Abstract
Interferon-producing cells (IPCs) secrete high levels of type I interferon in response to certain viruses. The lack of lineage markers, the expression of major histocompatibility complex (MHC) class II and the capacity to stimulate allogeneic T cells have led these cells to be classified as a subset of dendritic cells (DCs), called plasmacytoid DCs (PDCs). However, the role of IPCs/PDCs in initiating primary immune responses remains elusive. Here we examined the antigen presenting capacity of murine IPCs in antigen specific systems. While CD8α+ and CD11b+ DCs induced logarithmic expansion of naive CD4 and CD8 T cells, without conferring T helper commitment at a first encounter, primary IPCs lacked the ability to stimulate naive T cells. However, when antigen-experienced, nonpolarized T cells expanded by classical DC subsets, were restimulated by IPCs, they proliferated and produced high amounts of IFN-γ. These data indicate that IPCs can effectively stimulate preactivated or memory-type T cells and exert an immune-regulatory role. They also suggest that expansion of naive T cells and acquisition of effector function during antigen-specific T cell responses may involve different antigen-presenting cell (APC) types. Independent and coordinated control of T cell proliferation and differentiation would provide the immune system with greater flexibility in regulating immune responses.
Keywords: interferon-producing cells, dendritic cells, Th1 cells, unpolarized T cells
Introduction
Natural IFN-producing cells (IPCs)* were originally identified in humans as a small subset of blood leukocytes specialized in the secretion of high levels of type I IFN in response to viruses (1–4).
The absence of lineage markers, the expression of MHC class II molecules, the acquisition of a Dendritic Cell (DC)-like morphology upon activation, as well as their presence in secondary lymphoid organs, led to the concept of human IPCs being a distinct subset of DCs (1). This idea was also supported by the evidence that viruses, bacterial products or T cell contact can trigger in IPCs a developmental pathway closely resembling the maturation process of a genuine DC (1, 2). Human IPCs undergoing activation gain some T cell stimulatory function in allogeneic mixed lymphocyte reactions (MLRs; reference 5). However, due to the low frequency of antigen-specific T cells, the capacity of IPCs to present antigen to naive T cells, an essential requisite to fulfill the definition of DC (6, 7), has not yet been fully elucidated.
Recent studies have identified the murine counterpart of human IPCs. In mouse spleen and lymph nodes, IPCs are a CD11c low, Ly6C/G+ CD11b− cell population (8–10). These phenotypical features allow them to be distinguished from other DC subsets, as classical DCs express high levels of CD11c, are either CD11b+ or CD8α+, and lack Ly-6G/C, at least in an immature state (7).
Here we have examined the capacity of murine IPCs to present antigens to naive CD4+ and CD8+ T cells, as compared with the classical CD11b+/CD8α− and CD11b−/CD8α+ DC subsets (1). IPCs failed to stimulate a strong proliferation of naive T cells even after in vivo viral stimulation, questioning their belonging to the DC system (11).
However, IPCs could still promote proliferation of antigen-primed T cells, suggesting that they can exert some basic APC function for memory-type T cells. In our system, CD4 T cells expanded by classical DCs remained largely nonpolarized, preserving a high capacity for IL-2 production. These antigen-primed, nonpolarized T cells (12), when restimulated by IPCs, not only efficiently proliferated, but also differentiated to a Th1 effector type.
Together, these results are consistent with a model in which immune responses are regulated by a network of different APCs, each having distinct roles in controlling proliferation and differentiation of antigen-specific naive or memory T cells.
Materials and Methods
Media and Reagents.
Media were used as described previously (4, 5). Influenza virus PR8 was grown in MDCK cells, purified by gradient centrifugation with 20% sucrose and resuspended in PBS at a titer of 7.4 × 107 PFU/ml as determined by MDCK plaque assay. PR8 was used at 1 MOI. Recombinant vesicular stomatitis virus (VSV) was grown on BHK cells. The titer of clarified virus-containing supernatant was 4 × 109 PFU/ml as determined by plaque assay using BHK cells. The IL-12p70 ELISA kit was obtained from R&D Systems. CpG ODN 1826 (5′-tccatgacgttcctgacgtt-3′) was obtained from Operon Technologies.
Mice.
WT-mHEL (hen egg lysozyme) mice express membrane-bound HEL under the I-E promoter and are backcrossed to the B10.BR strain. CD4+ T cells from the 3A9 TCR transgenic mouse recognize the HEL peptide 48–62 in the context of I-Ak (13). HY-specific TCR transgenic mice recognize the HY male antigen restricted to MHC class I H-2Db. B6 and 129X1/SvJ mice were obtained from The Jackson Laboratory. All mice used in experiments were 6–12 wk in age. Handling of mice and experimental procedures were conducted in accordance with institutional guidelines for animal care and use. Mice were maintained at the Washington University small animal facility. For in vivo stimulation of IPCs, mice were infected with 2 × 108 PFU of VSV intravenously and killed after 9 to 12 h.
Preparation and Culture of Cells.
