Abstract
The integration site, attR, of the Shiga toxin-encoding phage 6220 (stx1ox3) has been determined. The phage integrates into the chromosome of its Escherichia coli host strain, CB6220, within a gene that is homologous to gene Z2577 and encodes an oxidoreductase. This new integration site was found in different Stx1ox3-producing enterohemorrhagic E. coli strains, which were analyzed by PCR.
Shiga toxin-producing Escherichia coli (STEC) strains such as E. coli O157:H7 and other non-O157:H7 STEC serotypes are associated with disease in humans, leading to diarrhea and frequently to hemorrhagic colitis and the hemolytic-uremic syndrome (9, 12, 15). STEC strains colonize the gastrointestinal tracts of cattle, sheep, and goats, which shed these organisms into the environment, and thus can be transmitted to humans via contaminated food (2, 10, 15). The major pathogenicity factors are Shiga toxins, which are generally phage borne (3, 7, 10, 14, 17, 20, 21, 25). Up to now, four Stx2-encoding phages (933W, VT2-Sa, VT2-Sakai, and φP27) and one Stx1-encoding phage (VT1-Sakai) have been sequenced (13, 18, 19, 20, 24, 27). The Stx2-converting phages VT2-Sakai and 933W integrate into the E. coli K-12 chromosome within wrbA (23.0 min), encoding the Trp repressor binding protein (13, 19). In contrast, the Stx2e-encoding phages φP27 and VT1-Sakai integrate into yecE (42 min) and yehV (47.7 min), respectively (4, 20, 27).
In this work, the integration site of the Shiga toxin Stx1ox3-encoding bacteriophage 6220 was determined. The phage was isolated from the human strain CB6220, which belongs to serogroup O146:H21 and has been previously investigated for its morphology, DNA similarity to other Stx phages, host range, lysogenization of Shigella and Salmonella strains, and stx genes (10). To determine the integration site of phage 6220, a cosmid library of its E. coli host strain was constructed with the Gigapack III XL packaging kit (Stratagene). Clones containing phage 6220 DNA were identified by colony hybridization using EcoRI-digested phage 6220 DNA as the probe (22). Positive clones were further investigated by dot blot hybridization with DNA probes comprising the attL or attR region of phage 933W (accession no. AF125520). Previous experiments had shown that this phage is closely related to phage 6220 (10). The probes were generated by PCR with primers (Table 1) yielding products of 315 bp (attL) and 350 bp (attR). Two cosmid clones hybridized with the attR probe (data not shown). In contrast, no specific hybridization signals were detected with the attL probe. Of the two positive attR cosmid clones, only one clone (H42) was further investigated.
TABLE 1.
Primers and PCR conditions used in this study
| Primer | Nucleotide sequence | Template | PCR product size (bp) | Conditions (denaturing; annealing; extension)a |
|---|---|---|---|---|
| 933attL-F | 5′-TTCATCCTCCTGGTCACTTTGG-3′ | Phage 933W | 315 | 94.0°C, 30 s; 57.0°C, 60 s; 72.0°C, 60 s |
| 933attL-R | 5′-CGTTCGCAAGAATCACAAGGC-3′ | |||
| 933attR-F | 5′-AGTGGTTTCGTTGGCGATAGTG-3′ | Phage 933W | 350 | 94.0°C, 30 s; 57.0°C, 30 s; 72.0°C, 45 s |
| 933attR-R | 5′-TTTCTGGATGAGGCATTTACCG-3′ | |||
| 6220attR-F | 5′-AGTGGTTTCGTTGGCGATAGTG-3′ | E. coli CB6220 | 472 | 94.0°C, 30 s; 58.5°C, 30 s; 72.0°C, 60 s |
| 6220attR-R | 5′-AGATAGCCCGTCATTTCTGCG-3′ | |||
| Z2577F | 5′-AACCCCATTGATGCTCAGGCTC-3′ | E. coli EDL933 | 909 | 94.0°C, 60 s; 55.0°C, 90 s; 72.0°C, 60 s |
| Z2576R | 5′-TTCCCATTTTACACTTCCTCCG-3′ |
All PCRs were run for 30 cycles with a final extension step of 5 min at 72°C; all PCRs were created in this study.
