Abstract
Human thymic stromal lymphopoietin (TSLP) is a novel epithelial cell–derived cytokine, which induces dendritic cell (DC)-mediated CD4+ T cell responses with a proallergic phenotype. Although the participation of CD8+ T cells in allergic inflammation is well documented, their functional properties as well as the pathways leading to their generation remain poorly understood. Here, we show that TSLP-activated CD11c+ DCs potently activate and expand naive CD8+ T cells, and induce their differentiation into interleukin (IL)-5 and IL-13–producing effectors exhibiting poor cytolytic activity. Additional CD40L triggering of TSLP-activated DCs induced CD8+ T cells with potent cytolytic activity, producing large amounts of interferon (IFN)-γ, while retaining their capacity to produce IL-5 and IL-13. These data further support the role of TSLP as initial trigger of allergic T cell responses and suggest that CD40L-expressing cells may act in combination with TSLP to amplify and sustain pro-allergic responses and cause tissue damage by promoting the generation of IFN-γ–producing cytotoxic effectors.
Keywords: TSLP, CD11c+ DC, CD8+ T lymphocyte, allergy, cytotoxicity
Introduction
Thymic stromal lymphopoietin (TSLP) is a novel IL-7–like cytokine, cloned from a murine thymic stromal cell line (1). While mouse TSLP supports early B and T cell development, human TSLP (hTSLP) only activates CD11c+ dendritic cells (DCs), but not other antigen presenting cells, B, T, NK cells, neutrophils, nor mast cells (2, 3). This is in accordance with the coexpression of mRNA for the IL-7Rα chain and TSLPR by CD11c+ DCs but not other cell types (2). Unlike most DC activators such as CD40-ligand and ligands for Toll-like receptors (LPS, CPG-DNA, poly I:C), hTSLP does not induce DCs to produce proinflammatory cytokines (IL-1, IL-6, TNF-α, and IL-12), but TH2-attracting chemokines TARC and MDC (3). Interestingly, hTSLP-activated DCs prime CD4+ T helper cells to produce proallergic cytokines IL-4, IL-5, IL-13, and TNF-α, while down-regulating IL-10 and IFN-γ. hTSLP is highly expressed by epithelial cells of inflamed tonsils and keratinocytes of atopic dermatitis and its expression is associated with Langerhans cell migration and activation (3). hTSLP represents the first epithelial cell–derived cytokine that directly triggers DC-mediated TH2 allergic inflammation.
Allergic inflammation is the result of a complex immunological cascade involving the production of TH2 cyto-kines IL-4, IL-5, and IL-13, which leads to eosinophilia and IgE-production by B cells (4, 5). The production of these proallergic TH2 cytokines was initially ascribed to CD4+ T cells (6, 7). There is now broader evidence that CD8+ T cells, once regarded solely as potent cytotoxic effectors in antiviral and antitumoral immunity, are also able to secrete TH2 cytokines (8–10). Substantial numbers of CD8+ T cells participate in allergic inflammation (11, 12). However, the functional properties of such CD8+ T cells as well as the pathways leading to their generation remain poorly understood.
In this study we demonstrate that human TSLP primes DCs to induce naive CD8+ T cell activation and differentiation into IL-5– and IL-13–producing T cells. Additional CD40 ligand stimulation of TSLP-DCs induced the differentiation of CD8+ T cells into effectors that exhibit potent cytolytic activity and produce large amounts of IFN-γ, in addition to IL-5 and IL-13, two types of cyto-kines that were considered to be mutually exclusive (13). Our data suggest that TSLP-activated DCs play an important role in inducing both CD4+ and CD8+ T cell–mediated allergic inflammation.
Materials and Methods
DC Purification and Culture.
CD11c+ DCs were purified from buffy coats of HLA-A2–positive volunteer blood donors after separation of PBMCs by Ficoll-Hypaque centrifugation (Amersham Biosciences) and immunomagnetic depletion using mouse anti-CD3 (OKT3), anti-CD14 (RPA-M1), anti-CD19 (Leu-12), anti-CD56 (Leu-19), and anti-glycophorin-A (10F7MN) followed by magnetic beads coated with goat anti–mouse IgG (Dynabeads M-450; Dynal). Depleted cells were further stained with PE-Cy5–conjugated anti-CD4 (Caltag), PE-conjugated anti-CD11c and FITC-conjugated anti-CD3, CD14, CD16, and CD20 (all Becton Dickinson). FITC−CD11c+ DCs were isolated with a Vantage FACsorter™ (Becton Dickinson) to reach a purity >99% .
