Abstract
Natural killer T (NKT) cells are a unique immunoregulatory T cell population that is positively selected by CD1d-expressing thymocytes. Previous studies have shown that NKT cells exhibit autoreactivity, which raises the question of whether they are subject to negative selection. Here, we report that the addition of agonist glycolipid α-galactosylceramide (α-GalCer) to a fetal thymic organ culture (FTOC) induces a dose-dependent disappearance of NKT cells, suggesting that NKT cells are susceptible to negative selection. Overexpression of CD1d in transgenic (Tg) mice results in reduced numbers of NKT cells, and the residual NKT cells in CD1d-Tg mice exhibit both an altered Vβ usage and a reduced sensitivity to antigen. Furthermore, bone marrow (BM) chimeras between Tg and WT mice reveal that CD1d-expressing BM-derived dendritic cells, but not thymic epithelial cells, mediate the efficient negative selection of NKT cells. Thus, our data suggest that NKT cells developmentally undergo negative selection when engaged by high-avidity antigen or abundant self-antigen.
Keywords: NKT cells, CD1, negative selection, avidity model, lipid antigen
Introduction
Natural killer T cells (NKT cells)* are a unique T lymphocyte subset that coexpress NK receptors and TCRs (1, 2). Two main subsets of NKT cells have been identified: those positively selected by CD1d (the major subset), and those that are CD1d-independent (less than 20% in thymus and liver; references 3 and 4). The majority of CD1d-restricted NKT cells express a Vα14-Jα18 (Vα14+) TCR rearrangement preferentially associated with either Vβ8, Vβ7, or Vβ2 (5–8). These cells are either CD4+ or CD4−CD8− (DN) and respond to stimulation by a marine sponge-derived glycolipid, α-galactosylceramide (α-GalCer; reference 9). The most striking characteristic of CD1d-restricted NKT cells is their ability to promptly produce large amounts of cytokines, in particular IL-4 and IFN-γ, upon primary stimulation through the TCR complex (10). The rapid release of cytokines implies that NKT cells may play a critical role in modulating the upcoming immune responses. In support of this notion, several recent studies showed that NKT cells are involved in prevention of autoimmune disease (11–14) and protection against bacterial or parasitic infections (15–17).
The lineage relationship between NKT cells and conventional T cells is controversial. Several lines of evidence suggest that CD1d-restricted NKT cells represent a unique lineage of T cells. First, the positive selection of NKT cells is mediated by CD4+CD8+ double-positive (DP) thymocytes (18, 19), while thymic epithelial cells are responsible for positive selection of conventional T cells. Second, NKT cell development is less dependent than conventional T cells on signaling intermediates in the Ras pathway (20), but instead requires a functional tyrosine kinase, Fyn (21, 22). Third, several factors are exclusively required for the development of NKT cells but not conventional T cells, including preTCRα (23), Ets-1 (24), and membrane lymphotoxin (25, 26). However, recent data suggested that, like conventional T cells, NKT cells might be derived from a DP thymic subset. Once the rearrangement has occurred, the DP cells that express the canonical Vα14 gene are positively selected by CD1d molecules and develop into NKT cells (27). Using an intrathymic transfer approach, Benlagha et al. (28) and Pellicci et al. (29) further identified a NK1.1− thymic population as precursors of NK1.1+ NKT cells. As NKT cells might diverge from conventional T cells at the DP precursor stage, it is of interest to study whether any rules that govern the selection of conventional T cells can also apply to NKT cells and whether novel mechanisms might be employed for the selection/development of this unique subset of T cells.
For conventional T cells, a key factor in determining whether a DP thymocyte undergoes positive or negative selection is the avidity and affinity of the TCR–ligand interaction (30–32). T cells with low-to-intermediate avidity for self-peptide/MHC class I or class II are positively selected and differentiate into mature CD8+ or CD4+ T cells while other T cells with high avidity for self-peptide/MHC molecules are eliminated by negative selection. The negative selection is finely tuned to create a window of T cell avidity that will be devoid of dangerous (high avidity) autoreactive T cells, but will still allow survival of self-reactive (low avidity) T cells. Consistent with this notion, several studies have shown that modulating the avidity of thymocytes for ligand expressing cells in the thymus by altering TCR affinity, coreceptor expression, or ligand density influences the outcome of negative selection (30, 33–35). Recently, it has been found that populations of T lymphocytes, including CD4+CD25+ regulatory T cells (36), intraepithelial lymphocytes that express CD8αα (37), and some CD1d-independent NK1.1+ T cells (38–40) can be positively selected by self-agonists that negatively select conventional T cells. It has been proposed that CD1d-restricted NKT cells also are selected by self-agonists (2), leading to the question of whether NKT cells are indeed subject to negative selection. If this is the case, how might this process differ from the negative selection of conventional T cells? Here we have addressed these questions in vitro using an agonist for the NKT cell TCR, and in vivo by transgene-mediated increases in CD1d expression. The data obtained are consistent with an avidity-dependent negative selection process that is performed by dendritic cells (DCs).
Materials and Methods
Antibodies, CD1 Tetramers, and Glycolipids.
Antibodies used in this study include: FITC-conjugated mAbs specific for hamster IgG, I-Ab (M5114), CD4 (RM4–5), CD5 (53–7.3), CD11c (HL3), CD24 (HSA; M1/69), Ly49A (A1), TCRβ (H57–597), Vβ2 (B20.6), Vβ6 (RR4–7), Vβ7 (TR310), and Vβ8 (MR5–2); PE-conjugated mAbs specific for CD3ɛ (145–2C11), CD8α (53–6.7), and NK1.1 (PK136); Cy-Chrome-conjugated mAbs specific for TCRβ (H57–597), and CD4 (RM4–5; BD Biosciences). The CD1d-specific mAbs 5C6 (hamster IgG) has been described previously (41). The generation of CD1d/α-GalCer tetramer has been described previously (42). PE- and APC-conjugated tetramers were used in these experiments. α-GalCer and β-GalCer were kindly provided by Dr. Koezuka (Kirin Brewery, Gunma, Japan).
Mice.
C57BL/6 and RAG-deficient mice were purchased from The Jackson Laboratory. Generation of CD1d-deficient mice has been described previously (43). CD1d-deficient mice used in this study were backcrossed onto C57BL/6 background for 12 generations. All animal work was approved by the Institutional Animal Care and Use Committee of the University of Chicago and the La Jolla Institute for Allergy and Immunology.
Cell Preparations and FACS® Analysis.
Single cell suspensions from thymus and spleen were prepared by mechanical disruption in RPMI 1640 medium containing 10% FBS (HyClone), 2 mM l-glutamine, 20 mM HEPES, 50 μM 2-mercaptoethanol, nonessential amino acids, sodium pyruvate, penicillin, and streptomycin (RPMI-10). The lymphocytes from perfused liver were isolated according to the method described by Goossens et al. (44). Red blood cells from single cell suspensions were removed when necessary by hypotonic lysis before cell surface staining. Thymic stromal cell suspensions were prepared by digestion with 0.1% trypsin, 0.5 mM EDTA for 40 min at 37°C. Digestion was stopped by addition of immunofluorescence (IF) buffer (HBSS containing 2% FBS and 0.1% NaN3) buffer. After mechanical disruption of the lobe, cells were harvested and washed two times with IF buffer before cell surface staining experiments. Unless noted otherwise, cell culture reagents were purchased from Life Technologies. After staining with various combination of mAbs, cells were analyzed by flow cytometry using a FACSCaliber™ (Becton Dickinson) with CELL Quest™ or FlowJo software. Cell sorting was performed using a MoFlo cell sorter (Beckman Coulter) and were typically of >95% purity.
Generation of Kb-mCD1d1 Transgenic (CD1d Tg) Mice.
The full-length CD1d1 gene fragment was amplified from a CD1d1-containing plasmid (provided by Dr. Luc Van Kaer, Vanderbilt University) by PCR using the following primers: 5′-GGGGGACTAGTATGCGGTACCTACCATGGCTGTTGCTGT-GGG-3′, forward; 5′-GGGGGATCGATTCATCACCGGATGTCTTGATAAGCGCTTCTCC-3′, reverse. The chimeric gene was generated by ligating the 2.9 kb SpeI-ClaI CD1d1 fragment with a 2 kb NotI/SpeI fragment containing the promoter of the H-2K b gene, and a 0.2 kb ClaI–XhoI fragment containing bovine growth hormone poly-adenylation gene. The entire construct was cloned into the NotI and XhoI sites of the pBluescript KS (Stratagene). Microinjection of chimeric DNA fragment into the pronuclei of fertilized oocytes of (BALB/c × B6) F1 mice was performed by The Jackson Laboratory. Transgenic mice were identified by PCR analysis using primers specific for the H-2K b promoter (5′-GCCGCGGACGCTGGATATAAAGTC-3′) and the CD1d1 gene (5′-CCTGGGACCATGGCTTCGTGAAGC-3′). A line of CD1d Tg mice was established by backcrossing Tg founder mice with B6 mice for five to six generations. All subsequent screening for transgenic mice was done by PCR as described above. CD1d Tg mice and littermates were analyzed at the age of 10–16 wk.
