected with pcDNA3.1-EGFP (MC57-EGFP; Fig. 1 A). An SIY expression construct was made by fusing three copies of the SIY minigene to the EGFP gene, which was used to quantify the level of antigen expression (Fig. 1 C). The SIY peptides were separated by three amino acid spacers to favor correct processing of this antigen (22–24 ). MC57-SIY expression of EGFP and MC57-Ld expression of Ld were confirmed by FACS® (Fig. 1 D). Ld expression on MC57-Ld was comparable to that found naturally on mastocytoma P815 (unpublished data). In B6 mice (haplotype H-2b), adoptively transferred 2C T cells can recognize MC57-Ld only via direct antigen presentation, whereas they can recognize MC57-SIY via both direct and indirect pathways (17, 18).
Figure 1.
Subcutaneous challenge of MC57-SIY, but not MC57-Ld, induced proliferation and activation of 2C T cells. MC57G tumor cells were transfected with pcDNA-EGFP (A), pcDNA-Ld (B), or pcDNA-SIY-EGFP (C). MC57-Ld and MC57-SIY expressed Ld and EGFP, respectively (D). 3 × 106 CFSE-labeled 2C T cells were transferred into B6 mice. 24 h later, these mice were challenged with 106 MC57-SIY, MC57-Ld, or MC57-EGFP above the tail base. MC57-SIY–induced proliferation (G), up-regulation of CD44 (H), and secretion of INF-γ (I) of 2C T cells 96 h after tumor challenge in the DLN, NDLN, and spleen. However, MC57-EGFP (E) and MC57-Ld (F) failed to cause any proliferation, up-regulation of CD44 (H), and production of INF-γ (I).
To trace the location and the progress of T cells priming, CFSE-labeled 2C T cells were transferred into B6 mice followed by a subcutaneous challenge with MC57-Ld cells 24 h after T cell transfer. 2, 4, and 7 d after the tumor challenge, very limited proliferation of CFSE-labeled 2C T cells was observed in the DLN, other NDLN, or spleen (Fig. 1 F). In contrast, subcutaneous inoculation of MC57-SIY induced vigorous antigen-specific 2C T cell proliferation in the DLN 4 d after the challenge (Fig. 1 G). Activated 2C T cells migrated to other NDLNs and spleen to continue proliferation. The proliferative response of CFSE-labeled 2C T cells to MC57-SIY was not due to EGFP because mice identically challenged with MC57-EGFP showed no proliferation (Fig. 1 E). Consistent with these findings, we noted that 2C T cells responded to MC57-SIY, but not MC57-Ld, by proliferation, up-regulation of activation marker CD44 (Fig. 1 H), and secretion of IFN-γ (Fig. 1 I). Using CFSE-labeled 2C T cells, we find that the priming of these CD8+ T cells in the lymphoid tissues is antigen- and TCR-specific: (a) MC57-SIY does not induce proliferation of OT-I transgenic T cells (unpublished data); and (b) 2C T cells do not respond to MC57-EGFP (Fig. 1 E). Thus, direct presentation alone was not sufficient to prime antigen-specific CD8+ T cells, whereas indirect presentation was required to specifically prime T cells leading to proliferation and gain of effector function.
Only the Tumor Expressing Antigen That Can Be Cross-presented Was Rejected.
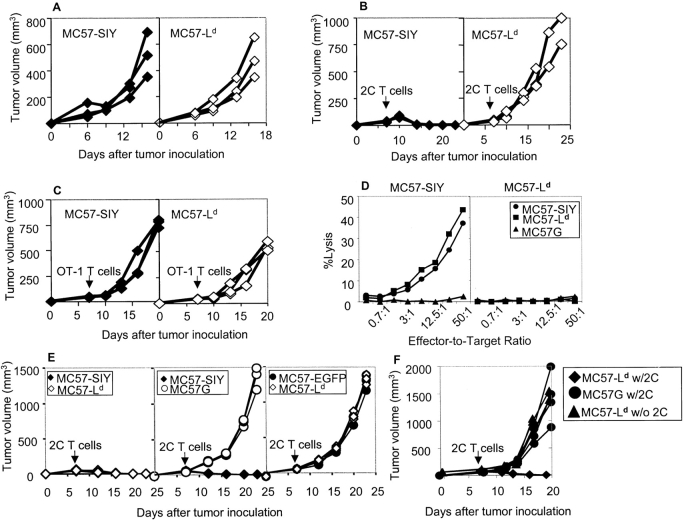
CD8+ T cells are critical for the rejection of MC57G because MC57G tumor cells grow progressively in CD8+ T cell–depleted B6 mice and CD8-deficient mice. Most tumors, including MC57G, bear multiple antigens and induce polyclonal CD8+ T cell responses that obfuscate clear analysis of contribution by direct or indirect presentation for tumor rejection. To ensure a clearly dissectable monoclonal response, we used CD8-deficient B6 mice reconstituted with 7–9 × 105 naive 2C T cells. In contrast to Rag-1−/− recipients, CFSE-labeled naive 2C T cells did not undergo homeostasis proliferation in the CD8−/− mice within 2 wk of constant observation after adoptive transfer (unpublished data). MC57-SIY and MC57-Ld growth was equally aggressive in the CD8-deficient mice (Fig. 2 A and Table I). Activated 2C T cells lysed MC57-Ld and MC57-SIY with similar efficiency in vitro, demonstrating comparable levels of direct presentation (unpublished data). To better trace the priming status of monoclonal tumor-specific CD8+ T cells in response to a defined antigen and their ability to reject the tumors, 107 MC57-SIY or MC57-Ld tumors were inoculated into CD8-deficient B6 mice, followed by adoptive transfer of 7–9 × 105 2C T cells 7 d later. Consistent with the earlier priming experiments, only MC57-SIY was rejected when 2C T cells were transferred (Fig. 2 B and Table I), whereas the MC57-Ld grew progressively even in the presence of 2C T cells (Fig. 2 B and Table I). Thus, direct antigen presentation alone appears insufficient for tumor rejection. The rejection of MC57-SIY by 2C T cells is antigen-specific because the same number of, or more irrelevant, OT-I T cells did not reject MC57-SIY tumor cells (Fig. 2 C). Furthermore, tumor-specific antigen SIY is required for the rejection by 2C T cells because antigen-negative control tumors, MC57G and MC57-EGFP, grew progressively in the 2C T cell–reconstituted CD8-deficient mice (Table I). This demonstrates that only antigen, expressed on a tumor that can be cross-presented, leads to tumor rejection. Moreover, antigen on tumor cells that can only be directly presented was not sufficient to prime T cells nor cause rejection.
