Abstract
In Paracoccus denitrificans, electrons pass from the membrane-bound cytochrome bc1 complex to the periplasmic nitrite reductase, cytochrome cd1. The periplasmic protein cytochrome c550 has often been implicated in this electron transfer, but its absence, as a consequence of mutation, has previously been shown to result in almost no attenuation in the ability of the nitrite reductase to function in intact cells. Here, the hypothesis that cytochrome c550 and pseudoazurin are alternative electron carriers from the cytochrome bc1 complex to the nitrite reductase was tested by construction of mutants of P. denitrificans that are deficient in either pseudoazurin or both pseudoazurin and cytochrome c550. The latter organism, but not the former (which is almost indistinguishable in this respect from the wild type), grows poorly under anaerobic conditions with nitrate as an added electron acceptor and accumulates nitrite in the medium. Growth under aerobic conditions with either succinate or methanol as the carbon source is not significantly affected in mutants lacking either pseudoazurin or cytochrome c550 or both these proteins. We concluded that pseudoazurin and cytochrome c550 are the alternative electron mediator proteins between the cytochrome bc1 complex and the cytochrome cd1-type nitrite reductase. We also concluded that expression of pseudoazurin is mainly controlled by the transcriptional activator FnrP.
The electron transport system of the facultative organism Paracoccus denitrificans is one of the best-characterized electron transport systems of bacteria (6, 15). Many of its components are closely related to their counterparts in the mitochondrial respiratory chain. There is a good understanding of how nonmitochondrial features, such as oxidases that are alternatives to cytochrome aa3, along with the apparatus for catalyzing oxidation of C1 compounds or reduction of nitrate through to nitrogen, are connected to the underlying mitochondrion-type respiratory chain (6, 32). A key component in such connections is often considered to be cytochrome c550 (analogous to cytochrome c2 in photosynthetic bacteria). This protein has the same tertiary structure as mitochondrial cytochrome c and a similar reduction potential (32). Thus, it has been reasonable to propose that it is an electron acceptor from the cytochrome bc1 complex and therefore the electron donor to periplasmic components of the electron transport system that receive electrons via this complex. One such component is the cytochrome cd1-type nitrite reductase, with which cytochrome c550 has been shown to interact in vitro (27, 39, 42). However, if cytochrome c550 does indeed carry electrons from the cytochrome bc1 complex to this enzyme, it cannot be the only component of the electron transfer system capable of this reaction. This is because a mutant of P. denitrificans that lacks the gene (cycA) encoding cytochrome c550 is still able to reduce nitrite; indeed, no respiratory activity of P. denitrificans is lost as a result of interruption of the cycA gene (44). The role of the cytochrome bc1 complex in electron delivery to cytochrome cd1 has been established through studies with both inhibitors (2) and mutants specifically deficient in this complex (33). In the case of nitrite reduction, it was proposed by Moir and Ferguson (30) that the absence of cytochrome c550 was compensated for by another protein that was able to act as an electron donor to cytochrome cd1. On the basis of the finding that nitrite respiration was much more sensitive to the copper chelator diethyldithiocarbamate in the cytochrome c550-deficient mutant than in the wild type (30) and because cytochrome cd1 does not contain copper, it was suggested that the substitute protein might be pseudoazurin. The latter protein is a molecule with a single type I copper center per polypeptide chain and is a member of a family of Greek-key fold proteins that includes bacterial azurins and plant plastocyanins. However, another possible interpretation has been put forward on the basis of the observations made with diethyldithiocarbamate (24). A further indication of the importance of a copper protein in the absence of cytochrome c550 came from the observation that the mutant grew anaerobically much more poorly in growth media with low copper contents than did the wild-type organism (33, 45). Furthermore, the nitrite reductase exhibits activity in vitro with pseudoazurin as an electron donor (29). A critical and definitive test of the proposal that cytochrome c550 and pseudoazurin are alternative electron donors to cytochrome cd1-type nitrite reductase would be provided by an examination of the properties of a mutant of P. denitrificans that has interruptions in both the cytochrome c550 (cycA) and pseudoazurin (pazS) genes. This is the subject of the present paper.
MATERIALS AND METHODS
Bacterial strains and growth.
The bacterial strains and plasmids used in this study are described in Table 1. Escherichia coli strains were grown aerobically in Luria-Bertani medium at 37°C. P. denitrificans strains were routinely grown at 37°C in minimal succinate medium (9). Media used for anaerobic growth contained KNO3 at a concentration of 100 mM unless specified otherwise. Tests for aerobic growth of P. denitrificans strains on methanol or methylamine were performed by using the media described by Alefounder and Ferguson (1). Antibiotics were added as required at the following final concentrations: ampicillin, 100 μg ml−1; kanamycin, 25 μg ml−1; rifampin, 40 μg ml−1; spectinomycin, 25 μg ml−1; and streptomycin, 25 μg ml−1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Organism or plasmid | Characteristics | Reference or source |