Fetal Thymic Organ Cultures and Reaggregated Thymic Organ Culture.
Thymus lobes were harvested on E15-E16 and placed on Transwell filters (Costar) or nitrocellulose filters (Millipore). Thymuses were cultured in DMEM (Hyclone), supplemented with 20% FCS (Hyclone), glutamine, sodium pyruvate, nonessential amino acids, 2-β mercaptoethanol, and antibiotics, in the presence or absence of the indicated concentrations of glycolipids, at 37°C, 7% CO2 for 21 d unless otherwise indicated. Reaggregated thymic organ culture (RTOC) experiments were performed as described by Jenkinson et al. (45). Thymic stromal cells (TSCs) from B6 were prepared by disaggregating fetal thymic lobes previously cultured for 5 d in 1.35 mM deoxyguanosine (Sigma-Aldrich) using 0.05% trypsin and 0.02% EDTA. CD4+CD8+ (DP) thymocytes from WT and CD1d Tg mice were obtained by gently grinding freshly isolated newborn thymus lobes. The resulting suspensions were sorted for DP thymocytes using FITC-anti-CD4 and PE-anti-CD8. Reaggregates were formed by mixing together the desired TSC and thymocytes at 1:1 cell ratio (absolute number, 5 × 105 of each cell type). After pelleting the cells by centrifugation, the cell mixture was placed as a standing drop on the upper membrane surface, and incubated for 5 d at 37°C in RPMI 1640–10.
Cytokine Assay.
Thymocytes, splenocytes, or hepatic lymphocytes (5 × 105 cells/well) were stimulated either with 100 ng/ml of α-GalCer or with anti-CD3 (2C11) coated 96-well plate in a final volume of 200 μl of RPMI-10. After 48 h, the culture supernatants were harvested, and the levels of IL-4 and IFN-γ were quantitated by sandwich ELISA (BD Biosciences). To compare the affinity of NKT cells, equal numbers of tetramer-positive NKT cells (2 × 104 cells/well) from both WT and Tg+ splenocytes were stimulated with serial dilution of α-GalCer. After 48 h, the culture supernatants were harvested, and the levels of IL-4 and IFN-γ were quantitated by ELISA.
Adoptive Transfer.
Bone marrow (BM)-derived cells were depleted of T cells by anti-Thy-1.2 (J1j.10; American Type Culture Collection) coupled with rabbit complement (Hornby). Recipient mice received 980 rad (700 rad in the case of RAG KO mice) 4 h before injection with donor BM cells. A total of 107 cells were injected intravenously into recipient mice. In the case of mixed BM chimeras, the appropriate BM cells and DCs were mixed at the stated percentages and then injected. BM-derived DCs (BM-DCs) were propagated from BM progenitor in culture medium supplemented with 10 ng/ml of rmGM-CSF (R&D Systems) and 1 ng/ml of rmIL-4 (R&D Systems). Culture medium was renewed every other day. Maximal yield of DCs was obtained between days 6 and 8 of culture. 10 wk after adoptive transfer, splenocytes, and hepatic lymphocytes were collected and the numbers of NKT cells were monitored by flow cytometry as described above.
Statistical Analysis.
Mean values were compared using Student's t test for independent variables. Significant differences at 95% confidence are depicted with an asterisk * on each graph.
Results
Fetal Thymic Organ Culture with α-GalCer Suggests that NKT Cells Are Susceptible to Negative Selection.
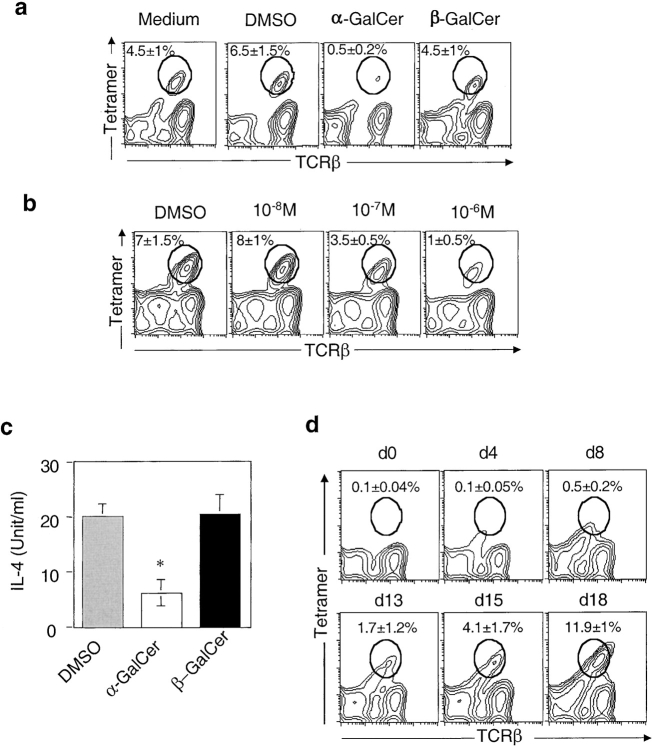
Conventional T cells undergo negative selection in the thymus to eliminate T cells bearing high-affinity TCR to self-peptide/MHC complexes. However, several NKT cell–derived hybridomas recognize CD1d in the absence of exogenous antigens. This apparent autoreactivity of NKT cells raises the question as to whether NKT cells also undergo negative selection in the thymus. To investigate this, we examined the effect of a potent NKT cell activator, α-GalCer, on NKT cell development in fetal thymic organ culture (FTOC). Fetal thymic lobes were harvested from gestational day 15 B6 mice and incubated either with α-GalCer, vehicle control (0.1% DMSO), or β-GalCer, which differs from α-GalCer only by the anomeric conformation of the sugar moiety (46). β-GalCer is not antigenic but binds to CD1d. After 18 d of culture, thymocytes were harvested and analyzed by flow cytometry. Fig. 1 a showed that no CD1d/α-GalCer tetramer-positive (tetramer+) cells could be detected in the thymic lobes cultivated in the presence of α-GalCer while ∼4–8% of tetramer+ cells were present in cultures with β-GalCer or DMSO. This effect is specific for NKT cells, as α-GalCer treatment had no effect on the number of CD4+CD8+, CD4+, and CD8+ thymocytes (unpublished data). The effect of α-GalCer is dose-dependent (Fig. 1 b), with intermediate levels of deletion obtained with a 10−7 M dose, suggesting an avidity-dependent mechanism. Consistent with the lower percentage of NKT cells, thymocytes isolated from α-GalCer–treated lobes produced lower amounts of IL-4 in response to stimulation with plate-bound anti-CD3 (Fig. 1 c).
Figure 1.
CD1d-restricted NKT cell development is decreased in α-GalCer-treated thymic lobes. (a) Thymic lobes were harvested from gestational day 15 B6 mice and incubated with solvent (0.1% DMSO), α-GalCer (10−6 M), or β-GalCer (10−6 M). After 18 d of culture, thymocytes were harvested and stained with FITC-anti-TCRβ and PE-CD1d/α-GalCer tetramers. The plots are representative of three experiments involving a total of 4 thymic lobes for each condition, with the percentage of tetramer+ cells indicated. No significant differences in the total number of thymocytes were observed between α-GalCer–treated lobes and controls. In addition, α-GalCer treatment did not affect the levels of CD1d surface expression. (b) Thymic lobes were incubated with α-GalCer at indicated concentrations, and the numbers of tetramer+ cells were analyzed by flow cytometry. Representative data from one of two experiments are shown. (c) Thymocytes from each treated group (n = 4) were pooled and stimulated with plate-bound anti-CD3 (2C11, 10 μg/ml) for 48 h. The amount of IL-4 secretion was determined by sandwich ELISA. (d) α-GalCer (10−6 M) was added to FTOC at different time points after the beginning of the culture. After 20 d, thymic lobes were harvested and analyzed by flow cytometry for the presence of tetramer+ cells. Representative data from one of two experiments are shown.
The activation of NKT cells by α-GalCer in vivo induces the rapid secretion of IFN-γ and IL-4 by hepatic and splenic NKT cells followed by their disappearance from these organs most likely due to activation-induced cell death (AICD) (42, 47, 48). Hence, the addition of α-GalCer into the FTOC could potentially induce AICD of mature NKT cells generated in the culture without having any effect on the selection of these cells. Although unlike cells in the liver, thymic NKT cells are not subject to AICD in vivo after α-GalCer injection (42, 49). To test for this possibility, α-GalCer was added to FTOC at different time points after the initiation of in vitro culture. After 20 d, thymic lobes were harvested and analyzed by flow cytometry for the presence of tetramer+ cells. As seen in Fig. 1 d, tetramer+ cells were readily detected in FTOC that received α-GalCer antigen 13 d after the beginning of culture. However, tetramer+ cells were absent when α-GalCer was added at earlier time points. Because tetramer+ cells are not detected before day 9 of the culture (unpublished data), this result suggests that the addition of α-GalCer early in the culture induces negative selection of developing NKT cells, not AICD of mature NKT cells. Taken together, these data suggest that developing CD1d-reactive NKT cells can undergo negative selection in the thymus.
