Changes in the state of a cell as it responds to signals delivered by soluble mediators as well as by engagement of ligands displayed on cell surfaces or on extracellular matrices are the basis of differentiation, development, and the activation of effector functions. The molecular mechanisms by which ligands of cell surface receptors elicit such changes occupy not only a large proportion of the time and interest of cell and molecular biologists, but also account for an extensive fraction of the prokaryotic and eukaryotic genomes and proteomes. Complex systems that lend themselves to a deep analysis of such cell-signaling events include the network by which the immune system communicates within and among its major components: T cells, B cells, NK cells, and APCs. The study of T cells and the means of their activation have been augmented by our understanding of the primary sensor of the activating signal, the multichain TCR complex, which consists of an MHC/peptide-specific αβ receptor, CD3 δɛ and γɛ heterodimers, and the ζζ homodimer (1). Here, ligand recognition is concentrated in the αβ pair, and signal transduction results from structural modification (phosphorylation) of specific tyrosine residues in the cytoplasmic domains of γ, δ, ɛ, and ζ. In addition, accessory molecules (CD4 and CD8) and costimulatory molecules (CD28) may properly be considered components of the complete signaling machinery (2–5). Interest in CD28 as well as its inhibitory homologue, CTLA-4, has been bolstered not only by a fundamental quest for the rules that govern complex signaling pathways, but also by the attractive likelihood that these molecules might well serve as targets for pharmacological intervention to augment immunotherapy for tumors and infections, or to temper autoimmune events that contribute to a host of acute and chronic diseases (6, 7).
The most recent of a series of investigations on the mechanism of CD28-mediated costimulation, based primarily on the detailed characterization of a number of anti-CD28 mAbs, reported by Lühder et al. (8) in this issue, not only offers insight into a means for stimulating T cells via a pathway that bypasses the TCR, but also illuminates the molecular mechanism by which ligation of CD28 leads to activation, lends support to other recent studies on the stoichiometry and affinity of CD28–ligand interactions (9), and raises the possibility that conformational alternatives of CD28 structure may contribute to the transduction of differential signals.
Under physiological conditions, optimal activation of a mature resting T lymphocyte requires that its clonally expressed αβ TCR bind its complementary MHC/peptide complex as a primary signal (known as “signal 1”) and also that its CD28 molecule bind a ligand, CD80 or CD86 (otherwise known as B7-1 and B7-2, respectively), as “signal 2” (10). Engagement of the αβ TCR in the absence of CD28 engagement results in a failure to fully activate the T cell, but also, for naive cells, may lead to a state of T cell unresponsiveness (11). Under controlled experimental conditions, immunologists frequently substitute mAbs directed against the TCR complex to replace the MHC/peptide ligand as signal 1. Similarly, signal 2 can be mimicked by engagement of CD28 with a suitable mAb directed against this cell surface signal transducer.
The novel results reported by Lühder et al. (8) derive initially from a set of mAbs raised in the mouse against rat CD28 (12). Characterized functionally, these mAbs fall into two groups: (a) those mAbs that provide the costimulation to T cells concomitantly exposed to a TCR-mediated signal (called by the authors “conventional” mAb), and (b) those mAbs (designated “superagonistic”) that fully activate primary resting T cells both in vivo and in vitro in the absence of signal 1. Because they were raised in the mouse against rat molecules, which differ at only nine positions in the exposed part of the molecule, these antibodies must be focused on surface epitopes defined by these differences. By straightforward engineering of recombinant chimeric and mutant CD28 molecules, and analysis of their antibody reactivity after expression in L929 cells, the precise location of the residues in the linear sequence of the rat CD28 was mapped. The conventional antibodies were focused on a region around phenylalanine residue 98, which lies adjacent to the highly conserved “MYPPPY” loop found in both CD28 and CTLA-4. The superagonist antibodies localized to residues on what is known as the C”D loop, comprising amino acids 60–65. These results were strengthened by the observation that the reactivity of a conventional hamster anti–mouse mAb was lost with the mutation at position 98. Although the X-ray structure of CD28 has not yet been solved, structures have been determined of mouse (13) and human (14) CTLA-4 and of human CTLA-4 in complex with both CD80 (B7-1; reference 15) and CD86 (B7-2; reference 16). CTLA-4, and by homology, CD28, contain a single Ig-like Ig V set domain linked to transmembrane and cytoplasmic domains. Amino acid identity in the extracellular Ig-like domains of CD28 and CTLA-4 is sufficient to allow precise sequence alignment and the generation of a respectable molecular model of the CD28 monomer (8, 17). Thus, the sites on the molecular surface of CD28 that define the focus of the two different groups of antibodies can be located. The C”D loop, on which the superagonist mAbs are focused, lies proximal to the cell surface and seems inaccessible for large ligands whereas the MYPPPY loop, which has been confirmed to be the precise site of B7 (CD80/CD86) binding in the crystal structure of the two different CTLA-4/B7-1 (15) and B7-2 (16) complexes, lies at the tips of an antibody-like “Y” poised for interaction with CD80/CD86 ligands and cross-linking (illustrated schematically in Fig. 1) . The consistent observation that the site of the epitope dictates the functional outcome of mAb binding is further underlined by identification of anti–human CD28 mAbs that fall into the same categories. Mouse CD28 molecules with grafted rat or human C”D sequences expressed in a T cell transfection system showed the predicted functionality: they contributed a superagonist signal when expected. Finally, the biochemical product of superagonist signaling was evaluated in the human cell system. Costimulation of TCR with anti-CD28 conventional mAb resulted in phosphorylation of both ZAP-70 and ζ as expected whereas superagonist stimulation bypassed the proximal TCR signaling but resulted in activation of the nuclear factor κB family, leading to nuclear translocation of c-Rel and p50.
