Abstract
Splenectomized and asplenic patients have a high incidence of infections by encapsulated bacteria and do not respond to polysaccharide vaccines. To understand whether the absence of the spleen is associated with a defined B cell defect, we analyzed B cell subsets in the peripheral blood. We found that a population of B cells known as immunoglobulin (Ig)M memory is lacking in patients without spleen. The absence of IgM memory B cells correlates with an impaired immune response to encapsulated bacteria not only in splenectomized patients, but also in individuals with an intact spleen. We show that the physiological and transient predisposition to pneumococcal infections of young children (0–2 yr) is associated with the lack of circulating IgM memory B cells and of serum antipolysaccharide IgM. We also demonstrate that IgM memory B cells are undetectable in a fraction of patients with common variable immunodeficiency, who have recurrent and invasive infections by encapsulated bacteria. IgM memory B cells, therefore, require the spleen for their generation and/or survival and are responsible for the protection against encapsulated bacteria.
Keywords: B cells, asplenia, polysaccharide vaccines, Streptococcus pneumoniae, infants
Introduction
Patients with anatomical or functional asplenia suffer from recurrent and invasive infections of the respiratory tract caused by encapsulated bacteria (mostly Streptococcus pneumoniae), which develop into overwhelming postsplenectomy infections (OPSI) in 5% of the adults and 20% of the children. OPSI is characterized by intravascular disseminated coagulation, septic shock, and multi-organ failure and is lethal in 50% of the adults and in almost all of the children (1). The incidence of congenital asplenia is very low and most children born without spleen die of infection during the first months of life (2). Vaccines containing bacterial capsular polysaccharides do not induce antibodies in splenectomized patients (3).
Infections with S. pneumoniae kill one million children each year worldwide (4). Before 2 yr of age, S. pneumoniae causes 50% of otitis media and most of pneumonia and meningitis. Polysaccharide vaccines are ineffective in infants but protective in older children (5).
Recurrent infections by encapsulated bacteria are the most common clinical manifestations in patients with common variable immunodeficiency (CVID), a heterogeneous immune disorder of unknown pathogenesis characterized by the inability to mount protective antibody responses in the presence of normal numbers of circulating B cells. Despite the profound hypogammaglobulinemia, a minority of the patients are asymptomatic (6).
We analyzed B cell subsets in three groups of individuals who have different medical conditions but share an increased susceptibility to infections by encapsulated bacteria, to answer the question of whether a high risk of infection is associated with the absence of a defined B cell pool. We found that in the absence of the spleen the so-called IgM memory B cells are undetectable in the blood. IgM memory B cells are also lacking in infants and CVID patients.
IgM memory B cells, therefore, need the splenic microenvironment for their generation and/or survival and are the B cell subset indispensable for the protection against encapsulated bacteria in humans.
Materials and Methods
Subjects.
Splenectomized and asplenic patients and healthy donors were recruited from the University Hospitals of Freiburg, Düsseldorf, Clermont-Ferrand, and from the Ospedale Pediatrico Bambino Gesù in Rome. Blood from 39 healthy infants and CVID patients was obtained from the University of Rome “La Sapienza” and the University of Brescia. All samples were collected with parental or patients' informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Flow Cytometry Analysis.
Mononuclear cells were stained with the appropriate antibody combinations of fluorescein, phycoerythrin, APC, cychrome, or biotin-labeled antibodies followed by streptavidin-cychrome and streptavidin-APC (Caltag). Monoclonal antibodies UCHT2 (anti-CD5), HIB19 (anti-CD19), HIB22 (anti-CD22), M-T271 (anti-CD27), G20-127 (anti-IgM), and IA6-2 (anti-IgD) were obtained from BD Biosciences and anti-IgM Fc5μ fragment specific was obtained from Jackson ImmunoResearch Laboratories.
Dead cells were excluded from analysis by side/forward scatter gating. All analyses were performed on a FACSCalibur® (Becton Dickinson) interfaced to a Macintosh CellQuest™ computer program.
Polysaccharides and Sera.
