Abstract
CD4+CD25+ regulatory T cells inhibit organ-specific autoimmune diseases induced by CD4+CD25−T cells and are potent suppressors of T cell activation in vitro. Their mechanism of suppression remains unknown, but most in vitro studies suggest that it is cell contact–dependent and cytokine independent. The role of TGF-β1 in CD4+CD25+ suppressor function remains unclear. While most studies have failed to reverse suppression with anti–transforming growth factor (TGF)-β1 in vitro, one recent study has reported that CD4+CD25+ T cells express cell surface TGF-β1 and that suppression can be completely abrogated by high concentrations of anti–TGF-β suggesting that cell-associated TGF-β1 was the primary effector of CD4+CD25+-mediated suppression. Here, we have reevaluated the role of TGF-β1 in CD4+CD25+-mediated suppression. Neutralization of TGF-β1 with either monoclonal antibody (mAb) or soluble TGF-βRII-Fc did not reverse in vitro suppression mediated by resting or activated CD4+CD25+ T cells. Responder T cells from Smad3−/− or dominant-negative TGF-β type RII transgenic (DNRIITg) mice, that are both unresponsive to TGF-β1–induced growth arrest, were as susceptible to CD4+CD25+-mediated suppression as T cells from wild-type mice. Furthermore, CD4+CD25+ T cells from neonatal TGF-β1−/− mice were as suppressive as CD4+CD25+ from TGF-β1+/+ mice. Collectively, these results demonstrate that CD4+CD25+ suppressor function can occur independently of TGF-β1.
Keywords: CD4+ suppressor T cells, autoimmunity, tolerance, immunoregulation, IL-2 receptor
Introduction
Although clonal deletion of autoreactive T cells in the thymus and the induction of T cell anergy in the periphery are the major mechanisms responsible for T cell tolerance, the ability to detect autoreactive T cells in normal individuals strongly suggests that neither of these mechanisms is fail-safe and that additional mechanisms of dominant immune tolerance must exist (1–3). Studies in a number of experimental animal models have demonstrated the existence of regulatory T cell populations that prevent the activation of autoreactive T cells (4). CD4+CD25+ T cells represent one of the best characterized populations of regulatory T cells and prevent the development of autoimmune disease that occurs after day 3 thymectomy, the transfer of disease mediated by autoreactive T cell clones, as well as disease induced by CD4+ T cell populations that are depleted of regulatory T cells (e.g., CD4+CD25− or the CD4+CD45RBhigh subsets; references 5 and 6). Furthermore, a partial deficiency of CD4+CD25+ T cells may also contribute to the genetic susceptibility of autoimmunity as seen in NOD mice, which spontaneously develop insulin-dependent diabetes mellitus (7). CD4+CD25+ T cells suppress CD4+ CD25− or CD8+ T cell proliferation and cytokine production (IL-2 and IFN-γ) in vitro (8, 9). However, the mechanism by which CD4+CD25+ T cells mediate their suppressive effects is poorly understood. In most studies, suppression is cell contact dependent and not mediated by IL-4 or IL-10 as CD4+CD25+ T cells from IL-4–deficient (−/−) and IL-10−/− mice are as effective as CD4+CD25+ T cells from wild-type (WT)* mice in vitro and in mediating suppression of autoimmune gastritis (AIG) in vivo (8, 10).
TGF-β1 plays an important role in maintaining immune homeostasis in general, and in regulating T cells, in particular. Global disruption of the TGF-β1 gene in mice has clearly illustrated the importance of this specific TGF-β isoform in regulating immune cell differentiation and function. TGF-β1−/− mice manifest a spontaneous autoimmune-like syndrome, with aberrant expression of MHC class I and II antigens, circulating SLE-like IgG antibodies to nuclear antigens, pathogenic glomerular IgG deposits, and a progressive infiltration of mononuclear cells into multiple organs (11–15). This syndrome can be adoptively transferred to lethally irradiated TGF-β1+/+ recipients with TGF-β1−/− bone marrow, suggesting an important role for autocrine production of TGF-β1 in immune cells. The importance of T cells in the genesis of this syndrome is supported by the observation that T cell–specific disruption of TGF-β signaling leads to a similar, though less aggressive syndrome (16–19). In this model, T cell–specific expression of a dominant-negative TGF-β type RII transgenic (DNRIITg) results in spontaneous activation of T cells, lymphocyte infiltration into multiple organs, and autoantibody production. Specific intracellular intermediates that regulate these responses have also been globally disrupted in mice, including the genes encoding Smad2 and Smad3 (14, 18). Loss of Smad3 has been associated with chemotaxis defects, altered T cell responses to TGF-β, and invasive mucosal infection with typically nonpathogenic microorganisms.