Figure 2.
MC57-SIY but not MC57-Ld tumors were rejected by naive 2C T cells. MC57 tumors were inoculated to CD8-deficient mice. 7–9 × 105 2C, OT-1, or no T cells as indicated were transferred into these mice 1 wk after tumor injection. (A) MC57-SIY and MC57-Ld both grew progressively in CD8-deficient mice. (B) Adoptively transferred 2C T cells rejected MC57-SIY but not MC57-Ld. (C) Both MC57-SIY and MC57-Ld grew progressively in the presence of OT-1 T cells. (D) CTL from MC57-SIY–challenged mice lysed both MC57-SIY and MC57-Ld equally well in 51Cr release assay. However, CTL from MC57-Ld–challenged mice lysed neither. (E) MC57-SIY and MC57-Ld tumors were inoculated to each flank of the same mouse. As controls, MC57-SIY and MC57G, or MC57-EGFP and MC57-Ld, were inoculated the same way to the mouse. MC57-Ld tumors were rejected by 2C T cells in the presence of MC57-SIY. MC57G grew progressively when MC57-SIY tumors on the same mouse were rejected by 2C T cells. MC57-EGFP and MC57-Ld both grew in the presence of 2C T cells. (F) MC57-Ld tumors were rejected when 2C T cells were primed by intrasplenic injection of 106 MC57-Ld cancer cells.
Table I.
Incidence of MC57 Tumors in Experiments
| Treatment
|
||||
|---|---|---|---|---|
| Mice | Tumor injected | 2C | Tumor incidence (%)a | 2C priming |
| CD8−/− | MC57G | 6/6 (100) | ||
| EGFP | 6/6 (100) | |||
| Ld | 12/12 (100) | |||
| SIY | 12/12 (100) | |||
| MC57G | Yes | 6/6 (100) | No | |
| EGFP | Yes | 6/6 (100) | No | |
| Ld | Yes | 12/12 (100) | No | |
| SIY | Yes | 0/12 (0) | Yes | |
| Ldw/SIYb | Yes | 0/9 (0) | Yes | |
| Ldw/EGFPb | Yes | 6/6 (100) | No | |
| MC57Gw/SIYb | Yes | 6/6 (100) | No | |
| CD4 depletion
|
||||
| WT | MC57G | 0/6 (0) | ||
| EGFP | 0/6 (0) | |||
| Ld | 0/6 (0) | |||
| SIY | 0/12 (0) | |||
| SIY | Yes | 0/12 (0) | ||
| Splenectomized WTc | SIY | 0/9 (0) | ||
| Splenectomized LT−/− c | SIY | 0/9 (0) | ||
| LT−/−-WT BM chimera | SIY | 0/6 (0) | ||
| Splenectomized LT−/−-WT BM chimerac |
SIY | 0/3 (0) | ||
| LT−/− | SIY | 0/12 (0) | ||
| SIY | Yes | 9/9 (100) | ||
| LN-less WT | SIY | 0/9 (0) | ||
| SIY | Yes | 6/6 (100) | ||
| Splenectomized LN-less WTc |
SIY | 0/3 (0) |
The number in parentheses reflects the pooled experiments with three mice per group.
Mice were challenged with tumor indicated on opposite flanks.
Splenectomized mice were used in the experiment at least 10 days after surgery.
Directly Presented Antigen on Tumors Is Sufficient for the Rejection by Activated T Cells.
