Abstract
Leishmania, a protozoan parasite, lives and multiplies as amastigote within macrophages. It is proposed that the macrophage expressed CD40 interacts with CD40 ligand on T cells to induce IFN-γ, a Th1-type cytokine that restricts the amastigote growth. Here, we demonstrate that CD40 cross-linking early after infection resulted in inducible nitric oxide synthetase type-2 (iNOS2) induction and iNOS2-dependent amastigote elimination. Although CD40 expression remained unaltered on L. major–infected macrophages, delay in the treatment of macrophages or of mice with anti-CD40 antibody resulted in significant reduction in iNOS2 expression and leishmanicidal function suggesting impaired CD40 signaling in Leishmania infection. The inhibition of CD40-induced iNOS2 expression by SB203580, a p38-mitogen activated protein kinase (p38MAPK)-specific inhibitor, and the reversal of the inhibition by anisomycin, a p38MAPK activator, suggested a crucial role of p38MAPK in CD40 signaling. Indeed, the CD40-induced p38MAPK phosphorylation, iNOS2 expression and anti-leishmanial function were impaired in Leishmania-infected macrophages but were restored by anisomycin. Anisomycin's effects were reversed by SB203580 emphasizing the role of p38MAPK in CD40-induced iNOS2-dependent leishmanicidal function. Anisomycin administration in L. major–infected BALB/c mice resulted in significant reduction in the parasite load and established a host-protective Th1-type memory response. Also implicated in these findings is a scientific rationale to define novel anti-parasite drug targets and to bypass the problem of drug resistance.
Keywords: Leishmaniasis, immune evasion mechanism, CD40 signaling, p38MAPK, T cell memory
Introduction
Macrophages are the primary target cells for Leishmania major, the causative agent of the disease cutaneous leishmaniasis. As activated macrophages eliminate the intracellular amastigotes (1) and play a crucial role in the initiation and regulation of host-protective anti-leishmanial T cell response, the parasite deploys different immune evasion strategies to survive within the macrophages (2). Categorically, the strategies include direct interference with IFN-γ–mediated macrophage activation (3) and indirect interference with macrophage activation by skewing the T-helper (Th) cells to Th2 type, the disease-promoting Th subset that suppresses the host-protective Th1 subset (4). It was proposed that the interaction between CD40, a costimulatory molecule expressed on macrophages, B cells, and dendritic cells (5), and its ligand, CD40 ligand (CD154) on T cells (6) results in Th subset skewing. The CD40-deficient mice develop a Th2-skewed response and are susceptible to Leishmania infection (7). The susceptibility to Leishmania infection can be prevented by IL-12 administration in these mice suggesting that CD40–CD154 interaction is required for the production of IL-12, which polarizes the Th cells to Th1 type (7, 8). Thus, the host-protective function of CD40 was attributed to setting a Th1 bias (7–9). However, these reports (7–9) neither explain the mechanism of the failure of CD40 to prevent the progression of the disease in susceptible mouse strains nor do they implicate modulation of CD40 effector functions in macrophages as the parasite's immune evasion strategy. Therefore, we tested whether or not CD40 signaling alone results in Leishmania killing in macrophages. We have also studied the regulation of CD40 signaling in Leishmania-infected macrophages to explore the nature of host-pathogen interaction and finally, based on these experiments; we have tested whether or not a pharmacological manipulation of CD40 signaling results in a host-protective immune response.
Materials and Methods
Leishmania major and Infection of Animals.
BALB/c mice were infected subcutaneously with 2 × 106 Leishmania major (MHOM/Su73/5ASKH) promastigotes in saline (25 μl). The disease was scored by measuring the footpad swelling by a digital micrometer (Mitituyo) and by limiting dilution analysis of parasite burden (10). In some experiments, BALB/c mice were primed with 5 × 102 promastigotes with or without anisomycin.
Reagents.
AMT, anisomycin, and SB203580 were procured from Sigma-Aldrich. The FITC and PE-labeled anti-CD40 and control isotypes, the purified anti-inducible nitric oxide synthetase type-2 (iNOS2) antibodies and the cytokine Opt-EIA kits were procured from BD Biosciences. Anti-CD40 antibody for macrophage activation and Western blotting was purified from a hybridoma (clone 3/23; kindly gifted by Dr. Gerry Klaus, National Institute of Medical Research, London, UK) secreting the antibody (11) by using a protein G–coupled sepharose affinity column (Amersham Biosciences).
Leishmania Infection of Macrophages.