|---|---|---|
| Paracoccus denitrificans Pd1222 | Spr Rifr, restriction deficient | 13 |
| Paracoccus denitrificans Pd21.31 | Pd1222 cycA | 44 |
| Paracoccus denitrificans Pd77.71 | Pd1222 nnr | 47 |
| Paracoccus denitrificans Pd29.31 | Pd1222 fnrP::Kmr | 47 |
| Paracoccus denitrificans Pd92.30 | Pd1222 fnrP::Kmrnnr | 47 |
| Paracoccus denitrificans DPΩNOSR | Pd1222 nosR::Smr | This study |
| Paracoccus denitrificans IP1013 | Pd1222 pazS::Kmr | This study |
| Paracoccus denitrificans IP1121 | Pd21.31 pazS::Kmr | This study |
| Escherichia coli XL1-Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac F′ [proAB+lacIqlacZΔM15 Tn10(tetr)] | 8 |
| Escherichia coli GM2163 | F′ dam-13::Tn9 dcm-6 hsdR2 leuB6 his-4 thi-1 ara-14 lacY1 galK2 galT22 xyl-5 mtl-1 rpsL136 tonA31 tsx-78 supE44 McrA− McrB− | 41 |
| Escherichia coli S17-1 | pro thi hsdR− M+recA Strr with integrated RP4 2-(tet::Mu) (kan::Tn7) | 41 |
| Escherichia coli NM554 | F−recA13 araD139 Δ(ara-leu) Δ(lac)X74 galU galK hsdR2 mcrB1 rpsL | 38 |
| pBluescript SK(+) | General cloning vector; Apr | Stratagene |
| pUC18 | General cloning vector; Apr | Amersham Pharmacia Biotech |
| pUC4K | Kmr cassette vector; Apr Kmr | Amersham Pharmacia Biotech |
| pWE16 | Cosmid cloning vector | Stratagene |
| pGRPd1 | Mobilizable suicide vector; Apr Spr/Smr | 44 |
| pHP45Ω | Spr/Smr Ω cassette vector; Apr Spr/Smr | 36 |
| pAR0181 | Mobilizable suicide vector; Kmr | 35 |
| pRK2013 | Helper plasmid, carries tra genes; Kmr | 16 |
| pBK11.paz | 1.9-kb EcoRI-SphI fragment containing the 5′ region of the P. denitrificans pseudoazurin gene, cloned in pBK11a | R. J. M. van Spanning, Vrije Universiteit, Amsterdam, The Netherlands |
| pIP0724 | EcoRI-SphI fragment from pBK11.paz excised with EcoRI-HindIII and cloned in pBluescript SK(+) | This study |
| pDP0725 | EcoRI-SphI fragment from pHP45Ω cloned into MunI-cut pIP0724 | This study |
| pDP0726 | nosR::Ω from pDP0725 ligated to EcoRI-cut pAR0181 | This study |
| cIP0727A | Approximately 50 kb of genomic DNA from P. denitrificans DPΩNOS cloned in pWE16 | This study |
| cIP0728 | cIP027A cut with EcoRI and self-ligated | This study |
| pIP0729 | XhoI-EcoRI fragment from cIP0728 cloned in pBluescript SK(+) | This study |
| pIP0730 | pIP0729 cut with SmaI and self-ligated | This study |
| pIP0731 | BamHI-Acc65I fragment from pIP0729 cloned BamHI-KpnI in pUC18 | This study |
| pIP0515 | pIP0731 cut with BclI and ligated to the BamHI-excised Kmr cassette from pUC4K | This study |
| pIP0516 | BamHI-EcoRI fragment from pIP0515 cloned in pGRPd1 | This study |
See reference 21.
General DNA manipulations.
General DNA manipulations were performed as described by Sambrook et al. (40). DNA sequencing was performed by the PNACL facility at the University of Leicester, Leicester, United Kingdom. Southern blotting was performed by using the digoxigenin labeling system (Boehringer Mannheim) according to the manufacturer's instructions.
Cloning and disruption of the pazS gene.
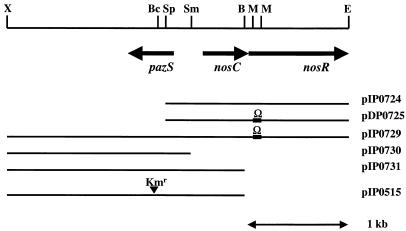
The P. denitrificans DNA fragment cloned in pBK11.paz was excised with EcoRI plus HindIII and cloned in EcoRI-HindIII-digested pBluescript SK(+) to generate pIP0724 (Fig. 1). For construction of DPΩnosR, pIP0724 was digested with MunI and ligated to the Ω cassette isolated from EcoRI-cut pHP45Ω. The resulting plasmid, pDP0725, was digested with EcoRI, and the DNA fragment containing the Ω-disrupted nosR gene was cloned in pARO181. The resulting plasmid, pDP0726, was transferred to P. denitrificans by triparental conjugation by using the helper plasmid pRK2013 (14). Smr Kms exconjugants were designated DPΩnosR. DNA contiguous with the introduced Ω cassette in DPΩnosR was isolated by cosmid cloning essentially as described by Viebrock and Zumft (48). DPΩnosR genomic DNA was digested with Sau3A to obtain a fragment length of approximately 50 kb and was ligated to BamHI-cut and dephosphorylated pWE16. Recombinant molecules were packaged into λ phage by using the Gigapack III XL system (Stratagene) and were transfected into E. coli NM554. Clones carrying the Ω cassette were selected as Apr Spr. One of the cosmids obtained, cIP0727A, was digested with EcoRI and self-ligated. Transformed E. coli cells were plated onto media containing spectinomycin in order to isolate clones carrying the Ω cassette. The resulting cosmid, cIP0728, consisted of a single EcoRI fragment of P. denitrificans DNA (approximately 30 kb) cloned in pWE16. Cosmid cIP0728 was digested with EcoRI plus XhoI, and the resulting restriction fragments were isolated and ligated to EcoRI-XhoI-digested pBluescript SK(+). Transformed E. coli cells were again plated onto media containing spectinomycin. This procedure yielded plasmid pIP0729, which contained the complete pazS gene together with nosC, the 5′ region of nosR, and the Ω cassette. pIP0729 was then digested with SmaI and self-ligated to obtain pIP0730, which contained pazS plus approximately 100 bp of upstream DNA and 1.4 kb of downstream DNA (Fig. 1).
FIG. 1.
Physical and restriction map of the P. denitrificans chromosomal DNA region containing the pazS gene. The map at the top is a restriction map. B, BamHI; Bc, BclI; E, EcoRI; M, MunI; Sm, SmaI; Sp, SphI; X, XhoI. The arrows indicate the positions of the pazS and nosC genes and the nosR 5′ region. The lines below the map are the P. denitrificans DNA fragments cloned in this study. Ω and Kmr indicate the positions of Ω (spectinomycin-streptomycin) and kanamycin resistance cassettes, respectively.