We hypothesized that failure to reject MC57-Ld tumor is likely to be due, at least in part, to the lack of priming. To further dissect whether it is the priming or the effector phase at which the MC57-Ld tumor cells fail to be rejected by 2C T cells, MC57-SIY, and MC57-Ld tumor cells were inoculated on the two flanks of a single CD8-deficient mouse. Given earlier findings, MC57-SIY tumor cells initiate robust 2C T cell priming, and if indeed MC57Ld progression is due to its failure to prime 2C T cells, then under these conditions MC57Ld should be rejected with the same kinetics as MC57-SIY. As controls, we injected MC57-SIY and MC57G, or MC57-EGFP and MC57Ld on the two flanks of the control mice. 2C T cells from TCR transgenic mice were purified and 7–9 × 105 were transferred on day seven into the mice bearing MC57-SIY and MC57-Ld tumors on each flank or the control mice. Remarkably, both MC57 tumors, expressing either SIY or Ld antigen, were rejected (Fig. 2 E and Table I). The MC57G parental tumor on the opposite flank of the MC57-SIY tumor grew progressively (Fig. 2 E and Table I), whereas MC57-Ld injected on the opposite flank of MC57-EGFP grew at the same rate as MC57-Ld (Fig. 2 E and Table I). To determine whether these two lines (MC57-SIY and MC57-Ld) are equally sensitive to 2C T cell effector functions, the splenocytes from the 2C T cell–reconstituted CD8-deficient mice primed with MC57-SIY for 4 wk were collected and cultured with irradiated MC57-SIY or MC57-Ld for 6 d for an in vitro 51Cr release assay. We found that T cells from mice that had been primed with MC57-SIY lysed MC57-SIY and MC57-Ld equally well, though MC57G parental cell lines were undisturbed (Fig. 2 D). However, the T cells from mice challenged with MC57-Ld could not lyse either MC57-Ld or MC57-SIY in vitro (Fig. 2 D). In our work, we found the priming and expansion of antigen-specific T cells paralleled with functional maturation measured by IFNγ production. Given the earlier finding that MC57-Ld caused very limited proliferation of 2C T cells, the failure of this recall response is likely due to the lack of priming, expansion, and functional maturation at an early stage of T cell activation, rather than only deficiency in elicitation of memory activity during the in vitro restimulation. The results of these experiments demonstrate both the necessity of cross-presentation for tumor antigen–specific CD8+ T cell activation, and directly presented antigen on tumors is sufficient for the rejection by activated T cells.
Direct Priming of CD8+ T Cells Inside the Secondary Lymphoid Organ.
Lack of priming and failure of rejection of MC57-Ld tumor via subcutaneous injection can be attributed to either poor direct antigen presentation or the lack of proper antigens on tumor cells to prime 2C T cells for rejection. To exclude the second possibility, we investigated whether direct priming of 2C T cells by MC57-Ld can occur under ideal conditions, i.e., when T cells encounter significant numbers of MC57-Ld tumor cells in the secondary lymphoid tissue. Mice were inoculated subcutaneously with 107 MC57-Ld, or MC57G parental tumor cells. 7–9 × 105 2C T cells were adoptively transferred 7 d after tumor inoculation. 24 h later, 106 MC57-Ld tumor cells were directly inoculated into the spleen of these mice. As controls, we injected 106 MC57G parental tumor cells into the spleens of the mice with or without 2C T cell transfer. Transferred 2C T cells primed intrasplenically with 106 MC57-Ld cells indeed were able to reject the subcutaneous MC57-Ld tumors (Fig. 2 F). The tumors in the control groups all grew out progressively (Fig. 2 F). These results are consistent with previous findings (9, 25) and demonstrate that large numbers of the MC57-Ld tumor cells are able to directly prime 2C T cells in the secondary lymphoid tissue. Thus, there is no intrinsic defect in these tumor cells to prime 2C T cells and the failure of rejection of MC57-Ld were likely attributed to ineffective priming by subcutaneously growing tumors.
Secondary Lymphoid Organs Were Not Essential for Tumor Rejection.
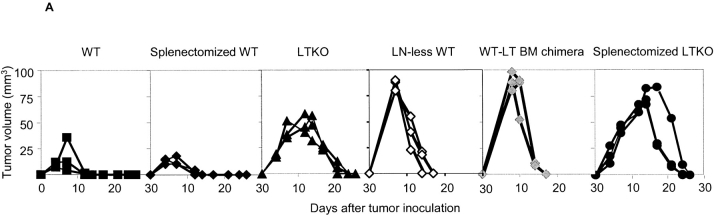
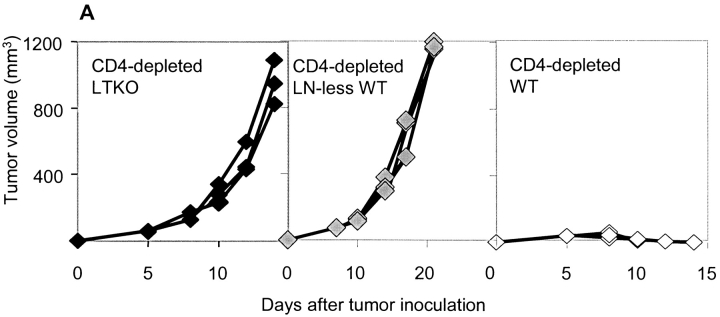
Draining lymph nodes have been proposed to be essential for the rejection of MC57G (9). To examine if draining lymph nodes are essential for immune responses when tumor antigens can be cross-presented, lymphotoxin (LT) α-deficient B6 mice were used. LTα-deficient mice lack all the lymph nodes and Peyer's patches and have disorganized splenic structures. We challenged B6 wild-type (WT) and LTα-deficient mice with titrated doses of MC57G, MC57-EGFP, MC57-Ld, or MC57-SIY tumor cells ranging from 107 to 108, and then compared their growth. Even though tumors were eventually rejected in the LTα-deficient mice (Table I), the rejection was noticeably delayed relative to WT mice (Fig. 3 A). To isolate the site of priming in mice devoid of all the lymph nodes, CFSE-labeled 2C T cells were adoptively transferred into LTα-deficient mice and MC57-SIY tumor cells were inoculated the next day. 2C T cell proliferation in LTα-deficient mice is antigen-dependent because SIY-negative control tumor cells MC57-EGFP failed to induce it (Fig. 3 C). We found proliferating 2C T cells in the spleen of LTα-deficient mice (Fig. 3 B). The priming of 2C T cells in the spleen of LTα-deficient was slightly delayed in comparison with the WT mice in the draining lymph nodes (Fig. 3 B). To exclude the possibility that cell-intrinsic defects in LTα-deficient mice, rather than lymph node agenesis, is responsible for delayed rejection, LTα-deficient mice were lethally irradiated and reconstituted with congenic marker Ly5.1+ WT BM. The reconstitution of LTα−/− BM by WT BM is approaching 100%, as estimated by the percentage of Ly5.1+ cells present in the peripheral blood in comparison to Ly5.1+ B6 donors (unpublished data). These WT-LTα−/− BM chimeras lack all the LNs and Peyer's patches but have LT from WT BM–derived cells. These chimeric mice were inoculated with MC57-SIY tumor cells. Similar to LTα-deficient mice, they also rejected tumors in a slightly delayed fashion compared with WT mice or WT-WT BM chimeras controls (Fig. 3 A and Table I). We also generated mice lacking peripheral lymph nodes (LN-less WT mice) by blocking LTβR signaling during gestation days with LTβ R–Ig. These mice have retained only mucosal lymph nodes, though they have normal splenic structure and immune response (21, 26). These LN-less WT mice were also able to reject MC57-SIY with kinetics similar to LTα−/− mice (Fig. 3 A and Table I). Together, the data showed that draining lymph nodes are not essential for priming T cells when the antigen can be efficiently cross-presented in secondary lymphoid tissues other than DLN, such as the spleen.
