Abstract
Invariant natural killer T (NKT) cells are a highly conserved subset of T lymphocytes expressing a semi-invariant T cell receptor (TCR), which is restricted to CD1d and specific for the glycosphingolipid antigen α-galactosylceramide. Their ability to secrete a variety of cytokines, which in turn modulate the activation of cells of both innate and acquired immune responses, suggests that invariant NKT cells exert a regulatory role mainly via indirect mechanisms. A relevant question is whether invariant NKT cells can directly help B cells. We document here that human invariant NKT cells are as efficient as conventional CD4+ Th0 lymphocytes in promoting proliferation of autologous memory and naive B lymphocytes in vitro, and in inducing immunoglobulin production. Help to B cells by invariant NKT cells is CD1d-dependent and delivered also in the absence of α-galactosylceramide, suggesting that NKT cells recognize an endogenous ligand presented by CD1d on B cells. The two major subsets of invariant NKT cells, CD4+ and double negative (CD4−CD8−), express comparable levels of CD40 ligand and cytokines, but differ in helper functions. Indeed, both subsets induce similar levels of B cell proliferation, whereas CD4+ NKT cells induce higher levels of immunoglobulin production. These results suggest a direct role for invariant NKT cells in regulating B lymphocyte proliferation and effector functions.
Keywords: autoreactivity, cytokine, α-galactosylceramide, antibodies, helper assay
Introduction
NKT cells are a heterogeneous subset of T lymphocytes, displaying a CD4+ or CD4−CD8− double negative (DN)* phenotype, and coexpressing the natural killer receptor NK1.1/NKRP1A (CD161) and a semi-invariant TCR, encoded in mice and humans by the homologue invariant Vα14-Jα281 and Vα24-JαQ rearrangements, respectively (1–4).
Both mouse and human invariant NKT (inv. NKT) cells recognize the highly conserved MHC class I–like molecule CD1d (5). Although natural antigens presented by CD1d to inv. NKT cells are still unknown, α-galactosylceramide (α-GalCer), a glycosphingolipid isolated from marine sponges, specifically binds CD1d, and activates mouse and human inv. NKT cells (6, 7).
Because of their homogeneous TCR repertoire and activated/effector phenotype, inv. NKT cells are regarded as actors of innate immune response (8, 9). Results obtained so far point toward a regulatory role for inv. NKT cells, determined by their capacity to promptly secrete high amounts of diverse cytokines upon TCR engagement, leading in turn to a variety of effects on the immune system (1). These effects go from NK cell activation (10) to helper T cell differentiation (11). A still unresolved question is whether inv. NKT lymphocytes help B lymphocytes proliferation and antibody production. Most mouse and human B cells express CD1d and data obtained in mice indeed suggest that interactions between CD1d expressed on B cells and CD1d-restricted T cells may play a role in determining amount, isotype, and specificity of the antibodies produced (3, 12, 13). Nevertheless, attempts to demonstrate specifically that inv. NKT cells help CD1d-dependent antibody responses have produced conflicting results in mice (14, 15) while nothing is known in humans.
As antibodies are an essential part of many immune responses, to address this question, we have generated a panel of inv. NKT cell clones and tested their ability to trigger autologous B cells proliferation and effector functions.
Here we show that human inv. NKT cells promote proliferation of memory and naive B lymphocytes, and immunoglobulin production. Help is mediated by CD1d recognition on B cells, and occurs also in the absence of α-GalCer.
Materials and Methods
Antibodies and Flow Cytometry.
FITC, PE, PerCP, APC, Cy5, or biotin-conjugated anti-Vβ11 (C21), anti-Vα24 (C15) (Immunotech); anti-CD1d (CD1d42), anti-CD3 (SK7), anti-CD4 (RPA-T4), anti-CD8 (SK1), anti-CD14 (M5E2), anti-CD62L (DREG56), anti-CD20 (L27), anti-CD27 (L128), anti-CD161 (BX12), anti-IFN-γ (25723.11), anti-IL-4 (3010.211), anti-IL-13 (JES10–5A2), anti-TNF-α (6401.1111), and isotype controls were from BD Biosciences; PE-anti-CCR7 (150503) was from R&D Systems; PECy7-streptavidin was from CALTAG.
Four- and five-color flow cytometric analysis was performed using a LSR® or a FACSVantageSE DiVaOption® instrument (Becton Dickinson), respectively.