Overall, these in vivo models support the importance of TGF-β in maintaining immune homeostasis, and raise the possibility that TGF-β may play a critical role in immune suppression mediated by CD4+CD25+ suppressor T cells. Neutralizing antibodies to TGF-β have been shown to reverse suppression mediated by regulatory T cells (enriched in CD25+ T cells) of inflammatory bowel disease (IBD) in mice (20) and thyroiditis (21) in rats. However, the cellular source and specific isoform of the TGF-β was not addressed in these studies but was assumed to be TGF-β1 derived from the regulatory T cells. Considerable controversy exists on the role TGF-β1 plays in the induction and execution of CD4+CD25+-mediated suppressor function in vitro. Most studies have concluded that suppression is not mediated by secreted TGF-β1 because of the requirement for cell contact and the failure to reverse suppression with anti–TGF-β1 in murine (8, 22, 23) as well as human systems (24–29). Nakamura et al. have recently proposed a novel model in which CD4+CD25+ T cells express cell surface latent TGF-β1 and mediate suppression via a cell contact–dependent presentation/activation of latent TGF-β1 to a TGF-βR on target CD4+CD25− T cells (30). This conclusion was based on the ability to detect the expression of cell surface TGF-β1 by flow cytometry and to completely abrogate suppression with high concentrations of anti–TGF-β. However, this study did not conclusively show functional evidence for a direct effect of TGF-β1 on responder T cells, nor did it consider the possibility that cell surface TGF-β1 mediated its effects by acting on the CD4+CD25+ suppressor T cells.
In this paper, we have taken advantage of the genetic models described above to critically evaluate whether CD4+CD25+-mediated suppression of T cell activation is dependent on the ability of CD4+CD25+ to produce TGF-β1 or on the ability of target T cells to respond to TGF-β1. Neutralization of TGF-β1 with either mAb or soluble TGF-βRII-Fc chimera did not reverse in vitro suppression mediated by resting or activated CD4+CD25+ T cells. Then, we demonstrate that both CD4+ and CD8+ responder T cells from Smad3−/− and DNRIITg mice, while unresponsive to exogenous rhTGF-β1, are as susceptible to suppression by CD4+CD25+ T cells as WT T cells. Furthermore, we demonstrate that CD4+CD25+ T cells isolated from neonatal TGF-β1−/− mice are as effective as CD4+CD25+ T cells from WT mice in their capacity to suppress proliferative responses of T cells from WT mice. Finally, we show that neutralization of TGF-β1 in vivo did not abrogate CD4+CD25+-mediated protection of AIG. These results formally demonstrate that CD4+CD25+ suppressor activity can occur in the functional absence of TGF-β1 production or responsiveness.
Materials and Methods
Mice.
TGF-β1−/− and Smad3−/− mice (C57BL/6×Sv129) were generated by targeted gene disruption in murine embryonic stem cells by homologous recombination (18, 31). Control littermates were bred and maintained in a pathogen-free animal facility. Female C57BL/6 and BALB/c mice were obtained from the National Cancer Institute. DNRIITg mice (C57BL/6 genetic background) were provided by Philip Lucas (National Cancer Institute, National Institutes of Health). Unless specified, the mice used in these experiments were 6–8 wk of age.
Reagents.