Primary splenic IPCs and DCs, were preenriched by magnetic sorting with CD11c microbeads (Miltenyi Biotec) after collagenase treatment of disrupted spleens. Cells were then stained with anti-Ly-6G/C-PE (RB6–8C5), anti-CD11b-FITC, and biotinylated anti-CD11c (BD Biosciences) followed by Streptavidin-APC (Molecular Probes) and sorted into CD11c+/CD11b−/Ly-6G/C+, CD11c+/CD11b+/Ly-6G/C−, and CD11c+/CD11b−/ Ly-6G/C− cells (purity >98%) on a MoFlo cytometer. CD4+ T cells were magnetically isolated with CD4-dynabeads (Dynal) from pooled spleens and lymph nodes of 3A9 TCR transgenic mice (90% purity, <2% MHC class II+ cells). CD4-beads were subsequently detached (DETACHaBEAD; Dynal). 60 to 90% of CD4+ cells stained positive with the 1G12 anti-clonotype monoclonal antibody. CD8 T cells were isolated from pooled spleens and lymph nodes of female homozygous HY-specific TCR transgenic mice using CD8α microbeads (Miltenyi Biotec, purity >90%).
T Cell Assays.
IPCs or DCs freshly isolated or cultured overnight with PR8 (1 MOI), were irradiated (3,000 rad), serially diluted, and cocultured with naive CD4 3A9 T cells (104/well) or naive CD8 HY-specific TCR transgenic T cells (5 × 104/well) for 72 h. H3-thymidine was added in the last 18 h of culture. 3A9 hybridoma T cells were added to APCs at 105/well and cultured for 20 h. Hybridoma reactivity was determined by IL-2 secretion. IL-2 was measured in supernatants as proliferative response of the IL-2 dependent cell line CTLL. For T cell polarization experiments, naive CD4 3A9 T cells were cocultured with different DC populations (2 × 104 T cells, 2 × 104 DC, 100 μl medium per well) in 96-well flat bottom plates for 5–7 d. Cells were restimulated with 100 nM PMA and 0.5 μg/ml ionomycin (Sigma-Aldrich) for 6 h. Monensin (2 μg/ml; Sigma-Aldrich) was added in the last 4 h. Cells were fixed, permeabilized, and stained intracellularly with anti–IFN-γ-FITC, anti–IL-2-APC, and anti–IL-4-PE (BD Biosciences). 3A9 T cells initially stimulated with splenic CD11b+ DCs were expanded with 50 U/ml IL-2 for 9–10 d. They were then collected for RNA preparation, stained for IL-12Rβ (BD Biosciences) and IL-18Rα (R&D Systems) expression, and restimulated with freshly isolated APCs in graded numbers for proliferation experiments or at 1:1 ratio (2 × 104) for polarization experiments. Antibodies against IL-18Rα (R&D Systems), IFN-β (PBL Biomedical Laboratories), and/or IL-12 (TOSH) were used to neutralize cytokine activity.
Flow Cytometry.
Magnetically enriched splenic DCs were stained with anti-Ly-6G/C-PE and biotinylated anti-CD11c plus Streptavidin–APC and analyzed for expression of CD40, CD80, CD86, I-Ak, and H-2Db using FITC-labeled antibodies (BD Biosciences). Membrane-bound HEL was detected with FITC-labeled F10.6.6 monoclonal antibody and the 48–62 HEL peptide bound to I-Ak was detected using the Aw3.18 monoclonal antibody.
Chemotaxis Assay.
Chemotaxis of 3A9 T cells was measured in a 2 h transwell migration assay using 24-well Costar transwell chambers (5 μ pore size; Corning). Recombinant mCCL19 was obtained from PeproTech).
Type I IFN Bioassay.
Type I IFN was measured by evaluating the inhibition of proliferation of murine BW 5147 T cell lymphoma cells with reference to a standard curve with recombinant murine IFN-α (Biosource International). The lower detection limit was 10 U/ml IFN-α. In initial experiments bioassay results were confirmed by measuring IFN-α by ELISA in parallel (mouse IFN-α ELISA kit; PBL Biomedical Laboratories). Briefly, cell culture supernatants and IFN-α standard were serially diluted in 96-well round-bottom plates and 5 × 103 BW 5147 cells were added per well. After 36 h of incubation cells were pulsed with H3-Thymidine and incorporation was measured after 8 h.
RT-PCR and Primers.
RNA extraction and cDNA synthesis were performed according to standard protocols. The following specific primers were used: IL-12Rβ1: 5′-gccaacattaagttcttggtg-3′, 5′-attcttggggttcttggaggc-3′; IL-12Rβ2: 5′-gggagtacatagtggaatgga-3′, 5′-gcgtcggtactgaatttcgca-3′; IL-23R 5′-tgaaagagaccctacatcccttga-3′, 5′-cagaaaattggaagttgggatatgtt-3′; IL-12p40: 5′-gacacgcctgaagaagatgac-3′, 5′-gccattccacatgtcactgc-3′; IL-12p35: 5′-atgatgaccctgtgccttgg-3′, 5′-cctttggggagatgagatgt-3′; IL-23p19: 5′-tggcatcgagaaactgtgaga-3′, 5′-tcagttcgtattggtagtcctgtta-3′; β-actin: 5′-ggagaagagctatgagctgcct-3′, 5′-gtggtaccaccagacaacact-3′. PCR conditions: 5 min, 94°C; 30 s, 94°C; 30 s annealing; 60 s 72°C (40 cycles; β-actin: 30 cycles), and 5 min final extension at 72°C. The annealing temperature was 62°C for IL-12Rβ1 and IL-12Rβ2, 60°C for IL-23R, IL-12p35, IL-23p19, and 55°C for IL-12p40. The PCR products were analyzed on 1.5% Nusieve 3:1 (BioWhitaker) agarose gels.