Invariant NKT and T Cell Clones.
PBMCs, obtained from peripheral blood of three healthy volunteers, were plated at 106 cells/ml in RPMI (GIBCO BRL) containing 10% FCS (HyClone), α-GalCer (50 ng/ml; KRN7000, Kirin Brewery Co., Gumna, Japan, supplied to P. Dellabona) and 100 U/ml of recombinant h-IL-2 (100 U/ml; Chiron Corp.). At day 15, Vα24+Vβ11+CD4+CD8−, Vα24+Vβ11+CD4−CD8−, or Vα24−Vβ11−CD4+CD8− cells were cloned with a FACSVantageSE® using CloneCytePlus® software, in Terasaki plates (Robbins) containing irradiated PBMC, PHA (0.5 μg/ml; Sigma-Aldrich), and recombinant h-IL-2. Clones were restimulated with the same reagents every 15–20 d.
Screening of Inv. NKT and T Cell Clones for α-GalCer Specificity.
NKT or T cells (2.5 × 105/ml) and 2.5 × 105/ml irradiated autologous CD19+ cells were cultured with 0.2 μg/ml anti-CD3 (UCHT1; BD Biosciences), 50 ng/ml of α-GalCer or equivalent amounts of DMSO or mouse IgG1 (Sigma-Aldrich). After 48 h, cell proliferation was assessed by adding 1 μCi/well of 3[H]-thymidine for additional 16 h. 6 Vα24−Vβ11−CD4+ α-GalCer-unreactive T cell clones and 12 Vα24+Vβ11+ α-GalCer–reactive NKT cell clones (6 CD4+, 6 CD4−) were expanded for this study. The presence of canonical Vα24-JαQ rearrangement in Vα24+Vβ11+ NKT cell clones was confirmed by PCR-heteroduplex analysis as described (9).
B Lymphocyte's Purification.
Autologous CD19+ cells were purified from PBMCs of the three healthy donors, with anti-CD19 magnetic beads (Miltenyi Biotec) following manufacturer's instructions. All preparations were 99% CD20+, the few contaminating cells being CD14+.
Helper Assays.
B lymphocytes (2.5 × 105/ml) were cultured alone or with irradiated NKT cells (2.5 × 105/ml), with 0.2 μg/ml anti-CD3, 50 ng/ml of α-GalCer, or equal amounts of mouse IgG1 or DMSO. After 5 d 3[H]-thymidine incorporation by B cells was assessed. Immunoglobulin concentration in supernatants was determined at day 10 by ELISA using the following antibodies (BD Biosciences): clones JDC-15 and G20–127 (for IgM); clones G18–145 and G17–1 (for IgG1); clones G7–18 and G7–26 (for IgE). Standard human IgG1 and IgM were from Sigma-Aldrich; IgE from Calbiochem.
Carboxyfluorescein-Diacetate-Succinimidyl Ester Dilution.
B cells were loaded with carboxyfluorescein-diacetate-succinimidyl ester (CFDA-SE; Molecular Probes) and cultured at 5 × 105/ml in the presence or in the absence of 2.5 × 104/ml non-irradiated inv. NKT cells and the following reagents: α-GalCer (50 ng/ml); anti-CD40 (10 μg/ml, agonistic, 626.1), IL-4 (5 ng/ml; R&D System), IL-2 (100 U/ml), anti-CD1d (10 μg/ml, CD1d42), anti–IL-4 and/or anti–IL-13 (10 μg/ml, MP425D2 and JES105A2; R&D Systems), anti-CD40 mAb (10 μg/ml, antagonistic, mu5D12; reference 16). The presence of dead cells was excluded by running parallel propidium iodide-stained samples.
Statistical Analysis.
Statistical analysis was performed using the paired Student's t test. * and ** indicate P < 0.05 and P < 0.01 against medium, respectively.
Results
Human CD4+ Inv. NKT Cell Clones Promote Activation and Proliferation of Both Naive and Memory B Lymphocytes Even in the Absence of α-GalCer.