CD11c+ DCs were cultured immediately after sorting in RPMI containing 10% FCS, 1% pyruvate, 1% HEPES, and penicillin-streptomycin. Cells were seeded at 0.5 × 106/ml in flat-bottomed 96-well plates in the presence of medium alone, TSLP (15 ng/ml), CD40L-transfected L fibroblasts (2.5 × 104/well), or a combination of TSLP and CD40L-transfected L fibroblasts. DCs were harvested after 24 h of culture and stained with FITC-labeled anti-CD80 and anti-CD86 (Becton Dickinson) to assess their state of maturation.
Isolation of Naive CD8+ T Lymphocytes from Adult Blood.
Naive CD8+ T cells were enriched from PBMCs of HLA-A2–negative healthy donors by immunomagnetic depletion using a cocktail of mouse anti-CD4, anti-CD14, anti-CD16, anti-CD19, anti-HLA-DR, and anti-CD45RO mAb, followed by magnetic beads coated with goat anti–mouse IgG. These cells were stained with PE-Cy5–conjugated anti-CD45RA, PE-conjugated anti-CD27 (both BD Biosciences), and a cocktail of FITC-conjugated anti-CD4, anti-γδTCR, anti-CD14, anti-CD16, and anti-CD20 mAbs (Becton Dickinson). CD27+CD45RA+lineage− cells were isolated by fluorescence activated cell sorting and were >98% CD8+ T cells. CD8+CD27+CD45RA+ have been previously described as naive CD8+ T cells.
DC/CD8 T Cell Cocultures.
CD11c+ DCs were collected after 24 h of culture under different conditions, washed twice to remove any residual cytokine, and cultured with 5 × 104 freshly isolated naive CD8+ T cells in round-bottomed 96-well plates at a ratio of 1:5 DC:T cell. After 6 d, cultures were harvested and numbers of viable cells determined by trypan blue exclusion.
Analysis of Cytokine Production.
To test their capacity to secrete cytokines, harvested CD8+ T cells (106/ml) were restimulated with plate-bound anti-CD3 mAb (10 μg/ml) plus 1 μg/ml soluble anti-CD28 mAb for 24 h. IFN-γ, TNF-α, IL-4, IL-5, IL-13, and IL-10 in the culture supernatants were measured by Quantikine ELISA kits (R&D Systems).
Cytotoxicity Assay and Intracellular Perforin Expression.
Harvested CD8+ T cells were tested for the capacity to lyse 51Cr-labeled HLA-A2–positive EBV-B lymphoblastoid cell line JY. The HLA-A2–negative EBV-LCL RSB cell line (DNAX) was used as negative control target. The assay was performed by incubating 2,500 51Cr-labeled target cells per well with indicated numbers of effectors at 37°C for 5 h before collecting the supernatants and measuring 51Cr release with a gamma counter. We used Triton X to determine the maximum lysis, and target cells alone to determine the spontaneous lysis. The percentage lysis was calculated as follows: (spontaneous cpm − experimental cpm)/spontaneous cpm. For quantification of perforin expression by CD8 T cells, harvested cells were stained with PE-labeled anti-CD8, fixed with 2% formaldehyde (Sigma-Aldrich), and permeabilized with PBS supplemented with 2% FCS, 0.1% NaN3, and 0.5% saponin (Sigma-Aldrich). Cells were then stained with FITC-labeled anti-perforin mAb (for 20 min) and analyzed on a FACScan™ flow cytometer.
Results
TSLP-DCs Induce Strong CD8+ T Cell Expansion.