Thioglycolate-elicited peritoneal macrophages from BALB/c mice were infected with Leishmania promastigotes at a ratio of 1:10 for 6 h (10). The extracellular parasites were washed out and the macrophages were cultured with or without anti-CD40 antibody and anisomycin or SB203580 treatment at the indicated doses for 72 h. The macrophages were then fixed, Giemsa-stained, and counted to calculate the number of amastigotes per 100 macrophages (10).
CD40-induced Nitrite Production by Leishmania-infected and Uninfected Macrophages.
BALB/c-derived peritoneal macrophages (5 × 105 cells/ ml in IMDM), uninfected or Leishmania-infected, were treated with the anti-CD40 antibody (10 μg/ml) for 48 h. The culture supernatants were collected. The nitrite concentration was measured by Griess reagent (ICN Biomedicals) following Soong et al. (9).
RT-PCR for Analyzing iNOS2 and CD40 Expression.
BALB/c-derived peritoneal macrophages, uninfected or Leishmania-infected, were treated with the anti-CD40 antibody or anisomycin in presence or absence of SB203580 for 6 h. Total RNA was extracted using TRIZOL (Life Technologies). For cDNA synthesis, 1 μg of total RNA from each sample was incubated with random primer, 0.1 M dithiothreitol, 500 μM dNTPs, 40 U RNase inhibitor, and 1 μl (200 U) of MMLV-reverse transcriptase (Life Technologies). Samples were then incubated at 37°C for 1 h followed by 5 min incubation at 95°C. cDNA from each sample was amplified with Taq DNA Polymerase (GIBCO BRL) in 50 μl under the following conditions: 95°C for 2 min, 94°C 1 min, 65°C for 1 min, and 72°C for 1 min for a total of 35 cycles. Specific primers (GenoMechanix) were designed to amplify the mouse CD40 (sense: 5′-TCCCTGCCCAGTCGGCTTCT-3′; antisense: 5′-CTGTCTTGGCTCATCTCAAA-3′) and iNOS2-coding regions (sense: 5′AGCTCCTCCCAGGACCACAC-3′; antisense: 5′ ACGCTGAGTACCTCATTGGC 3′). Each sample was amplified for mouse DHFR to ensure equal cDNA input.
Preparation of Cell Lysates and Western Blot for iNOS2 and p38-Mitogen-activated Protein Kinase.
Cell lysates were prepared as described earlier (12). Macrophages (1–2 × 107/ sample) were washed twice with ice-cold TBS (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 100 mM Na-ortho-vanadate) and harvested. The cells were lysed in lysis buffer (20 mM HEPES, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 50 μg each of leupeptin, aprotinin, and PMSF) by incubation on ice for 30 min. Lysates were centrifuged (13,000 rpm; 30 min). The supernatants were collected and the protein contents were determined by using the Bradford reagents (Bio-Rad Laboratories). Obtained samples were boiled for five minutes in Laemmli sample buffer, electrophoresed on 10% SDS-polyacrylamide, and transferred to the nitrocellulose using a trans-blot system (Hoeffer Scientific). The nitrocellulose filter was washed, blocked with 5% BSA for 1 h at room temperature, and incubated with anti-iNOS2 antibody for 1 h. The blots were washed and incubated with an HRP-conjugated secondary antibody (1:1,000 in wash buffer containing 1% BSA) for 1 h at room temperature. Immunoreactive bands were visualized by the enhanced chemiluminescence system (Amersham Biosciences). Each sample was blotted with anti-β-actin antibody (Santa Cruz Biotechnology, Inc.) to ensure equal input of protein samples onto the gel.
Similar protocols were followed for detecting the phospho- and dephospho–p38-mitogen-activated protein kinase (p38MAPK) in differently treated macrophages using phospho or dephospho-p38MAPK-specific antibodies. The same blot was used to probe for phospho-p38AMPK and reprobe for dephospho-p38MAPK.
CD40 Expression by Flow Cytometry.
BALB/c-derived macrophages, uninfected or L. major-infected, as described above, were stained with PE-labeled anti-CD40 antibody. The cells were analyzed by a FACSVantage™ flow cytometer (Becton Dickinson).
Cytokine ELISA.
CD4+ T cells from the lymph nodes of the mice, naive or treated, were incubated with anti-CD3 (1 μg/ml) and anti-CD28 (5 μg/ml) antibodies for 72 h. The culture supernatants were assayed for the cytokines by using Opt-EIA kits following manufacturer's instructions.
Statistical Analysis.