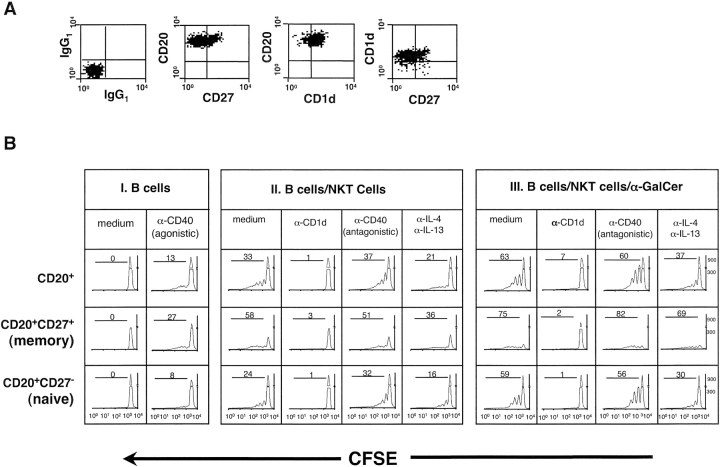
To assess whether CD4+ inv. NKT cells were able to help B lymphocytes, autologous B cells were purified from peripheral blood and analyzed for the expression of the memory marker CD27 (17) and of the NKT cell restriction molecule CD1d. No differences in CD1d expression were observed between naive (CD27−) and memory (CD27+) B cell subsets (Fig. 1 A). Purified B cells were then cultured with cytokines or an agonistic anti-CD40 mAb or autologous CD4+ inv. NKT cell clones with or without α-GalCer. B cell proliferation was determined after 5 d, by assessing the dilution of the CFDA-SE dye in CD27+ and CD27− B cell subsets. No significant B cell proliferation was observed in response to IL-2 plus IL-4, indicating that B cells were not preactivated (unpublished data). In the presence of an agonistic anti-CD40 mAb, B cell division was detected, but was mainly restricted to the memory subset (Fig. 1 B, panel I). When B cells were cocultured with inv. NKT cell clones, both CD27+ and CD27− B lymphocytes divided (Fig. 1 B, panels II and III). Although maximal proliferation was observed in the presence of both inv. NKT cells and α-GalCer (Fig. 1 B, panel lII), we also observed significant cell division when B cells and inv. NKT cells were cocultured without α-GalCer (Fig. 1 B, panel II). B cell proliferation induced by inv. CD4+ NKT cells, with and without α-GalCer, was almost completely inhibited by a neutralizing anti-CD1d antibody.
Figure 1.
Human CD4+ inv. NKT cell clones induce CD1d-dependent proliferation of naive and memory B lymphocytes in the presence and in the absence of α-GalCer. (A) Expression of CD1d and CD27 on freshly isolated B lymphocytes from one representative healthy donor out of three analyzed. (B) CFDA-SE-dilution on CD27+ and CD27− B lymphocytes (CD20+CD3−) after 5 d in culture with the indicated reagents, alone (panel I) or together with CD4+ inv. NKT cells in the absence (panel II) or in the presence (panel III) of α-GalCer. Samples run in the presence of isotype-matched control mAbs gave no differences above medium. Results are from one experiment out of three, in which three CD4+ NKT cell clones were tested in parallel giving comparable results. For each sample, 3 × 105 events in the lymphocyte region were acquired.
The role played by cytokines and CD40-CD40L in B-NKT cell interactions was analyzed by adding neutralizing antibodies (Fig. 1 B, panels II and III). When added individually, anti-IL-4 and IL-13 mAbs failed to exert any effect (unpublished data), while they inhibited B cell proliferation when combined, both in the presence and in the absence of α-GalCer (Fig. 1 B, panel II and III). Conversely, an antagonistic anti-CD40 mAb did not reduce the total number of proliferating B lymphocytes, although it induced some reduction in the number of cells undergoing three or more cell divisions (Fig. 1 B, panel II and III).
From the above experiments we conclude that CD4+ inv. NKT cells induce proliferation of both naive and memory B cells even in the absence of α-GalCer in a CD1d-restricted manner.
Human CD4+ Inv. NKT Cells Help Immunoglobulin Production.