Overexpression of CD1d Leads to Diminished Number of NKT Cells in CD1d Transgenic Mice.
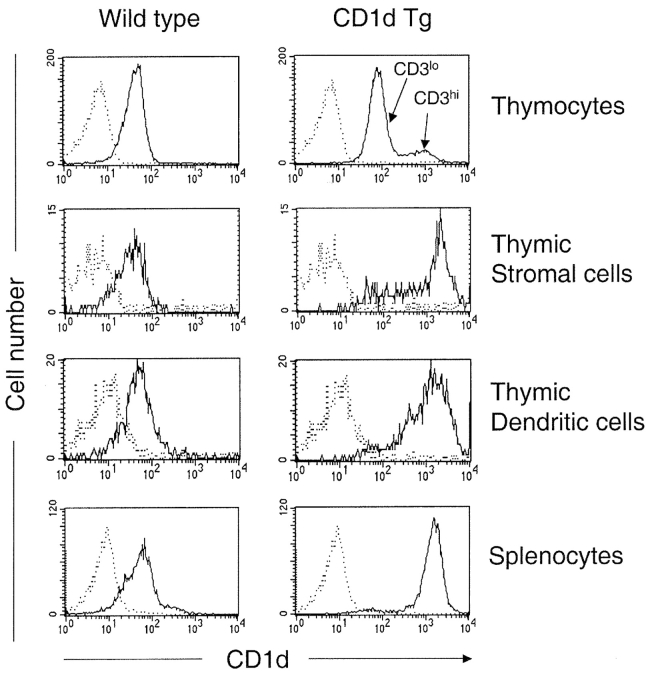
While the experiments described above demonstrate that NKT cells have the capability to undergo negative selection in organ cultures using a high affinity agonist, they do not demonstrate that this can occur in vivo with the glycolipids naturally presented by CD1d. Using TCR transgenic models, it has been shown that the density of MHC class I and class II expressed at the cell surface affects the outcome of thymic selection (50, 51). Limited diversity of the TCR repertoire of NKT cells allows us to study the effect of CD1d surface density on the NKT cell development in a non-TCR transgenic system. Toward this end, we have generated a CD1d transgenic mouse model, Kb-mCD1d1 Tg (CD1d Tg), which directs CD1d expression under the control of a classical MHC class I gene promoter. Fig. 2 shows that a high level of CD1d expression can be detected from a variety of cell types in CD1d Tg mice. The level of CD1d expression on Tg+CD3low thymocytes and Tg+CD3high thymocytes was increased approximately threefold and 8–10-fold, respectively. Thymic stromal cells (MHC class IIhigh) and thymic dendritic cells (DCs, CD11chi) express 20–40-fold higher levels of CD1d. Therefore, this animal model allows us to investigate whether increased levels of CD1d expression affect NKT cell development and which cell type(s) mediate the effect.
Figure 2.
CD1d expression in Kb-mCD1d1 transgenic (CD1d Tg) mice. Flow cytometric analysis of cell surface expression of CD1d in WT control and Tg mice. Cells were stained with FITC-anti-CD1d mAb (5C6) and PE-conjugated anti-CD3, anti-CD11c, and anti-I-Ab. Specific fluorescence profiles (thin line) obtained with anti-CD1d were overlayed onto background profiles (dotted line) obtained with isotype control Ab (hamster anti-TNP). Similar results were obtained with the other anti-CD1d mAb, 3H3 (unpublished data). Results are representative of two separate experiments.
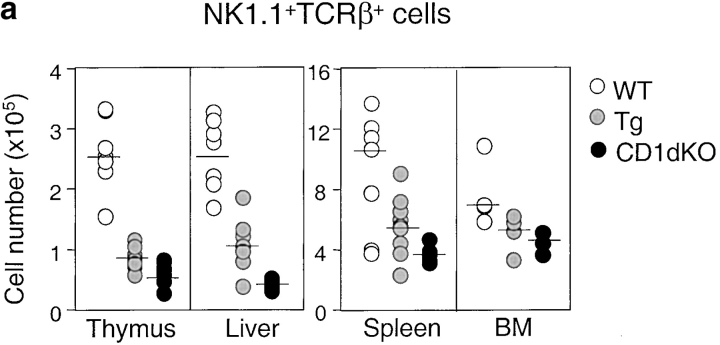
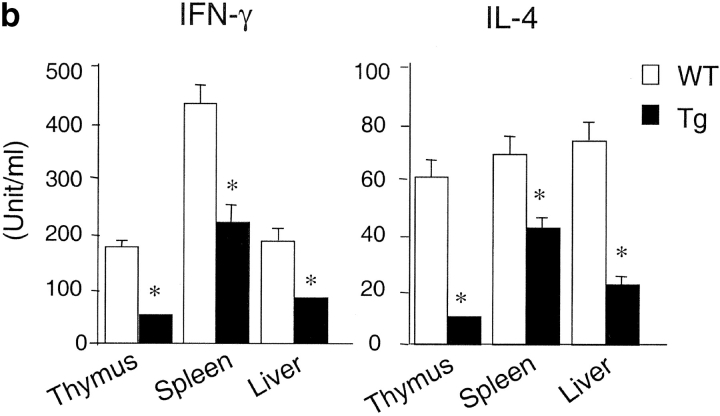
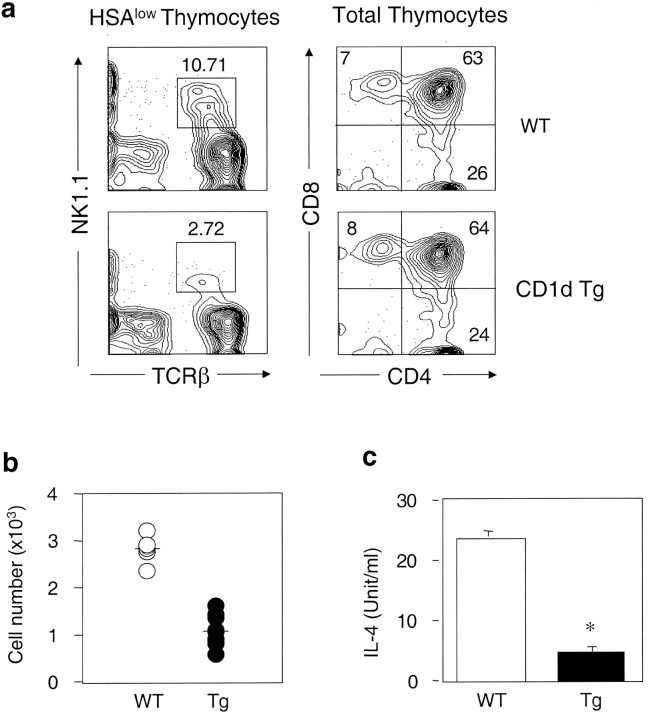
Although CD1d/α-GalCer tetramers react with the majority of invariant Vα14+ NKT cells, there are some CD1d-restricted NKT cells that do not recognize α-GalCer (42, 52, 53). Therefore, we used both TCR-β/NK1.1 and CD1d/α-GalCer tetramer staining to examine NKT cell development in the CD1d Tg mice. Compared with the nontransgenic littermate controls, the number of NK1.1+ T cells decreased significantly in the CD1d Tg mice. The reduction of the absolute number of NK1.1+ T cells in Tg mice was ∼50–65% in the thymus and liver, but was less severe in the spleen and bone marrow (∼30–40%). Notably, the number of NK1.1+ T cells is greater in Tg mice than that in CD1dKO mice, suggesting the presence of residual CD1d-restricted NK1.1+ T cells in Tg mice (Fig. 3 a). The amount of cytokine production in response to α-GalCer stimulation was also significantly diminished in Tg mice as compared with WT mice (Fig. 3 b), consistent with the notion of impaired development of CD1d-restricted NKT cells. Similar results were obtained when lymphocytes from WT (WT), Tg, and CD1dKO mice were stained with the CD1d/α-GalCer tetramers (Fig. 3 c). Calculation of the absolute number of NK1.1+ T cells and tetramer+ cells in Tg and control mice indicated that the loss of tetramer+ cells could account for almost all the decreased number of NK1.1+ T cells in Tg mice (Fig. 3 d). Thus, overexpression of CD1d might have a lesser effect on the tetramer− CD1d-reactive NKT cells.
Figure 3.