The in vitro cultures were in triplicates and a minimum of five mice was used per group for in vivo experiments. The data, presented as mean ± standard deviation, is from one experiment which was performed at least three times. Student's t test was employed to assess the significance of the differences between the mean values of control and experimental groups.
Results
CD40 Signaling Results in iNOS2-dependent Killing of Amastigotes in Early-infected but Not in Late-infected Macrophages.
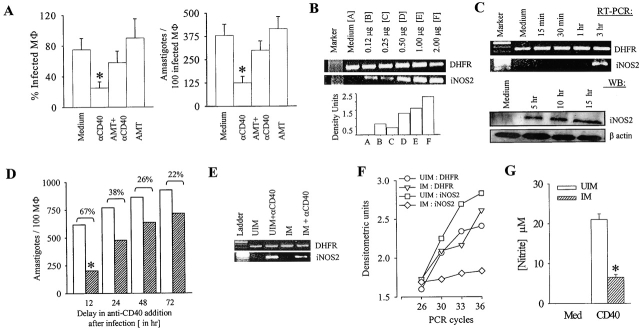
The anti-CD40 antibody treatment of the macrophages 6 h after infection resulted in significant reduction in percent infection and in the amastigote number per hundred infected macrophages (Fig. 1 A; P < 0.01) suggesting that the CD40 signal regulates amastigote survival. AMT, an iNOS2 inhibitor (13), inhibited the CD40-induced anti-leishmanial function suggesting an iNOS2-dependent anti-leishmanial function of CD40 (Fig. 1, A–C). It was observed by RT-PCR and Western blot that the anti-CD40 antibody treatment of macrophages resulted in iNOS2 induction in a dose- and time-dependent manner (Fig. 1, B and C).
Figure 1.
CD40 stimulation results in the induction of iNOS2 expression and iNOS2-dependent amastigote elimination. (A) CD40 induces leishmanicidal activity in BALB/c-derived peritoneal macrophages. The macrophages were infected with L. major promastigotes at a ratio of 1:10 for 6 h at 33°C and anti-CD40 antibody (clone 3/23; 2 μg/ml), with or without AMT, were added to the cultures. After 72 h, the macrophages were fixed, Giemsa-stained, and the percent-infected macrophages and the number of amastigotes per 100 infected macrophages were counted under a microscope. Values are expressed in mean ± SD. Anti-CD40 antibody treatment reduced infection significantly (*, P < 0.01). (B) CD40 induces iNOS2 expression. The BALB/c-derived peritoneal macrophages were incubated with different doses of the anti-CD40 antibody as indicated. RNA was extracted 3 h after stimulation and the RT-PCR for iNOS2 expression was performed as described in Materials and Methods. The density units shown in the bar diagram are arbitrary units obtained from the densitometric analysis using the Quantity-One software. (C) Time-kinetics of CD40-induced iNOS2 expression. Macrophages were stimulated with anti-CD40 antibody (2 μg/ml) as described above. Cells were lysed at different time points after anti-CD40 antibody treatment to obtain RNA (RT-PCR) or protein (WB). RNA was used for iNOS2 RT-PCR and the protein was used for iNOS2 Western blot (WB) analysis as described in Materials and Methods. (D) Delay in anti-CD40 antibody treatment results in the loss of its anti-leishmanial function. BALB/c-derived macrophages were infected with promastigotes at a 1:10 ratio in 16-well tissue culture slides for the indicated period. The cultures were treated with anti-CD40 or left untreated for another 24 h. The macrophages were fixed, Giemsa-stained, and 500 cells were counted as described under Materials and Methods. Values are expressed in mean ± SD (*, P < 0.01). (E) iNOS2 induction by CD40 is impaired in Leishmania-infected macrophages. Macrophages were cultured uninfected or Leishmania-infected for 72 h, followed by anti-CD40 antibody (2 μg/ml) treatment for 3 h. RNA was isolated from these macrophages and RT-PCR for DHFR and iNOS was performed. (F) RNA was isolated from the macrophages as described in the panel (E). RT-PCR for iNOS2 was performed for the indicated cycles and densitometric analysis was done. (G) CD40-induced nitrite production is impaired in Leishmania-infected macrophages. The macrophage cultures, as stated above, were maintained for 72 h. Culture supernatants were collected and nitrite concentration was measured using Griess reagent and calculated by comparison to NaNO2 as a standard. The data (mean ± SD) showing significant reduction of CD40-induced nitrite production (*, P < 0.005) in Leishmania infection is from one of three experiments.