Cosmid DNA was isolated with a plasmid maxi kit (Clontech) following the instructions of the supplier. The cosmid was digested with EcoRI, resulting in seven to eight restriction fragments. To identify the EcoRI fragment containing the attR integration site of phage 6220, we hybridized the EcoRI digest with a 1.1-kb PCR product obtained using the attR primers (Table 1) and CB6220 DNA as the template. Two EcoRI restriction fragments of 7.0 and 6.5 kb hybridized with the probe. However, the signal of the 7-kb EcoRI fragment was much stronger.
In order to isolate the insert from cosmid H42, plasmid DNA was digested with NotI. The cloned DNA fragment was separated from SuperCos1 vector DNA by agarose gel electrophoresis. The insert of approximately 19 kb was excised from the gel and isolated by β-agarase treatment (Gibco BRL). Recovered DNA was subcloned by digestion with EcoRI, ligation to the EcoRI-digested vector pLitmus28 (New England Biolabs), and transformation of E. coli DH5α. Bacteria were plated on Luria-Bertani agar containing 100 μg of ampicillin per ml, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and IPTG (isopropyl-β-d-thiogalactopyranoside) according to standard methods (22). Transformants were screened by restriction analysis. One clone harbored the 7-kb EcoRI fragment of cosmid H42 and was further investigated.
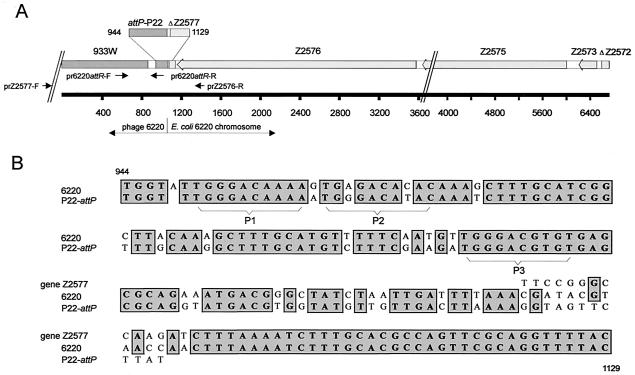
We determined the complete nucleotide sequence (6,803 bp) of this EcoRI fragment by primer walking. The sequence was aligned with known DNA sequences in the National Center for Biotechnology Information databases, confirming that it harbors the integration site of phage 6220 (Fig. 1A). We identified phage-related sequences on this fragment as well as sequences belonging to the E. coli CB6220 chromosome. A stretch of 879 bp located at the left end of the restriction fragment is very similar (94%) to the attR region of the phages VT2-Sakai (accession no. AP000422) and 933W (accession no. AF125520). However, the approximately 300 terminal nucleotides of the VT2-Sakai and 933W genomes that contain the attR site (GTTTCAA) important for the specific integration of these phages into the E. coli wrbA gene could not be identified in the 6220 prophage DNA. Instead a stretch of 118 bp (positions 944 to 1061) was detected at the terminus of the prophage DNA that shows strong homology (82.2%) to the attP region of phage P22 (accession no. M10893). The attP site of P22 comprises approximately 260 bp and contains two integration host factor binding sites and five arm-type binding sites for Int (23). In the attR region of phage 6220, we identified DNA sequences homologous to the arm-type binding sites P1 (5′-TGGGACAAAA-3′), P2 (5′-TGGGACATAC-3′), and P3 (5′-TGGGACGTGT-3′) (Fig. 1B). The sequences of the P1 and P3 homologs are 100% identical to the sequences of phage P22, while the P2-related sequence of phage 6220 exhibited two substitutions at positions 3 (A instead of G) and 8 (C instead of T). The integration site (ataA) of phage P22 in Salmonella enterica serovar Typhimurium is known. P22 integrates within thrW, located in the E. coli K-12 chromosome at approximately 6.0 min (1, 26). The sequence analysis of the cloned CB6220 EcoRI fragment revealed that phage 6220 integrates at a position different from those of other E. coli Stx phages and phage P22. Adjacent to the P22-related sequence, we found a stretch of 5,728 bp (positions 1076 to 6803) almost identical to chromosomal DNA of E. coli strains EDL933 (98% homology) (accession no. AE005382) and MG1655 (99% homology) (accession no. D90802). The related DNA region in the chromosome of strain MG1655 is located at 35.7 to 36.1 min. The attR region of phage 6220 was identified next to a DNA sequence homologous to gene Z2577 of strain EDL933 that was obviously interrupted by integration of the phage genome. The exact transition point from the 6220 prophage to the CB6220 chromosome still has to be determined. There are 14 nucleotides that match neither phage P22 nor the E. coli chromosome (Fig. 1B). It can be assumed that the transition point is located within this region. Adjacent to the interrupted gene Z2577, we identified three complete open reading frames corresponding to the genes Z2576, Z2575, and Z2573 of EDL933 and a sequence corresponding to the 5′ end of gene Z2572. The gene order in CB6220 turned out to be identical to that in EDL933.