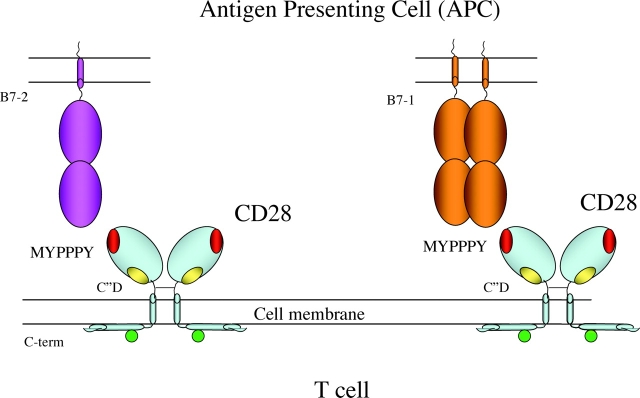
Figure 1.
Location of sites of interaction of CD80 (B7-1) and CD86 (B7-2) and of superagonist mAbs mapped to the surface of CD28. The structure of CD28 as a disulfide-linked Ig-like homodimeric domain on the surface of the T cell is illustrated schematically, depicting interactions with either the monomeric B7-2 or the homodimeric B7-1. The surface exposure the MYPPPY loop that has been shown crystallographically to interact with both B7-2 and B7-1 is colored red and the C”D loop that interacts with the superagonistic antibodies is shown in yellow. The cytoplasmic carboxylterminus of CD28 is indicated and the potential phosphorylation site(s) is indicated as a green circle.
Recent results examining the stoichiometry, kinetics, and affinities of the CTLA-4 and CD28 interactions with CD80 (B7-1) and CD86 (B7-2; reference 9) as well as an expanding understanding of the mobilization of TCR and costimulatory molecules to a distinct membrane domain known as the central supramolecular activation complex (c-SMAC; reference 18), suggest several models to explain how ligation of a CD28 in one topological orientation may give rise to a cellular signal distinct from that elicited when bound in a second orientation.
First, we need to review the most crucial results of the recent experiments. Collins et al. (9) demonstrate that despite the homodimeric structure of CD28, it interacts with both B7-1 and B7-2 as though it has a single binding site. Thus, it is functionally monovalent. B7-2, a monomer with only one binding site for either CD28 or CTLA-4, is induced on the APC early in the immune response. Therefore, the earliest interaction is of the dimeric but monovalent CD28, with the monomeric B7-2. This is an interaction of modest affinity and thus might be assumed to offer a modest costimulatory signal. As the immune response proceeds, B7-1 on the APC and CTLA-4 on the responding T cell, are expressed after ∼24–48 h. B7-1 seems to be in a monomer–dimer equilibrium at the APC surface, permitting maintenance of the CD28 signal and perhaps even a more powerful signal (assuming that the measured affinities read out more or less directly as the strength of signal) results. Concomitant with the B7-1 induction on the APC, the T cell expresses its inhibitory CD28 analogue, CTLA-4, which not only has a higher affinity for the B7-2 ligand expressed earlier and therefore may effectively compete for binding there, but also has an even higher affinity for B7-1. The salient features of the stoichiometry and affinity measurements are that CD28 interacts essentially monovalently with B7-2 early in the response and that the later B7-1 interaction may cause some forced dimerization of the CD28 monovalent dimers, but does not cross-link CD28 further. The inherently divalent CTLA-4 interactions, both with monomeric B7-2 and dimeric B7-1, provide a clear explanation for predominance of the later CTLA-4–driven inhibition of the T cell.