Pneumococcal antigens, types 3, 9, and 22, were obtained from the American Type Culture Collection and pneumococcal C polysaccharide was obtained from the Statens Seruminstitut. Hyperimmune sera from blood donors immunized with pneumococcal vaccine was used to establish the different polysaccharide serotype ELISAs. A standard serum from a vaccinated adult was included in every ELISA. ELISA procedures were previously described (7).
Results
B Cell Subsets in the Human Peripheral Blood.
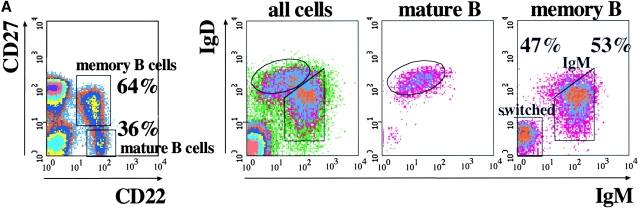
Two populations of B cells have been identified in human peripheral blood: mature and memory B cells. These populations can be distinguished by the combined use of CD22 and CD27. CD22 is a B cell marker (8) and CD27 is expressed on memory B and T cells (9). Mature B cells are CD27− and circulating memory B cells are CD27+ (Fig. 1 A, left). Based on the relative expression of IgM and IgD, memory B cells can be further subdivided into ∼50% IgM memory (IgMbright IgDdull) and 50% switched memory B cells (IgM− IgD−; Fig. 1 A).
Figure 1.
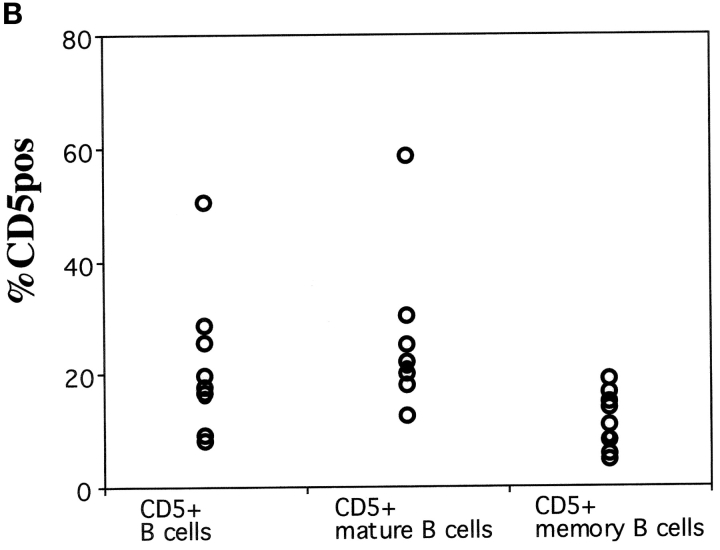
(A) Phenotypic analysis of B cells in the human peripheral blood. Donor PBLs were stained with Abs to CD22, CD27, IgM, and IgD and analyzed by four-color flow cytometry. Data is presented as density plots. CD22+ B cells were separated into CD27+ memory B cells (60%) and CD27− mature B cells (40%; left). In the right panels, IgD and IgM staining of all cells in the lymphocyte gate and of mature and memory B cells, identified as indicated in the left panel, is shown. Mature B cells are IgM+ IgDbright. Two populations of memory B cells can be identified, IgMbright IgDdull IgM memory B cells and switched memory B cells lacking the expression of both IgM and IgD. (B) Frequency of CD5+ B cells in the mature and memory compartments of eight donors. The expression of CD5 in IgM and switched memory B cells was calculated based on the staining for CD22, CD27, IgM, and CD5.
In the mouse the expression of CD5 identifies B-1a B cells (10). In humans, CD5+ B cells have also been described. We found that CD5+ cells represented a highly variable but minor fraction of both mature (from 12 to 58%) and memory B cell (4–19%) pools (Fig. 1 B). CD5, therefore, does not define a population of B cells in humans.
IgM Memory B Cells Are Reduced in the Blood of Splenectomized and Asplenic Patients.