Human rIL-2 was purchased from Peprotech. Human rTGF-β1 was obtained from R&D Systems. The following antibodies were used for in vitro stimulation/neutralization and flow cytometry experiments: biotin anti-CD25 (7D4 clone); PE-CD25 (PC61 clone); PE-streptavidin; PE anti-CD4; PE anti-CD8; PE anti-CD69; and purified anti-CD3ε (2C11) were purchase from BD PharMingen. Tricolor CD4 was purchased from Caltag Laboratories. Anti–TGF-β antibodies (clones 1D11, 9016.2, and R&D Systems model AB-101-NA) and soluble TGF-βRII-Fc were purchased from R&D Systems.
Cell Purification.
CD4+CD25+ T cells were isolated on a FACStar™ Cell Sorter. In brief, fresh lymph nodes (axillary, inguinal, brachial, and mesenteric) were collected from adult BALB/c, C57BL/6, or appropriate knockout mice and single cell suspensions were prepared by passing cells through a sterile wire mesh. Cells were resuspended in HBSS/5% FCS, gently layered on a Lympholyte-M gradient (Cederlane), and centrifuged according to the manufacturer's specifications to remove dead cells. The resulting cell preparation was stained with biotin-conjugated anti-CD25 (15 μg/108 cells) in PBS/2% FCS for 20 min at 4°C, washed, and then incubated with PE-streptavidin (15 μg/108 cells) and Tricolor-CD4 (15 μg/108 cells) in PBS/2% FCS for 20 min at 4°C. The cells were then washed and resuspended in RPMI 1640/10% FCS (no phenol red) for FACS® sorting. The purity of the final CD4+CD25+ preparation was typically >98%. T cell–depleted spleen cells were used as APC and were prepared by lysing the RBCs with ACK lysing buffer, followed by negative selection (depletion of T cells with Thy1.2 magnetic beads) on the AutoMACS® magnetic separation system according to the manufacturer's protocol. The cells were then washed extensively and irradiated at 3,000 rads. CD4+ and CD8+ responder T cells were prepared from spleens or lymph node of appropriate adult mice. Single cell suspensions were prepared as described above, and responder T cells were purified by either negative (depletion of B220+, CD4, or CD8 and I-Ab-positive cells) or positive selection (using CD4 or CD8 magnetic beads) on the AutoMACS® magnetic separation system. In some experiments, cells were labeled with CFSE as described previously (32).
Proliferation Assays.
Proliferation assays were performed by culturing CD4+ or CD8+ T cells (5 × 104) in 96-well, flat-bottomed microtiter plates (0.2 ml) in RPMI 1640 (Biofluids) supplemented with 10% heat-inactivated FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mM l-glutamine, 10 mM Hepes, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (all obtained from Biofluids) and 50 μM 2-ME (Sigma-Aldrich) with irradiated, T cell–depleted spleen cells (1–2 × 105 cells) for 72 h at 37°C in 7% CO2. Cell cultures were pulsed with 1 μCi 3[H]TdR for the last 6–12 h. All the data represents the average counts per minute of triplicate determinations. All proliferation experiments were repeated at least three times.
To generate CD4+CD25+ short-term cell lines, cell sorted CD4+CD25+ cells were cultured with irradiated APC (1:1), 0.5 μg/ml anti-CD3 and 5 ng/ml (100 U/ml) hIL-2 for 72 h and were then split and maintained in IL-2 media for ∼5–7 d. In some experiments, CD4+CD25+ cells were prepared by stimulation with plate-bound anti-CD3 (5 ug/ml) and IL-2 (5 ng/ml) for 72 h.
Flow Cytometry.
Cells were collected and stained with PE-CD4, PE-CD8, FITC-CD69, or FITC-CD25 and analyzed with FACScan™ flow cytometer (Becton Dickinson) using the CELLQuest™ software program.
Induction and Suppression of AIG.