To assess the ability of CD4+ inv. NKT cells to support immunoglobulin production, autologous purified B lymphocytes were cultured with irradiated CD4+ inv. NKT cell clones in the presence of the polyclonal T cell stimulus anti-CD3 or of the inv. NKT–specific antigen α-GalCer. After 10 d, we measured the presence of IgM, IgG1, and IgE in culture supernatants. As shown in Fig. 2 , IgM (Fig. 2 A) and IgG1 (Fig. 2 B) production was induced by CD4+ inv. NKT cell clones activated by anti-CD3 or by α-GalCer, while IgE were never detected (data not depicted). When inv. NKT cells were activated by α-GalCer, but not by anti-CD3, the subsequent immunoglobulin production by B cells was inhibited by an anti-CD1d antibody. Interestingly, CD4+ inv. NKT cells helped low but significant levels of IgM (but not IgG1) production by autologous B cells in the absence of any T cell activation stimuli. Also in this case, antibody production was inhibited by a neutralizing anti-CD1d antibody.
Figure 2.
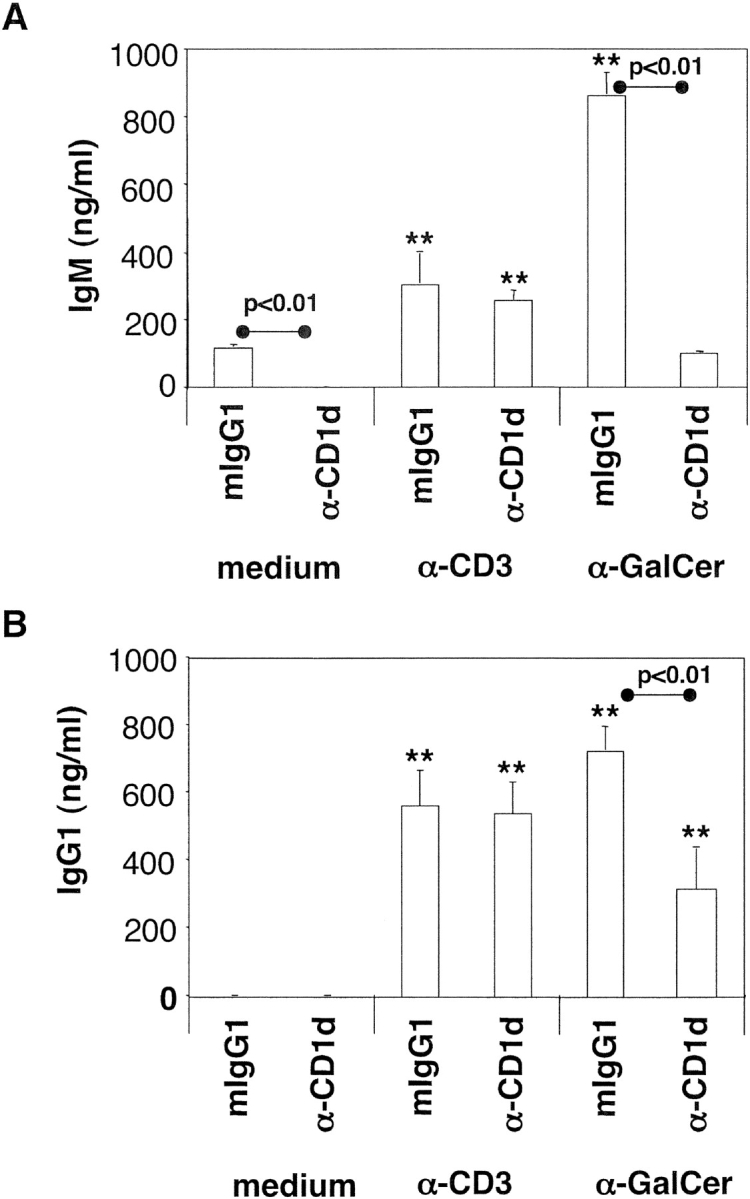
Human CD4+ inv. NKT cell clones provide CD1d-dependent help to B lymphocytes for immunoglobulin production. IgM (A) and IgG1 (B) released by B lymphocytes cultured with irradiated CD4+ inv. NKT cell clones, the indicated stimuli and a neutralizing anti-CD1d or an isotype control mAb. Values are the mean ± SE from three independent experiments, each performed with three CD4+ inv. NKT cell clones. P values between anti-CD1d- and isotype control-treated samples are indicated above the lines.
These results further demonstrated that human inv. NKT cells, probably recognizing a yet unidentified ligand, can help autologous B cells in the absence of α-GalCer.
Human CD4+ and DN Inv. NKT Cells Provide Similar Help for B Cell Proliferation, Whereas CD4+ NKT Cells Preferentially Sustain Immunoglobulin Production.