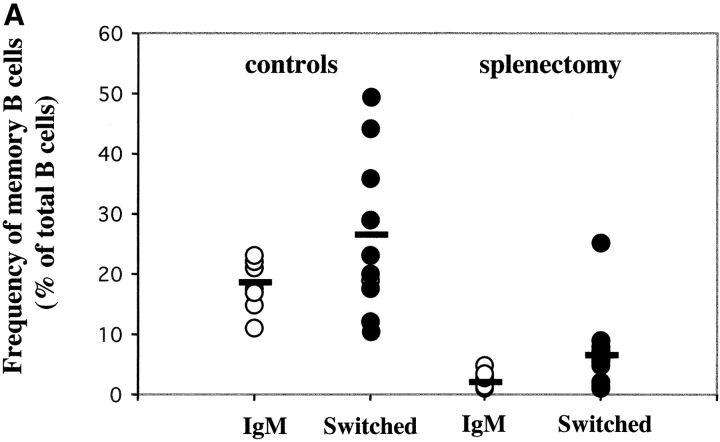
We compared the frequency of B cells in the blood of normal donors and of 14 patients splenectomized 6 mo to 9 yr before. There was a highly significant difference in the frequency of memory B cells (P < 0.001; Cochran-Cox t test) between the two groups. Memory B cells were 45.9% (SD = 12.5) of the B cells in the control group but were strongly reduced (7.1%, SD = 2.2) in the patients. IgM and switched memory B cells were equally reduced in all patients independently of the cause of splenectomy (Fig. 2 A). None of the patients suffered from ongoing bacterial infections or were under treatment at the time of analysis.
Figure 2.
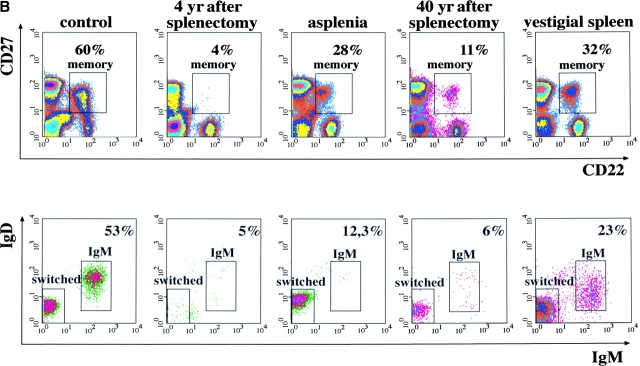
(A) Frequency of IgM and switched memory B cells in 11 controls and 14 splenectomized patients. (B) PBLs from the indicated subjects were stained and analyzed as described in Fig. 1 A. On the top, the expression of CD22 and CD27 is shown. The numbers indicate the frequency of memory B cells, calculated as a percentage of CD22+ B cells. On the bottom, IgM and switched memory B cells were identified as described in Fig. 1 A.
Congenital asplenia is a rare malformation associated to life-threatening infections with encapsulated bacteria (2). We compared peripheral blood B cells of a 62-yr-old woman with congenital asplenia (11) with a splenectomized patient with a normal control. The splenectomized patient had a low frequency of both IgM and switched memory B cells. In contrast, only IgM memory B cells were undetectable in the blood of the congenitally asplenic patient and switched memory B cells were present at normal frequency (Fig. 2 B). Because switched memory B cells were also found in a patient splenectomized 40 yr before, the spleen is indispensable for the generation or survival of IgM memory B cells whereas switched memory B cells can also develop in other lymphoid tissues.
The Presence of IgM Memory B Cells in the Blood Correlates with Protection from Pneumococcal Infections.
The asplenic patient had suffered from recurrent respiratory tract and middle ear infections all her life and was hospitalized for overwhelming septicemia caused by S. pneumoniae (11) 2 yr before our analysis. The patient splenectomized 40 yr before also had recurrent pneumococcal infections. The clinical history of these patients confirms the association between absence of the spleen and recurrent infections. The lack of IgM memory but not of switched memory B cells suggests a protective role of IgM memory B cells against pneumococcal infections.