Results
Primary IPCs Are Inefficient in Expanding Antigen-specific Naive CD4 T Cells.
To examine the antigen-presenting function of primary IPCs to naive CD4 T cells we used, as a model, mHEL transgenic mice, which express membrane bound HEL as antigen, and CD4+ T cells from 3A9 TCR-transgenic mice, that recognize the HEL peptide 48–62 in the context of MHC class II I-Ak molecules.
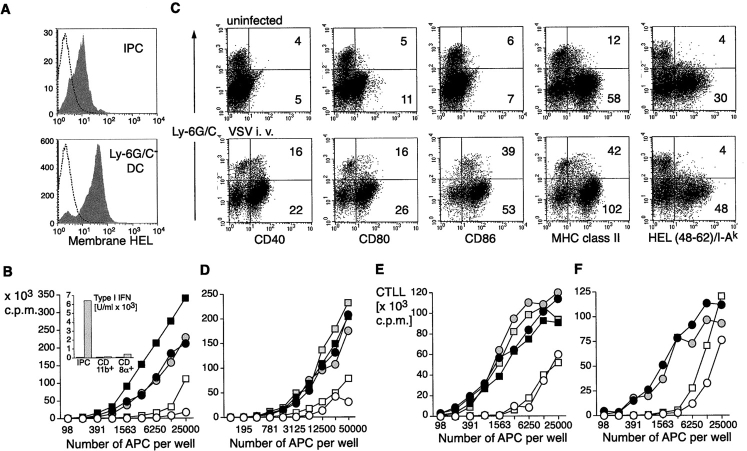
CD11c+ cells from spleens of mHEL mice were prepared, and IPCs as well as CD11b+ and CD11b−CD8α+ DCs were sorted at high purity (>98%, not shown) and analyzed for mHEL expression by flow cytometry. All subsets expressed substantial levels of HEL antigen at the cell surface (Fig. 1 A). We tested the ability of each subset to stimulate naive CD4+ T cells derived from 3A9 TCR transgenic mice in vitro. As shown in Fig. 1 B, IPCs did not induce proliferation of HEL-specific T cells, as compared with other DCs.
Figure 1.
HEL expression and antigen presentation to HEL-specific naive CD4 T cells and 3A9 T cell hybridoma by primary mHEL-transgenic DCs and IPCs. (A) IPCs and Ly-6G/C− DCs from the mHEL mouse were stained with an antibody specific for HEL to measure antigen expression at the cell surface (gray histograms). Open histograms indicate staining with control antibody. (B) Proliferative response of HEL-specific naive 3A9 CD4 T cells (104) cultured with graded numbers of purified IPCs (open symbols), CD11b+(black filled symbols), and CD8α+ (gray filled symbols) DCs derived from mHEL mice. IPCs and DCs were untreated (circles) or treated (squares) in vitro for 12 h with PR8 (1 MOI) before addition of T cells. Supernatants were then collected and type I IFN production was measured (insert to B). Proliferation was measured after 72 h. (C) Expression of costimulatory molecules, MHC class II and HEL48–62/IAk specific complexes in IPCs and DCs derived from mHEL-mice. Mice were either uninfected or challenged with VSV for 12 h. MFI for IPCs and Ly-6G/C− DCs are indicated. (D) Proliferation of 3A9 naive CD4 T cells cocultured with graded numbers of IPCs or DCs purified ex vivo from mHEL mice uninfected or infected with VSV (intravenous, 2 × 108 PFU) 12 h before. (E and F) HEL-specific 3A9 cell hybridoma T cells (105) were cocultured with graded numbers of IPCs and DCs sorted from mHEL mice and stimulated in vitro with influenza virus PR8 (E) or activated in vivo by VSV infection (F). Supernatants were collected after 48 h and the IL-2 produced upon activation was measured as proliferation of CTLL cells after 20 h.
To test whether the antigen presenting function increased with IPC activation, IPCs and other DCs were sorted and incubated with influenza virus for 12 h before coculture with HEL-specific T cells in vitro. Viral stimulation of IPCs was sufficient to induce high secretion of type I IFN (insert to Fig. 1 B), but their T cell stimulatory capacity was only minimally increased. Other DC subsets stimulated T cell proliferation 10- to 50-fold more efficiently than IPCs, and incubation with influenza virus further increased their T cell stimulatory capacity.