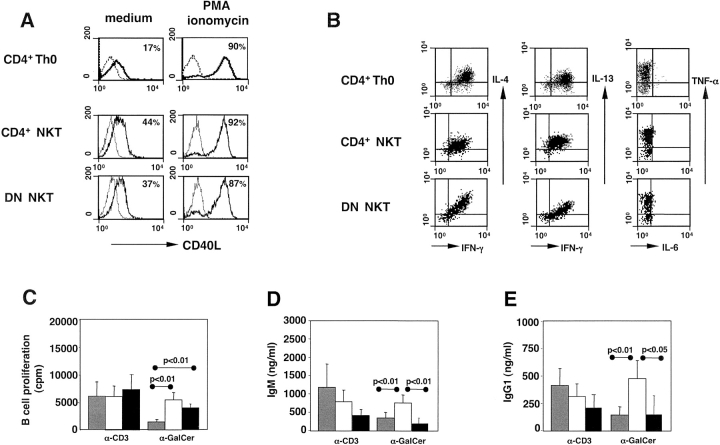
It has been described that CD4+ and DN human inv. NKT cells display different cytokine profiles, with the DN subset producing higher levels of IFN-γ, TNF-α, and perforin, but little IL-4 and IL-13 (2). To assess whether these two NKT cell subsets display different helper activity, we first compared phenotype and cytokine production of CD4+ and DN inv. NKT cell clones with those of conventional CD4+ Th0 cell clones obtained from the same individuals. When activated with PMA and ionomycin, the three T cell subsets expressed similar levels of CD40L (Fig. 3 A) and comparable patterns and levels of cytokines (Fig. 3 B). Consistently, the two subsets of inv. NKT cells induced similar levels of B cell proliferation (Fig. 3 C) when activated by anti-CD3 or α-GalCer. By contrast, CD4+ inv. NKT cells were significantly more efficient than the DN ones in eliciting IgM and IgG1 production (Fig. 3, D and E).
Figure 3.
Human CD4+ and DN inv. NKT cell clones help similarly B cell proliferation, but only CD4+ NKT cell clones sustain immunoglobulin production. The expression of CD40L (A) and intracellular cytokines (B) was compared on conventional CD4+ Th0 cells, CD4+, and DN inv. NKT cells. For all samples, 104 events in the lymphocyte region were acquired. Results are from one clone representative of six for each group. B lymphocytes proliferation (C), IgM (D), and IgG1 (E) production after stimulation by irradiated CD4+ T cells (gray bars), CD4+ (open bars), or DN (black bars) inv. NKT cells in the presence of the indicated stimuli (basal levels have been subtracted). Values are the mean ± SE from three independent experiments in which six clones for each group were tested. P values between samples activated with the three groups of clones are indicated.
We conclude that CD4+ and DN NKT cells differ in their ability to support immunoglobulin production despite their comparable CD40L expression and cytokine production.
Human CD4+CCR7+CD62L+ NKT Cells Expand in Response to α-GalCer.
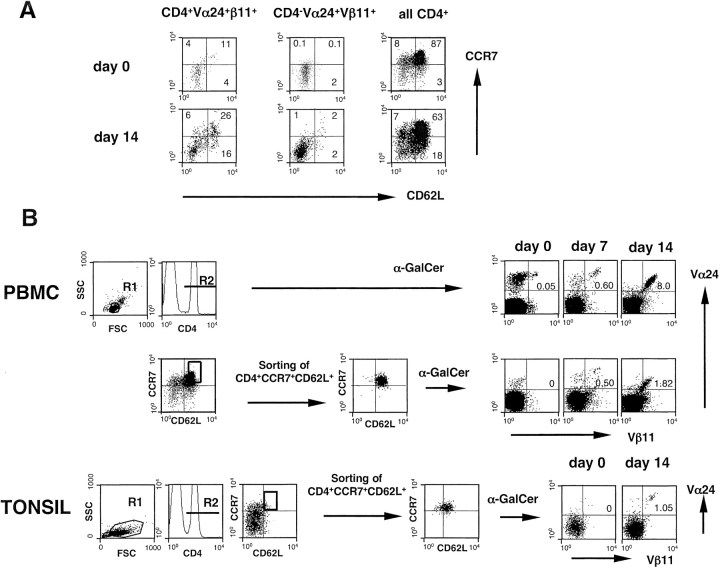
One possible site, where inv. NKT and B cells can interact, is lymphoid tissue. As recently described, very few circulating human Vα24+Vβ11+ T cells express the lymphoid tissue homing receptors CCR7 and CD62L, and these cells belong only to the CD4+ subset (18). To assess whether CCR7+CD62L+Vα24+Vβ11+ cells were “canonical” inv. NKT cells, we determined the expression of CCR7 and CD62L on Vα24+Vβ11+ CD4+ and CD4− cell subsets, before and after stimulation with α-GalCer. As shown in Fig. 4 A, CD4+ Vα24+Vβ11+ cells coexpressed CCR7 and CD62L at similar levels ex vivo (day 0) and after 2 wk of culture in the presence of α-GalCer. No coexpression of CCR7 and CD62L was found in the CD4− subset of Vα24+Vβ11+ cells at any time point (Fig. 4 A).