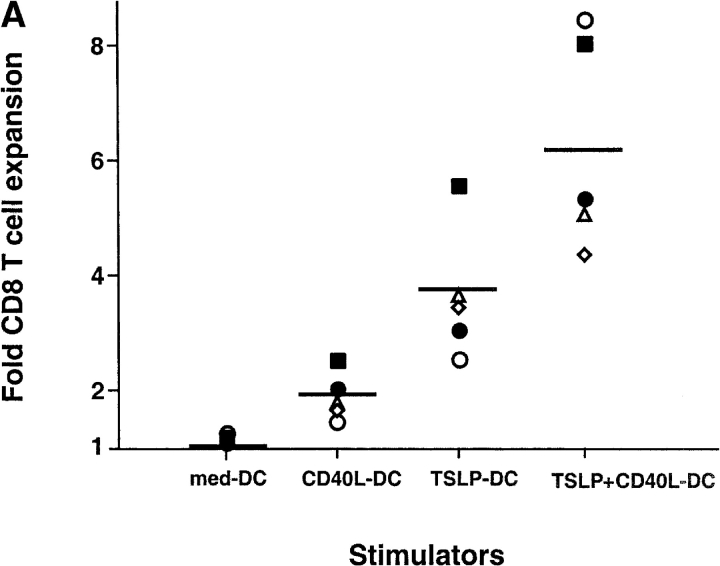
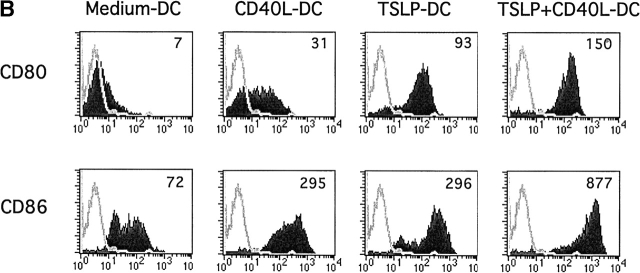
The capacity of DCs cultured with human TSLP (TSLP-DC) to activate and differentiate naive CD8+ T cells was determined in comparison to DCs cultured either with medium alone, CD40L (CD40L-DC), or with a combination of TSLP and CD40L (CD40L+TSLP-DC). Whereas DCs cultured with medium alone did not induce significant CD8+ T cell expansion, CD40L-DCs induced a 1.4 to 2.8-fold expansion of CD8+ T cells after 6 d of coculture (Fig. 1 A). TSLP-DCs induced a 2.8 to 5.6-fold increase in CD8+ T cell number, which was 2 times more than that induced by CD40L-DCs (Fig. 1 A). CD40L and TSLP had a strong synergistic effect in activating DCs, as shown by the very high levels of CD80 and CD86 expression (Fig. 1 B), and the ability to induce the strongest CD8+ T cell expansion (4.5 to 8.2-fold). Thus, TSLP, either alone or in combination with CD40L, represents a very potent DC activation factor with a marked capacity to induce DC-mediated activation and expansion of naive CD8+ T cells.
Figure 1.
CD11c+ DCs activated by TSLP alone or in combination with CD40L induce a strongly expansion of naive CD8+ T cells. (A) Naive CD8+ T cells were cultured for 6 d with CD11c+ DCs activated for 24 h with medium, CD40L, TSLP, or TSLP + CD40. TSLP-DCs induced stronger expansion of naive CD8+ T cells than CD40-L-DCs. The combination of TSLP and CD40L showed an additive effect on the capacity of activated DCs to induce CD8+ T cell expansion. Data represent five independent experiments; horizontal bars represent the median value. (B) Surface expression of CD80 and CD86 by medium-DCs, CD40L-DCs, TSLP-DCs, and TSLP+CD40L-DCs. TSLP-DCs showed significantly higher levels of CD80 than CD40L-DCs, TSLP+CD40L-DCs showed the highest levels of both CD80 and CD86. Filled histograms represent staining of costimulatory molecules CD80 and CD86; open histograms represent the isotype control. Numbers indicate the mean fluorescence intensity.
TSLP-DCs Prime CD8+ T Cells to Produce Large Amounts of IL-5 and IL-13.