In vitro activation of IPCs may not be sufficient to reveal their T cell stimulatory function, and in vivo viral infections might provide more complex and integrated signals, which can be essential to this task. Thus, we tested the antigen presenting function of IPCs stimulated in vivo with VSV. It has been previously shown, that 9 to 12 h after VSV infection, IPCs are activated and secrete IFN-α (14). We therefore infected mHEL transgenic mice with VSV, and, after 12 h, sorted IPCs and DC subsets, analyzed their surface expression of MHC class II and costimulatory molecules (Fig. 1 C), as well as their ability to stimulate HEL-specific naive T cells (Fig. 1 D). Upon VSV infection, IPCs increased cell surface expression of CD40, CD80, MHC class II, and especially CD86 as compared with IPCs from uninfected mice (Fig. 1 C). Careful analysis of CD11c expressing cells, after viral infection, failed to show changes in the expression of the cell surface markers used for IPC identification (CD11b and Ly6C/G), although Ly6C/G expression was up-regulated on some CD11b expressing DCs (not shown).
Even in the context of an in vivo viral infection, IPCs always expressed lower levels of MHC class II and costimulatory molecules than other DCs (Fig. 1 C). In particular, when we measured cell surface expression of the specific peptide–MHC class II complexes consisting of HEL-derived peptide 48–62 bound to I-Ak, by using the monoclonal antibody Aw3.18, IPCs did not stain, either before or after in vivo viral stimulation. In contrast, DCs expressed high levels of specific peptide–MHC complexes (Fig. 1 C). In agreement with this observation, IL-2 production by the 3A9 hybridoma T cell line, which is activated by as few as 30 peptide-MHC complexes on APC and exhibits a linear antigen dose response curve between 30 and 200 peptide MHC complexes, was less efficient when primary IPCs were used as stimulator as compared with other DCs. In vitro (Fig. 1 E) or in vivo (Fig. 1 F) stimulation of IPCs with viruses had no or little effect on IL-2 production, suggesting that IPC activation does not lead to a cell surface increase of specific peptide–MHC complexes. Similarly, viral stimulation of other DC subsets did not result in an increased stimulation of IL-2 production. However, in this case, it is likely that both CD8α+ and CD11b+ DCs display sufficient peptide–MHC complexes to reach maximal activation of the 3A9 hybridoma, even in the absence of stimulation.
All together our data demonstrate that IPCs dramatically differ from classical DCs in their ability to assemble peptide–MHC class II complexes and to stimulate T cells. In addition they did not gain this function after in vitro or in vivo viral stimulation (Fig. 1 C). Similar results were obtained by in vivo injection of LPS and CpG (ODN 1585), stimuli that markedly enhanced the antigen presenting capacity of classical DCs (not shown).
Primary IPCs Are Poor Antigen-presenting Cells for Naive CD8+ T Cells.
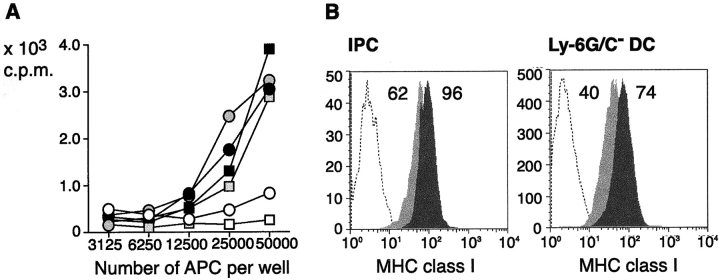
It has been shown that type I IFN promotes survival of CD8+ T cells. As IPCs secrete high levels of type I IFN, they may be specialized in presenting antigen to naive CD8 T cells rather than CD4 T cells. We assessed the ability of IPCs to stimulate CD8 T cells specific for the male minor transplantation antigen HY, which is restricted to the MHC H-2Db class I molecule. IPCs and other DCs were derived from spleens of either uninfected or VSV-infected male B6 mice. HY-specific CD8+ T cells were prepared from female mice bearing a rearranged α/β-TCR transgene specific for the HY male antigen. As shown in Fig. 2 A, IPCs were inefficient in inducing proliferation of HY-specific CD8 T cells, as compared with other DCs. This occurred despite the expression of high levels of class I molecules on IPCs (Fig. 2 B). Thus, IPCs are inadequate in presenting antigens to naive CD8 T cells.
Figure 2.
Antigen presentation to HY-specific naive CD8 T cells by HY-transgenic IPCs and DCs. (A) Proliferation of naive CD8 T cells (5 × 104) purified from HY-transgenic mice to IPCs (open symbols) or DCs (filled symbols) derived from uninfected or VSV infected H-2Db B6 males. VSV infection was performed as in Fig. 1. Proliferation was measured after 72 h. (B) Expression of H-2Db class I molecules in IPCs or DCs of uninfected mice (light gray histograms) or VSV infected mice (dark gray histograms). Open histograms show staining with a control antibody. Numbers indicate MFI.
DCs Expand Antigen-primed Nonpolarized T Cells which Proliferate and Produce IFN-γ upon Restimulation with IPCs.