As Leishmania suppresses host-protective immune response (2), we tested if the anti-leishmanial function of CD40 persists in late-infected macrophages. Therefore, the macrophages were infected for 12 to 72 h before they were treated with the anti-CD40 antibody for another 24 h. It was observed that the anti-CD40 antibody treatment resulted in 67% decrease in the number of amastigotes per 100 12-h infected macrophages as compared with the 22–38% decrease in 24 to 72 h infected macrophages (Fig. 1 D) implying a significant impairment in CD40-induced anti-leishmanial function in macrophages parasitized for more than 12 h. The decrease in anti-leishmanial function was possibly due to impaired CD40-induced iNOS2 expression (Fig. 1 E), as confirmed by the analysis in the exponential phase of PCR amplification (Fig. 1 F). Corroborating to the RT-PCR data, the CD40-induced nitrite production was significantly reduced in the late-infected macrophages (Fig. 1 G).
Leishmania Infection of Macrophages Does Not Down-regulate CD40 Expression.
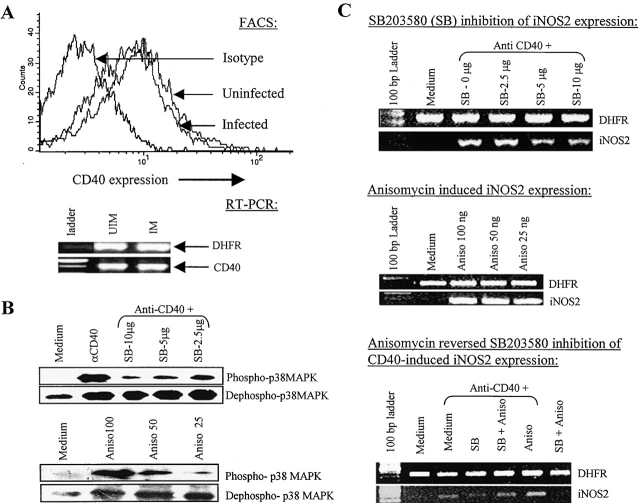
The interference with the CD40-induced anti-leishmanial activity might be executed by two different mechanisms: down-regulated CD40 expression resulting in a quantitative decrease in CD40 signaling or impaired CD40 signaling despite unaltered CD40 expression. Therefore, we tested whether or not Leishmania infection resulted in reduced CD40 expression. We observed that in striking contrast with the deregulated expression of other costimulatory molecules like CD80 or ICAM-1 (14), CD40 expression was not significantly altered on Leishmania-infected macrophages (Fig. 2 A). Therefore, the loss of anti-leishmanial function in late-infected macrophages was perhaps due to impaired CD40 signaling.
Figure 2.
CD40 signals through p38MAPK in macrophages and its expression remain unchanged in Leishmania-infected macrophages. (A) CD40 expression is not down-regulated in the Leishmania-infected macrophages. BALB/c-derived macrophages were cultured uninfected or Leishmania-infected for 72 h. The macrophages were stained with anti-CD40-PE antibody and CD40 expression was analyzed with a FACSVantage™ flow cytometer. CD40 expression by RT-PCR was performed as described in Materials and Methods. (B) Anisomycin (Aniso), a p38MAPK activator, and CD40 cross-linking induces p38MAPK phosphorylation and SB203580 (SB), an inhibitor of p38MAPK, inhibits the CD40-triggered p38MAPK phosphorylation. Macrophages were stimulated with anti-CD40 (2 μg/ml) alone or with the indicated doses of SB203580 or anisomycin for 15 min. Cell extracts were prepared and Western blot was performed to detect phospho- and dephospho-p38MAPK as described earlier. (C) SB203580 inhibits while anisomycin induces the CD40-triggered iNOS2 expression. RNA was isolated from BALB/c macrophages, which were stimulated with anti-CD40 antibody alone (2 μg/ml) or with the indicated doses of SB203580 or anisomycin alone or in combination, as indicated, for 3 h and RT-PCR for iNOS2 message was performed as described earlier. The data shown is from one of three individual experiments.
p38MAPK Plays an Important Role in CD40 Signaling in Macrophages.
As Leishmania infection is proposed to alter LPS-induced p38MAPK activity (15), we tested whether or not CD40 signals through p38MAPK in macrophages and if it does, whether or not the regulation of p38MAPK activity by its pharmacological inhibitor, SB203580 (16), or activator, anisomycin (17), modulates the CD40-induced leishmanicidal activity. It was observed that SB203580 prevented CD40-induced p38MAPK phosphorylation and iNOS2 expression while anisomycin induced p38MAPK phosphorylation and iNOS2 expression in a dose-dependent manner (Fig. 2, B and C). Together with these observations, the reversal of SB203580-mediated inhibition of CD40-induced iNOS2 expression by anisomycin (Fig. 2 C) suggested that CD40 signals through p38MAPK in macrophages regulating iNOS2 expression and that anisomycin directly activates p38MAPK, as anisomycin reverses the inhibitory activities of SB203580.