Figure 3.
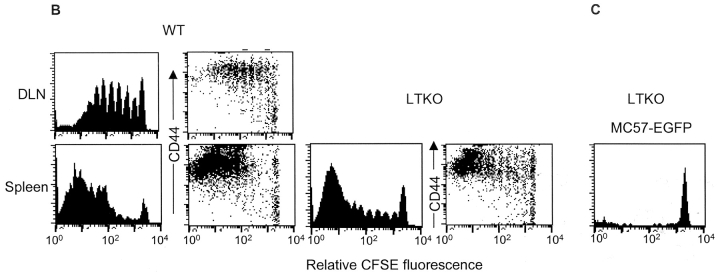
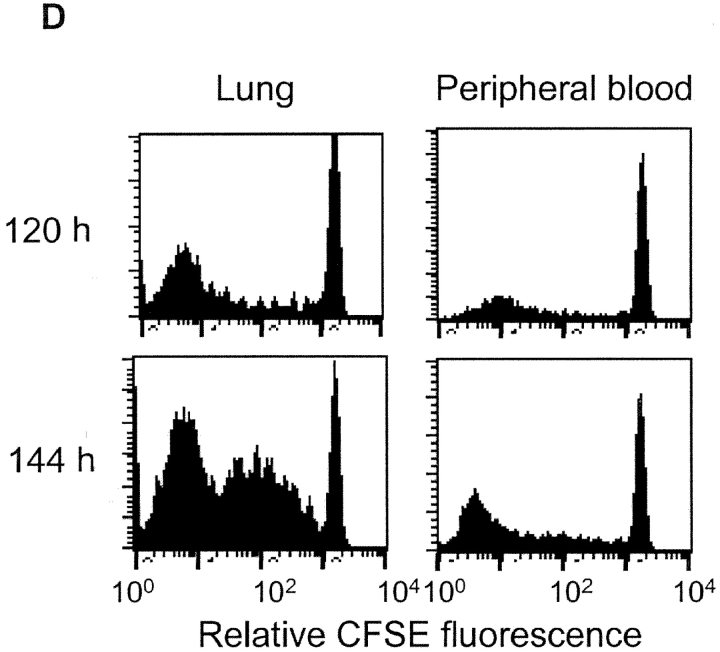
Tumor antigen–specific T cells proliferated and rejected the tumors in the absence of LN and spleen. 2 × 107 MC57-SIY tumors were inoculated to WT, splenectomized WT, LT-deficient (LTKO), LN-less WT, WT′LT-deficient BM chimeric mice, and splenectomized LT-deficient mice and rejected by these mice (A). 3 × 106 CFSE-labeled 2C T cells were transferred to B6, LT-deficient B6, or splenectomized LT-deficient B6 mice. MC57-SIY tumor cells were inoculated 24 h later and induced 2C T cell proliferation and up-regulation of CD44 in both WT and LT-deficient mice. 2C T cells proliferated in the spleen of LT-deficient mice with reduced kinetics comparing to those in WT mice (B). MC57-EGFP tumor cells, which were inoculated the same way as MC57-SIY, did not cause proliferation of 2C T cells in the LT-deficient mice (C). Proliferated 2C T cells can be detected in the peripheral blood of splenectomized LT-deficient mice and some proliferating 2C T cells were present in the lung of these mice (D).