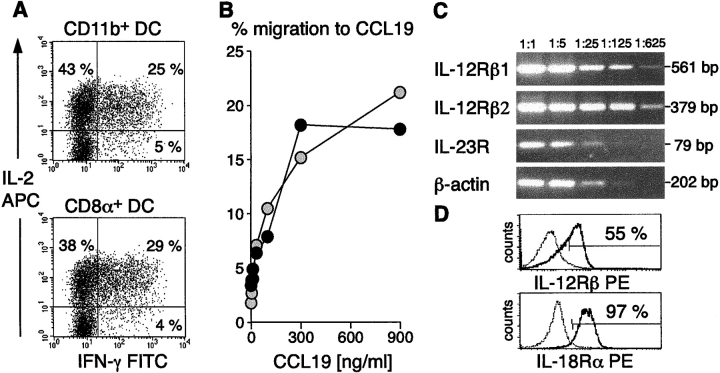
DCs not only stimulate naive T cells but also play a key role in the regulation of CD4 T cell differentiation into Th1 and Th2 cells (15–17). To compare the capacity of the different APC subsets to polarize T cell responses, CD4 T cells derived from 3A9 transgenic mice were cocultured with primary DCs purified from mHEL mice. After 5 to 7 d, T cells expanded by CD8α+ and CD11b+ DCs were analyzed for their capacity to produce IL-2, IL-4, and IFN-γ at single cell level, by intracellular staining (Fig. 3 A). IPCs generally failed to expand sufficient numbers of T cells for subsequent analysis at this stage. Despite the high proliferation rate, ∼70–80% of T cells primed by classical DCs remained nonpolarized, and still secreted high amounts of IL-2, as naive T cells. These T cells responded to CCL19/ELC in transwell migration assays (Fig. 3 B), expressed both IL-12 receptor (IL-12R) β1 and β2 chains as well as the specific IL-23R chain and IL-18Rα chain (Fig. 3, C and D), suggesting that they have lymph node homing capacity and they are prepared to respond to IFN-γ–inducing cytokines.
Figure 3.
DCs induce expansion of antigen experienced uncommitted T cells which have lymph node homing potential and can respond to IFN-γ–inducing cyto-kines. (A) CD4+ 3A9 T cells expanded by freshly isolated CD11b+ or CD8α+ mHEL mouse-derived DCs were analyzed for IFN-γ and IL-2 production, 5–7 d after the first stimulation. (B) In vitro migration to CCL19 of 3A9 T cells expanded by CD11b+ DCs (black filled symbols) or CD8α+ DCs (gray filled symbols) is dose dependent. (C and D) 3A9 T cells expanded by CD11b+ DCs express IL-12, IL-23, and IL-18 receptors thus having the capacity to respond to all these IFN-γ–inducing cytokines. IL-12Rβ1, IL-12Rβ2, IL-23R, and β-actin were detected by RT-PCR. (C) Numbers indicate fold dilutions of cDNAs. IL-12Rβ (β1 and β2 chains), and IL18Rα chain expression were measured at the cell surface of 3A9 T cells expanded by CD11b+ DCs by flow cytometry at the same time of restimulation. Identical results were obtained with 3A9 T cells expanded by CD8α+ DCs.
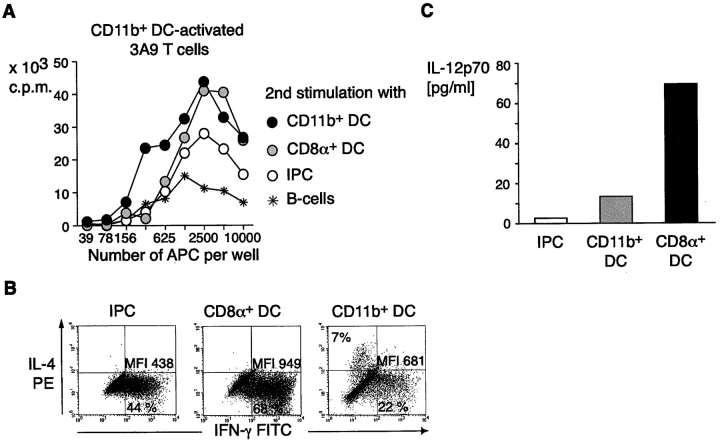
To test whether these antigen-experienced but still largely unpolarized T cells expanded by regular DCs could further proliferate, and differentiate, thus acquiring effector function, they were restimulated a second time, using graded numbers of different APC subsets from the mHEL mouse. Thymidine incorporation was measured at day 3 after restimulation. All CD11c+ APCs, including IPCs, now induced rapid division of antigen-primed T cells while CD11c−/CD19+ splenic B cells were less effective (Fig. 4 A). T cells restimulated with IPCs and CD8α+ DCs produced higher levels of IFN-γ as compared with T cells restimulated by CD11b+ DCs, which still remained largely unpolarized (Fig. 4 B), with a small fraction of cells acquiring IL-4 producing capacity. To test whether the Th1 polarization induced by IPCs was dependent on the production of IL-12, we measured bioactive IL-12 in the supernatants of preactivated T cell/APC cocultures. Substantial amounts of IL-12p70 were detected only when CD8α1 DCs were used as stimulators (17), indicating that Th1 polarization induced by IPCs, in these specific culture conditions, was IL-12 independent.