CD40 Signaling Is Impaired in Leishmania-infected Macrophages but Rescued by Anisomycin.
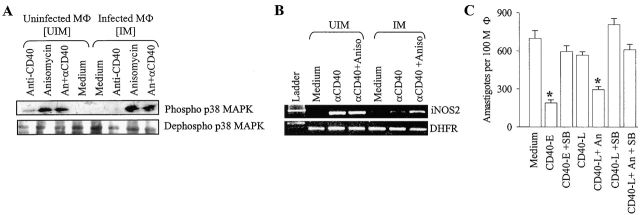
We tested whether or not CD40 signaling through p38MAPK is impaired in late-infected (72 h infected) macrophages and if it is, whether or not anisomycin restored CD40 signaling. It was observed that anti-CD40-induced p38MAPK phosphorylation (Fig. 3 A) and iNOS2 expression (Fig. 3 B) were impaired in the late-infected macrophages. Treatment of these macrophages with anisomycin alone or in combination with anti-CD40 resulted in the restoration of p38MAPK phosphorylation and iNOS2 expression (Fig. 3, A and B). Similarly, CD40-induced anti-leishmanial function was significantly reduced in late-infected macrophages but rescued by anisomycin (P < 0.01; Fig. 3 C). Therefore, these results indicate that Leishmania interrupts the CD40 signaling by inhibiting p38MAPK phosphorylation. As anisomycin does not kill promastigotes (data not shown) and, as predicted by docking simulation, fits into p38MAPK (unpublished data), it is suggested that the anti-leishmanial effect of anisomycin is through p38MAPK.
Figure 3.
CD40-induced p38MAPK phosphorylation, iNOS2 induction, and anti-leishmanial function are impaired during Leishmania infection but restored by anisomycin. The uninfected and the L. major–infected macrophages, treated as indicated, were lysed to obtain protein, for studying p38MAPK phosphorylation (A) or RNA, for studying iNOS2 expression (B), as described in Materials and Methods. In some experiments (C), macrophages were treated with anti-CD40 antibody either at the beginning of infection (CD40-E) with or without SB203580 or 72 h after infection (CD40-L) with or without anisomycin. Macrophages were fixed, stained and counted to record the number amastigotes per 100 macrophages. The data (mean ± SD) showed that anisomycin rescued the CD40-induced anti-leishmanial effect (CD40-L+An) significantly (*, P < 0.01).
Anisomycin Retains Its Anti-leishmanial Activity In Vivo and Establishes a Host-protective Memory T Cell Response.
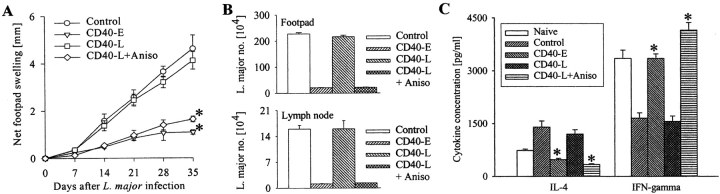
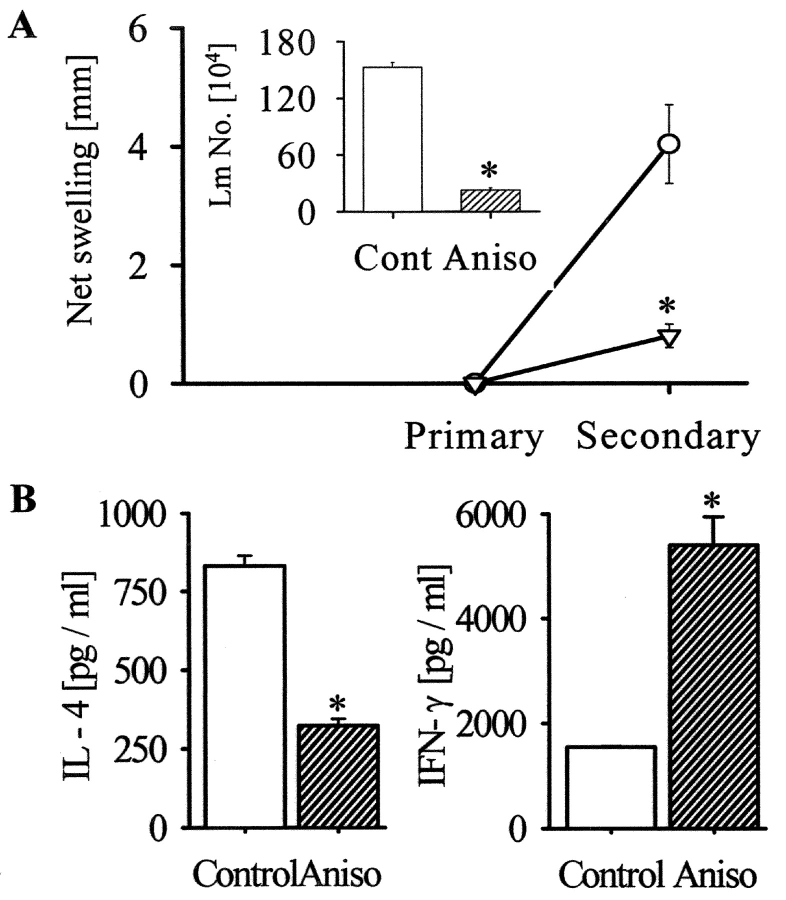
As anisomycin rescued the CD40 signaling defect in vitro, we administered this drug in Leishmania-infected BALB/c mice to test its therapeutic potential. Four groups of L. major–infected mice were either left untreated or treated with anti-CD40 antibody alone from the beginning or 7 d after infection or with the antibody plus anisomycin 7 d after infection. It was observed that the delay in beginning the treatment with anti-CD40 antibody alone failed to reduce the infection but when treated in combination with anisomycin, the disease, as assessed by foot-pad swelling and parasite load, was significantly less severe (P < 0.001) compared with controls (Fig. 4, A and B) . A comparison between the cytokine profiles in the anisomycin-treated and control mice demonstrated that IFN-γ was