FIG. 1.
Integration site of phage 6220. (A) Schematic illustration of the DNA sequences located on the cloned EcoRI fragment containing the phage 6220 attR site. Similarities to the attachment regions of the phages 933W and P22 and to the E. coli genes Z2572 to Z2577 are shown. The transition region analyzed in panel B is depicted schematically. The positions of primers (pr) used in this study are indicated. (B) Transition region (positions 944 through 1129) from the 6220 prophage to the E. coli chromosome. The alignment shows homologies to attP of phage P22 (accession no. M10893) and to the E. coli gene Z2577 (accession no. AE005382). The positions of the related arm-type binding sites P1, P2, and P3 of phage P22 are indicated.
In order to determine whether other STEC strains producing the Stx1ox3 toxin might harbor a similar prophage within gene Z2577, several E. coli strains were analyzed by PCR (Table 2). All wild-type strains had been characterized previously for the production of Stx by the verocytotoxin-producing E. coli reverse passive latex agglutination test (10). The PCR was performed with the primers Z2577F and Z2576R (Table 1), which bind in the E. coli EDL933 chromosome immediately upstream and downstream from the phage 6220 integration site (Fig. 1A). In strains not harboring a prophage at that position, a product of 909 bp was expected. In all Stx1 wild-type strains investigated, strain EDL933 (Stx1, Stx2), and E. coli C600 including its lysogenized derivatives (933W and H-19B), a product of approximately 909 bp was obtained. DNA sequencing confirmed that gene Z2577 was amplified in these strains. In contrast, most of the Stx1ox3-strains investigated revealed no PCR product, suggesting that these strains might contain a prophage at that position. A faint band was detected in the preparation of strain DG128/1, and we also found a product in strain C600 lysogenized with phage 6220. We suspect that the phages in these strains are rather unstable, yielding bacteria cured of their prophages.
TABLE 2.
E. coli strains investigated for the occurrence of the phage Stx1ox3 integration site
| Strain | Origin | Year | Serotype | stx gene | Reference | Product of PCR
|
|
|---|---|---|---|---|---|---|---|
| 909 bp | 472 bp | ||||||
| C600 | Laboratory strain | 22 | − | NDa | |||
| C600-H-19B | 1 | 2 | + | − | |||
| C600-933W | 2 | 2 | + | − | |||
| C600-6220 | 2000 | 1ox3 | 10 | (+)b | + | ||
| CB6220 | Human | 1989 | O146:H21 | 1ox3 | This work | − | + |
| EDL933 | Human | 1982 | O157:H7 | 1, 2 | 16 | + | − |
| DG137/4 | Sheep | 1989 | O146:H21 | 1ox3 | This work | − | + |
| CB6618 | Human | 1996 | O146:H21 | 1ox3 | This work | − | + |
| DG128/1 | Sheep | 1989 | O128:H2 | 1ox3 | This work | (+) | + |
| CB7050 | Human | 1997 | O128:H− | 1ox3 | This work | − | + |
| CB6660 | Human | 1997 | O22:H8 | 1ox3 | This work | − | + |
| CB7151 | Human | 1997 | O111:H− | 1ox3 | This work | − | + |
| DG92/3 | Cattle | 1989 | O22:H8 | 1 | This work | + | − |
| CB4536 | Cattle | 1995 | O22:H8 | 1 | This work | + | − |
| CB7263 | Human | 1997 | O156:H25 | 1 | This work | + | − |
| DG33/1 | Cattle | 1989 | O156:H21 | 1 | This work | + | − |
| CB6595 | Human | 1996 | O76:H19 | 1ox3 | This work | − | + |
| CB8008 | Human | 1999 | O76:H+ | 1ox3 | This work | − | + |
| DG85/2 | Cattle | 1989 | O76:H21 | 1 | This work | + | − |
| DG87/1 | Cattle | 1989 | O76:H21 | 1 | This work | + | − |
| 1404-83 | Human | O157:H7 | 1 | RKIc | + | − | |
| H19 | Human | 1977 | O26:H11 | 1 | 11 | + | − |
ND, not determined.