Figure 4.
IPCs induce proliferation and IFN-γ production by antigen experienced unpolarized T cells. (A) Proliferative response of 3A9 T cells expanded by CD11b+ DCs to graded numbers of primary unstimulated DCs, IPCs, or B cells. 3A9 T cells were restimulated 9–10 d after the first stimulation. (B) IFN-γ and IL-4 production in 3A9 T cells restimulated with primary unstimulated IPCs, CD8α+, and CD11b+ DCs. The percentage of positive cells and mean fluorescence intensity of IFN-γ–producing cells are indicated. (C) Quantitation of IL-12p70 in supernatants of T cell-DC and T cell–IPC cocultures. Supernatants were collected after 24 h and IL-12p70 was measured by ELISA.
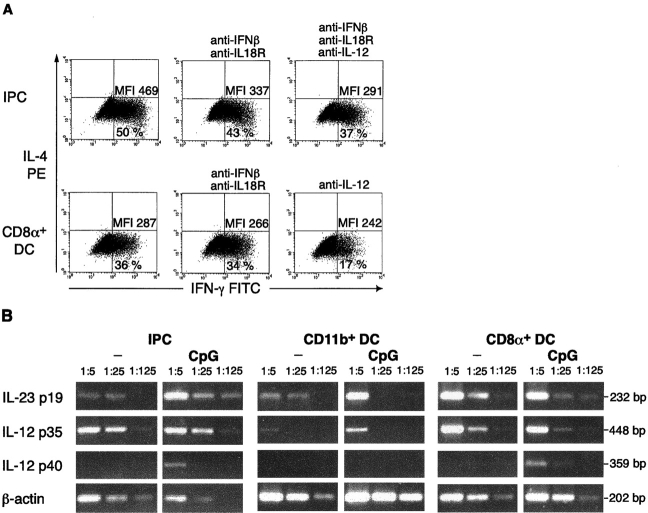
Recent work has shown that in the mouse as in the human system, IFN-γ production can be elicited through an IL-12 independent pathway which involves type I IFN and IL-18 (18, 19). To investigate if Th1 polarization driven by IPC was mediated by production of type I IFN and/or IL-18, experiments were performed with neutralizing antibodies. A modest reduction in the level of IFN-γ production induced by restimulation with IPCs was detected by neutralizing IL-18 and IFN-β activity (Fig. 5 A). A direct role of IFN-α in this context could not be determined because of the lack of good neutralizing reagents against all mouse IFN-α species. However, we failed to detect high levels of type I IFN in supernatants of T cell/IPC cocultures. Neutralizing antibodies to IL-12 effectively reduced the number of IFN-γ producing cells induced upon restimulation with CD8α+ DCs (Fig. 5 A). As IL-23 has been shown to promote proliferation and IFN-γ production in memory-type and preactivated T cells (20), we also investigated if IPCs have the intrinsic ability to produce IL-23. The specific p19 subunit of IL-23, as well as the IL-12/p35 and the IL-12/IL-23 common p40 subunit were amplified from freshly isolated primary IPCs and from primary IPCs stimulated in vitro for 3 h with CpG oligonucleotides. As shown in Fig. 5 B, a remarkable induction of p40 and p19 was detectable in CpG-stimulated IPCs, suggesting that IPCs could represent a major in vivo source of this cytokine, and indicating that IL-23 may take part in the IFN-γ production observed in IPC-restimulated T cells.
Figure 5.
Th1 polarization induced by IPCs is IL-12 independent and only partially reverted by neutralizing antibodies to IFN-β and IL-18. (A) IFN-γ and IL-4 production in 3A9 T cells restimulated with freshly isolated unstimulated IPCs or CD8α+ DCs in the absence or in the presence of neutralizing antibodies to IFN-β and IL-18Rα and/or IL-12. The concomitant neutralization of IFN-β and IL-18 slightly reduce the level of IFN-γ production in T cells restimulated with IPCs; neutralization of IL-12 greatly reduced the number of IFN-γ–producing cells in T cells restimulated by CD8α+ DCs. The percentage of positive cells and mean fluorescence intensity of IFN-γ–producing cells are indicated. (B) Expression of IL-23p19, IL-12p35, and IL-12/IL-23p40 common subunit in freshly isolated and CpG (ODN 1826) stimulated IPCs and DCs measured by RT-PCR. Induction of p40 and p19 can be observed in stimulated IPCs.
Together, our results indicate that “professional” APCs, such as DCs, potently stimulate naive T cells and preferentially expand unpolarized T cells at a first encounter. These antigen-experienced T cells can recirculate to the lymph node and, during a second antigen encounter, start to acquire T helper commitment. In this context, even “non-professional APCs,” such as IPCs, may drive proliferation and rapid differentiation to a Th1 phenotype. At this stage, the cytokines involved in IFN-γ induction are likely to depend on both, the nature of the APC offering the antigen and its activation state.