To investigate whether (a) the spleen is essential, (b) the spleen is the only alternative site, or (c) secondary lymphoid organs are required for cross-priming, splenectomized LTα−/− and WT mice were generated and challenged subcutaneously with 2 × 107 MC57-SIY. Splenectomy did not hinder tumor rejection by WT mice, suggesting that the spleen is dispensable for tumor rejection in the presence of DLNs (Fig. 3 A and Table I). Splenectomized LTα−/− mice lacking all secondary lymphoid tissues were still able to reject the tumors (Table I), though with delayed kinetics relative to eusplenic LTα−/− mice (Fig. 3 A). To exclude the possibility that cell-intrinsic defects in splenectomized LTα−/− mice, rather than a lack of secondary lymphoid organs, are responsible for the delayed and ultimate tumor rejection, we challenged splenectomized WT-LTα−/− BM chimeras with 2 × 107 MC57-SIY tumor cells. These mice that were deficient of all the secondary lymphoid organs were able to reject the tumor similarly to splenectomized LTα−/− mice (Table I). We also generated mice lacking all the lymph nodes, including peripheral and mucosal ones, by blocking LTβR and TNF receptor-I signaling at the same time during early gestation days with LTβR–Ig and TNFR55–Ig. These mice did not have any lymph nodes, though they have normal splenic structure and immune response (21, 26). These splenectomized LN-less WT mice were also able to reject MC57-SIY similarly to splenectomized LTα−/− mice (Table I). Thus, we conclude that secondary lymphoid organs are not an absolute requirements for the initial priming of protective anti-tumor responses in immune competent individuals.
Proliferating T Cells Can Be Detected in the Lung in the Absence of Secondary Lymphoid Organs.
The rejection of MC57-SIY is dependent on the activation of CD8+ T cells. To address if and where CD8+ T cells are primed in the absence of secondary lymphoid tissues, we traced the priming of adoptively transferred 2C T cells as a marker for endogenous tumor antigen–specific T cell clones responding to MC57-SIY. 10 d after LTα-deficient mice were splenectomized, they received 3 × 106 CFSC-labeled 2C T cells. MC57-SIY tumor cells were inoculated subcutaneously 1 d later. Lung tissues were collected 2, 5, and 6 d after tumor inoculation and digested by collagenase. Purified leukocytes were subjected to flow cytometry analysis. Interestingly, proliferating 2C cells could be readily detected (Fig. 3 D), suggesting that antigen-specific CD8+ T cells were primed in the absence of all the secondary lymphoid tissues. Also, there is the possibility that indirect priming of CD8+ T cells might happen in the peripheral tissues such as lung in the absence of secondary lymphoid organs. It is possible that delayed proliferation in the periphery may lead to delayed rejection, as seen in Fig. 3 A. Therefore, secondary lymphoid organs, although perhaps providing the ideal environment for cross-priming, can in some cases be dispensable.
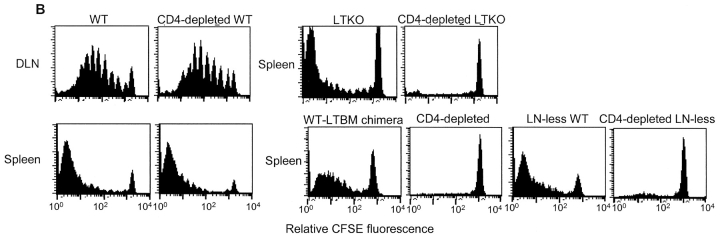
The Role of CD4 Help for Cross-priming of CD8 + T Cells in the Spleen versus LN. The importance of CD4+ T cells in promoting an effective CD8+ T cell response is well established. Recent studies suggest that the mechanism by which CD4+ T cells accomplish such “help” is through the activation of APCs (13–15). The rejection of MC57-SIY by CD8+ T cells can be CD4 help–independent (Fig. 4 A and Table I), and substantively independent of secondary lymphoid tissues. Because the priming of CD8+ T cells in this model is predominantly dependent on the cross-presentation of antigen by host APCs, this suggests to us that antigen-carrying APCs were able to migrate not only to the DLN but also to the spleen and some other peripheral tissues to prime CD8+ T cells. To investigate if CD4 help is essential for this process, CD4+ T cells were depleted in WT and LTα-deficient mice by anti-CD4 antibody GK1.5, followed by subcutaneous inoculation of 107 MC57-SIY tumor cells. CD8+ T cells were able to reject MC57-SIY tumor cells independently of CD4+ T cell help in WT mice (Fig. 4 A and Table I). However, in LTα-deficient mice, 107 MC57-SIY tumor cells grew unchecked with the depletion of CD4+ T cells (Fig. 4 A and Table I). To further address whether it is the priming or effector phase that is compromised, CFSE-labeled 2C T cells were adoptively transferred into B6 and LTα-deficient mice after CD4+ T cell depletion, and recipients were challenged with MC57-SIY the next day. 2, 4, and 5 d after the tumor challenge, spleens from these mice were collected and CFSE-labeled 2C T cell proliferation was evaluated. We found that whereas 2C T cells proliferated robustly in the spleen of LTα-deficient mice, they failed to do so in the absence of CD4+ T cells (Fig. 4 B). In contrast, 2C T cells proliferated in the DLN of WT mice at comparable levels with or without CD4 help (Fig. 4 B). We compared the rate of proliferation of CFSE-labeled 2C T cells in the WT DLN and CD4 knockout mice to similar results (unpublished data).
Figure 4.
Tumor antigen–specific T cells failed to proliferate and reject the tumors in the absence of DLN and CD4+ T cells. (A) 107 MC57-SIY tumor cells were rejected in WT mice but not in the LT-deficient mice (LTKO) or LN-less WT mice in the absence of CD4+ T cells. (B) 3 × 106 CFSE-labeled 2C T cells were transferred to WT, CD4-depleted WT, LT-deficient, CD4-depleted LT-deficient, WT-LT−/− BM chimera, CD4-depleted WT-LT−/− BM chimera, LN-less, or CD4-depleted LN-less B6 mice. MC57-SIY tumor cells were inoculated 24 h later. 2C T cells proliferated in WT, CD4-depleted WT, LT-deficient, WT-LT−/− BM chimera, and LN-less B6 mice but failed to do so in the CD4-depleted LN-devoid mice.