For pazS disruption, the pazS-containing BamHI-Acc65I fragment from pIP0729 was isolated and cloned in BamHI-KpnI-digested pUC18. The resulting plasmid, pIP0731, was digested with BclI and ligated to the Kmr cassette isolated from BamHI-cut pUC4K to produce pIP0515. This construct was digested with BamHI plus EcoRI, and the DNA fragment containing the disrupted pazS gene was ligated to BamHI-EcoRI-digested pGRPd1 to obtain pIP0516. This plasmid was transformed into E. coli S17-1 and transferred to P. denitrificans Pd1222 (wild type) and Pd2131 (cycA) by biparental mating (5).
Analytical methods.
Preparation of total soluble protein fractions from P. denitrificans strains, determination of protein contents, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting were performed as described previously (34). Pseudoazurin was polymerized with glutaraldehyde as described by Hennig and Neupert (20), and antibodies were raised in sheep by the Scottish Antibody Production Unit, Law Hospital, Carluke, Scotland. Nitrite accumulation in the medium of growing cultures was determined as described by Nicholas and Nason (31).
Nucleotide sequence accession number.
The P. denitrificans pazS sequence, together with approximately 1,500 flanking base pairs, has been deposited in the GenBank database under accession no. AF334183.
RESULTS
Cloning and sequencing of the P. denitrificans pseudoazurin structural gene, pazS.
The starting material for the cloning of pazS from P. denitrificans was a DNA fragment (pBK11.paz) that contained (GenBank accession no. AJ010260) 135 bp of the 5′ end of pazS (Fig. 1). This fragment had been found unexpectedly, oriented in the opposite direction, adjacent to the nosC and nosR genes in a cluster of nos genes that are required for formation of nitrous oxide reductase (N. F. W. Saunders and R. J. M. van Spanning, unpublished observations). Because previous attempts to clone pazS had proved to be problematic, the strategy adopted in the present work was to generate a marked mutation in the chromosomal nosR gene and then to isolate DNA containing the marker and the flanking region containing the full-length pazS gene. The cosmid-based cloning procedure leading to plasmid pIP0730 is described in Materials and Methods.
The protein sequence translated from the P. denitrificans pazS structural gene sequence was in exact agreement with that obtained by direct protein sequencing (25). These gene and protein sequences are very similar to their counterparts in the closely related organism (37) Paracoccus pantotrophus (11, 25). However, immediately beyond the structural gene region the DNA sequences of P. pantotrophus and P. denitrificans had considerable differences. At the 5′ end, these differences not only were apparent in the noncoding region but also reflected much variation in the periplasmic targeting sequences, although this variation was conservative (Fig. 2). Nevertheless, in both organisms the pazS gene clearly had an anaerobox of the Fnr type centered at bp 80.5 upstream of the translational initiation point and most likely at a similar position, bp 41.5, relative to the transcriptional start point, if the latter was assumed to be identically positioned in P. denitrificans and P. pantotrophus (6, 25) (Fig. 2).
FIG. 2.
Nucleotide sequences of the 5′ ends of the pazS genes of P. denitrificans and P. pantotrophus together with the amino acid sequences of the signal peptides and the N-terminal regions of the mature proteins. The plus sign indicates the experimentally determined transcriptional initiation site for P. pantotrophus pazS. The shaded area is a presumed binding site for the transcriptional activator FnrP. Asterisks indicate DNA sequence identity.
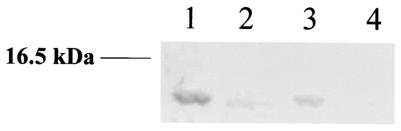
Two transcription factors, NNR and FnrP, bind differentially to anaeroboxes in P. denitrificans (46, 47). The synthesis of pseudoazurin by mutants of P. denitrificans deficient in either FnrP or NNR was therefore examined. The former protein, along with anaerobic conditions, could be shown to be essential for significant expression of pseudoazurin, as judged by immunoblotting (Fig. 3), whereas the presence of NNR appeared to be less significant for pazS expression. The attenuated expression in the NNR mutant may be explained by the poor growth of the cells under anaerobic conditions, a consequence of their inability to reduce nitrite or nitric oxide (46).
FIG. 3.
Pseudoazurin expression in P. dentrificans nnr and fnrP mutant strains. Proteins were separated by SDS-PAGE, and pseudoazurin was detected by immunoblotting. Lane 1, Pd1222 (wild type); lane 2, Pd29.31 (fnrP::Kmr); lane 3, Pd77.71 (nnr); lane 4, Pd92.30 (fnrP::Kmr nnr). The lanes contained approximately 20 μg of total protein. The position of a 16.5-kDa molecular mass marker is indicated on the left.
Targeted disruption of pazS in P. denitificans Pd1222 (wild type) and Pd21.31 (cycA).
The cloned pazS gene was disrupted by insertion of a kanamycin resistance cassette, and the disrupted gene was cloned in the mobilizable suicide vector pGRPd1. The resulting plasmid, pIP0516, was transferred by conjugation into P. denitrificans strains Pd1222 (wild type) and Pd21.31 (carrying an unmarked mutation in cycA). Kanamycin-resistant mutants of P. denitrificans, putatively carrying interruptions in either pazS alone or in pazS and cycA, were analyzed to identify mutants that had undergone the required double homologous recombination event leading to a genomic kanamycin-marked pazS gene. A single homologous recombination event would have resulted in both the marked pazS gene and the original intact pazS gene being present. There was no antibiotic resistance marker that could be used for this discrimination (the BamHI/EcoRI component of pGRPd1 present in pIP0516 did not contain the spectinomycin-streptomycin resistance locus), and so the presence of a large region of DNA originating from the vector pGRPd1 was used as a probe to detect the unwanted cells that had undergone only a single recombination event rather than the required double recombination event.