much higher in the former whereas IL-4 was higher in the latter groups (P < 0.01; Fig. 4 C). The observation suggested that the anti-leishmanial activity of anisomycin was associated with the Th subset skewing. Importantly, the mice, which were primed with anisomycin plus ultra-low dose of L. major, developed significantly less severe disease during reinfection as compared with the control mice (P < 0.001; Fig. 5 A). Restimulation of the T cells from the draining lymph nodes of these mice resulted in more IFN-γ and less IL-4 production in the primed/anisomycin-treated group than the untreated group (Fig. 5 B). Although we do not know whether or not anisomycin treatment in vivo established the host-protective memory by working on T cells or macrophages or both, nevertheless, our results suggest that p38MAPK plays an important role in establishing host-protective memory T cell response.
Figure 4.
Anisomycin treatment results in significant decrease in infection (A and B) and is associated with a Th1-type response (C). BALB/c mice were infected with L. major promastigotes (2 × 106) and were treated either at the beginning of infection with saline (Control) or anti-CD40 (CD40-E) or 7 d after infection with anti-CD40 antibody alone (CD40-L) or in combination with anisomycin (Aniso). The footpad swelling was measured weekly (A) and the parasite load was measured on day 35 post-infection (B). CD4+ T cells from lymph nodes of naive or infected mice treated with saline, anti-CD40 antibody or anisomycin were stimulated in vitro with anti-CD3 + anti-CD28 for 36 h. The cell culture supernatants were assayed for IL-4 and IFN-γ (C). The data (mean ± SD) represents one of three individual experiments. Anti-CD40 or anisomycin treatment reduced infection significantly (*, P < 0.001), decreased IL-4 and increased IFN-γ production significantly (P < 0.01).
Figure 5.
Priming of BALB/c mice with ultra-low dose of L. major plus anisomycin establishes a host-protective Th1-type memory response. (A) BALB/c mice, primed with 5 × 102 Leishmania promastigotes alone (Circle) or with anisomycin (inverted triangle), showed no demonstrable infection after 6 wk (Primary). The mice were then challenged with L. major promastigotes (2 × 106). The net footpad swelling in the control (open bar) or anisomycin-treated (hatched bar) mice was measured 6 wk after infection (Secondary). Limiting dilution assay assessed parasite load in the footpad (Inset). Values are expressed in mean ± SD. Anisomycin priming reduced the infection significantly (*, P < 0.001). (B) CD4+ T cells from popliteal lymph nodes of the control (open bar) or anisomycin-treated (hatched bar) mice were stimulated with anti-CD3+anti-CD28 for 36 h. IL-4 and IFN-γ were measured in the supernatants by ELISA. The data (mean ± SD) representing one of three experiments shows that anisomycin priming reduced IL-4 production but increased IFN-γ production significantly (*, P < 0.005).