We also analyzed the blood of a 56-yr-old patient thought to be asplenic because of thrombocytosis and presence of Howell-Jolly bodies (12) but without history of recurrent infections. In the peripheral blood, memory B cells were found. 77% of them were switched and only 23% were IgM memory (Fig. 2 B, vestigial spleen). Ectopic spleen has been described (13). In this patient, accurate studies revealed an ectopic vestigial spleen, which was not sufficient to fulfil its physiological function of removing Howell-Jolly bodies and sequestering platelets from the blood but efficiently supported the generation and/or survival of IgM memory B cells. The presence of these cells was correlated with the lack of recurrent and chronic infections in this patient.
IgM Memory B Cells Are Undetectable in Infants. Their Frequency Correlates with the Level of Antipneumococcal Polysaccharides IgM.
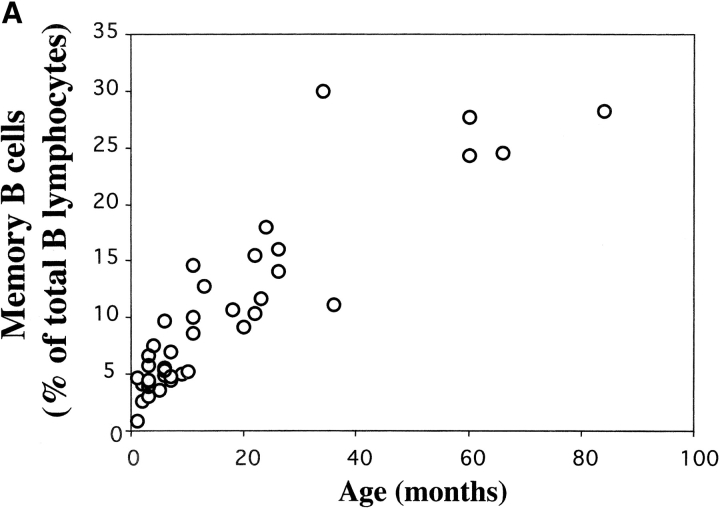
To confirm the association between IgM memory B cells and susceptibility to S. pneumoniae, we analyzed B cell populations in the peripheral blood of infants, which represent a group with a high risk of infection (4). We analyzed B cell populations in 39 healthy children of different ages (0–7 yr). We found that memory B cells were undetectable in cord blood, slowly increased during the first year of life, and reached 10–20% of the B cells at 2 yr. Higher frequencies were found in older children and adults (Fig. 3 A). In young children >99% of CD27+ B cells were IgM memory B cells.
Figure 3.
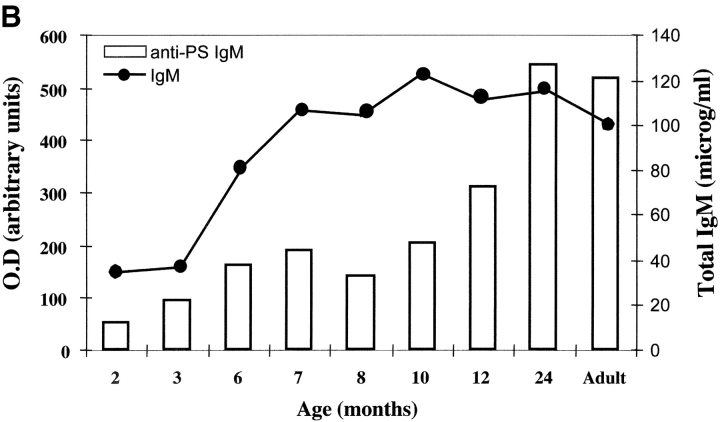
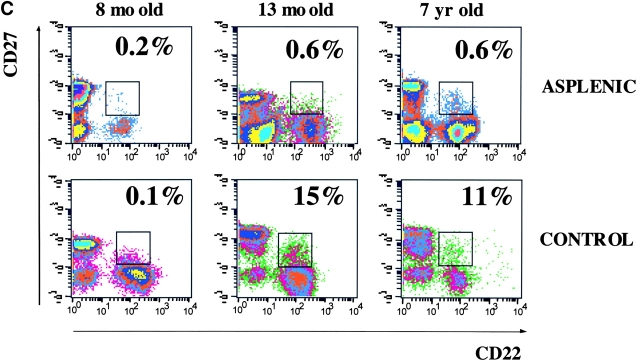
(A) Frequency of memory B cells in children. PBLs from 0–7-yr-old healthy children were stained and analyzed as described in Fig. 1 A. The frequency of CD22+ CD27+ memory B cells as a percentage of total CD22+ B cells is shown. The age in months is indicated on the x axis. (B) The level of total IgM in eight children of different ages and in one adult control were measured by ELISA and compared with the concentration of IgM specific for pneumococcal polysaccharide serotype 22. Total IgM is indicated by • (scale on the right y axis in micrograms/ml) and polysaccharide-specific IgM is indicated by the columns (scale in OD on the left y axis). The age in months is shown on the x axis. (C) Flow cytometric analysis of PBL from three asplenic and three control children. Samples were stained and analyzed as described in Fig. 1 A. The density plots show the CD22 and CD27 distribution. The frequency of memory B cells (as a percentage of total B cells) and the age of the children are indicated.