The role of CD28 with respect to the physical reorganization of the c-SMAC is somewhat controversial, with several reports indicating the importance of CD28 in the temporal reorganization of the TCR and the late persistence of kinases (lck and PKC-θ) in the immunological synapse (19, 20), and some suggesting that raft accumulation is independent of CD28 costimulation (21). For the purposes of the model described below, I will take the view that CD28 plays a role in the c-SMAC and contributes to more stable and prolonged activation.
How can we explain the clear-cut correlation of epitope mapping and functional outcome of the two different classes of anti-CD28 mAbs? Lühder et al. (8) lay out four possible models, succinctly described as: preferential engagement of signaling-competent CD28, differential cross-linking, proximity effects, and antibody-induced conformational changes. They favor a model that bivalent binding and lattice formation of CD28 by the superagonist antibodies forms an array distinct from that formed by the interaction of CD28 with B7-2 and B7-1 and this therefore delivers a stronger signal.
I think that it is helpful to consider the differential effects of the two classes of mAbs not only in terms of their ability to cross-link, but also in terms of the possibility that they stabilize two distinct conformations of the CD28 homodimer. First, we hypothesize that the CD28 molecule, a disulfide-linked covalent dimer, has the structural flexibility to dimerize in two different conformations that are in dynamic equilibrium with each other. One dimeric form is “relaxed” and the other “parallel.” The relaxed form has a dimeric structure that mimics the known structure of CTLA-4, with the MYPPPY B7 binding loops available at the tips of the Y. The parallel form provides a structure akin to an antibody or TCR Fv, with the MYPPPY loops away from the cell membrane, but each loop so close to its dimeric partner as to preclude divalent binding by B7. In the relaxed conformation the cytoplasmic tail of the molecule is largely unavailable for interaction with other molecules, such as kinases lck and PI-3 kinase, or the SH2 adaptor protein, GRB-2 (22). Although the precise sequence of activation events conveyed by CD28 ligation remains another controversial area, I would take the view that the COOH-terminal cytoplasmic proline-rich region of CD28 is mobilized by interactions of the extracellular domain with either B7 or antibodies, that this first interacts with an SH3 domain of a kinase such as lck (23), which then contributes to the phosphorylation of the tyrosine of the YMNM sequence of the CD28 cytoplasmic domain. The phospho YMNM sequence is then available for the wide variety of molecular interactions known for CD28, including those with PI-3 kinase, GRB-2, and ITK.
How then can we account for the different signals delivered by the different sets of anti-CD28 mAbs? Some insight can be gained by consideration of popular models for transmembrane signaling, best articulated by Ottemann et al. (24) in their discussion of the mechanism of action of the aspartate receptor. They described five possible models: association/dissociation, piston, rotation, scissor, and seesaw, by which ligation of the extracellular domains of a simple dimeric receptor could change the topological relationships of the cytoplasmic domains ultimately leading to changes in associated enzymatic activities. They argued, based on electron proton resonance spectra of molecules spin labeled at strategic positions, that the aspartate receptor exploits the piston model. Recent crystallographic evidence suggests that a screw-like movement involving both rotational and piston movement (of ∼1.6 Å) might be involved in a similar receptor, the microbial light sensory rhodopsin (25). Although the covalent disulfide bond that stabilizes the CD28 homodimer may limit aspects of the molecular motion that might be transduced from extracellular ligation of the molecule, this may not preclude changes in the exposure of the cytoplasmic domain dependent on either lattice formation or lipid environment. A model for ζ-mediated signaling based on differential exposure of cytoplasmic phosphorylation sites in different lipid microenvironments is relevant here (26), because ζ too is a disulfide-linked covalent dimer.