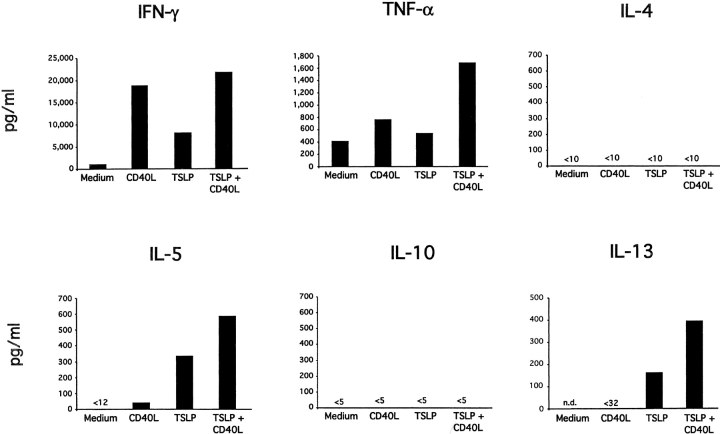
To determine the capacity of TSLP-DCs to polarize naive CD8+ T cells into cytokine-producing cells, DC-primed CD8+ T cells were restimulated for 24 h with anti-CD3 and anti-CD28 and cytokine production was measured in the culture supernatant by ELISA (Fig. 2) . CD8+ T cells primed with medium-DCs produced low levels of IFN-γ and TNF-α, and did not produce detectable amounts of IL-4, IL-5, or IL-10. CD8+ T cells primed with CD40L-DCs produced large amounts of IFN-γ and TNF-α, low amounts of IL-5 and undetectable amounts of IL-13, IL-4, or IL-10. Interestingly, CD8+ T cells primed by TSLP-DCs produced large amounts of IL-5 (up to 350 pg/ml) and IL-13 (up to 170 pg/ml), and moderate amounts of IFN-γ and TNF-α, but did not produce detectable amounts of IL-4 and IL-10. DCs activated by TSLP together with CD40L primed CD8+ T cells to produce the highest levels of IL-5, IL-13, TNF-α, and IFN-γ, but undetectable amounts of IL-4 and IL-10.
Figure 2.
TSLP-activated DCs induce IL-5 and IL-13–producing CD8+ T cells. Cytokine production by naive CD8+ T cells primed for 6 d with medium-DCs, CD40L-DCs, TSLP-DCs, or TSLP+CD40L-DCs. Harvested CD8+ T cells were restimulated for 24 h with anti-CD3 and anti-CD28 and IFN-γ, TNF-α, IL-4, IL-5, IL-10, and IL-13 were measured in the culture supernatant by ELISA. TSLP-DCs and TSLP+CD40L-DCs both induced IL-5 and IL-13 producing CD8+ T cells. However, in contrast to TSLP-DCs, TSLP+CD40L-DCs primed CD8+ T effector cells to produce high levels of IFN-γ. Data represent one of three independent experiments.
TSLP-DC Activated in the Presence of CD40L Induce Highly Cytotoxic CD8+ T Cells and Secrete IL-5, IL-13, and IFN-γ.
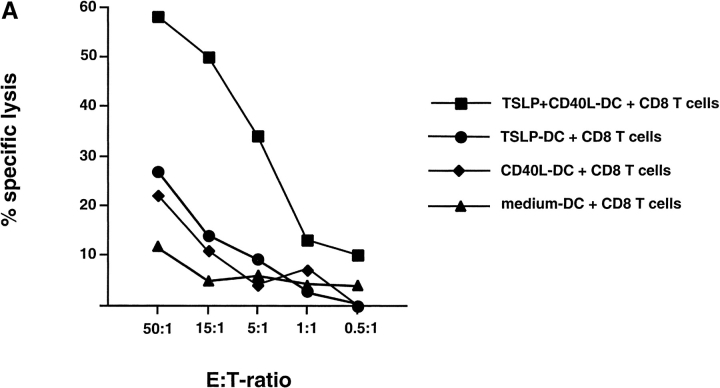
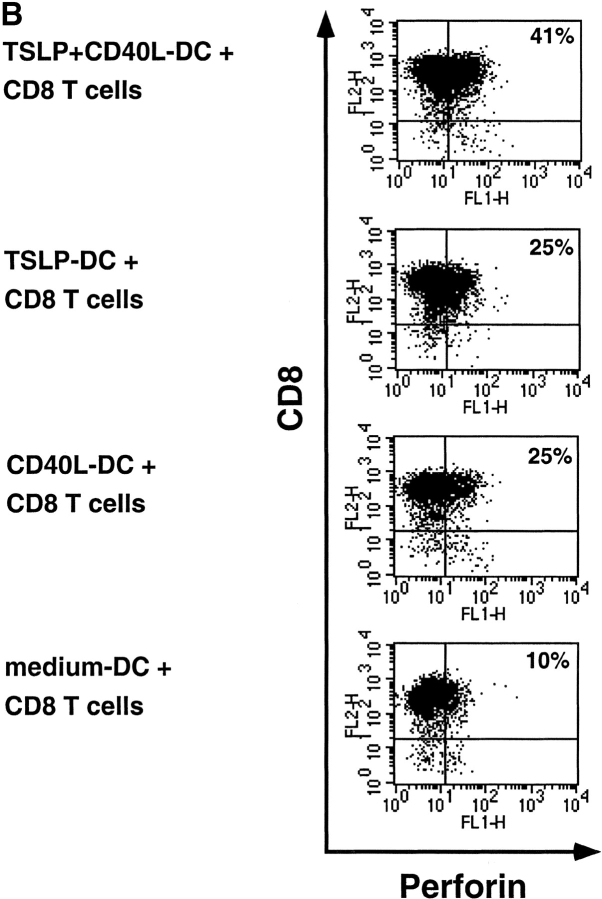
Next, we asked whether these polarized effector CD8+ T cells had characteristics of cytotoxic T cells and could kill allogeneic targets (Fig. 3 A). DCs cultured for 24h in medium alone did not induce cytotoxic CD8+ T cell activity (cytolytic activity was not significantly different from cytolytic activity against autologous targets). Low levels of cytotoxic activity were detected in both CD8+ T cells primed with CD40L-DC or TSLP-DCs (cytolytic activity detectable at the lowest ratio of 15 effector to 1 target cell). In contrast, CD40L-TSLP-DCs induced very potent of CD8+ cytotoxic T cells, detectable at ratios as low as 1 effector to 1 target (Fig. 3 A). The cytotoxic activity correlated with the intracellular perforin expression (Fig. 3 B).