Natural antibodies make up most of the IgM in the serum and have the function to limit the growth and dissemination of pathogens during the early phases of infection and potentiate the acquired immune response (14). In the mouse they are produced by B-1a B cells (15).
We measured the levels of IgM binding pneumococcal polysaccharides of 3, 9, and 22 serotypes in the serum of children. Antibodies were undetectable at birth and progressively increased. There was a statistically significant correlation between the level of polysaccharide-binding serum IgM and age (P < 0.0001) and between the level of specific antibodies and IgM memory B cells in the blood (P < 0.0001). 2-yr-old children had the same amount of polysaccharide-specific antibodies as adults. Because adult levels of total IgM were reached earlier, by 7–8 mo of age (Fig. 3 B), polysaccharide-specific IgM is produced relatively late.
Our data shows that in infants the absence of IgM memory B cells is associated with a low level of polysaccharide-specific IgM. During the second year of life children have a reduced risk of pneumococcal infections and are able to respond to polysaccharide vaccines. At this time they have about half of the memory B cells of the adult and the same amount of polysaccharide-specific IgM.
The age-related development of IgM memory B cells does not occur in children with congenital asplenia, as shown by the analysis of three asplenic children (Fig. 3 C). A reduction of the memory B cell pool could be seen in the two older children. IgM memory B cells were undetectable and all CD27+ B cells were switched memory. In contrast, changes in the frequency of memory B cells were not evident in the youngest child because this population is also rare in healthy controls at this age. These data confirm the correlation between the presence of the spleen and IgM memory B cells in the blood.
IgM Memory B Cells in Asymptomatic CVID Patients.
Systemic respiratory infections by encapsulated bacteria are the most important clinical manifestation leading to permanent organ damage and eventually death in CVID (16). Surprisingly, despite the profound hypogammaglobulinemia, some CVID patients have no infections.
To investigate whether the different susceptibility to infections was correlated to a defined B cell defect, we analyzed the B cell compartment in patients with or without recurrent infections. All patients had a normal frequency of B cells and a functional spleen, but those with infections lacked IgM memory B cells whereas those who did not have infections had a normal frequency of IgM memory B cells (Table I). IgM memory B cells, therefore, are the B cell subset indispensable for the protection against encapsulated bacteria.
Table I.