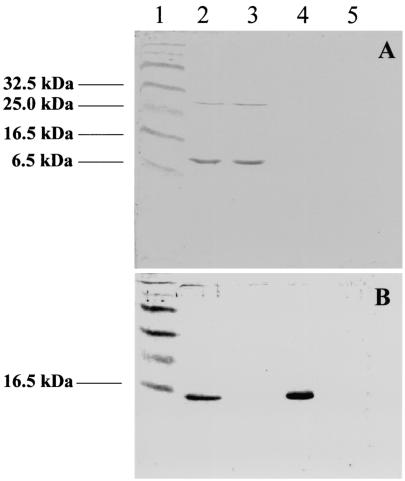
PvuII digests of genomic DNA from one in five putative pseudoazurin single mutants did not hybridize to the pGRPd1-specific probe, suggesting that each of these mutants was a product of a double recombination event. PvuII was used because there are no restriction sites for this enzyme within either the cloned pazS DNA or the kanamycin resistance cassette. The pazS gene was used as a second probe for the appearance in the P. denitrificans genome of a pazS gene interrupted by the kanamycin resistance cartridge. This proved to be problematic as only very weak probes could be generated in the present study when paz alone was labeled. Thus, we labeled pIP0731, which is a pUC18-derived construct containing a BamHI/Acc65I pazS fragment. Southern blotting with this probe showed that the putative mutants indeed had an interrupted pazS gene. One of these mutants, IP1013, was selected for further characterization. Immunoblotting (Fig. 4) confirmed the absence of the pseudoazurin protein from cells of this mutant after growth under anaerobic conditions.
FIG. 4.
Detection of pseudoazurin and cytochrome c550 in total soluble extracts from wild-type and mutant strains of P. denitrificans. Proteins were separated by SDS-PAGE, and pseudoazurin (A) or cytochrome c550 (B) was detected by immunoblotting. Lane 1, molecular weight markers; lane 2, Pd1222 (wild type); lane 3, Pd21.31 (cycA); lane 4, IP1013 (pazS::Kmr); lane 5, IP1121 (cycA pazS::Kmr). The lanes contained approximately 20 μg of total protein.
Coincidentally, one in five putative mutants with mutations in the pazS gene that resulted from a double recombination event were again isolated following conjugation of pIP0516 into P. denitrificans Pd21.31 (the cycA mutant). One of these mutants, designated IP1121, for which Southern blotting clearly demonstrated the absence of the vector pGRPd1 but the presence of the kanamycin insertion in pazS, was selected for further study.
The absence of pseudoazurin from the double cytochrome c550 and pseudoazurin mutant, IP1121, was further confirmed by Western blotting. It is clear (Fig. 4) that whereas wild-type cells or the strain with the unmarked mutation for cytochrome c550 contained pseudoazurin, both types of pazS mutants lacked this protein. It was noticed that the absence of cytochrome c550 did not result in an increase in the amount of pseudoazurin in the P. denitrificans Pd21.31 (cycA) cells. As expected, cytochrome c550 was still expressed in the single pazS mutant, but strikingly, it was expressed at a higher level than in the wild-type cells (Fig. 4), suggesting there was some compensatory effect of the absence of pseudoazurin on expression of this cytochrome.
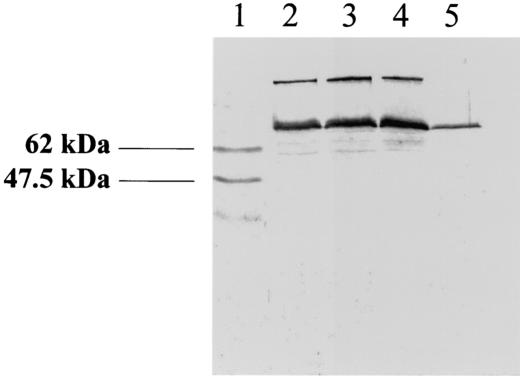
The construction of the pazS cycA double mutant was designed to show whether in vivo nitrite reductase activity was compromised. In this context it was important to show that loss of both pseudoazurin and cytochrome c550 from the cell had not caused a loss of cytochrome cd1 from the cell. The Western blot in Fig. 5 shows that the latter cytochrome was still present after anaerobic growth, but at lower levels than those observed for the wild type or the pazS and cycA single mutants. The explanation for this is that maximal transcription of the nirS gene, coding for cytochrome cd1-type nitrite reductase, requires production of the enzyme's reaction product, nitric oxide, in order to activate the transcription factor, NNR, that stimulates the expression of nirS. (46). Clearly, in a mutant unable to form nitric oxide the amount of cytochrome cd1-type nitrite reductase synthesized is considerably attenuated. Thus, the decrease in the amount of cytochrome cd1-type nitrite reductase as a consequence of the absence of both cytochrome c550 and pseudodazurin is itself evidence that these molecules are the two physiological electron donors.
FIG. 5.
Detection of cytochrome cd1 in total soluble extracts from wild-type and mutant strains of P. denitrificans. Proteins were separated by SDS-PAGE, and cytochrome cd1 was detected by immunoblotting. Lane 1, molecular weight markers; lane 2, Pd1222 (wild type); lane 3, Pd21.31 (cycA); lane 4, IP1013 (pazS::Kmr); lane 5, IP1121 (cycA pazS::Kmr). The lanes contained approximately 20 μg of total protein.
Growth characteristics.
It was not thought that aerobic growth on a heterotrophic carbon and free energy source should require either cytochrome c550 or pseudoazurin, because cytochrome c552 is a protein that accepts electrons from the cytochrome bc1 complex and delivers them to the cytochrome aa3 oxidase (33), while cytochrome ba3, which is a quinol oxidase, functions independent of either pseudoazurin or cytochrome c550 (15, 33). This expectation was confirmed by the finding that aerobic growth with succinate as a carbon and free energy source was not affected in any of the mutants. Thus, the stationary phase was reached after approximately 14 h with a turbidity (optical density at 650 nm [OD650]) between 2.5 and 3 for both wild-type cells and cells having mutations in the gene(s) encoding either cytochrome c550 or pseudoazurin or both.