Discussion
A systematic analysis of the antigen-presenting function of distinct murine APC/DC subsets, isolated ex vivo from the spleen, showed that IPCs were at least 10–50-fold less efficient than typical DCs in expanding both CD4 and CD8 naive T cells. In vivo and in vitro stimulation of IPCs with viruses or CpG oligonucleotides did not significantly improve their T cell stimulatory capacity, despite increased cell surface expression of MHC class II and costimulatory molecules. However, IPCs could still present antigens to antigen-experienced T cells, a cell type less demanding than virgin T cells, in terms of activation requirements. The intrinsic lack of professionalism in initiating an immune response raises the issue of the DC nature of IPCs. However, the ability to stimulate and differentiate antigen-primed T cells suggests that IPCs may indeed exert a more ordinary antigen-presenting function but still potently modulate and shape immune responses (1, 2).
The striking disparity in T cell stimulatory capacity between IPCs and classical DCs could be the result of differences in the antigen processing and presenting machinery. Staining of IPCs and DCs with a monoclonal antibody that can specifically detect the HEL48–62 peptide/I-Ak complex showed a great discrepancy in the number of complexes displayed at the cell surface of IPCs as compared with DCs. While HEL antigen was delivered to the plasma membrane in IPCs, either lack of internalization or insufficient processing, resulted in low numbers of specific complexes to be assembled. Indeed, human IPCs, have been shown to express low levels of mature cathepsins (21) and their antigen-presenting capacity to antigen-specific CD4 or CD8 T cells was comparable to that of a “non-professional” APCs such as monocytes (not shown). In addition, capture and uptake of exogenous antigens, via receptor-mediated endocytosis or macropinocytosis were poorly accomplished by human (22–24), as well as mouse primary or in vitro-derived IPCs (not shown). IPCs were also ineffective in presenting MHC class I–restricted antigens to naive CD8 T cells. As the expression of MHC class I molecules was identical to other DCs, IPCs may be less capable than DCs in processing and loading stable peptides onto MHC class I molecules. Proliferation in IPC/T cell cocultures might also be affected by soluble factors produced by IPCs, such as type I IFN. However, addition of exogenous IFN-α to CD4 T cells stimulated by CD11b+ DCs, did not result in inhibition of T cell proliferation (not shown). IPCs inability to stimulate naive T cells could also reflect a lack of expression of DC specific costimulatory or adhesion molecules essential to trigger naive T cell proliferation.
Professional APCs, such as CD8α+ and CD8α− DCs, showed a powerful capacity of driving proliferation of naive T cells (7, 25). However, over 80% of these T cells did not undergo T helper polarization after the first stimulation, preserving the ability to produce high levels of IL-2 typical of naive T cells. These nonpolarized memory type T cells effectively proliferated and differentiated to IFN-γ–producing Th1 cells upon restimulation with CD8α+ DCs or IPCs. CD11b+ DCs preferentially expanded unpolarized T cells even after a second round of stimulation.
Recent evidence indicates that a normal immune response includes substantial numbers of uncommitted T cells that produce IL-2 but not IFN-γ, IL-4, or IL-5 (26). They seem to provide an expanded pool of antigen-specific T cells that may differentiate into Th1 or Th2 cells later during the primary response or subsequent immune responses, if needed (26, 27). In addition, they have been shown to express CCR7, respond to its ligands and thus easily recirculate to the lymph nodes, where they can further proliferate or differentiate (28). Although it has been reported that TGF-β (26) or brief antigenic stimulation (29), in vitro, promote expansion of unpolarized T cells, the in vivo mechanisms regulating the generation of antigen-experienced uncommitted versus Th1/Th2 effector cells are only partially understood. Our results would suggest that expansion of naive CD4 T cells and acquisition of effector function could be uncoupled processes, performed, when necessary, by distinct APC types. DCs could accomplish the difficult task of expanding rare antigen specific T cells. IPCs, which are likely to be recruited at high numbers to the lymph node during inflammatory conditions (2), could rather promote T helper differentiation of antigen-primed nonpolarized T cells recirculating through high endothelial venules (HEVs). However, conditions may occur in which the two functions can be performed by the same APC type.
The Th1 differentiation effect of CD8α+ DCs during the second restimulation is dependent on the presence of IL-12 (30), which in our system, can be secreted by APCs upon contact with activating T cells via CD40L–CD40 interaction or through cross-linking of other TNF-TNF receptor superfamily members. On the contrary, IPC–induced Th1 polarization seems to involve an IL-12–independent pathway. While IL-18 and IFN-β appear to play a modest role, other IFN-γ–inducing cytokines, such as IL-23, which is indeed produced by CpG-activated IPCs, could have major effects.