(+), weak PCR product.
RKI, Robert Koch Institut strain collection.
We also looked for phage 6220-related sequences in the STEC strains investigated by performing PCR with primers deduced from the determined 6220 attR region (Fig. 1A). In phage 6220, the forward primer 6220attR-F and reverse primer 6220attR-R (Table 1) yielded a fragment of 472 bp. As expected, products of this size were obtained with the strains CB6220 and C600 lysogenized with 6220. In addition, similar-sized products were detected with all Stx1ox3 strains. None of the investigated Stx1 and Stx2 strains revealed a PCR product. We determined the DNA sequences of all products obtained with Stx1ox3 strains and found sequences identical to those in phage 6220.
In conclusion, the data presented here demonstrate that phage 6220 integrates into the E. coli chromosome at a position different from the integration sites of other Stx phages. This result was a little surprising, since phage 6220 was shown to be closely related to Stx2 phage 933W (10). Moreover, part of the 6220 attR region shows nearly 100% sequence identity to the attR region of 933W. However, adjacent to this DNA sequence, the phage 6220 attR region shows strong homology to a part of the attP region of phage P22. We speculate that the phage 6220 attR region is a product of recombination between two phage genomes resulting in a new integration site. It confirms the notion that the genomes of lambdoid phages are mosaics arising by multiple recombination events (5, 6, 8).
Nucleotide sequence accession number.
The nucleotide sequence determined in this study has been deposited in the EMBL gene bank under accession no. AJ576011.
Acknowledgments
We thank Lothar Beutin for supplying E. coli strains and Jens Hammerl for his support in preparing the manuscript.
REFERENCES
- 1.Bachmann, B. J. 1987. Linkage map of Escherichia coli K-12, edition 7, p. 807-876. In J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and E. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 2. American Society for Microbiology, Washington, D.C.