Frequency of B Cells, Memory B Cells, and IgM Memory B Cells in CVID Patients
| Patients | Age(years) | PercentB cells | PercentCD27+
B cells |
PercentIgM memory | Infections byencapsulatedbacteria |
|---|---|---|---|---|---|
| 1 | 24 | 8.7 | 2.12 | undetectable | yes |
| 2 | 20 | 17.1 | 3.5 | undetectable | yes |
| 3 | 27 | 7.2 | 3.3 | undetectable | yes |
| 4 | 32 | 14.92 | 8 | 94.5 | no |
| 5a | 43 | 8.4 | 72.7 | 88.8 | no |
| 6a | 51 | 11.9 | 53 | 85.4 | no |
All patients were diagnosed as having CVID and received intravenous Ig treatment. Percent of B cells was calculated in the lymphocyte gate, percent of CD27+ B cells was calculated considering B cells as 100% positive, and percent of IgM memory B cells was calculated considering the CD27+ B cells as 100%.
These patients have a major T cell defect.
Discussion
We have recently shown that in mice the spleen is indispensable for the generation or survival of B-1a B cells (17). B-1a B cells are the major source of natural antibodies and rapidly produce Igs in response to encapsulated bacteria (15). Mice lacking B-1a B cells have a low level of natural IgM and do not produce antibodies in response to pneumococcal polysaccharide vaccines.
We now demonstrate that in humans the so-called IgM memory B cells have similar survival requirements and function as murine B-1a B cells. Similarly to B-1a B cells, IgM memory B cells cannot be detected in splenectomized and asplenic individuals and their absence is associated with an increased frequency of pneumococcal infections and impaired response to polysaccharide vaccines.
Memory B cells are classically described as highly specific and long-lived cells, generated in response to infectious agents or vaccines in the germinal centers after somatic mutation, selection, and class switch (18). They persist in the organism and rapidly produce antibodies upon a second challenge with the same pathogen.
In humans 30–60% of the B cells express CD27 and are considered memory B cells. Half of them are IgM memory and the rest are switched memory B cells. IgM memory B cells have few somatic mutations and switched memory B cells express highly mutated VH sequences (9).
Our data suggest that IgM memory B cells have different origins and functions from switched memory B cells. They are undetectable in splenectomized and asplenic patients. In contrast, switched memory B cells are only transiently depleted after splenectomy and are present at normal frequencies in asplenic patients. Because IgM memory B cells carrying somatic mutations can be detected in patients with X-linked HyperIgM syndrome, it has been suggested that only switched but not IgM memory B cells originate from the germinal centers (19). IgM memory B cells, therefore, may originate in the spleen, in extrafollicular sites, and independently of T cells. Because switched memory B cells only derive from the germinal centers, they represent the “true” memory pool.
Infections of the respiratory tract by encapsulated bacteria remain localized and might be asymptomatic in normal individuals. They are far more frequent, severe, and invasive in patients with anatomical or functional asplenia. The spleen has the function of entrapping circulating pathogens during systemic infections. Our data suggest that it also plays a fundamental role in preventing the spreading of localized infection by the generation of IgM memory B cells. This is supported by the observation that localized infections are more frequent and often develop into invasive diseases (e.g., pulmonitis, meningitis) in individuals lacking IgM memory B cells but with an intact spleen, such as young children and CVID patients. OPSI never occurs in these cases, underlining that splenic components other than B cells remove circulating bacteria and avoid systemic infections that become overwhelming.
The traditional vaccine against S. pneumoniae, which contains a mixture of capsular polysaccharides from the 23 most frequent bacterial serotypes, induces antibody production only if administered before splenectomy, but is ineffective if the spleen has been already removed (3). Similarly, it is not protective in children before 2 yr of age. Thus, IgM memory B cells produce antibodies in response to bacterial polysaccharides.
Based on our data, IgM memory B cells functionally resemble murine B-1a B cells but do not express CD5 and may have a different tissue distribution. The frequency of CD5+ B cells, which varies between donors, does not identify any B cell subset. Paradoxically, CD5+ B cells are particularly frequent in young children (unpublished data), the age group most susceptible to pneumococcal infections. Splenectomy had no effect on the frequency of CD5+ B cells. Although CD5 may also have a relevant function in humans, its expression cannot be used to identify human B-1a B cells. The great majority of B-1a B cells is in the mouse peritoneal cavity. We did not find IgM memory B cells in peritoneal exudates (unpublished data). This could be explained by differences in mouse and human anatomy, B cell recirculation, and pathogen tropism. Accordingly, it has been shown that CD5+ B cells cannot be found in the peritoneal cavity of rats, but rat fetal liver stem cells transplanted in mouse generate CD5+ B cells, which populate the peritoneal cavity (20).