Therefore, our best model is illustrated in Fig. 2 . The binding of the relaxed form of the CD28 homodimer by B7 or by conventional mAbs causes a sufficient perturbation of the cytoplasmic domain to allow the carboxylterminal peptide to be exposed to the SH3 domain of a kinase such as lck. For the sake of argument, I would favor a “piston” model as described above (24). In the context of TCR ligation, where lck is juxtaposed to the TCR complex by its interaction with CD4 or CD8, the activated lck may then initiate phosphorylation of the YMNM tyrosine of the CD28 cytoplasmic domain. This then becomes the focus for the dynamic cascade of subsequent signaling events. The binding of the superagonist antibodies, by forcing a greater perturbation of the cytoplasmic domain through the transmitted effect, perhaps by a scissors model and enforced by the greater spatial distribution of CD28 resulting from the ∼150 Å distance from the first to the second binding site of mouse IgG1 (27), releases the membrane-associated domain from a sequested to an available conformation, results in a more accessible, and thus more active CD28. This might function in the absence of TCR signal 1 by interactions of the carboxylterminal peptide with the SH3 domain of a kinase other than lck. One feature of this model that is inconsistent with available data is that it proposes both a bivalent and a monovalent form of the CD28 homodimer for B7 or conventional antibody ligation. The data of Collins et al. (9) indicate that the recombinant form of CD28 is monovalent. Perhaps the cell surface form may reveal two conformations. Tests of the model lie in further exploration of the signaling intermediates that result from the two kinds of antibodies, and in the determination of the CD28 structure and further clarification of its dimerization and valency properties. One additional prediction of the model is that physiological ligands that bind the C”D site other than the superagonist mAb exist and play a functional role in vivo. Perhaps these have been overlooked because they play an infrequent or minor role. The stage is set for more experiments. Let the play begin.
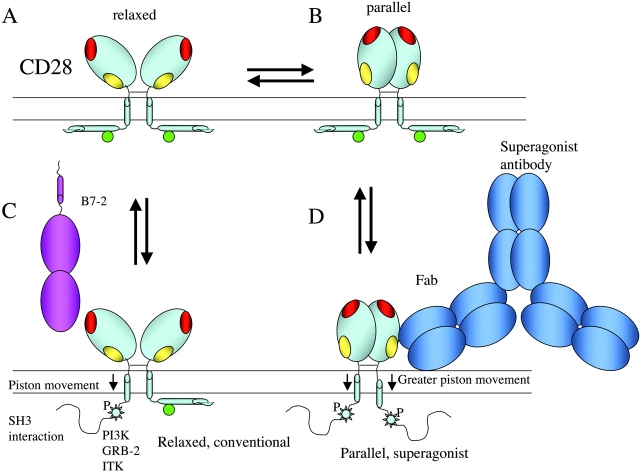
Figure 2.
A differential conformation model for CD28-mediated conventional and superagonist signaling. The unliganded disposition of the hypothesized relaxed (A) and parallel (B) conformations of CD28 is indicated. Color coding of interacting sites is as in Fig. 1. The conventional or costimulatory binding of B7-2 to CD28 results in the liberation of the carboxylterminus of CD28, making this proline-rich domain available for SH3 domain interaction. This would result in the kinase-dependent phosphorylation of the potential PI3-kinase, GRB-2, and ITK binding site (C). Drawn approximately to scale, the binding of a full antibody to the C”D superagonist site promotes the more complete liberation of the cytoplasmic domains of CD28, making them more available for both SH3 and kinase and adaptor interaction (D).
Acknowledgments
I thank I. Stefanova and the members of my laboratory for comments and discussion.
References
- 1.Call, M.E., J. Pyrdol, M. Wiedmann, and K.W. Wucherpfennig. 2002. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 111:967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alegre, M.L., K.A. Frauwirth, and C.B. Thompson. 2001. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 1:220–228. [DOI] [PubMed] [Google Scholar]
- 3.Blair, P.J., J.L. Riley, R.G. Carroll, D.C. St. Louis, B.L. Levine, B. Saha, K.P. Lee, P.J. Perrin, D.M. Harlan, and C.H. June. 1997. CD28 co-receptor signal transduction in T-cell activation. Biochem. Soc. Trans. 25:651–657. [DOI] [PubMed] [Google Scholar]
- 4.Frauwirth, K.A., and C.B. Thompson. 2002. Activation and inhibition of lymphocytes by costimulation. J. Clin. Invest. 109:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salomon, B., and J.A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19:225–252. [DOI] [PubMed] [Google Scholar]
- 6.Egen, J.G., M.S. Kuhns, and J.P. Allison. 2002. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 3:611–618. [DOI] [PubMed] [Google Scholar]