Aerobic growth on methanol or methylamine is more dependent on the cytochrome bc1-dependent oxidase, cytochrome aa3, than growth on succinate is. In Methylotrophus methylophilus a pseudoazurin has been implicated in electron transfer to oxidases from the methylamine dehydrogenase (4). However, growth of P. denitrificans on either of these two one-carbon sources was also not affected by the absence of both pseudoazurin and cytochrome c550, implying that neither of these molecules has an obligatory role in this case, a conclusion for pseudoazurin that is consistent with the presence of the upstream anaerobox (Fig. 2).
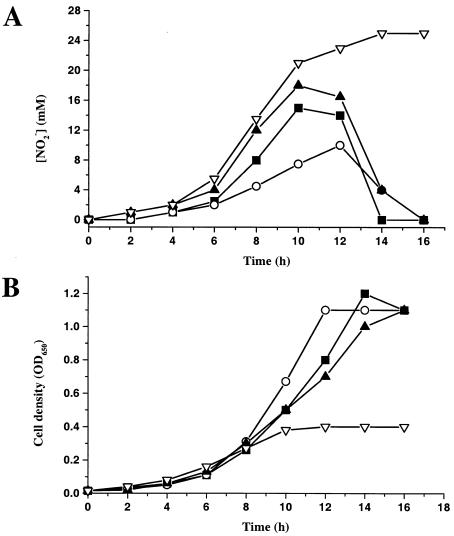
Growth under anaerobic conditions with nitrate as an added electron acceptor and succinate as a carbon and free energy source gave a different result. The double mutant (cycA pazS) grew to a lower final turbidity than the other organisms; nitrite accumulated and remained in the medium (Fig. 6). When both the cell density and the nitrite concentration in the medium were measured under these conditions, it was clear that the nitrite concentration in wild-type and single mutant cultures rose and then declined to zero (Fig. 6); gas production resulting from denitrification proceeding beyond nitrite could clearly be observed in the medium. In contrast, the double mutant cells converted nitrate to nitrite almost quantitatively (Fig. 6), and production of gas did not occur. The formation of 25 mM nitrite correlated with cessation of cell growth, which occurred at an OD650 of 0.4, compared with an OD650 of 1.2 for the wild-type and single mutants (Fig. 6). This was expected, because once all the added nitrate had been reduced to nitrite, there was no useable electron acceptor available in such anaerobic cultures (i.e., the nitrite could not be used owing to the double mutation, which in turn meant that nitric and nitrous oxides could not be generated for respiration). All types of cells took approximately 12 h, after any lag phases, to reach the final OD650, showing that the double mutant had a similar time course of growth but produced less biomass. These observations clearly imply that the double mutant was unable to reduce nitrite, in contrast to the wild-type strain and strains having a single mutation in either cycA or pazS.
FIG. 6.
Nitrite accumulation (A) and growth curves (B) for cultures of the P. denitrificans wild type and cycA, pazS, and cycA pazS mutants. The data are typical of data for three replicates for each strain; in each case variable lag phases following inoculation were omitted to permit comparisons between strains. For these experiments the concentration of nitrate initially present was lowered to 25 mM in order to facilitate measurement of the stoichiometry of nitrite accumulation; concentrations of accumulated nitrite higher than 25 mM are toxic. Symbols: ▪, wild type; ▴, pazS mutant; ○, cycA mutant; ▿, cycA pazS mutant.
The transient accumulation of nitrite (Fig. 6) in cultures of anaerobically grown wild-type P. denitrificans has been observed previously (7; Ferguson, unpublished observations) and is thought to reflect the later onset of nitrite reductase synthesis compared to the onset of nitrate reductase synthesis. The presence of nitrite at millimolar levels disrupts the pattern of growth away from the ideal behavior (33). In the wild-type and single mutant cells nitrite formation correlated with the early stage of growth following any lag phase, but why different strain-dependent nitrite concentrations were reached (Fig. 6) before the concentrations declined to zero is not clear. Thus, lower nitrite concentrations were attained in the media of the cycA mutant cells than in the media of either the wild type or the pazS mutant. This observation suggests that there may be subtly different consequences of the absence of either cytochrome c550 or pseudoazurin. The reason for this behavior is not clear but may be related to the requirement for nitric oxide formation to in turn stimulate the production of more nitrite reductase (see above). Thus, there may be a relationship between the rate of nitrite reduction in the early stages of growth and the subsequent formation of more nitrite reductase.
DISCUSSION
Whereas the absence of either pseudoazurin or cytochrome c550 has no major effect on anaerobic respiration of nitrite in P. denitrificans, this process is clearly severely compromised in the P. denitrificans mutant that lacks both these periplasmic electron transfer proteins. Thus, either cytochrome c550 or pseudoazurin is required for one, or most probably the only, step of the electron transfer pathway from the cytochrome bc1 complex to cytochrome cd1. This conclusion is in agreement with the recent report that addition of pseudoazurin stimulated the rate of electron transfer from succinate to nitrite in right-side-out vesicles, which must have retained externally bound cytochrome cd1-type nitrite reductase, from cytochrome c550-deficient P. denitrificans (23). A previous proposal (28) that one or more additional c-type cytochromes act as alternatives to cytochrome c550 in electron transfer to nitrite reductase is not supported by the present work. This conclusion is enhanced by the demonstration (33) that another c-type cytochrome, cytochrome c552, functions only in electron transfer from the cytochrome bc1 complex (or from amicyanin during growth on methylamine) to cytochrome aa3 oxidase. Cells of a P. denitrificans mutant deficient in cytochrome c552 denitrify as well as cells carrying a disrupted cytochrome c550 gene, but the former, unlike the latter, do not require copper in the growth medium for denitrification to occur. The implication is again that only a copper-containing protein can substitute for cytochrome c550 in nitrite reduction during denitrification. As might be predicted on this basis, a double deletion mutant with deletion of both cytochrome c550 and cytochrome c552 also requires copper in the medium for anaerobic growth on succinate plus nitrate (33). Cytochrome c552 is expressed constitutively, and its globular heme-containing domain has a structure very similar to that of cytochrome c550, although it possesses an acidic patch and overall has a less positive charge (18). Nevertheless, although it shares a capability with cytochrome c550 and (as implied by the present work) pseudoazurin to interact with the cytochrome bc1 complex, it seems to be specific as an electron donor to cytochrome aa3 (33). The acidic patch or the transmembrane anchor of cytochrome c552 may prevent its productive interaction with cytochrome cd1.