Figure 4.
Human CD4+CCR7+CD62L+ inv. NKT cells expand in response to α-GalCer. CCR7 and CD62L (A) expression on CD4+Vα24+Vβ11+ and CD4−Vα24+Vβ11+ PBMCs, before and after 2 wk in culture with α-GalCer. Expression of CCR7 and CD62L on total CD4 lymphocytes is also shown. Expansion of inv. NKT cells (B) from the CD4+CCR7+CD62L+ subset sorted from PBMCs or tonsils and stimulated with α-GalCer and irradiated autologous B lymphocytes. Sorting was performed according to the indicated gates. For each sample 2 × 105 to 2 × 106 events in the lymphocyte region were acquired. Results are from one out of three representative donors.
To confirm that the CD4+CCR7+CD62L+ Vα24+ Vβ11+ subset expands in response to α-GalCer stimulation, and not because of bystander activation mechanisms, the CD4+CCR7+CD62L+ lymphocyte subset was sorted from PBMCs of three healthy subjects, and analyzed for the presence of NKT cells before and after α-GalCer stimulation. While Vα24+Vβ11+ cells were virtually undetectable in the freshly sorted population, they were found in a sizeable fraction after one and two weeks of culture with α-GalCer (Fig. 4 B).
From these results we conclude that a small fraction of circulating CD4+ inv. NKT cells is equipped with appropriate lymphoid tissue–homing receptors and can theoretically encounter B cells in lymph nodes.
Discussion
This study demonstrates that human inv. NKT cells can provide direct help to autologous B lymphocytes, inducing proliferation of naive and memory B cells and immunoglobulin production.
Productive interaction between inv. NKT cells and B lymphocytes requires CD1d molecules on B cell surface. Notably, inv. NKT cells react to CD1d expressed on primary B lymphocytes even in the absence of α-GalCer. This “auto-reactivity” toward CD1d molecules, although new for NKT-B cell interactions, has been described for NKT cells interacting with tumor cell lines transfected with CD1d, when NKT lymphocytes are pretreated with suboptimal doses of PMA (3, 4). The use of autologous inv. NKT and B cells allowed to exclude any form of alloreactivity in NKT–B cell interactions occurring in the absence of α-GalCer. Although we cannot formally rule out carry over of α-GalCer used in the original stimulation of peripheral blood lymphocytes from which NKT cell clones were generated, two reasons make this “carry over” extremely unlikely. First, inv. NKT cell clones were used after at least 20 d of culture without any exogenous α-GalCer. Second, and most importantly, all clones used were CD1d negative. Therefore, we favor the hypothesis that B cells express an endogenous glycolipid other than α-GalCer, associated to CD1d and recognized by NKT cells.
What is the functional relevance of inv. NKT cell help? The requirement of CD1d as restriction element for NKT–B cell interactions parallels MHC class II requirement observed in T-B cell cognate interactions. The classical MHC-II–dependent “cognate help” requires BcR-mediated antigen internalization and presentation of specific epitopes to antigen-specific T cells (19). Possibly, interactions with inv. NKT cells are restricted in vivo to B cells producing antibodies against glycosphingolipid antigens that would be captured, internalized, and presented in the CD1d context.