Figure 3.
CD40L-TSLP-DCs induced the generation of CD8+ cytotoxic effector T cells. (A) Naive CD8+ T cells were primed for 6 d with medium-DCs (triangles), CD40L-DCs (diamonds), TSLP-DCs (circles), or TSLP+CD40L-DCs (squares), collected and tested for their ability to lyse allogeneic HLA-A2–positive target cells. Specific lysis of autologous HLA-A2–negative target cells remained below 15%. Whereas both TSLP-DCs and CD40L-DCs induced CD8+ T cells with poor cytolytic activity, TSLP+CD40L-DCs induced CD8+ T cells with potent cytolytic activity. Data represent one of three independent experiments. (B) Two color staining for intracellular perforin and surface CD8 of T cells primed with medium-DCs, CD40-L-DCs, TSLP-DCs, or TSLP+CD40-L-DCs for 6 d.
Discussion
In this study, we report that DCs activated by TSLP have a marked ability to expand naive CD8+ T cells and induce their differentiation into IL-5 and IL-13 secreting CD8+ T cells. Furthermore, we show that the addition of CD40L to the TSLP-mediated DC activation induced CD8+ T cell differentiation into potent cytolytic effectors secreting high levels of IFN-γ, while retaining their ability to produce high levels of IL-5 and IL-13.
The importance of CD8+ T cells in the pathogenesis and exacerbation of allergic diseases has been recently highlighted by the identification of allergen-specific CD8+ T cells (14). In addition, large numbers of CD8+ T cells secreting IL-5 and IL-13 have been observed in the inflammatory infiltrate of allergic asthma (15) and atopic dermatitis (12) as well as in the blood of patients with drug allergies (16). CD8+ T cell–derived IL-5 and IL-13 were shown to exert their proallergic functions by inducing eosinophilia and increased IgE production, both in-vitro and in-vivo (12, 17). Interestingly, IL-10, a cytokine also produced by Th2 clones, was shown to be decreased in allergic diseases (14, 18), and can suppress allergic inflammation (19, 20). In our study, CD8+ T cells induced by TSLP-activated DCs produced high levels of IL-5 and IL-13, but low levels of IL-10, thus exhibiting a typical proallergic cytokine profile. This parallels our data on CD4+ T cells, which can be instructed to produce high levels of proallergic cytokines but low levels of IL-10, when cultured with TSLP-activated DCs (3).