In an early in vitro study it was concluded that cytochrome c550, but not pseudoazurin, is the electron donor to cytochrome cd1-type nitrite reductase (26, 42). However, recent work with P. pantotrophus proteins has shown that pseudoazurin is an effective electron donor to Paracoccus cytochrome cd1, provided that the latter molecule is first activated by reduction (3, 39). Presumably, this explains why Martinkus et al. (26) observed little activity with pseudoazurin, in contrast to Moir et al. (29), who in retrospect fortuitously activated cytochrome cd1 before their assay. Since the presence of at least cytochrome c550 or pseudoazurin is required for in vivo nitrite respiration, any activator for this cytochrome cd1 must be a different protein for which the midpoint potential is less than 60 mV and thus able to change the inactive oxidized protein into the active reduced state (3, 22, 39).
One conclusion of the present work is that not only must pseudoazurin and cytochrome c550 be alternative electron donors to cytochrome cd1, but they must also be alternative acceptors from the cytochrome bc1 complex. This conclusion leads to the question of how two such structurally distinct proteins as pseudoazurin and cytochrome c550 can interact with the same reductant and oxidant. In the case of interaction with cytochrome cd1-type nitrite reductase, it has been proposed that pseudoazurin and cytochrome c550 each interact with a hydrophobic patch on the surface of the enzyme, with the reaction promoted by patches of positive charges on the cytochrome c550 or pseudoazurin and negative charges on cytochrome cd1 (50). There is at least one precedent for the cytochrome bc1 complex interacting with a copper protein. In the green sulfur bacterium Chloroflexus aurantiacus there is no evidence that there are water-soluble c-type cytochromes analogous to cytochrome c550, and the cytochrome bc1 complex is thought to use auracyanin, a copper protein related to pseudoazurin (43), as its electron acceptor. In thylakoids of both cyanobacteria and Arabidopsis, it is known that either cytochrome c6 or plastocyanin, which is related to pseudoazurin, is an alternative molecule for transfer of electrons from the cytochrome bf complex to photosystem I (12, 17). Negatively charged patches on cytochrome c6 or plastocyanin are thought to present a recognition feature to complementary charged regions on partner proteins. Thus, there are several good examples of an organism that is able to use a type I copper protein instead of a c-type cytochrome in an electron transfer step from membrane-bound electron transfer complexes that occur in both respiratory and photosynthetic systems.
It has been deduced that only cytochrome c551, and not azurin, could act as an electron donor to the cytochrome cd1-type nitrite reductase from Pseudomonas aeruginosa (49); this contrasts with the present work. There is much evidence which shows that azurin can function as an electron donor to the P. aeruginosa nitrite reductase in vitro, and thus the data are slightly surprising. The apparent failure of azurin to sustain substantial nitrite respiration in this organism in the absence of cytochrome c551 might be attributed to several factors. Among these factors could be a failure of azurin to accept electrons from the cytochrome bc1 complex. An explanation based on the possibility of a failure to express cytochrome cd1-type nitrite reductase in the absence of azurin was eliminated by Vijgenboom et al. (49). In fact, in P. aeruginosa it appears that denitrifying conditions do not promote the expression of azurin (49), and thus in this organism only one electron-carrying protein, cytochrome c551, can act between the cytochrome bc1 complex and the nitrite reductase. The situation in P. aeruginosa has been further complicated by the subsequent finding that a second c-type cytochrome, NirC, can also act in vitro as a donor to cytochrome cd1-type nitrite reductase (19), a result that contrasts with the data of Vijgenboom et al. (49).
The characteristic anaerobox sequence that is found upstream of the pazS gene in P. denitrificans could be a binding site for FnrP or NNR, two molecules which bind to similar sequences, although the context of these sequences must confer specificity (e.g., for NNR binding rather than FnrP binding upstream of nirS [47]) for one of the two transcription factors. The results in the present paper suggest that it is FnrP that is the more important of these transcription factors for activating transcription of pazS. Stimulation of pseudoazurin production by anaerobiosis signaled via FnrP rather than by NNR signaled by nitric oxide would be consistent with the finding (N. F. W. Saunders and S. J. Ferguson, unpublished data) that microanaerobic growth conditions stimulate production of not only the cytochrome c peroxidase, which is known to be under FnrP control (47), but also pseudoazurin. The −10 regions of the pazS genes of the two Paracoccus species have a CCTA sequence in common. The TA pair, in conjunction with the presence of an anaerobox at position −41.5, is diagnostic of the class II-type CAP family of promoters, in which the −10 region is likely to be dependent upon a σ70 factor (10).
Acknowledgments
This work was supported by the BBSRC through a studentship to I.P. and grants 43/B07079 and 43/B11988 to S.J.F.
We thank James Allen and Julie Stevens for their advice on the manuscript.