To exclude any BM cell-intrinsic defects from LTα deficiency, both WT-LTα−/− BM chimeras and LN-less WT mice that lacking all the LN were used and yielded the same results. CFSE-labeled 2C T cells failed to proliferate in the spleen of WT-LTα−/− chimeras without CD4+ T cells (Fig. 4 B). Similarly, in the LN-less WT mice, we detected a drastically diminished proliferation of CFSE-labeled 2C T cells in the absence than in the presence of CD4 help (Fig. 4 B). The findings in the proliferation assays paralleled the tumor rejection by endogenous normal repertoire of CD8+ T cells in these lymphoid organ-deficient mice. LN-less WT mice lacking all the LN failed to reject 107 MC57-SIY tumor cells after depletion of CD4+ T cells. Even LN-less WT mice with mucosal LN and normal splenic structure were not able to reject tumors without CD4 help (Fig. 4 A and Table I). Therefore, we conclude that CD4+ cells play an essential role in the cross-priming of CD8+ cells in the spleen, but not in the DLN.
Discussion
We used a unique tumor model in which direct antigen presentation by tumor cells can be distinguished from cross-presentation to study the relative contributions of the two pathways to tumor antigen–specific T cell priming for tumor rejection. We show that direct presentation is very inefficient for this process when examined in isolation of cross-priming. Interestingly, we find in this cross-priming dominant model that helper T cells are not required for the priming of CD8+ T cells in the draining lymph nodes but are essential for that in the spleen. Therefore, we would argue that the interactions of CD4+ T cells, CD8+ T cells, and APCs are orchestrated uniquely to facilitate efficient priming disparate microenvironments (like the DLN), other secondary lymphoid tissues, or peripheral sites.
The importance of different modes of antigen presentation for the priming of CD8+ T cells is still vigorously debated. The low frequency of antigen-specific T cells has made analysis difficult. Previous studies on importance of direct versus cross-priming of CD8+ T cells evaluated effector and memory responses assayed in in vitro culture (6, 8, 9). Other factors may complicate such conclusions. CD4 help is important for the maintenance of CD8+ T cell memory response (16, 27). The production of CD8+ effector and memory response differs in their requirements (28). Moreover, the irradiation BM chimera model used by other papers (6, 9) may be complicated by the fact that naive T cells may require the restricting MHC element to survive (29). Furthermore, it is difficult to study the relative contribution of direct and indirect presentation on a background of polyclonal CD8+ T cell responses to multiple undefined antigens. Further study using monoclonal transgenic T cells clearly showed that both direct and indirect presentations of the same antigenic epitope derived from P1A can lead to activation of tumor antigen P1A–specific CD8+ T cells (10). However, whether such activation sufficiently leads to ultimate tumor rejection is not clear. To be able to trace a monoclonal population of tumor antigen–specific naive CD8+ T cells, visualized beginning at the very early stages and every step of their response, and followed to eventual tumor rejection, we have developed a tumor system that permits monoclonal responses either in whole, or in isolation of cross-priming. We studied how and where these monoclonal CD8+ T cells (2C) were primed in response to a defined antigen leading to tumor rejection that was dependent on their activation. We have revealed that subcutaneous challenge of MC57G-bearing antigen SIY, which can be cross-presented, induced proliferation and maturation of 2C T cells leading to tumor rejection. However, subcutaneously growing MC57G-bearing antigen Ld, which can only be direct-presented, failed to do so. It has been reported that MC57G tumor cells migrate to DLN (7–9). We could detect tumor cells in the DLN by RTPCR (unpublished data). However, these metastasized tumor cells were not sufficient to induce priming. Only when we injected a large amount of MC57Ld tumor cells directly into the secondary organs did they prime 2C T cells. Thus, our data suggest that direct priming alone is very inefficient to prime T cells. Cross-priming is dominant in this model and may be dominant under the physiological conditions.
Secondary lymphoid organs are widely understood to be the nexus of initial antigen presentation to various lymphocytes for adaptive immune reaction to antigens (11, 12). Dependence of various subsets of lymphocytes and APCs on lymphoid tissues has not been well-dissected. We observed that whereas secondary lymphoid tissues increased the efficiency of priming, they were not absolutely required. In our model, T cells were likely to be predominantly primed by APCs but not by tumor cells. Thus, our data implies that APCs are able to carry antigens and migrate to other peripheral sites to prime T cells.
Secondary lymphoid tissues were shown to be critical for the rejection of MC57G tumor using alymphoplastic (aly/aly) mice (9). However, our data indicated that they are important but not essential using an identical tumor line. However, apart from the absence of lymph nodes, aly/aly mice host a variety of other serious immune defects that may confound the findings, including depressed baseline immunoglobulin production and isotype switching, defective T cell function, and faulty homing response to secondary lymphoid tissues (31–33). The inability to reject the MC57G tumor in aly/aly mice might be explained by additional defects in T cell functions, which might be consistent with unchecked tumor growth in the absence of CD4 help in our case. Although secondary lymphoid organs undoubtedly play an important role in certain immune responses, they may not play an essential role in others (33). Using three murine models that are devoid of lymph nodes, we have shown that tumor antigen–specific CD8+ T cells can still proliferate in the spleen in the absence of lymph nodes. CD8+ T cells are able to proliferate in response to and reject the tumor even when the hosts lack all the secondary lymphoid organs, including the LN and spleen. Interestingly, 2C T cell proliferation kinetics was delayed but clearly detectable in the lungs of mice that lack secondary lymphoid tissues, suggesting the peripheral tissue could be alternative sites for indirect priming. Early divisions of CFSE-labeled 2C T cells from collagenase-digested lung tissues suggest that naive 2C cells might be primed there. Unlike most other tissues, bronchial-associated lymphoid tissues may allow constitutive homing of immune cells, including APCs and the naive T cell. Such delayed priming of 2C cells in the lung may contribute to eventual tumor rejection. Therefore, lymphoid tissues may be more efficient in indirect priming but not absolute required in all cases.