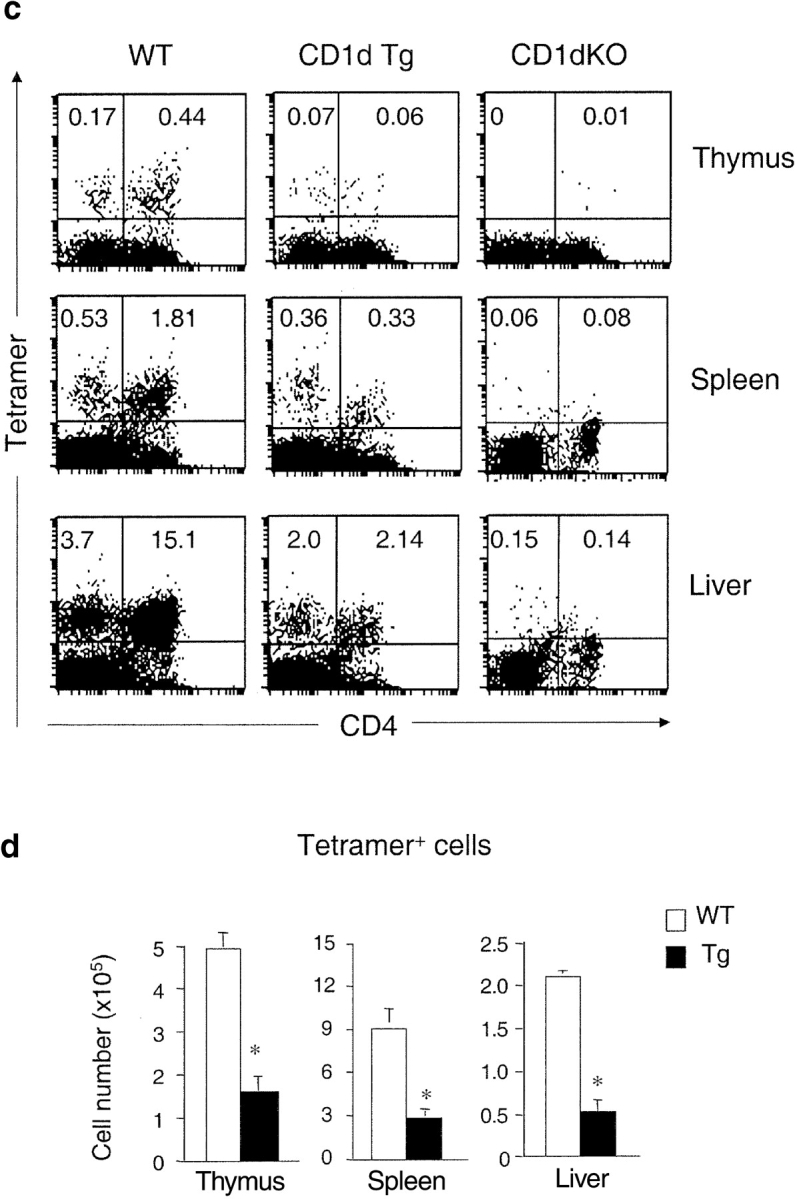
The number of NKT cells and cytokine production capacity are reduced in CD1d Tg mice. (a) Lymphocytes isolated from thymus, spleen, and liver of indicated mice were stained with FITC-anti-TCRβ and PE-anti-NK1.1 and analyzed by flow cytometry. The absolute number of NKT cells was calculated by percentage of NK1.1+ T cells x total number of cells. Circles represent number of NKT cells from indicated tissues, and the horizontal bar denotes the mean value. (b) Reduced cytokine production by NKT cells from Tg mice in response to α-GalCer. Lymphocytes from WT (n = 5) and Tg (n = 5) were cultured in the presence of α-GalCer for 48 h. IL-4 and IFN-γ levels in the supernatant were detected by ELISA. Lymphocytes from CD1dKO mice did not produce detectable amounts of cytokines upon α-GalCer stimulation. (c) The number of CD1d/α−GalCer tetramer+ cells is reduced in Tg mice. Lymphocytes from WT, Tg, and CD1dKO mice were stained with APC conjugated-CD1d/α-GalCer tetramers, FITC-anti-CD4, and PE-anti-CD8 and analyzed by flow cytometry. Results are representative of three experiments. (d) Compared with WT mice, the absolute number of CD1d/α-GalCer tetramer+ cells in thymus, spleen and liver of Tg mice are significantly diminished. Data shown represent mean ± SE of seven mice in each group.
We have not yet eliminated the possibility that overexpression of CD1d in Tg mice might result in peripheral deletion of high-affinity CD1d-restricted NKT cells. However, the diminished numbers of NKT cells are already apparent in the thymus of Tg mice. To demonstrate that thymic negative selection contributes to the observed changes of NKT cells in CD1d Tg mice, we performed FTOC from both Tg and WT mice. Compared with the WT controls, total NKT cells generated in FTOC of Tg thymic lobes were decreased about threefold (Fig. 4 , a and b), similar to the observed NKT cell reduction in the Tg adult mice. The overexpression of CD1d has no noticeable effect on the development of other thymocyte subsets in FTOC (Fig. 4 a). Stimulation with anti-CD3 resulted in lower amounts of IL-4 production from cells harvested from Tg thymic lobes as compared with WT lobes (Fig. 4 c). Collectively, these data suggest that high levels of CD1d expression leads to increased negative selection in Tg thymus, which may be largely responsible for the drastic reduction of NKT cells in Tg mice.
Figure 4.
Impaired NKT cell development in CD1d Tg fetal thymic lobes. (a) Thymic lobes from both Tg (n = 10) and littermate control mice (n = 5) were harvested on gestational day 16.5. After 10 d of culture, thymocytes were stained with either FITC-anti-HAS, CyChrome-anti-TCRβ, and PE-anti-NK1.1; or FITC-anti-CD4 and PE-anti-CD8α. The percentage of NKT cells in HSAlow gated population, and the percentage CD4+, CD8+, and DP thymocytes are shown from a representative experiment. (b) Decreased total number of NKT cells in Tg thymic lobes. Circles represent pairs of thymic lobes, and the horizontal bar denotes the mean value. (c) Thymocytes from Tg and control thymic lobes were pooled and stimulated with anti-CD3 (2C11, 10 μg/ml) for 48 h. The amount of IL-4 secretion was determined by sandwich ELISA.
Phenotypic and Functional Analysis of NKT Cells in CD1d Transgenic Mice.
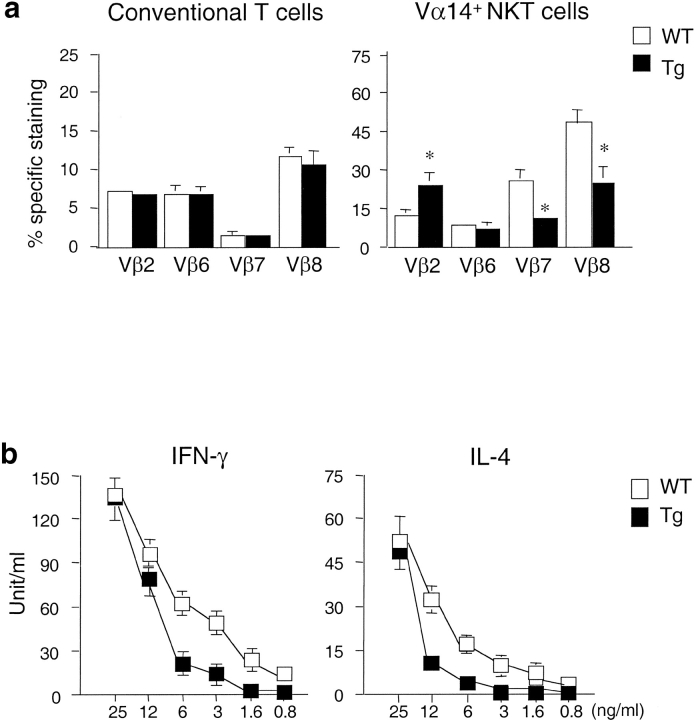
It has been shown that the Vβ segments used by CD1d-restricted Vα14+ NKT cells are skewed toward Vβ8, Vβ7, and Vβ2 (5–8). To examine whether high levels of CD1d expression alter the Vβ usage in Vα14+ NKT cells from CD1d Tg mice, we stained splenocytes from Tg and control mice with APC-conjugated CD1d/α-GalCer tetramer and FITC-conjugated mAb specific for different TCR β segments. As shown in Fig. 5 a, Vβ usage of tetramer+ cells in Tg mice differs from WT controls. Although Vβ8, Vβ7, and Vβ2 are still preferentially used by tetramer+ cells in Tg mice, the usage of Vβ8 and Vβ7 by the residual NKT cells in Tg mice is significantly reduced while the usage of Vβ2 is increased. There is no significant difference in the Vβ usage of conventional T cells between Tg and controls.
Figure 5.
Altered Vβ usage and affinity to α-GalCer of CD1d-restricted NKT cells in CD1d Tg mice. (a) Splenocytes from indicated mice were stained with APC conjugated-CD1d/α-GalCer tetramer, PE-anti-TCRβ, and FITC-anti-Vβ2, Vβ6, Vβ7, and Vβ8. Percentage of Vβ usage was calculated within gated TCRβ+ cells (conventional T cells) and CD1d/α-GalCer tetramer+ cells (Vα14+ cells). Data shown represent mean ± SE of four mice in each group. (b) Reduced cytokine production by CD1d/α-GalCer tetramer+ cells from CD1d Tg mice in response to α-GalCer. 2 × 104 tetramer+ cells from CD1d Tg and WT mice were cultured in the presence of various concentration of α-GalCer for 48 h. IL-4 and IFN-γ levels in the supernatant were detected by ELISA. Results are representative of two separate experiments.