Alternatively, based on our observations in vitro, inv. NKT cells may recognize B cells expressing a “yet unknown” endogenous ligand associated to CD1d, regardless of their BcR specificity. This possibility would require strict control to avoid generalized B cell activation. For instance, inv. NKT cells might help B cells in which CD1d presents endogenous ligands that are synthesized by B cells upon stimuli such as inflammatory/innate signals via Toll–like receptors (20).
Secondary lymphoid organs are the anatomical sites were T helper and B cells meet (21). Circulation of T cells within lymphoid tissues depends on the expression of specific sets of homing receptors, i.e., chemokine receptors CXCR5, CCR7, and the adhesion molecule CD62L (22). We show here that a small fraction of CD4+ inv. NKT cells expresses CCR7 and CD62L, and retains them after α-GalCer–specific activation. Therefore, intranodal interactions may occur between CD4+ inv. NKT cells and a subset of CD1d-expressing B cells. Interestingly, the frequency of inv. NKT cells in lymph nodes, spleen, and peripheral blood is similar, and a significant percentage of intranodal B cells expresses CD1d (our unpublished observations). Conversely, given that the great majority of inv. NKT cells is equipped with the CXCR3 and CCR5 homing receptors for inflamed tissues (18), extranodal interaction between inv. NKT cells and B cells is also likely to occur in inflamed nonlymphoid tissues. In this case, inv. NKT cells activated by B cells that have migrated into inflammation sites may contribute to the local immune response, even if the inflamed tissue is CD1d negative. Consequently, CD1d dependent interactions between inv. NKT and B cells would result also in activation of B cells proliferation and/or immunoglobulin production. Ectopic lymphoid follicles containing activated B cells are often found in tissues chronically inflamed because of autoimmune diseases (23) or viral infections (24). It is tempting to speculate that inv. NKT cells play an active role in B cell activation at the inflammation sites. Indeed, lymphoid aggregates detected in the liver peri-portal spaces of patients with chronic hepatitis C, contain many B cells expressing CD1d (our unpublished observations).
CD4+ and DN inv. NKT cells show comparable ability to promote B cell proliferation, whereas the CD4+ subset is superior in helping immunoglobulin production. This finding may imply a different role for these two inv. NKT cell subsets in the regulation of B cell responses. While CD4+ NKT cells can help true humoral responses under physiological conditions, DN NKT cells might play a regulatory role on B lymphocyte activation at inflammation sites. Accordingly, the few circulating inv. NKT cells equipped with appropriate lymphoid tissue-homing receptors are CD4+ (18), further underscoring the possibility that, physiologically, this subset and not the DN one contains NKT cells endowed with full helper effector functions.
While inv. NKT cells produce both IFN-γ and IL-4, it is unclear whether their effector functions influence adaptive immune responses toward Type 1 or Type 2 responses. In our experimental system, inv. NKT cells, like conventional CD4+ Th0 cells, did not induce detectable IgE production (the hallmark of a Th2 response). This finding is in agreement with the reduction of IgE levels observed in sera of mice injected with α-GalCer (25), but contrast with other studies showing that repeated inoculation of α-GalCer in mice increases IgE production in the context of a global Th2-switch of the immune response (11, 26). These discrepancies probably reflect differences in the various experimental settings that could favor costimulatory pathways required by inv. NKT cells to preferentially produce Th1 or Th2 cytokines (27).
The role played by NKT cells in regulating B cell responses should be now investigated in depth in different models, especially as our finding that NKT cells help B cells in the absence of α-GalCer suggests a regulatory role of NKT cells on B cell physiology.
Acknowledgments
This study has been funded by the Italian Association for Cancer Research (AIRC), the Human Frontiers Science Program (HFSP, RG00168/200-M), European Union (QLK2-CT-2001-01205), and Italian Ministry of Health.
Footnotes
Abbreviations used in this paper: α-GalCer, α-galactosylceramide; CFDA-SE, carboxyfluorescein-diacetate-succinimidyl ester; DN, double negative; inv. NKT, invariant NKT.