CD4+ T cells can help the priming of CD8+ T cells in many ways, such as providing soluble cytokines or membrane ligands. They can recognize cognate antigens with CD8+ T cells during a helper-dependent CD8+ T cell response (30). We have evidence that MC57-SIY tumor cells induce specific helper response that can be detected as early as 3–4 d after a tumor challenge measured by DTH and specific antibody response (unpublished data). However, bystander CD4 help can also contribute to the activation of CD8+ T cells (31).
The CD4+ T cell–dependent CD8+ T cell response revealed an important role for CD4+ T cells in the activation of APCs. Such activation on dendritic cells (DC) may enhance their expression of costimulatory molecules, production of inflammatory cytokines, and migratory capacity out of their resident peripheral compartments (32–34). Our work has revealed that CD4+ T cells are required for the priming in the spleen but dispensable for that in the DLN. There are several possible explanations. First, there may be different types of APCs that carry antigens for subcutaneously growing tumors. For example, it has been reported that epidermal DC can only migrate to DLNs but not the spleen (26). Other APCs that migrate to the spleen or other peripheral sites might be dependent on CD4+ T cell activation. Second, enhanced migratory capacity of DCs might be required for them to prime T cells in sites other than DLN. This could be due to the differential structures of LN and spleen or the unique position of the DLN. Third, CD4+ T cells may also provide some critical cytokines and membrane ligands for the activation and survival of CD8+ T cells (16). When the conditions for priming are not optimal, activation and survival signals provided by CD4+ T cells become critical for CD8+ T cell priming. Thus, helper T cells may be required for efficient priming of CD8+ T cells when antigens are limiting or when DCs cannot efficiently interact with CD8+ T cells. Our preliminary findings suggested that CD40 signaling can partially replace CD4+ T cell function and restore the proliferation of antigen-specific CD8+ T cells in the spleen of LTα−/− mice using agonistic anti-CD40 antibody in CD4+ T cell–depleted mice (unpublished data). CD40 signal functioning directly on CD8+ T cells has been implicated to be essential for generation of memory but not for initial proliferation (16). Thus, it is possible that the complementary role of CD4+ T cells for lack of DLN involves activation of APCs.
In our tumor model, cross-priming of tumor antigen–specific T cells is essential for efficient priming that leads to tumor rejection. With the presence of helper T cells, priming of tumor antigen–specific CD8+ T cells can efficiently occur in the spleen. However, in the absence of CD4+ T cell help, local draining lymph nodes become essential for the priming of CD8+ T cells. Therefore, our paper reveals the distinct interplay between T cells for cross-priming CD8+ T cells in the LN and the spleen.
Acknowledgments
We thank Robert Chin for critical comment and editing; we thank Yang Wang for technical assistance.
This research was supported by grants from the National Institutes of Health (NIH; R01-HD37104, DK58897, R01-CA22677, and P01-CA09296-01) and Biogen, Inc. P. Yu is a recipient of an NIH training grant (5T32DK07074).
Footnotes
Abbreviations used in this paper: BM, bone marrow; CFSE, carboxyfluorescein diacetate succinimidyl ester; CTL, cytolytic T lymphocytes; DC, dendritic cells; DLN, draining LN; LN, lymph node; LT, lymphotoxin; LTβR-Ig, LTβ receptor-Ig; NDLN, nondraining lymph node; SIY, SIYRYYGL; TNFR55-Ig, TNF receptor-Ig; WT, wild-type.