To characterize the functional properties of the residual NKT cells in Tg mice, we analyzed the cytokine secretion capacity of NKT cells from Tg mice upon α-GalCer stimulation. Equal numbers of tetramer+ T cells from Tg or WT mice were incubated with various concentrations of α-GalCer for 48 h. As shown in Fig. 5 b, NKT cells from both Tg and WT mice were able to produce comparable amounts of IL4 and IFN-γ in response to a high concentration of α-GalCer. However, NKT cells from Tg+ mice secreted less amounts of cytokines than those of WT mice when stimulated with lower concentrations of α-GalCer. These results suggest that the residual NKT cells may escape negative selection by means of modified TCR usage and decreased affinity to CD1d and its ligand.
Thymic Stromal Cells Are Required for the Negative Selection of NKT Cells
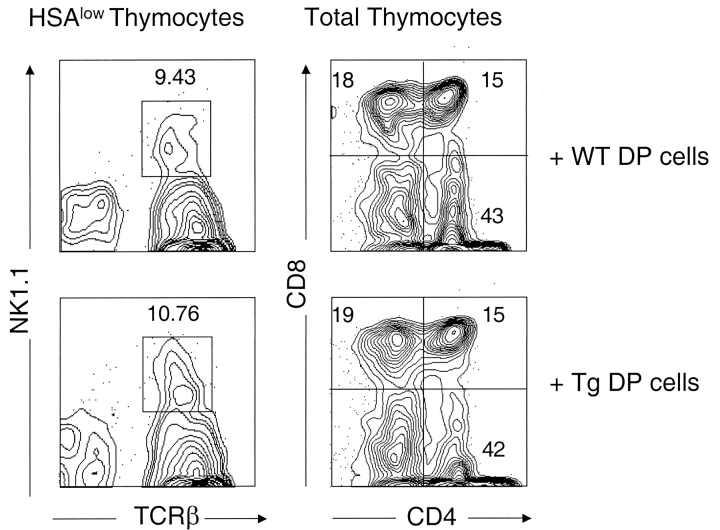
Next, we performed RTOC between thymic stromal cells of WT mice and DP thymocytes from either wild type or Tg mice to determine whether Tg+ stromal cells are required for the negative selection of NKT cells, and whether expression of CD1 transgene on DP thymocytes influences the positive and/or negative selection of NKT cells. Purified DP thymocytes from Tg or WT newborn mice were mixed with equal numbers of stromal cells derived from deoxyguanosine-treated fetal thymic lobes from WT mice. After 5 d in culture, the number of NKT, CD4+, and CD8+ cells were analyzed by flow cytometric analysis. Fig. 6 shows that comparable numbers of NKT cells were generated in the culture containing either WT or Tg+ DP thymocytes with WT thymic stromal cells, suggesting that high levels of CD1d expression on thymic stromal cells are required for the negative selection of NKT cells in the CD1d Tg model. In addition, expression of CD1 transgene on DP thymocytes did not enhance negative selection nor affect the positive selection of NKT cells.
Figure 6.
Thymic stromal cells are required for the negative selection of NKT cells. Reaggregate thymic organ cultures were made from equal numbers of WT thymic epithelial cells and either DP thymocytes from WT (top panel) or Tg mice (bottom panel). Cultures were harvested after 5 d and analyzed by flow cytometry for the development of NKT cells and various thymocyte subsets. The percentage of NKT cells in HSAlow gated population, and the percentage CD4+, CD8+, and DP thymocytes are indicated from a representative experiment. Similar results were obtained from two separate experiments.
High Levels of CD1d Expression on Bone Marrow–derived Cells Is Critical for the Negative Selection of NKT Cells.
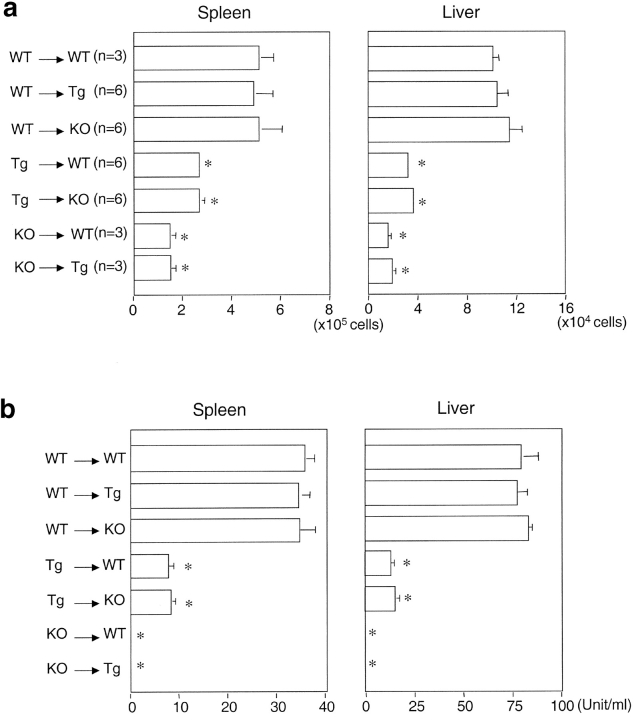
Thymic stromal cells consist of thymic epithelial cells (TECs), fibroblast-like cells, and some bone marrow–derived cells (45). To investigate the contribution of CD1d expression by hematopoietic cells or nonhematopoietic in the selection of CD1d-restricted NKT cells, we established bone marrow chimera between Tg and WT mice. The Tg→WT bone marrow chimeras had significantly lower numbers of NKT cells and significantly reduced capacity to secrete IL-4 than did WT→WT chimeras (Fig. 7 , a and b). The amounts of IFN-γ produced in Tg→WT bone marrow chimeras was also decreased (unpublished data). In contrast, WT→Tg chimeras yielded NKT cell populations similar in frequency to WT→WT chimeras, demonstrating overexpression of CD1d on nonhematopoietic cells (i.e., TECs) cannot mediate negative selection of NKT cells. In addition, CD1d-restricted NKT cells were undetectable in chimeras that received hematopoietic cells from the CD1dKO mice (KO→WT and KO→Tg), as reflected by lack of IL-4 (Fig. 7 b) and IFN-γ (data no shown) secretion upon stimulation with α-GalCer. Our results indicate that the level of CD1d expression on hematopoietic cells is critical in determining the outcome of NKT cell development. Overexpression of CD1d on hematopoietic cells may cause negative selection of CD1d-restricted NKT cells, while lack of CD1d expression on hematopoietic cells precludes the positive selection of NKT cells. This effect cannot be compensated for by overexpression of CD1d on nonhematopoietic cells. Thus, the level of CD1d expression on various cell types is likely to be tightly regulated in vivo to ensure the proper development and selection of NKT cells.
Figure 7.
CD1d expressed on bone marrow–derived cells are critical for the negative selection of NKT cells. 107 bone marrow-derived cells from WT, Tg, and CD1dKO mice were transferred into lethally irradiated recipient mice (980 rad) by intravenous injection. After 10 wk, spleen and liver lymphocytes from bone marrow chimera were isolated and analyzed by flow cytometry. (a) Absolute NKT cell numbers of liver lymphocytes and splenocytes from each group of bone marrow chimeras. (b) IL-4 production by liver lymphocytes and splenocytes from each group of bone marrow chimeras. 106 liver lymphocytes and splenocytes from indicated group of mice were cultured in the presence of 100 ng/ml of α-GalCer for 48 h. IL-4 levels in the supernatant were detected by ELISA.
CD1d-expressing DCs Can Mediate the Negative Selection of NKT Cells.