Allergic inflammation typically develops in two phases with a switch from the initial type 2 cytokine profile in acute lesions to increasing levels of IFN-γ and high numbers of cytolytic effectors in chronic lesions, while proallergic type 2 cytokines still remain at high levels (21–23). In atopic dermatitis, both the increased IFN-γ production as well as the presence of high numbers of cytotoxic T cells represent key pathogenic factors leading to epidermal injury and the formation of the eczematous lesion (24, 25). In allergic asthma, CD8+ T cell–derived IFN-γ has been linked to bronchial hyperresponsiveness (26, 27) and the number of lesional cytotoxic CD8+ T cells have been associated with the severity of the disease (28). Our data suggest that TSLP may represent the initial trigger of allergic inflammation, by instructing DCs to induce IL-5 and IL-13 production by CD8+ T cells. In a second step, CD40L triggering of TSLP-activated DCs could induce IFN-γ production and high cytotoxicity by CD8+ T cells, which would have characteristics of pathogenic T cells of the late phases of allergy. These IL-5, IL-13, and IFN-γ–producing cytolytic effectors might act to amplify and sustain the proallergic response, and to cause tissue damage. In this two-step process, an important question is the source of CD40L, in vivo. It is generally accepted that CD40L stimulation essentially comes from activated CD4+ T cells within secondary lymphoid organs, during the DC–T cell cross-talk (29). However, a number of CD40L-expressing cells are recruited to the site of allergic inflammation and could deliver a CD40 trigger to the TSLP-activated DCs, in situ. These include Ag-specific T helper cells, but also eosinophils and basophils, which express significant levels of CD40L (30).
Our study provides further evidence that human TSLP might represent a key early mediator of allergic inflammation. The central role of TSLP, which acts upstream of both CD4+ and CD8+ proallergic T cell responses, should encourage clinical studies with specific TSLP inhibitors in allergic diseases.
Acknowledgments
We thank J. Cupp for flow cytometry.
DNAX is supported by the Schering-Plough Corporation.
M. Gilliet and V. Soumelis contributed equally to this work.
References
- 1.Sims, J.E., D.E. Williams, P.J. Morrissey, K. Garka, D. Foxworthe, V. Price, S.L. Friend, A. Farr, M.A. Bedell, N.A. Jenkins, et al. 2000. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 192:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reche, P.A., V. Soumelis, D.M. Gorman, T. Clifford, M. Liu, M. Travis, S.M. Zurawski, J. Johnston, Y.J. Liu, H. Spits, et al. 2001. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167:336–343. [DOI] [PubMed] [Google Scholar]
- 3.Soumelis, V., P.A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673–680. [DOI] [PubMed] [Google Scholar]
- 4.Kay, A.B. 2001. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 344:30–37. [DOI] [PubMed] [Google Scholar]
- 5.Renauld, J.C. 2001. New insights into the role of cytokines in asthma. J. Clin. Pathol. 54:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Reijsen, F.C., C.A. Bruijnzeel-Koomen, F.S. Kalthoff, E. Maggi, S. Romagnani, J.K. Westland, and G.C. Mudde. 1992. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J. Allergy Clin. Immunol. 90:184–193. [DOI] [PubMed] [Google Scholar]
- 7.Robinson, D.S., Q. Hamid, S. Ying, A. Tsicopoulos, J. Barkans, A.M. Bentley, C. Corrigan, S.R. Durham, and A.B. Kay. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326:298–304. [DOI] [PubMed] [Google Scholar]
- 8.Salgame, P., J.S. Abrams, C. Clayberger, H. Goldstein, J. Convit, R.L. Modlin, and B.R. Bloom. 1991. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 254:279–282. [DOI] [PubMed] [Google Scholar]
- 9.Croft, M., L. Carter, S.L. Swain, and R.W. Dutton. 1994. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J. Exp. Med. 180:1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemeny, D.M., A. Noble, B.J. Holmes, and D. Diaz-Sanchez. 1994. Immune regulation: a new role for the CD8+ T cell. Immunol. Today. 15:107–110. [DOI] [PubMed] [Google Scholar]