Discussion
Successful invasion and survival of a parasite within an immunocompetent host depends on its ability to adapt itself to the anti-parasitic microenvironment and to subvert the host's immune response (2). Our data reveals a novel fact that Leishmania impairs the CD40 effector functions by interrupting the CD40 signaling through p38MAPK in macrophages. The impairment in p38MAPK in Leishmania-infected macrophages could be due to the inhibition of PI-3 kinase (6), ras-raf-rac system (18), or protein kinase C (19) that are known to be involved in CD40 signaling and p38MAPK phosphorylation. The signaling intermediates upstream of p38MAPK could be impaired by the parasite directly, where a parasite product interacts with one or more of these proteins (20) to inhibit phosphorylation or increase dephosphorylation (21) or, indirectly, by inducing a host-derived factor that may act in an autocrine manner to incapacitate the CD40 signaling. For example, a selective impairment of LPS-activated protein kinase C isotypes in Leishmania donovani–infected macrophages by leishmanial lipophosphoglycan and host-derived IL-10 is reported (19). We have observed that IL-10 impairs CD40-triggered iNOS2 expression and anti-leishmanial function (unpublished data). As CD40-CD40 ligand (CD154) interaction generates CD40 signals that play important roles in modulating Th1-dependent anti-leishmanial response (7–9), we have studied the effect of Leishmania infection on CD40 signaling in macrophages and the involvement of p38MAPK in the establishment of anti-leishmanial immune response. Our observations indicate that as an immune evasion strategy and for establishing infection, Leishmania inhibits CD40-triggered p38MAPK signaling. On the other hand, anisomycin's ability to restore CD40 signaling and eliminate amastigotes not only highlights the susceptibility of amastigotes to killing after p38MAPK activation but also suggests a potential use of anisomycin as an anti-leishmanial drug.
The two most important problems in designing an anti-parasitic drug are defining the target and the development of drug-resistance by the parasite. The current study suggests a strategy that addresses both the problems. First, since a parasite impairs the functions of the host cell proteins employed to eliminate the parasite, a drug target can in principle be identified by tracking the host cell proteins impaired by the parasite. An activator of such a crucial host cell protein, like anisomycin for the p38MAPK in the present report, may therefore be considered as a drug candidate. Second, the parasite biomolecules, which are absent in the host, are considered as drug targets. As these drugs inhibit the activities of the target parasite molecules, they exert a selection pressure on the parasite resulting in the emergence of drug resistance (22). As anisomycin controls the parasite by restoring the host immune system, the emergence of drug-resistance can be avoided. Furthermore, the amelioration of the disease is accompanied by Th1-predominance suggesting that the anisomycin treatment generates a bystander host-protective Th cell memory rendering the host relatively resistant to reinfection.
Acknowledgments
The work is supported by the Department of Science and Technology (DST), the Department of Biotechnology (DBT), and Life Science Research Board (LSRB), Government of India.
References
- 1.Murray, H.W., G.L. Spitalny, and C.F. Nathan. 1985. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J. Immunol. 134:1619–1622. [PubMed] [Google Scholar]
- 2.Russell, D.G., and P. Talamas-Rohana. 1989. Leishmania and the macrophage: a marriage of inconvenience. Immunol. Today. 10:328–333. [DOI] [PubMed] [Google Scholar]
- 3.Olivier, M., B.J. Romero-Gallo, C. Matte, J. Blanchette, B.I. Posner, M.J. Tremblay, and R. Faure. 1998. Modulation of interferon-gamma-induced macrophage activation by phosphotyrosine phosphatases inhibition. Effect on murine leishmaniasis progression. J. Biol. Chem. 273:13944–13949. [DOI] [PubMed] [Google Scholar]
- 4.Reiner, S.L., and R.M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151–177. [DOI] [PubMed] [Google Scholar]