References
- 1.Simpson, E., and R.D. Gordon. 1977. Responsiveness to HY antigen Ir gene complementation and target cell specificity. Immunol. Rev. 35:59–75. [DOI] [PubMed] [Google Scholar]
- 2.Bevan, M.J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross react in the cytotoxic assay. J. Exp. Med. 143:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan, M.J. 1987. Antigen recognition. Class discrimination in the world of immunology. Nature. 325:192–194. [DOI] [PubMed] [Google Scholar]
- 4.den Haan, J.M., and M.J. Bevan. 2001. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13:437–441. [DOI] [PubMed] [Google Scholar]
- 5.Seung, S., J.L. Urban, and H. Schreiber. 1993. A tumor escape variant that has lost one major histocompatibility complex class I restriction element induces specific CD8+ T cells to an antigen that no longer serves as a target. J. Exp. Med. 178:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, A.Y., P. Golumbek, M. Ahmadzadeh, E. Jaffee, D. Pardoll, and H. Levitsky. 1994. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 264:961–965. [DOI] [PubMed] [Google Scholar]
- 7.Kundig, T.M., M.F. Bachmann, C. DiPaolo, J.J. Simard, M. Battegay, H. Lother, A. Gessner, K. Kuhlcke, P.S. Ohashi, H. Hengartner, et al. 1995. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 268:1343–1347. [DOI] [PubMed] [Google Scholar]
- 8.Ochsenbein, A.F., P. Klenerman, U. Karrer, B. Ludewig, M. Pericin, H. Hengartner, and R.M. Zinkernagel. 1999. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc. Natl. Acad. Sci. USA. 96:2233–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochsenbein, A.F., S. Sierro, B. Odermatt, M. Pericin, U. Karrer, J. Hermans, S. Hemmi, H. Hengartner, and R.M. Zinkernagel. 2001. Roles of tumour localization, second signals, and cross priming in cytotoxic T-cell induction. Nature. 411:1058–1064. [DOI] [PubMed] [Google Scholar]
- 10.Bai, X.F., J.X. Gao, J. Liu, J. Wen, P. Zheng, and Y. Liu. 2001. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res. 61:6860–6867. [PubMed] [Google Scholar]
- 11.Fu, Y.X., and D.D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399–433. [DOI] [PubMed] [Google Scholar]
- 12.Cyster, J.G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science. 286:2098–2102. [DOI] [PubMed] [Google Scholar]
- 13.Toes, R.E., S.P. Schoenberger, E.I. van der Voort, R. Offringa, and C.J. Melief. 1998. CD40-CD40 ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity. Semin. Immunol. 10:443–448. [DOI] [PubMed] [Google Scholar]
- 14.Schoenberger, S.P., R.E. Toes, E.I. van der Voort, R. Offringa, and C.J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480–483. [DOI] [PubMed] [Google Scholar]
- 15.Ridge, J.P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 393:474–478. [DOI] [PubMed] [Google Scholar]
- 16.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 297:2060–2063. [DOI] [PubMed] [Google Scholar]
- 17.Sha, W.C., C.A. Nelson, R.D. Newberry, D.M. Kranz, J.H. Russell, and D.Y. Loh. 1988. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 336:73–76. [DOI] [PubMed] [Google Scholar]
- 18.Udaka, K., K.H. Wiesmuller, S. Kienle, G. Jung, and P. Walden. 1996. Self-MHC-restricted peptides recognized by an alloreactive T lymphocyte clone. J. Immunol. 157:670–678. [PubMed] [Google Scholar]
- 19.Spiotto, M.T., P. Yu, D.A. Rowley, M.I. Nishimura, S.C. Meredith, T.F. Gajewski, Y.X. Fu, and H. Schreiber. 2002. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 17:737–747. [DOI] [PubMed] [Google Scholar]
- 20.Ward, P.L., H. Koeppen, T. Hurteau, and H. Schreiber. 1989. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologous normal cells. J. Exp. Med. 170:217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rennert, P.D., D. James, F. Mackay, J.L. Browning, and P.S. Hochman. 1998. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 9:71–79. [DOI] [PubMed] [Google Scholar]
- 22.Velders, M.P., S. Weijzen, G.L. Eiben, A.G. Elmishad, P.M. Kloetzel, T. Higgins, R.B. Ciccarelli, M. Evans, S. Man, L. Smith, and W.M. Kast. 2001. Defined flanking spacers and enhanced proteolysis is essential for eradication of established tumors by an epitope string DNA vaccine. J. Immunol. 166:5366–5373. [DOI] [PubMed] [Google Scholar]
- 23.Theobald, M., T. Ruppert, U. Kuckelkorn, J. Hernandez, A. Haussler, E.A. Ferreira, U. Liewer, J. Biggs, A.J. Levine, C. Huber, et al. 1998. The sequence alteration associated with a mutational hotspot in p53 protects cells from lysis by cytotoxic T lymphocytes specific for a flanking peptide epitope. J. Exp. Med. 188:1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel, S., F. Levy, O. Burlet-Schiltz, F. Brasseur, M. Probst-Kepper, A.L. Peitrequin, B. Monsarrat, R. Van Velthoven, J.C. Cerottini, T. Boon, et al. 2000. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 12:107–117. [DOI] [PubMed] [Google Scholar]
- 25.Kedl, R.M., and M.F. Mescher. 1997. Migration and activation of antigen-specific CD8+ T cells upon in vivo stimulation with allogeneic tumor. J. Immunol. 159:650–663. [PubMed] [Google Scholar]
- 26.Rennert, P.D., P.S. Hochman, R.A. Flavell, D.D. Chaplin, S. Jayaraman, J.L. Browning, and Y.X. Fu. 2001. Essential role of lymph nodes in contact hypersensitivity revealed in lymphotoxin α–deficient mice. J. Exp. Med. 193:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Herrath, M.G., M. Yokoyama, J. Dockter, M.B. Oldstone, and J.L. Whitton. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilloux, Y., X.F. Bai, X. Liu, P. Zheng, and Y. Liu. 2001. Optimal induction of effector but not memory antitumor cytotoxic T lymphocytes involves direct antigen presentation by the tumor cells. Cancer Res. 61:1107–1112. [PubMed] [Google Scholar]
- 29.Brocker, T. 1997. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II–expressing dendritic cells. J. Exp. Med. 186:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett, S.R., F.R. Carbone, F. Karamalis, J.F. Miller, and W.R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tough, D.F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 272:1947–1950. [DOI] [PubMed] [Google Scholar]
- 32.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J. Exp. Med. 184:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: up-regulation via MHC class II and CD40 molecules and down-regulation by IL-4 and IL-10. J. Exp. Med. 184:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moodycliffe, A.M., V. Shreedhar, S.E. Ullrich, J. Walterscheid, C. Bucana, M.L. Kripke, and L. Flores-Romo. 1999. CD40-CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J. Exp. Med. 191:2011–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]