- 11.Wahlstrom, J., B. Dahlen, E. Ihre, H. Wigzell, J. Grunewald, and A. Eklund. 1998. Selective CD8+ T cells accumulate in the lungs of patients with allergic asthma after allergen bronchoprovocation. Clin. Exp. Immunol. 112:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akdis, M., H.U. Simon, L. Weigl, O. Kreyden, K. Blaser, and C.A. Akdis. 1999. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J. Immunol. 163:466–475. [PubMed] [Google Scholar]
- 13.Mosmann, T.R., and R.L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. [DOI] [PubMed] [Google Scholar]
- 14.Seneviratne, S.L., L. Jones, A.S. King, A. Black, S. Pwell, A.J. McMichael, and G.S. Ogg. 2002. Allergen-specific CD8+ T cells and atopic disease. J. Clin. Invest. 110:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiota, Y., H. Arikita, N. Horita, J. Hiyama, T. Ono, and M. Yamakido. 2002. Intracellular IL-5 and T-lymphocyte subsets in atopic and nonatopic bronchial asthma. J. Allergy Clin. Immunol. 109:294–298. [DOI] [PubMed] [Google Scholar]
- 16.Mauri-Hellweg, D., F. Bettens, D. Mauri, C. Brander, T. Hunziker, and W.J. Pichler. 1995. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J. Immunol. 155:462–472. [PubMed] [Google Scholar]
- 17.Coyle, A.J., F. Erard, C. Bertrand, S. Walti, H. Pircher, and G. Le Gros. 1995. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J. Exp. Med. 181:1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borish, L., A. Aarons, J. Rumbyrt, P. Cvietusa, J. Negri, and S. Wenzel. 1996. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97:1288–1296. [DOI] [PubMed] [Google Scholar]
- 19.Grunig, G., D.B. Corry, M.W. Leach, B.W. Seymore, V.P. Kurup, and D.M. Rennick. 1997. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 185:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akbari, O., G.J. Freeman, E.H. Meyer, E.A. Greenfield, T.T. Chang, A.H. Sharpe, G. Berry, R.H. DeKruyff, and D.T. Umetsu. 2002. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 21.Werfel, T., A. Morita, M. Grewe, H. Renz, U. Wahn, J. Krutmann, and A. Kapp. 1996. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J. Invest. Dermatol. 107:871–876. [DOI] [PubMed] [Google Scholar]
- 22.Grewe, M., C.A. Bruijnzeel-Koomen, E. Schopf, T. Thepen, A.G. Langeveld-Wildschut, T. Ruzicka, and J. Krutmann. 1998. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today. 19:359–361. [DOI] [PubMed] [Google Scholar]
- 23.Thepen, T., E.G. Langeveld-Wildschut, I.C. Bihari, D.F. van Wichen, F.C. van Reijsen, G.C. Mudde, and C.A. Bruijnzeel-Koomen. 1996. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J. Allergy Clin. Immunol. 97:828–837. [DOI] [PubMed] [Google Scholar]
- 24.Carroll, J.M., T. Crompton, J.P. Seery, and F.M. Watt. 1997. Transgenic mice expressing IFN-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J. Invest. Dermatol. 108:412–422. [DOI] [PubMed] [Google Scholar]
- 25.Yawalkar, N., S. Schmid, L.R. Braathen, and W.J. Pichler. 2001. Perforin and granzyme B may contribute to skin inflammation in atopic dermatitis and psoriasis. Br. J. Dermatol. 144:1133–1139. [DOI] [PubMed] [Google Scholar]
- 26.Hessel, E.M., A.J. Van Oosterhout, I. Van Ark, B. Van Esch, G. Hofman, H. Van Loveren, H.F. Savelkoul, and F.P. Nijkamp. 1997. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am. J. Respir. Cell Mol. Biol. 16:325–334. [DOI] [PubMed] [Google Scholar]
- 27.Hamelmann, E., A. Oshiba, J. Paluh, K. Bradley, J. Loader, T.A. Potter, G.L. Larsen, and E.W. Gelfand. 1996. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a murine model of airway sensitization. J. Exp. Med. 183:1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan, S., L. Cormican, J.L. Faul, S. Ichinohe, S.L. Johnston, C.M. Burke, and L.W. Poulter. Activated, cytotoxic CD8+ T lymphocytes contribute to the pathology of asthma death. Am. J. Respir. Crit. Care Med. 164:560–564. [DOI] [PubMed]
- 29.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 30.Van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2–17. [DOI] [PubMed] [Google Scholar]