References
- 1.Kronenberg, M., and L. Gapin. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557–568. [DOI] [PubMed] [Google Scholar]
- 2.Lee, P.T., K. Benlagha, L. Teyton, and A. Bendelac. 2002. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 5:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac, A., M.N. Rivera, S.H. Park, and J.K. Roark. 1997. Mouse CD1d-specific NK1.1+ T cells. Development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 4.Dellabona, P., E. Padovan, G. Casorati, M. Brockhaus, and A. Lanzavecchia. 1994. An invariant V alpha24-J alpha Q/V beta11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J. Exp. Med. 180:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porcelli, S.A., and R.L. Modlin. 1999. The CD1d system: antigen presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329. [DOI] [PubMed] [Google Scholar]
- 6.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 7.Brossary, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benlagha, K., and A. Bendelac. 2000. CD1d-restricted mouse Vα14 and human Vα24 T cells: lymphocyte of innate immunity. Semin. Immunol. 12:537–542. [DOI] [PubMed] [Google Scholar]
- 9.D'Andrea, A., D. Goux, C. De Lalla, Y. Koezuka, D. Montagna, A. Moretta, P. Dellabona, G. Casorati, and S. Abrignani. 2000. Neonatal invariant Vα24+ NKT lymphocytes are activated memory cells. Eur. J. Immunol. 30:1544–1550. [DOI] [PubMed] [Google Scholar]
- 10.Carnaud, C., D. Lee, O. Donnaris, S.-H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647–4650. [PubMed] [Google Scholar]
- 11.Singh, N., S. Hong, D.C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of Th2 phenotype. J. Immunol. 163:2373–2377. [PubMed] [Google Scholar]
- 12.Gumperz, J.E., and M.B. Brenner. 2001. CD1-specific T cells in microbial immunity. Curr. Opin. Immunol. 13:471–478. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto, T., A. Bendelac, C. Watson, J. Hu-Li, and W.E. Paul. 1995. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 270:1845–1847. [DOI] [PubMed] [Google Scholar]
- 14.Scoefield, L., M.J. McConville, D. Hansen, A.S. Campbell, B. Fraser-Reid, M.J. Grusby, and S.M. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 283:225–229. [DOI] [PubMed] [Google Scholar]
- 15.Molano, A., S.H. Park, Y.H. Chiu, S. Dossier, A. Bendelac, and M. Tsuji. 2000. The IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NKT cell activation and antimalarial responses. J. Immunol. 164:5005–5009. [DOI] [PubMed] [Google Scholar]
- 16.Laman, J.D., B.A. ‘t Hart, H. Brok, M. Meurs, M.M. Schellekens, A. Kasran, L. Boon, J. Bauer, M. Boer, and J. Ceuppens. 2002. Protection of marmoset monkeys against EAE by treatment with a murine antibody blocking CD40 (mu5D12). Eur. J. Immunol. 32:2218–2228. [DOI] [PubMed] [Google Scholar]
- 17.Agematsu, K., S. Hokibara, H. Nagumo, and A. Komiyama. 2000. CD27: a memory B cell marker. Immunol. Today. 21:204–206. [DOI] [PubMed] [Google Scholar]
- 18.Kim, C.H., B. Jhonston, and E.C. Butcher. 2002. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+) V beta 11 (+) NKT cell subsets with distinct cytokine producing capacity. Blood. 100:11–16. [DOI] [PubMed] [Google Scholar]
- 19.Lanzavecchia, A. 1985. Antigen-specific interaction between T and B cells. Nature. 314:537–539. [DOI] [PubMed] [Google Scholar]
- 20.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan, I.C.M. 1994. Germinal centers. Annu. Rev. Immunol. 12:117–139. [DOI] [PubMed] [Google Scholar]
- 22.Cyster, J.G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science. 286:2098–2102. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H.J., V. Krenn, G. Steinhauser, and C. Berek. 1999. Plasma cell development in synovial germinal centers in patient with rheumatoid and reactive arthritis. J. Immunol. 162:3053–3062. [PubMed] [Google Scholar]
- 24.Murakami, J., Y. Shimizu, Y. Kashii, T. Kato, M. Minemur, K. Okada, S. Nambu, T. Takahara, K. Higuchi, Y. Maeda, et al. 1999. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 30:143–150. [DOI] [PubMed] [Google Scholar]
- 25.Cui, J., N. Watanabe, T. Kawano, M. Yamashita, T. Kamata, C. Shimizu, M. Kimura, E. Shimizu, J. Koike, H. Koseki, et al. 1999. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer cells. J. Exp. Med. 190:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α−galactosylceramide polarizes CD1-reactive NKT cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014–2025. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa, Y., K. Takeda, H. Yagita, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166:6012–6018. [DOI] [PubMed] [Google Scholar]