- 5.Miga, A., S. Master, M. Gonzalez, and R.J. Noelle. 2000. The role of CD40-CD154 interactions in the regulation of cell-mediated immunity. Immunol. Invest. 29:111–114. [DOI] [PubMed] [Google Scholar]
- 6.Ren, C.L., T. Morio, S.M. Fu, and R.S. Geha. 1994. Signal transduction via CD40 involves activation of lyn kinase and PI-3-kinase, and phosphorylation of PLC-γ2. J. Exp. Med. 179:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamanaka, M., P. Yu, T. Yasui, K. Yoshida, T. Kawabe, T. Horii, T. Kishimoto, and H. Kikutani. 1996. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 4:275–281. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, K.A., P.J. Ovendale, M.K. Kennedy, W.C. Fanslow, S.G. Reed, and C.R. Maliszewski. 1996. CD40-ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 4:283–289. [DOI] [PubMed] [Google Scholar]
- 9.Soong, L., J. Xu, I.S. Grewal, P. Kima, J. Sun, B.J. Longley, N. Ruddle, D. McMahon-Pratt, and R.A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 4:263–273. [DOI] [PubMed] [Google Scholar]
- 10.Saha, B., A. Saini, R. Germond, P. Perrin, D. Harlan, and T. Davis. 1999. Susceptibility or resistance to Leishmania infection is dictated by bone marrow derived-macrophages developed under the influence of IL-3 or GM-CSF. Eur. J. Immunol. 29:2319–2329. [DOI] [PubMed] [Google Scholar]
- 11.Klaus, G.G., M.S. Choi, and M. Holman. 1994. Properties of mouse CD40. Ligation of CD40 activates B cells via a Ca++-dependent, FK506-sensitive pathway. Eur. J. Immunol. 24:3229–3235. [DOI] [PubMed] [Google Scholar]
- 12.Venuprasad, K., P. Banerjee, S. Sharma, S. Pal, P. Parab, D. Mitra, and B. Saha. 2002. Human neutrophil expressed CD28 interacts with macrophage expressed B7 to induce IFN-γ and restrict Leishmania growth. J. Immunol. 169:920–928. [DOI] [PubMed] [Google Scholar]
- 13.Nakane, M., V. Klinghofer, J. Kuk, J.L. Donnelly, G. Budzik, J.S. Pollock, F. Basha, and G.W. Carter. 1995. Novel potent and selective inhibitors of inducible nitric oxide synthase. Mol. Pharmacol. 47:831–834. [PubMed] [Google Scholar]
- 14.Saha, B., G. Das, H. Vohra, N. Ganguly, and G. Mishra. 1995. Macrophage-T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on Leishmania-infected macrophages and its implication in the suppression of cell-mediated immunity. Eur. J. Immunol. 25:2492–2498. [DOI] [PubMed] [Google Scholar]
- 15.Junghae, M., and J.G. Raynes. 2002. Activation of p38MAPK attenuates Leishmania donovani infection in macrophages. Infect. Immun. 70:5026–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saklatvala, J., L. Rawlinson, R.J. Waller, S. Sarsfield, J. Lee, L. Morton, M.J. Barnes, and R.W. Farndale. 1996. Role for p38MAPK in platelet aggregation caused by collagen or a thromboxane analogue. J. Biol. Chem. 271:6586–6589. [DOI] [PubMed] [Google Scholar]
- 17.Polverino, A.J., and S.D. Patterson. 1997. Selective activation of caspases during apoptotic induction in HL-60 cells. Effects of a tetrapeptide inhibitor. J. Biol. Chem. 272:7013–7021. [DOI] [PubMed] [Google Scholar]
- 18.Gulbins, E., B. Brenner, K. Schlottmann, U. Koppenhoefer, O. Linderkamp, K. Coggeshall, and F. Lang. 1996. Activation of the Ras-signaling pathway by the CD40 receptor. J. Immunol. 157:2844–2850. [PubMed] [Google Scholar]
- 19.Bhattacharyya, S., S. Ghosh, P.L. Jhonson, S. Bhattacharya, and S. Majumdar. 2001. Immunomodulatory role of interleukin-10 in visceral leishmaniasis: defective activation of protein kinase C-mediated signal transduction events. Infect. Immun. 69:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandan, D., T. Yi, M. Lopez, C. Lai, and N.E. Reiner. 2002. Leishmania EF-1 alpha activates the SH-2 domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J. Biol. Chem. 277:50190–50197. [DOI] [PubMed] [Google Scholar]
- 21.Blanchette, J., N. Racette, R. Faure, K. Siminovitch, and M. Olivier. 1999. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-γ-triggered JAK2 activation. Eur. J. Immunol. 29:3737–3744. [DOI] [PubMed] [Google Scholar]
- 22.Sereno, D., and J.L. Lemesre. 1997. In vitro life cycle of pentamidine-resistant amastigotes: stability of the chemoresistant phenotypes is dependent on the level of resistance induced. Antimicrob. Agents Chemother. 41:1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]