Our results are consistent with the following scenario: T cells constantly recirculate through a network of APCs in secondary lymphoid organs (7). During an immune response, DCs, which are equipped with an efficient antigen processing and presenting machinery, can present specific peptide–MHC complexes and rapidly expand rare antigen-specific naive T cells. However, only a minor fraction of these proliferating cells undergo T helper commitment and reach a mature effector function at a first encounter. Certain conditions, such as inflammation, may be critical in promoting the recruitment of other APC subsets, for example IPCs, which can deliver differentiation signals to the proliferating T cells, even in the absence of a second DC–T cell encounter. As a consequence, the efficiency of T helper commitment dramatically increases. Uncoupling the expansion of naive T cells from the acquisition of effector function provides the immune system with an additional regulatory checkpoint. This may be helpful in preventing uncontrolled autoimmune T cell responses and in generating a pool of sentinel antigen-experienced T cells that promptly respond to subsequent antigen encounters.
Acknowledgments
We thank William Eades (Siteman Cancer Center Core Sorting facility) for excellent cell sorting; Ken Murphy for helpful discussion and neutralizing anti–IL-12 antibodies and Tim Wilson for revising the manuscript.
A. Krug is supported by the Deutsche Forschungsgemeinschaft (KR 2199/1-1).
Footnotes
Abbreviations used in this paper: DC, dendritic cell; HEL, hen egg lysozyme; IPC, IFN-producing cell; VSV, vesicular stomatitis virus.
References
- 1.Liu, Y.J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585–589. [DOI] [PubMed] [Google Scholar]
- 2.Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14:373–379. [DOI] [PubMed] [Google Scholar]
- 3.Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 4.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. [DOI] [PubMed] [Google Scholar]
- 5.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 7.Steinman, R.M., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25–37. [DOI] [PubMed] [Google Scholar]
- 8.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 9.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 98:3520–3526. [DOI] [PubMed] [Google Scholar]
- 10.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galibert, L., C.R. Maliszewski, and S. Vandenabeele. 2001. Plasmacytoid monocytes/T cells: a dendritic cell lineage? Semin. Immunol. 13:283–289. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann, T.R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today. 17:138–146. [DOI] [PubMed] [Google Scholar]
- 13.Ho, W.Y., M.P. Cooke, C.C. Goodnow, and M.M. Davis. 1994. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J. Exp. Med. 179:1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. [DOI] [PubMed] [Google Scholar]
- 16.Swain, S.L. 1999. Helper T cell differentiation. Curr. Opin. Immunol. 11:180–185. [DOI] [PubMed] [Google Scholar]
- 17.Moser, M., and K.M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199–205. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, K.B., W.T. Watford, R. Salomon, S.R. Hofmann, G.C. Pien, A. Morinobu, M. Gadina, J.J. O'Shea, and C.A. Biron. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 297:2063–2066. [DOI] [PubMed] [Google Scholar]
- 19.Freudenberg, M.A., T. Merlin, C. Kalis, Y. Chvatchko, H. Stubig, and C. Galanos. 2002. Cutting edge: a murine, IL-12-independent pathway of IFN-gamma induction by gram-negative bacteria based on STAT4 activation by Type I IFN and IL-18 signaling. J. Immunol. 169:1665–1668. [DOI] [PubMed] [Google Scholar]
- 20.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 21.Fiebiger, E., P. Meraner, E. Weber, I.F. Fang, G. Stingl, H. Ploegh, and D. Maurer. 2001. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J. Exp. Med. 193:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito, T., M. Inaba, K. Inaba, J. Toki, S. Sogo, T. Iguchi, Y. Adachi, K. Yamaguchi, R. Amakawa, J. Valladeau, et al. 1999. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 163:1409–1419. [PubMed] [Google Scholar]
- 23.Kohrgruber, N., N. Halanek, M. Groger, D. Winter, K. Rappersberger, M. Schmitt-Egenolf, G. Stingl, and D. Maurer. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250–3259. [PubMed] [Google Scholar]
- 24.Robinson, S.P., S. Patterson, N. English, D. Davies, S.C. Knight, and C.D. Reid. 1999. Human peripheral blood contains two distinct lineages of dendritic cells. Eur. J. Immunol. 29:2769–2778. [DOI] [PubMed] [Google Scholar]
- 25.Steinman, R.M., and K. Inaba. 1999. Myeloid dendritic cells. J. Leukoc. Biol. 66:205–208. [DOI] [PubMed] [Google Scholar]
- 26.Akai, P.S., and T.R. Mosmann. 1999. Primed and replicating but uncommitted T helper precursor cells show kinetics of differentiation and commitment similar to those of naive T helper cells. Microbes Infect. 1:51–58. [DOI] [PubMed] [Google Scholar]
- 27.Wang, X., and T. Mosmann. 2001. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-gamma, and can subsequently differentiate into IL-4- or IFN-gamma-secreting cells. J. Exp. Med. 194:1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iezzi, G., D. Scheidegger, and A. Lanzavecchia. 2001. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J. Exp. Med. 193:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iezzi, G., E. Scotet, D. Scheidegger, and A. Lanzavecchia. 1999. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur. J. Immunol. 29:4092–4101. [DOI] [PubMed] [Google Scholar]
- 30.Moser, M. 2001. Regulation of Th1/Th2 development by antigen-presenting cells in vivo. Immunobiology. 204:551–557. [DOI] [PubMed] [Google Scholar]