REFERENCES
- 1.Alefounder, P. R., and S. J. Ferguson. 1981. A periplasmic location for methanol dehydrogenase from Paracoccus denitrificans: implications for proton pumping in cytochrome aa3. Biochem. Biophys. Res. Commun. 98:778-784. [DOI] [PubMed] [Google Scholar]
- 2.Alefounder, P. R., J. E. G. McCarthy, and S. J. Ferguson. 1981. The basis of the control of nitrate reduction by oxygen in Paracoccus denitrificans. FEMS Microbiol. Lett. 112:321-326. [Google Scholar]
- 3.Allen, J. W. A., N. J. Watmough, and S. J. Ferguson. 2000. A switch in heme axial ligation prepares Paracoccus pantotrophus cytochrome cd1 for catalysis. Nat. Struct. Biol. 7:885-888. [DOI] [PubMed] [Google Scholar]
- 4.Ambler, R. P., and J. Tobari. 1985. The primary structures of Pseudomonas AM1 amicyanin and pseudoazurin. Biochem. J. 232:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagdasarian, M., R. Lürz, B. Rückert, F. C. H. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 6.Baker, S. C., S. J. Ferguson, B. Ludwig, M. D. Page, O.-M. H. Richter, and R. J. M. van Spanning. 1998. Molecular genetics of the genus Paracoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol. Mol. Biol. Rev. 62:1046-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauman, B., M. Snozzi, A. J. B. Zehnder, and J. R. van der Meer. 1996. Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic-anaerobic changes. J. Bacteriol. 178:4367-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. A high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 9.Burnell, J. N., P. John, and F. R. Whatley. 1975. The reversibility of active sulphate transport in membrane vesicles in Paracoccus denitrificans. Biochem. J. 150:527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busby, S., and R. Ebright. 1994. Promoter structure, promoter recognition and transcription activation in prokaryotes. Cell 79:743-746. [DOI] [PubMed] [Google Scholar]
- 11.Chan, C., A. C. Willis, C. V. Robinson, R. T. Aplin, S. E. Radford, and S. J. Ferguson. 1995. The complete amino acid sequence confirms the presence of pseudoazurin in Thiosphaera pantotropha. Biochem. J. 308:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De la Cerda, B., A. Diaz-Quintana, J. A. Navarro, M. Hervas, and M. de la Rosa. 2002. Site-directed mutagenesis of cytochrome c6 from Synechocystis sp PCC6803: the heme protein possesses a negatively charged area that may be isofunctional with the acidic patch of plastocyanin. J. Biol. Chem. 274:13292-13297. [DOI] [PubMed] [Google Scholar]
- 13.De Vries, G. E., N. Harms, J. Hoogendijk, and A. H. Stouthamer. 1989. Isolation and characterisation of Paracoccus denitrificans mutants with increased conjugation frequencies. Arch. Microbiol. 152:52-57. [Google Scholar]
- 14.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning systems for Gram-negative bacteria: construction of a clone bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson, S. J. 1988. The Paracoccus denitrificans electron transfer system: aspects of organization, structures and biogenesis. NATO Adv. Study Inst. Ser. C 512:77-88. [Google Scholar]
- 16.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function derived in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, R., Z. He, and S. Luan. 2002. Functional relationship of cytochrome c6 and plastocyanin in Arabidopsis. Nature 417:567-571. [DOI] [PubMed] [Google Scholar]
- 18.Harrenga, A., B. Reincke, H. Ruterjans, B. Ludwig, and H. Michel. 2000. Structure of the soluble domain of cytochrome c552 from Paracoccus denitrificans in the oxidized and reduced states. J. Mol. Biol. 295:667-678. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa, N., H. Arai, and Y. Igarashi. 2001. Two c-type cytochromes, NirM and NirC, encoded in the nir gene cluster of Pseudomonas aeruginosa act as electron donors for nitrite reductase. Biochem. Biophys. Res. Commun. 288:1223-1230. [DOI] [PubMed] [Google Scholar]
- 20.Hennig, B., and W. Neupert. 1983. Biogenesis of cytochrome c in Neurospora crassa. Methods Enzymol. 97:261-274. [DOI] [PubMed] [Google Scholar]
- 21.Kessler, B., V. de Lorenzo, and K. N. Timmis. 1992. A general system to integrate lacZ fusions into the chromosome of Gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studies with all controlling elements in monocopy. Mol. Gen. Genet. 233:293-301. [DOI] [PubMed] [Google Scholar]
- 22.Koppenhöfer, A., K. L. Turner, J. W. A. Allen, S. K. Chapman, and S. J. Ferguson. 2000. Cytochrome cd1 from Paracoccus pantotrophus exhibits kinetically gated, conformationally dependent, highly cooperative two-electron redox behavior. Biochemistry 39:4243-42499. [DOI] [PubMed] [Google Scholar]
- 23.Koutny, M., I. Kucera, R. Tesarik, J. Turnek, and R. J. M. van Spanning. 1999. Pseudoazurin mediates periplasmic electron flow in a mutant strain of Paracoccus denitrificans lacking cytochrome c550. FEBS Lett. 448:157-159. [DOI] [PubMed] [Google Scholar]
- 24.Kucera, I. 1996. On the use of diethyldithiocarbamate for detection of electron-transferring copper proteins in bacterial respiratory chains. FEMS Microbiol. Lett. 135:93-96. [Google Scholar]
- 25.Leung, Y.-C. C. Chan, J. S. Reader, A. C. Willis, R. J. M. van Spanning, S. J. Ferguson, and S. E. Radford. 1997. The pseudoazurin gene from Thiosphaera pantotropha: analysis of upstream putative regulatory sequences and overexpression in Escherichia coli. Biochem. J. 321:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinkus, K., P. J. Kennelly, J. Rea, and R. Timkovich. 1980. Purification and properties of Paracoccus denitrificans azurin. Arch. Biochem. Biophys. 199:465-472. [DOI] [PubMed] [Google Scholar]
- 27.Mat'chova, I., and I. Kucera. 1991. Evidence for the role of soluble cytochrome c in the dissimilatory reduction of nitrite and nitrous oxide by cells of Paracoccus denitrificans. Biochim. Biophys. Acta 1058:256-260. [DOI] [PubMed] [Google Scholar]
- 28.Mat'chova, I., I. Kucera, O. Janicek, R. J. M. van Spanning, and L. F. Oltmann. 1993. The existence of an alternative electron transfer pathway to the periplasmic nitrite reductase (cytochrome cd1) in P. denitrificans. Arch. Microbiol. 159:272-275. [Google Scholar]
- 29.Moir, J. W. B., D. Baratta, D. J. Richardson, and S. J. Ferguson. 1993. The purification of a cd1-type nitrite reductase from, and the absence of a copper-type nitrite reductase from, the aerobic denitrifier Thiosphaera pantotropha: the role of psuedoazurin as an electron donor. Eur. J. Biochem. 212:377-385. [DOI] [PubMed] [Google Scholar]
- 30.Moir, J. W. B., and S. J. Ferguson. 1994. Properties of a Paracoccus denitrificans mutant deleted in cytochrome c550 indicate that a copper protein can substitute for this cytochrome in electron transport to nitrite, nitric oxide and nitrous oxide. Microbiology 140:389-397. [Google Scholar]
- 31.Nicholas, D. J. D., and A. Nason. 1957. Detection of nitrate and nitrite. Methods Enzymol. 3:981-984. [Google Scholar]
- 32.Nicholls, D. G., and S. J. Ferguson. 2002. Bioenergetics 3. Academic Press, London, United Kingdom.