- 2.Beutin, L., D. Gleier, S. Zimmermann, and H. Karch. 1995. Virulence markers of Shiga-like toxin-producing Escherichia coli strains originating from healthy domestic animals of different species. J. Clin. Microbiol. 33:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin, L., E. Strauch, and I. Fischer. 1999. Isolation of Shigella sonnei lysogenic for a bacteriophage encoding gene for production of Shiga toxin. Lancet 353:1498. [DOI] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 5.Hendrix, R. W., M. C. M. Smith, R. N. Burns, M. E. Ford, and G. F. Hatfull. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. USA 96:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrix, R. W., J. G. Lawrence, G. F. Hatfull, and S. Casjens. 2000. The origins and ongoing evolution of viruses. Trends Microbiol. 8:504-508. [DOI] [PubMed] [Google Scholar]
- 7.Jackson, M. P., R. J. Neill, A. D. O'Brien, R. K. Holmes, and J. W. Newland. 1987. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol. Lett. 44:109-114. [DOI] [PubMed] [Google Scholar]
- 8.Johansen, B. K., Y. Wasteson, P. E. Granum, and S. Brynestad. 2001. Mosaic structure of Shiga-toxin-2-encoding phages isolated from Escherichia coli O157:H7 indicates frequent gene exchange between lambdoid phage genomes. Microbiology 147:1929-1936. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, R. P., R. C. Clarke, J. B. Wilson, S. C. Read, K. Rahn, S. A. Renwick, K. A. Sandhu, D. Alves, M. A. Karmali, H. Lior, S. A. McEwen, J. S. Spika, and C. L. Gyles. 1996. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J. Food Prot. 59:1112-1122. [DOI] [PubMed] [Google Scholar]
- 10.Koch, C., S. Hertwig, R. Lurz, B. Appel, and L. Beutin. 2001. Isolation of a lysogenic bacteriophage carrying the stx1ox3 gene, which is closely associated with Shiga toxin-producing Escherichia coli strains from sheep and humans. J. Clin. Microbiol. 39:3992-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konowalschuk, J., N. Dickie, S. Stavric, and J. I. Speirs. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1997. Characterization of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli serotypes isolated from sheep. J. Clin. Microbiol. 35:892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino, K., K. Yokoyama, Y. Kubota, C. H. Yutsudo, S. Kimura, K. Kurokawa, K. Ishii, M. Hattori, I. Tatsuno, H. Abe, T. Iida, K. Yamamoto, M. Onishi, T. Hayashi, T. Yasunaga, T. Honda, C. Sasakawa, and H. Shinagawa. 1999. Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157:H7 derived from the Sakai outbreak. Genes Genet. Syst. 74:227-239. [DOI] [PubMed] [Google Scholar]
- 14.Muniesa, M., J. Recktenwald, M. Bielaszewska, H. Karch, and H. Schmidt. 2000. Characterization of a Shiga toxin 2e-converting bacteriophage from an Escherichia coli strain of human origin. Infect. Immun. 68:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien, A. D., T. A. Lively, M. E. Chen, S. W. Rothman, and S. B. Formal. 1983. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet i:702. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien, A. D., L. R. M. Marques, C. F. Kerry, J. W. Newland, and R. K. Holmes. 1989. Shiga-like toxin converting phage of enterohemorrhagic Escherichia coli strain 933. Microb. Pathog. 6:381-390. [DOI] [PubMed] [Google Scholar]
- 18.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grothbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. S. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 19.Plunkett, G., D. J. Rose, T. J. Durfee, and F. R. Blattner. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7 Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recktenwald, J., and H. Schmidt. 2002. The nucleotide sequence of Shiga toxin (Stx) 2e-encoding phage φP27 is not related to other Stx phage genomes, but the modular genetic structure is conserved. Infect. Immun. 70:1896-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rietra, P. J. G. M., G. A. Willshaw, H. R. Smith, A. M. Field, S. M. Scotland, and B. Rowe. 1989. Comparison of Vero-cytotoxin-encoding phages from Escherichia coli of human and bovine origin. J. Gen. Microbiol. 135:2307-2318. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Smith-Mungo, L., I. T. Chan, and A. Landy. 1994. Structure of the P22 att site. J. Biol. Chem. 269:20798-20805. [PubMed] [Google Scholar]
- 24.Strockbine, N. A., L. R. M. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unkmeir, A., and H. Schmidt. 2000. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect. Immun. 68:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vander Byl, C., and A. M. Kropinski. 2000. Sequence of the genome of Salmonella bacteriophage P22. J. Bacteriol. 182:6472-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama, K., K. Makino, Y. Kubota, M. Watanabe, S. Kimura, C. H. Yutsudo, K. Kurokawa, J. Ishii, M. Hattori, I. Tatsuno, H. Abe, M. Yoh, T. Iida, M. Ohnishi, T. Hayashi, T. Yasunaga, T. Honda, C. Sasakawa, and H. Shinagawa. 2000. Complete nucleotide sequence of the prophage VT1-Sakai carrying the Shiga toxin 1 genes of the enterohemorrhagic Escherichia coli O157:H7 strain derived from the Sakai outbreak. Gene 258:127-139. [DOI] [PubMed] [Google Scholar]