We show that circulating IgM memory B cells mirror the presence of functional splenic tissue, albeit ectopic and of small size, associated with a low risk of infection and OPSI. Thus, detection of IgM memory B cells could be used to discriminate between patients with a high or low risk of infection after splenectomy and consequent degree of a patient's invalidism.
In children, polysaccharide protein conjugate vaccines against S. Pneumoniae reduce the risk of systemic infection by 90%. By eliciting a classical T-dependent immune response they bypass the immunological impairment due to the lack of IgM memory B cells. The administration of conjugate vaccines should therefore reduce the risk of systemic disease and most likely OPSI after splenectomy.
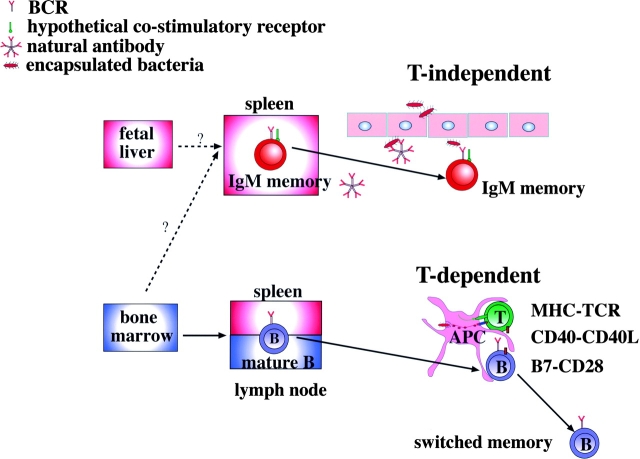
We propose a model in which IgM and switched memory B cells have different origins and functions. The spleen is indispensable to ensure the presence of circulating IgM memory B cells. It is not known whether the spleen supports IgM memory survival or is the site where IgM memory B cells are generated. B-1a B cells, which are functionally homologous to IgM memory B cells, derive from fetal liver precursors. IgM memory B cells produce natural antibodies and are necessary for the T-independent response against encapsulated bacteria. Mature B cells are generated in the bone marrow, populate spleen and lymph nodes, and in the germinal centers give rise to switched memory B cells (Fig. 4) .
Figure 4.
Our model on the origin and function of mature and IgM memory B cells. IgM memory B cells might be able to migrate to sites of inflammation and recognize invading pathogens through the B cell receptor (BCR) and possibly other pathogen-binding coreceptors. Switched memory B cells are generated from bone marrow–derived mature B cells after somatic mutation and class switch in the germinal centers.
Finally, because the first and most important sign of primary and secondary B cell immunodeficiency is a high incidence of infections by encapsulated bacteria, the defense against these pathogens might be the primary function of B cells and we show that this function is performed by IgM memory cells.
Acknowledgments
We thank Dr. P. Natali for critical reading of the manuscript.
M.M. Rosado was supported by an individual fellowship from Praxis XXI (Portugal) and S. Kruetzmann from the Hans-Hench-Foundation for Rheumatology. This work was supported in part by the European Union grant QLG1-CT-2001-01536 to A. Plebani and H.H. Peter.