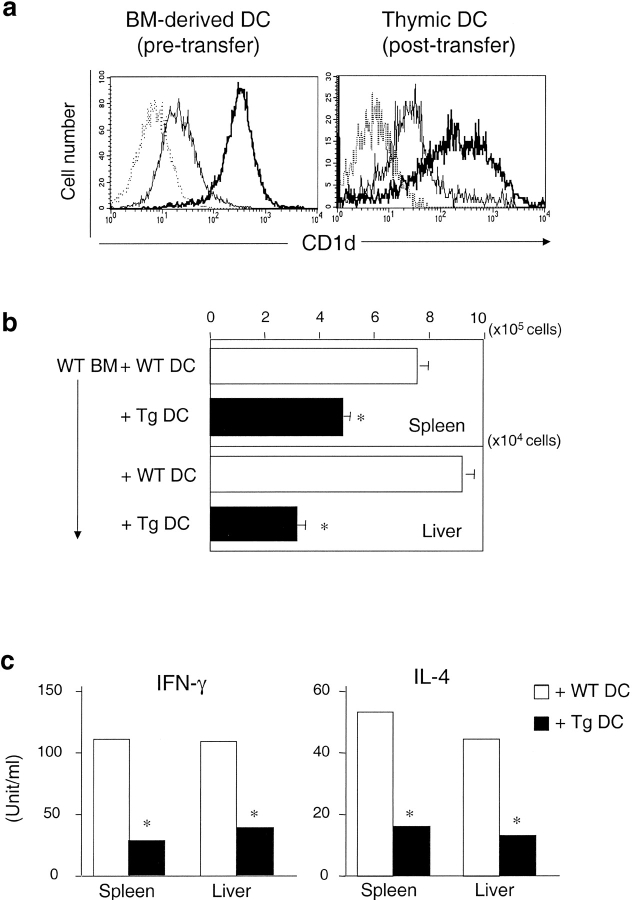
Several bone marrow–derived cell types have been shown to mediate the negative selection of conventional T cells, including DCs (54), thymic B cells (55), and thymocytes (56). Among these, DCs are the most potent mediator of negative selection for conventional T cells (57). To test whether overexpression of CD1d on DCs can induce the negative selection of NKT cells, bone marrow cells (107 cells) from WT mice were cotransferred with DCs (3.3 × 106 CD11c+ cells) from either WT or Tg mice to irradiated RAG-deficient mice. Flow cytometric analysis confirmed that DCs isolated from Tg mice express much higher levels of CD1d than DCs isolated from wild type controls (Fig. 8 a). 10 wk after adoptive transfer, splenocytes and hepatic lymphocytes from these chimeras were analyzed by flow cytometric analysis. Fig. 8 a shows that Tg+ DCs can be detected in the thymus of chimeras that received the cotransfer of WT bone marrow and Tg+ DCs, indicating these DCs are accessible during thymic selection. The number of NKT cells is significantly lower in the chimeras cotransferred with Tg+ DCs compared with the chimeras cotransferred with WT DCs (Fig. 8 b). In agreement with FACS® results, splenocytes and hepatic lymphocytes from chimeras cotransferred with Tg+ DCs secrete lower amounts of IFN-γ and IL-4 in response to α-GalCer stimulation (Fig. 8 c). These results indicate that CD1d expressed on DCs can mediate the negative selection of NKT cells.
Figure 8.
DCs can mediate the negative selection of NKT cells. 107 bone marrow–derived cells from WT mice and 3.3 × 106 DCs (CD11c+) from either WT or CD1dTg mice were cotransferred to irradiated RAG-deficient mice (700 rad) by intravenous injection. After 10 wk, spleen and liver lymphocytes from chimera were isolated and analyzed by flow cytometry. (a) Flow cytometry analysis of CD1d expression on bone marrow-derived DCs (CD11c+, greater than 80% are CD11b+CD8−) from donor mice and thymic DCs (CD11c+, ∼50–60% CD11b+CD8−, ∼30–40% CD11b−CD8+) from reconstituted mice. The levels of CD1d expression on bone marrow–derived DCs from Tg animals is ∼10–20 fold higher than those of WT controls. Specific fluorescence profiles (thin line: WT DCs and thick line: Tg DCs) obtained with anti-CD1d (5C6) were overlayed onto background profiles (dotted line) obtained with isotype control Ab. (b) Absolute NKT cell numbers of liver lymphocytes and splenocytes from each group of chimeras. The absolute number of NKT cells was calculated by percentage of NKT cells × total number of cells. Data shown represent mean ± SE of three mice in each group. (c) Cytokine production by NKT cells of liver lymphocytes and splenocytes from each group of chimeras was determined as described in Fig. 7.
Discussion
It has been suggested that NKT cells are biased toward CD1d autoreactivity. Consistent with this, NKT cells have an activated/effector or memory phenotype, even in germ free animals (58). Also, some NKT cell hybridomas exhibit CD1d autoreactivity (59), and freshly isolated NKT cells respond to CD1d transfectants and DCs (60). In light of this possible autoreactivity, it remained to be shown whether NKT cell precursors that encounter a strong signal during development undergo negative selection, and if so, what cell type(s) can mediate the negative selection of NKT cells. In this study, we showed that the addition of an agonist glycolipid into FTOC or increasing CD1d surface expression by transgenesis resulted in a drastic reduction of NKT cells, supporting the notion that NKT cells are susceptible to negative selection. This is the first demonstration that a glycolipid can induce negative selection of a T cell population. Although our models do not directly address whether NKT cells can be negatively selected in physiological conditions, our data provides a potential mechanism for the negative selection for these highly autoreactive T cells. Similar to conventional T lymphocytes, the negative selection of NKT cells is enhanced when overall avidity is increased, and is mediated by CD1d expressing DCs, but not TECs. Many characteristics distinguish the differentiation of NKT cells from conventional T cells in adult mice, including the cell type responsible for their positive selection. The data presented here, however, emphasize the similarity in the negative selection of the two types of T cells, including the importance of bone marrow–derived DCs in the negative selection process.
It has been shown previously that forced expression of a CD8αβ transgene expressed under the control of the CD2 promoter prevented the development of CD1d-dependent NKT cells (61). This result was interpreted as evidence for negative selection of NKT cells, because CD8 interaction with CD1d might lead to increased avidity of the canonical Vα14 TCR expressed by NKT cell precursors for CD1d. Although this is an attractive hypothesis, other interpretations are possible, as the forced expression of CD8 has been shown to partially inhibit the double negative to DP transition (62). NKT cells might be particularly affected by this, as their precursor frequency is likely to be very low on account of the low frequency of the Vα14-Jα18 rearrangement. Furthermore, results from preliminary experiments indicate that the affinity of CD1d for CD8 is very low and difficult to measure (unpublished data).
Our data suggest that the repertoire of NKT cells results from a balance between positive and negative selection mediated by endogenous ligands bound to CD1d molecules. The overexpression of CD1d in the transgenic mice may simply shift the balance such that an increased number of the Vα14 NKT cells exceed the threshold for negative selection and are deleted. The restricted TCR repertoire of CD1d-reactive NKT cells allowed us to examine the effect of CD1d density on the quantitative and qualitative changes in the NKT cells population, which would not be possible in a TCR Tg system. Despite their relatively restricted TCR repertoire, the affinity of NKT cells for α-GalCer plus CD1d complexes is heterogeneous. This has recently been demonstrated in three Vα14 NKT cell hybridomas by equilibrium staining with α-GalCer/CD1d tetramers (63). A similar heterogeneity might also be true for the TCR avidity for complexes of CD1d with endogenous glycolipids. Consistent with this, although tetramer+ NKT cells from CD1d Tg spleen displayed a similar bias toward expression of the Vβ2, -7, and -8 segments, the relative reduction of NKT cells was more prominent in the Vβ8-bearing NKT cells than the Vβ2-bearing NKT cells (Fig. 5 a). This suggests that most of the Vβ8-bearing NKT cells may have a higher affinity for the CD1d/glycolipid complexes than the Vβ2-bearing NKT cells. The heterogeneity of affinity makes it likely that the residual NKT cells that can escape negative selection in CD1d Tg mice have a reduced TCR affinity. Indeed, these residual NKT cells have a reduced response to low concentrations of α-GalCer in vitro. Nevertheless, the limited TCR repertoire employed by Vα14+NKT cells poses constraints on the extent to which NKT cells are able to escape the negative selection.
Studies using TCRαβ Tg mice have shown that several alternative mechanisms could prevent conventional T cells bearing autoreactive TCRs from being deleted, including down-regulation of TCR and coreceptor as well as altered expression of some surface molecules, such as CD5 (64–66). As for NKT cells, engagement of Ly49 receptors with ligand has been shown to negatively regulate autoreactivity of NKT cells (60), indicating expression of NK receptors on NKT cells may affect their selection. Our analysis of the surface phenotype of CD1d-restricted NKT cells showed that the number of CD4+ tetramer+ cells was reduced by 80–85%, while the number of CD4−CD8− tetramer+ cells was reduced by 25–40% in Tg mice (Fig. 3 c), suggesting that CD4+ Vα14+ NKT cells might have higher avidity to CD1d/glycolipid complexes and/or are more sensitive to negative selection. However, our immunofluorescence analysis showed that NKT cells in CD1d Tg and WT mice expressed similar levels of TCR, CD4, CD8, NK1.1, and Ly49A (unpublished data). This suggests that modulating the expression levels of TCR and other surface molecules is not the primary mechanism that allows residual NKT cells to escape negative selection in CD1dTg mice. Thus, it is likely that the residual NKT cells escape negative selection in CD1dTg mice primarily by altering the NKT cell repertoire.