- 33.Otten, M. F., J. van der Oost, W. N. M. Reijnders, H. V. Westerhoff, B. Ludwig, and R. J. M. van Spanning. 2001. Cytochromes c550, c552, and c1 in the electron transport network of Paracoccus denitrificans: redundant or subtly different in function? J. Bacteriol. 183:7017-7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page, M. D., and S. J. Ferguson. 1990. Apo forms of cytochrome c550 and cytochrome cd1 are translocated to the periplasm of Paracoccus denitrificans in the absence of haem incorporation caused by either mutation or inhibition of haem synthesis. Mol. Microbiol. 4:1181-1192. [DOI] [PubMed] [Google Scholar]
- 35.Parke, D. 1990. Construction of mobilizable vectors derived from plasmids RP4, pUC18 and pUC19. Gene 93:135-137. [DOI] [PubMed] [Google Scholar]
- 36.Prentki, P., and H. M. Kirsch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 37.Rainey, F. A., D. P. Kelly, E. Stackebrandt, J. Burghardt, A. Hiraishi, Y. Katayama, and A. P. Wood. 1999. A re-evaluation of the taxonomy of Paracoccus denitrificans and a proposal for the creation of Paracoccus pantotrophus comb. nov. Int. J. Sys. Bacteriol. 49:645-651. [DOI] [PubMed] [Google Scholar]
- 38.Raleigh, E. A., N. E. Murray, H. Revel, R. M. Blumenthal, D. Westaway, A. D. Reith, P. W. J. Rigby, J. Elhai, and D. Hanahan. 1988. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 16:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter, C. D., J. W. A. Allen, C. W. Higham, A. Koppenhofer, R. Zajicek, N. J. Watmough, and S. J. Ferguson. 2002. Cytochrome cd1: reductive activation and kinetic analysis of a multifunctional respiratory enzyme. J. Biol. Chem. 277:3093-3100. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 42.Timkovich, R., R. Dhesi, K. J. Martinkus, M. K. Robinson, and T. M. Rea. 1982. Isolation of Paracoccus denitrificans cytochrome cd1: comparative kinetics with other nitrite reductases. Arch. Biochem. Biophys. 215:47-58. [DOI] [PubMed] [Google Scholar]
- 43.Van Driessche, G., W. Hu, G. van de Werken, F. Selvaraj, J. D. McManus, R. E Blankenship, and J. J. van Beeumen. 1999. Auracyanin A from the thermophilic gliding bacterium Chloroflexus aurantiacus represents an unusual class of small blue copper proteins. Protein Sci. 8:947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Spanning, R. J. M., C. W. Wansell, N. Harms, F. Oltmann, and A. H. Stouthamer. 1990. Mutagenesis of the gene encoding cytochrome c550 of Paracoccus denitrificans and analysis of the resulting physiological effects. J. Bacteriol. 172:986-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Spanning, R. J. M., A. P. N. de Boer, W. N. M. Reijnders, J. W. L. de Gier, C. O. Delorme, A. H. Stouthamer, H. V. Westerhoff, N. Harms, and J. van der Oost. 1995. Regulation of oxidative phosphorylation: the flexible respiratory network of Paracoccus denitrificans. J. Bioenerg. Biomembr. 27:499-512. [DOI] [PubMed] [Google Scholar]
- 46.Van Spanning, R. J. M., A. P. N. de Boer, W. N. M. Reijnders, S. Spiro, H. V. Westerhoff, A. H. Stouthamer, and J. van der Oost. 1995. Nitrite and nitric oxide reduction in Paracoccus denitrificans is under control of NNR, a regulatory protein that belongs to the FNR family of transcriptional activators. FEBS Lett. 360:151-154. [DOI] [PubMed] [Google Scholar]
- 47.Van Spanning, R. J. M., A. P. N. de Boer, W. N. M. Reijnders, H. V. Westerhoff, A. H. Stouthamer, and J. van der Oost. 1995. FnrP and NNR of Paracoccus denitrificans are both members of the FNR family of transcriptional activators but have distinct roles in respiratory adaptation in response to oxygen limitation. Mol. Microbiol. 23:893-907. [DOI] [PubMed] [Google Scholar]
- 48.Viebrock, A., and W. G. Zumft. 1987. Physical mapping of transposon Tn5 insertions defines a gene cluster functional in nitrous oxide respiration by Pseudomonas stutzeri. J. Bacteriol. 169:4577-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vijgenboom, E., J. E. Busch, and G. W. Canters. 1997. In vitro studies disprove the obligatory role of azurin in denitrification in Pseudomonas aeruginosa and show that azu expression is under the control of RpoS and ANR. Microbiology 143:2853-2863. [DOI] [PubMed] [Google Scholar]
- 50.Williams, P. A., V. Fulop, Y.-C. Leung, C. Chan, J. W. B. Moir, G. Howlett, S. J. Ferguson, S. E. Radford, and J. Hajdu. 1995. Pseudospecific docking surfaces on electron transfer proteins as illustrated by pseudoazurin, cytochrome c550 and cytochrome cd1. Nat. Struct. Biol. 2:975-982. [DOI] [PubMed] [Google Scholar]