- 7.Alegre, M., F. Fallarino, P. Zhou, K. Frauwirth, J. Thistlethwaite, K. Newell, T. Gajewski, and J. Bluestone. 2001. Transplantation and the CD28/CTLA4/B7 pathway. Transplant. Proc. 33:209–211. [DOI] [PubMed] [Google Scholar]
- 8.Lühder, F., Y. Huang, K.M. Dennehy, C. Guntermann, I. Müller, E. Winkler, T. Kerkau, S. Ikemizu, S.J. Davis, T. Hanke, et al. 2003. Topological requirements and signaling properties of T cell–activating, anti-CD28 antibody superagonists. J. Exp. Med. 197:955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, A.V., D.W. Brodie, R.J. Gilbert, A. Iaboni, R. Manso-Sancho, B. Walse, D.I. Stuart, P.A. van der Merwe, and S.J. Davis. 2002. The interaction properties of costimulatory molecules revisited. Immunity. 17:201–210. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz, R.H. 1992. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 71:1065–1068. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz, R.H. 1997. T cell clonal anergy. Curr. Opin. Immunol. 9:351–357. [DOI] [PubMed] [Google Scholar]
- 12.Tacke, M., G. Hanke, T. Hanke, and T. Hunig. 1997. CD28-mediated induction of proliferation in resting T cells in vitro and in vivo without engagement of the T cell receptor: evidence for functionally distinct forms of CD28. Eur. J. Immunol. 27:239–247. [DOI] [PubMed] [Google Scholar]
- 13.Ostrov, D.A., W. Shi, J.C. Schwartz, S.C. Almo, and S.G. Nathenson. 2000. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 290:816–819. [DOI] [PubMed] [Google Scholar]
- 14.Metzler, W.J., J. Bajorath, W. Fenderson, S.Y. Shaw, K.L. Constantine, J. Naemura, G. Leytze, R.J. Peach, T.B. Lavoie, L. Mueller, et al. 1997. Solution structure of human CTLA-4 and delineation of a CD80/CD86 binding site conserved in CD28. Nat. Struct. Biol. 4:527–531. [DOI] [PubMed] [Google Scholar]
- 15.Stamper, C.C., Y. Zhang, J.F. Tobin, D.V. Erbe, S. Ikemizu, S.J. Davis, M.L. Stahl, J. Seehra, W.S. Somers, and L. Mosyak. 2001. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 410:608–611. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz, J.C., X. Zhang, A.A. Fedorov, S.G. Nathenson, and S.C. Almo. 2001. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 410:604–608. [DOI] [PubMed] [Google Scholar]
- 17.Bajorath, J., W.J. Metzler, and P.S. Linsley. 1997. Molecular modeling of CD28 and three-dimensional analysis of residue conservation in the CD28/CD152 family. J. Mol. Graph. Model. 15:135–139, 108–111 [DOI] [PubMed] [Google Scholar]
- 18.Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. [DOI] [PubMed] [Google Scholar]
- 19.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 283:680–682. [DOI] [PubMed] [Google Scholar]
- 20.Huang, J., P.F. Lo, T. Zal, N.R. Gascoigne, B.A. Smith, S.D. Levin, and H.M. Grey. 2002. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc. Natl. Acad. Sci. USA. 99:9369–9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burack, W.R., K.H. Lee, A.D. Holdorf, M.L. Dustin, and A.S. Shaw. 2002. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J. Immunol. 169:2837–2841. [DOI] [PubMed] [Google Scholar]
- 22.Rudd, C.E. 1996. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 4:527–534. [DOI] [PubMed] [Google Scholar]
- 23.Holdorf, A.D., J.M. Green, S.D. Levin, M.F. Denny, D.B. Straus, V. Link, P.S. Changelian, P.M. Allen, and A.S. Shaw. 1999. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 190:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottemann, K.M., W. Xiao, Y.K. Shin, and D.E. Koshland, Jr. 1999. A piston model for transmembrane signaling of the aspartate receptor. Science. 285:1751–1754. [DOI] [PubMed] [Google Scholar]
- 25.Gordeliy, V.I., J. Labahn, R. Moukhametzianov, R. Efremov, J. Granzin, R. Schlesinger, G. Buldt, T. Savopol, A.J. Scheidig, J.P. Klare, et al. 2002. Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature. 419:484–487. [DOI] [PubMed] [Google Scholar]
- 26.Aivazian, D., and L.J. Stern. 2000. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat. Struct. Biol. 7:1023–1026. [DOI] [PubMed] [Google Scholar]
- 27.Harris, L.J., E. Skaletsky, and A. McPherson. 1998. Crystallographic structure of an intact IgG1 monoclonal antibody. J. Mol. Biol. 275:861–872. [DOI] [PubMed] [Google Scholar]