S. Kruetzmann and M.M. Rosado contributed equally to this work.
References
- 1.Davidson, R.N., and R.A. Wall. 2001. Prevention and management of infections in patients without a spleen. Clin. Microbiol. Infect. 7:657–660. [DOI] [PubMed] [Google Scholar]
- 2.Feder, H.M., Jr., and H.A. Pearson. 1999. Assessment of splenic function in familial asplenia. N. Engl. J. Med. 341:210–212. [DOI] [PubMed] [Google Scholar]
- 3.Hosea, S.W., C.G. Burch, E.J. Brown, R.A. Berg, and M.M. Frank. 1981. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1:804–807. [DOI] [PubMed] [Google Scholar]
- 4.Stansfield, S.K. 1987. Acute respiratory infections in the developing world: strategies for prevention, treatment and control. Pediatr. Infect. Dis. J. 6:622–629. [DOI] [PubMed] [Google Scholar]
- 5.Overturf, G.D. 2002. Pneumococcal vaccination of children. Semin. Pediatr. Infect. Dis. 13:155–164. [DOI] [PubMed] [Google Scholar]
- 6.Spickett, G.P., J. Farrant, M.E. North, J.G. Zhang, L. Morgan, and A.D. Webster. 1997. Common variable immunodeficiency: how many diseases? Immunol. Today. 18:325–328. [DOI] [PubMed] [Google Scholar]
- 7.Quinti, I., A. Velardi, S. Le Moli, E. Guerra, R. D'Amelio, P. Mastrantonio, M.F. Martelli, and F. Aiuti. 1990. Antibacterial polysaccharide antibody deficiency after allogeneic bone marrow transplantation. J. Clin. Immunol. 10:160–166. [DOI] [PubMed] [Google Scholar]
- 8.Nitschke, L., R. Carsetti, B. Ocker, G. Kohler, and M.C. Lamers. 1997. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 7:133–143. [DOI] [PubMed] [Google Scholar]
- 9.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+ IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa, K., and R.R. Hardy. 2000. Development and function of B-1 cells. Curr. Opin. Immunol. 12:346–353. [DOI] [PubMed] [Google Scholar]
- 11.Germing, U., C. Perings, S. Steiner, A.J. Peters, M.P. Heintzen, and C. Aul. 1999. Congenital asplenia detected in a 60 year old patient with septicemia. Eur. J. Med. Res. 4:283–285. [PubMed] [Google Scholar]
- 12.Chanet, V., O. Tournilhac, V. Dieu-Bellamy, N. Boiret, P. Spitz, O. Baud, C. Darcha, P. Travade, and H. Laurichesse. 2000. Isolated spleen agenesis: a rare cause of thrombocytosis mimicking essential thrombocythemia. Haematologica. 85:1211–1213. [PubMed] [Google Scholar]
- 13.Heller, R.E., and S.K. Fernbach. 2000. Two apparent suprarenal masses. Two cases in children: heterotaxy syndrome with spleen lying in suprarenal space and gastric duplication cyst lying in suprarenal space. Pediatr. Radiol. 30:400–403. [DOI] [PubMed] [Google Scholar]
- 14.Ochsenbein, A.F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R.M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159. [DOI] [PubMed] [Google Scholar]
- 15.Forster, I., and K. Rajewsky. 1987. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 17:521–528. [DOI] [PubMed] [Google Scholar]
- 16.Ochs, H.D., S.H. Fischer, M.L. Lee, E.S. Delson, H.S. Kingdon, and R.J. Wedgwood. 1986. Intravenous immunoglobulin home treatment for patients with primary immunodeficiency diseases. Lancet. 1:610–611. [DOI] [PubMed] [Google Scholar]
- 17.Wardemann, H., T. Boehm, N. Dear, and R. Carsetti. 2002. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, Y.J., F. Malisan, O. de Bouteiller, C. Guret, S. Lebecque, J. Banchereau, F.C. Mills, E.E. Max, and H. Martinez-Valdez. 1996. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 4:241–250. [DOI] [PubMed] [Google Scholar]
- 19.Weller, S., A. Faili, C. Garcia, M.C. Braun, F.F. Le Deist, G.G. de Saint Basile, O. Hermine, A. Fischer, C.A. Reynaud, and J.C. Weill. 2001. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA. 98:1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deenen, G.J., P.M. Dammers, T. de Boer, and F.G. Kroese. 1997. Identification of a novel rat B cell subset in the peritoneal cavity of xenogeneic rat to mouse SCID chimeras. Transplant. Proc. 29:1752–1753. [DOI] [PubMed] [Google Scholar]