Our bone marrow chimera study shows that overexpression of CD1d on hematopoietic cells, but not on nonhematopoietic cells (i.e., TECs), was responsible for the reduction of NKT cells in CD1d Tg mice. Furthermore, cotransfer of Tg DCs with WT bone marrow cells leads to diminished NKT cell development, suggesting that similar to conventional T cells, CD1d expressing on DCs can mediate the negative selection of NKT cells. It remains possible that other cell types of hematopoietic origin, such as thymic B cells and macrophages, can also mediate the negative selection of NKT cells. In addition, although we detected no effect of Tg DP thymocytes in the negative selection of NKT cells (Fig. 6), the level of CD1d expressed on DP thymocytes in CD1d Tg mice is much lower than other cell types. Thus, we cannot eliminate the potential role of DP thymocytes in negative selection. We can conclude, however, that the inability of TEC to mediate negative selection was not due to insufficient levels of CD1d, as expression of CD1d by DCs and TECs was similar in Tg mice. Other factors then, such as accessory molecules, cell type–specific ligands, and antigen processing and presentation pathways, must account for the ability of DCs to play a role in the negative selection of NKT cells.
Recent studies showed that NKT cell development is impaired in mice expressing a CD1d mutant which lacks an endosomal targeting motif, and in mice deficient of cathepsin S, which disrupts the intracellular trafficking of CD1d (67, 68). These results indicated that the selection and maturation of NKT cells might require autologous CD1d-bound ligands generated from the endosomal processing pathway. Our finding that the addition of α-GalCer induces the negative selection of NKT cells in B6 FTOC raises some interesting questions about the potential role of self-glycolipids in shaping the repertoire of mature CD1d-restricted NKT cells. As the surface level of CD1d is unchanged in α-GalCer–treated thymocytes and DCs (unpublished results), qualitative and quantitative differences in TCR-CD1d recognition may be a critical determinant of NKT cell fate. Thus, it is possible that during infection or stress, the outcome of NKT cell selection might be affected by either modulating the levels of CD1d expression and/or varying the supply of glycolipids.
NKT cells are potent immunoregulatory cells that play a significant role in regulating autoimmunity and immune responses against microbes and tumors (69). As such, the repertoire of NKT cells must be tightly regulated to maintain a pool of functional NKT cells while at the same time eliminating dangerous autoreactive NKT cells. Our study suggests that negative selection plays a major role in eliminating the high affinity self-reactive NKT cells, while other factors might fine-tune the resulting NKT cell repertoire. Further investigation will be required to understand how these mechanisms are altered, or can be manipulated, in infection and autoimmunity.
Acknowledgments
We thank Drs. Y. Koezuka and L. Van Kaer for providing glycolipids and CD1d1 plasmid, respectively; A. Parson and M. Zimmer for critical review for the manuscript, and T. King for help in maintenance and genotyping the mice.
Supported by grants from National Institutes of Health (AI43407 to C.-R. Wang, AI42284 to S. Joyce, and CA52511 to M. Kronenberg), a grant from the Human Frontiers of Science Research Program (S. Joyce and M. Kronenberg), and grants from the Cancer Research Institute (T. Chun and L. Gapin).
T. Chun's present address is Department of Microbiology and Immunology, School of Medicine, Hanyang University, Seoul 133-791, South Korea.
Footnotes
Abbreviations used in this paper: α-GalCer, α-galactosylceramide; β-GalCer, β-galactosylceramide; BM, bone marrow; DC, dendritic cell; DP, double positive; FTOC, fetal thymic organ culture; NKT cell, natural killer T cell; RTOC, reaggregate thymic organ culture; TEC, thymic epithelial cell; Tg, transgenic.
References
- 1.MacDonald, H.R. 1995. NK1.1+ T cell receptor-α/β+ cells: New clues to their origin, specificity, and function. J. Exp. Med. 182:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 3.Eberl, G., R. Lees, S.T. Smiley, M. Taniguchi, M.J. Grusby, and H.R. MacDonald. 1999. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162:6410–6419. [PubMed] [Google Scholar]
- 4.Hammond, K.J., S.B. Pelikan, N.Y. Crowe, E. Randle-Barrett, T. Nakayama, M. Taniguchi, M.J. Smyth, I.R. van Driel, R. Scollay, A.G. Baxter, and D.I. Godfrey. 1999. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29:3768–3781. [DOI] [PubMed] [Google Scholar]
- 5.Arase, H., N. Arase, K. Ogasawara, R.A. Good, and K. Onoe. 1992. An NK1.1+ CD4+CD8- single-positive thymocyte subpopulation that expresses a highly skewed T cell antigen receptor family. Proc. Natl. Acad. Sci. USA. 89:6506–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice. J. Exp. Med. 180:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino, Y., R. Kanno, T. Ito, K. Higashino, and M. Taniguchi. 1995. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int. Immunol. 7:1157–1161. [DOI] [PubMed] [Google Scholar]
- 8.Ohteki, T., and H.R. MacDonald. 1996. Stringent Vβ requirement for the development of NK1.1+ T cell receptor-αβ+ cells in mouse liver. J. Exp. Med. 183:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto, T., and W.E. Paul. 1994. CD4+, NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi, F.D., M. Flodstrom, B. Balasa, S.H. Kim, K. Van Gunst, J.L. Strominger, S.B. Wilson, and N. Sarvetnick. 2001. Germ line deletion of the CD1 locus exacerbates diabetes in the NOD mouse. Proc. Natl. Acad. Sci. USA. 98:6777–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, B., Y.B. Geng, and C.R. Wang. 2001. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 194:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong, S., M.T. Wilson, I. Serizawa, L. Wu, N. Singh, O.V. Naidenko, T. Miura, T. Haba, D.C. Scherer, J. Wei, et al. 2001. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7:1052–1056. [DOI] [PubMed] [Google Scholar]
- 14.Sharif, S., G.A. Arreaza, P. Zucker, Q.S. Mi, J. Sondhi, O.V. Naidenko, M. Kronenberg, Y. Koezuka, T.L. Delovitch, J.M. Gombert, et al. 2001. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat. Med. 7:1057–1062. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, H., A. Belperron, S.W. Barthold, and L.K. Bockenstedt. 2000. CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165:4797–4801. [DOI] [PubMed] [Google Scholar]
- 16.Flesch, I.E., A. Wandersee, and S.H. Kaufmann. 1997. IL-4 secretion by CD4+ NK1+ T cells induces monocyte chemoattractant protein-1 in early listeriosis. J. Immunol. 159:7–10. [PubMed] [Google Scholar]
- 17.Schofield, L., M.J. McConville, D. Hansen, A.S. Campbell, B. Fraser-Reid, M.J. Grusby, and S.D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 283:225–229. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac, A. 1995. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coles, M.C., and D.H. Raulet. 2000. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164:2412–2418. [DOI] [PubMed] [Google Scholar]
- 20.Alberola-Ila, J., K.A. Hogquist, K.A. Swan, M.J. Bevan, and R.M. Perlmutter. 1996. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 184:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eberl, G., B. Lowin-Kropf, and H.R. MacDonald. 1999. NKT cell development is selectively impaired in Fyn-deficient mice. J. Immunol. 163:4091–4094. [PubMed] [Google Scholar]
- 22.Gadue, P., N. Morton, and P.L. Stein. 1999. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J. Exp. Med. 190:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberl, G., H.J. Fehling, H. von Boehmer, and H.R. MacDonald. 1999. Absolute requirement for the pre-T cell receptor alpha chain during NK1.1+ TCRαβ cell development. Eur. J. Immunol. 29:1966–1971. [DOI] [PubMed] [Google Scholar]
- 24.Walunas, T.L., B. Wang, C.R. Wang, and J.M. Leiden. 2000. The Ets1 transcription factor is required for the development of NK T cells in mice. J. Immunol. 164:2857–2860. [DOI] [PubMed] [Google Scholar]
- 25.Iizuka, K., D.D. Chaplin, Y. Wang, Q. Wu, L.E. Pegg, W.M. Yokoyama, and Y.X. Fu. 1999. Requirement for membrane lymphotoxin in natural killer cell development. Proc. Natl. Acad. Sci. USA. 96:6336–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elewaut, D., L. Brossay, S.M. Santee, O.V. Naidenko, N. Burdin, H. De Winter, J. Matsuda, C.F. Ware, H. Cheroutre, and M. Kronenberg. 2000. Membrane lymphotoxin is required for the development of different subpopulations of NK T cells. J. Immunol. 165:671–679. [DOI] [PubMed] [Google Scholar]
- 27.Gapin, L., J.L. Matsuda, C.D. Surh, and M. Kronenberg. 2001. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2:971–978. [DOI] [PubMed] [Google Scholar]
- 28.Benlagha, K., T. Kyin, A. Beavis, L. Teyton, and A. Bendelac. 2002. A thymic precursor to the NK T cell lineage. Science. 296:553–555. [DOI] [PubMed] [Google Scholar]
- 29.Pellicci, D.G., K.J. Hammond, A.P. Uldrich, A.G. Baxter, M.J. Smyth, and D.I. Godfrey. 2002. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashton-Rickardt, P.G., A. Bandeira, J.R. Delaney, L. Van Kaer, H.P. Pircher, R.M. Zinkernagel, and S. Tonegawa. 1994. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 76:651–663. [DOI] [PubMed] [Google Scholar]
- 31.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 32.Sebzda, E., V.A. Wallace, J. Mayer, R.S. Yeung, T.W. Mak, and P.S. Ohashi. 1994. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 263:1615–1618. [DOI] [PubMed] [Google Scholar]
- 33.Auphan, N., G. Schonrich, M. Malissen, M. Barad, G. Hammerling, B. Arnold, B. Malissen, and A.M. Schmitt-Verhulst. 1992. Influence of antigen density on degree of clonal deletion in T cell receptor transgenic mice. Int. Immunol. 4:541–547. [DOI] [PubMed] [Google Scholar]
- 34.Robey, E.A., F. Ramsdell, D. Kioussis, W. Sha, D. Loh, R. Axel, and B.J. Fowlkes. 1992. The level of CD8 expression can determine the outcome of thymic selection. Cell. 69:1089–1096. [DOI] [PubMed] [Google Scholar]
- 35.Liu, G.Y., P.J. Fairchild, R.M. Smith, J.R. Prowle, D. Kioussis, and D.C. Wraith. 1995. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 3:407–415. [DOI] [PubMed] [Google Scholar]
- 36.Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 37.Leishman, A.J., L. Gapin, M. Capone, E. Palmer, H.R. MacDonald, M. Kronenberg, and H. Cheroutre. 2002. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity. 16:355–364. [DOI] [PubMed] [Google Scholar]
- 38.Legendre, V., C. Boyer, S. Guerder, A. Bernd, G. Hämmerling, and A.-M. Schmitt-Verhulst. 1999. Selection of phenotypically distinct NK1.1+ T cells upon antigen expression in the thymus or in the liver. Eur. J. Immunol. 29:2330–2343. [DOI] [PubMed] [Google Scholar]
- 39.Capone, M., M. Troesch, G. Eberl, B. Hausmann, E. Palmer, and H.R. MacDonald. 2001. A critical role for the T cell receptor a-chain connecting peptide domain in positive selection of CD1-independent NKT cells. Eur. J. Immunol. 31:1867–1875. [DOI] [PubMed] [Google Scholar]
- 40.Curnow, S.J., C. Boyer, M. Buferne, and A.-M. Schmitt-Verhulst. 1995. TCR-associated ζ-FcɛRIγ heterodimers on CD4−CD8− NK1.1+ T cells selected by specific class I MHC antigen. Immunity. 3:427–438. [DOI] [PubMed] [Google Scholar]
- 41.Mandal, M., X.R. Chen, M.L. Alegre, N.M. Chiu, Y.H. Chen, A.R. Castano, and C.-R. Wang. 1998. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol. Immunol. 35:525–536. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.-R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, Y.H., N.M. Chiu, M. Mandal, N. Wang, and C.-R. Wang. 1997. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 6:459–467. [DOI] [PubMed] [Google Scholar]
- 44.Goossens, P.L., H. Jouin, G. Marchal, and G. Milon. 1990. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J. Immunol. Methods. 132:137–144. [DOI] [PubMed] [Google Scholar]
- 45.Jenkinson, E.J., G. Anderson, and J.J. Owen. 1992. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J. Exp. Med. 176:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naidenko, O.V., J.K. Maher, W.A. Ernst, T. Sakai, R.L. Modlin, and M. Kronenberg. 1999. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J. Exp. Med. 190:1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leite-de-Moraes, M.C., A. Herbelin, C. Gouarin, Y. Koezuka, E. Schneider, and M. Dy. 2000. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J. Immunol. 165:4367–4371. [DOI] [PubMed] [Google Scholar]
- 48.Hayakawa, Y., K. Takeda, H. Yagita, S. Kakuta, Y. Iwakura, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Critical contribution of IFN-γ and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur. J. Immunol. 31:1720–1727. [PubMed] [Google Scholar]
- 49.Eberl, G., and H.R. MacDonald. 1998. Rapid death and regeneration of NKT cells in anti-CD3ɛ- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 9:345–353. [DOI] [PubMed] [Google Scholar]
- 50.Cook, J.R., E.M. Wormstall, T. Hornell, J. Russell, J.M. Connolly, and T.H. Hansen. 1997. Quantitation of the cell surface level of Ld resulting in positive versus negative selection of the 2C transgenic T cell receptor in vivo. Immunity. 7:233–241. [DOI] [PubMed] [Google Scholar]
- 51.Fukui, Y., T. Ishimoto, M. Utsuyama, T. Gyotoku, T. Koga, K. Nakao, K. Hirokawa, M. Katsuki, and T. Sasazuki. 1997. Positive and negative CD4+ thymocyte selection by a single MHC class II/peptide ligand affected by its expression level in the thymus. Immunity. 6:401–410. [DOI] [PubMed] [Google Scholar]
- 52.Park, S.H., A. Weiss, K. Benlagha, T. Kyin, L. Teyton, and A. Bendelac. 2001. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruisbeek, A.M., and D. Amsen. 1996. Mechanisms underlying T-cell tolerance. Curr. Opin. Immunol. 8:233–244. [DOI] [PubMed] [Google Scholar]
- 55.Ferrero, I., F. Anjuere, P. Martin, G. Martinez del Hoyo, M.L. Fraga, N. Wright, R. Varona, G. Marquez, and C. Ardavin. 1999. Functional and phenotypic analysis of thymic B cells: role in the induction of T cell negative selection. Eur. J. Immunol. 29:1598–1609. [DOI] [PubMed] [Google Scholar]
- 56.Schonrich, G., G. Strauss, K.P. Muller, L. Dustin, D.Y. Loh, N. Auphan, A.M. Schmitt-Verhulst, B. Arnold, and G.J. Hammerling. 1993. Distinct requirements of positive and negative selection for selecting cell type and CD8 interaction. J. Immunol. 151:4098–4105. [PubMed] [Google Scholar]
- 57.Anderson, G., K.M. Partington, and E.J. Jenkinson. 1998. Differential effects of peptide diversity and stromal cell type in positive and negative selection in the thymus. J. Immunol. 161:6599–6603. [PubMed] [Google Scholar]
- 58.Park, S.H., K. Benlagha, D. Lee, E. Balish, and A. Bendelac. 2000. Unaltered phenotype, tissue distribution and function of Vα14+ NKT cells in germ-free mice. Eur. J. Immunol. 30:620–625. [DOI] [PubMed] [Google Scholar]
- 59.Bendelac, A., L. Lantz, M.E. Quimby, J.W. Yewdell, J.R. Bennink, and R.R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science. 268:863–865. [DOI] [PubMed] [Google Scholar]
- 60.Ikarashi, Y., R. Mikami, A. Bendelac, M. Terme, N. Chaput, M. Terada, T. Tursz, E. Angevin, F.A. Lemonnier, H. Wakasugi, and L. Zitvogel. 2001. Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: critical role for B7 in CD1d-dependent NKT cell interferon gamma production. J. Exp. Med. 194:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bendelac, A., N. Killeen, D.R. Littman, and R.H. Schwartz. 1994. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 263:1774–1778. [DOI] [PubMed] [Google Scholar]
- 62.Wack, A., M. Coles, T. Norton, A. Hostert, and D. Kioussis. 2000. Early onset of CD8 transgene expression inhibits the transition from DN3 to DP thymocytes. J. Immunol. 165:1236–1242. [DOI] [PubMed] [Google Scholar]
- 63.Sidobre, S., O.V. Naidenko, B.C. Sim, N.R. Gascoigne, K.C. Garcia, and M. Kronenberg. 2002. The Vα14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J. Immunol. 169:1340–1348. [DOI] [PubMed] [Google Scholar]
- 64.von Boehmer, H., J. Kirberg, and B. Rocha. 1991. An unusual lineage of α/β T cells that contains autoreactive cells. J. Exp. Med. 174:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sebzda, E., S. Mariathasan, T. Ohteki, R. Jones, M.F. Bachmann, and P.S. Ohashi. 1999. Selection of the T cell repertoire. Annu. Rev. Immunol. 17:829–874. [DOI] [PubMed] [Google Scholar]
- 66.Azzam, H.S., J.B. DeJarnette, K. Huang, R. Emmons, C.S. Park, C.L. Sommers, D. El-Khoury, E.W. Shores, and P.E. Love. 2001. Fine tuning of TCR signaling by CD5. J. Immunol. 166:5464–5472. [DOI] [PubMed] [Google Scholar]
- 67.Chiu, Y.H., S.H. Park, K. Benlagha, C. Forestier, J. Jayawardena-Wolf, P.B. Savage, L. Teyton, and A. Bendelac. 2002. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat. Immunol. 3:55–60. [DOI] [PubMed] [Google Scholar]
- 68.Riese, R.J., G.P. Shi, J. Villadangos, D. Stetson, C. Driessen, A.M. Lennon-Dumenil, C.L. Chu, Y. Naumov, S.M. Behar, H. Ploegh, et al. 2001. Regulation of CD1 function and NK1.1+ T cell selection and maturation by cathepsin S. Immunity. 15:909–919. [DOI] [PubMed] [Google Scholar]
- 69.Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. [DOI] [PubMed] [Google Scholar]