CD4+CD25+ T cells were depleted from BALB/c mice by treatment with anti-CD25 (PC61, 1 mg, intraperitoneally) every other day beginning on day 10 of life. Splenocytes from the depleted mice were harvested on day 24 and injected (20 × 106 cells) intravenously into BALB/c nu/nu or C.B-17 SCID mice. Some animals were coinjected with splenocytes (50 × 106) from normal BALB/c mice. One group of recipients was treated with anti–TGF-β (1D11.16, 2 mg intraperitoneally, on days –1 and 1 of cell transfer and weekly thereafter for 6 wk). Gastric pathology was evaluated as described previously (33) using disease scores ranging from normal gastric mucosa (0) to severe gastritis (6).
Results
Anti–TGF-β1 Antibodies and Soluble TGF-βRII-Fc Fail To Reverse Suppression Mediated by Resting or Activated CD4+CD25+ T Cells.
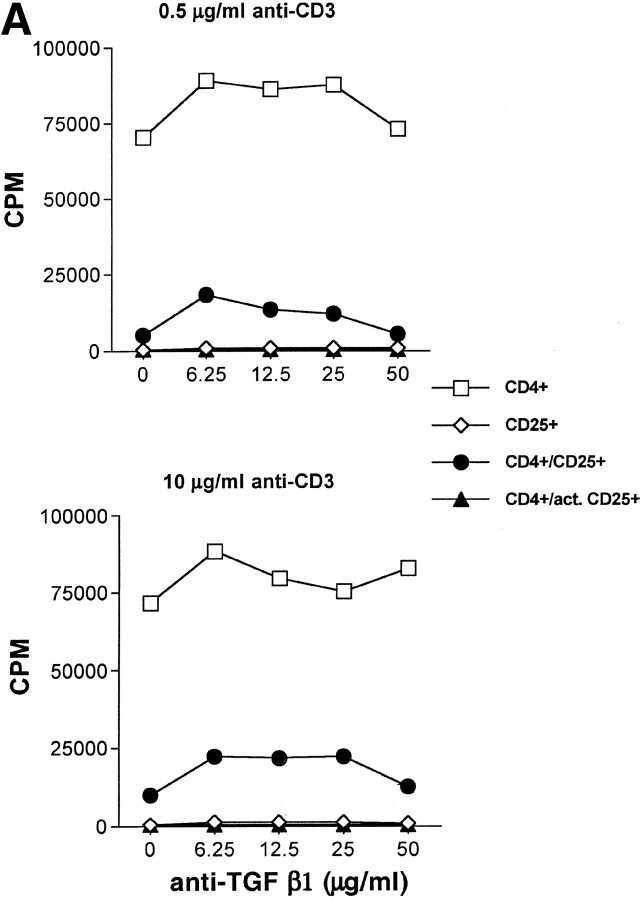
We and others (8, 22, 23) have previously demonstrated that TGF-β1 mAbs (10 μg/ml) failed to reverse suppression mediated by freshly isolated CD4+CD25+ T cells. Nakamura et al. (30) demonstrated that suppression could only be reversed with higher concentrations (>25 μg/ml) of anti–TGF-β1 and suggested that the higher concentration was needed to penetrate the physical interaction of the responder T cells with the suppressor T cell–bearing surface latent TGF-β1. We did not observe reversal of suppression of T cell responses to soluble anti-CD3 and APC even in the presence of high concentrations of anti–TGF-β1 (Fig. 1) . We previously demonstrated that activated CD4+CD25+ cells maintain their anergic phenotype and exhibit enhanced suppressor function in the absence of TCR reengagement (34). Nakamura et al. have demonstrated that activated CD4+ CD25+ T cells express high levels of membrane-bound latent TGF-β1 complexes and have hypothesized that this would account for the enhanced antigen nonspecific suppressive capacity of activated CD4+CD25+ T cells. However, suppression mediated by activated CD4+CD25+ T cells was readily observed even in the presence of high concentrations of anti–TGF-β (50 μg/ml) (Fig. 1 A). The inability to reverse suppression was also observed when the TCR signal strength was increased 20-fold (Fig. 1 A) demonstrating that the failure to abrogate suppression could not be explained by suboptimal T cell activation. Identical results were observed when activated CD4+ CD25+ T cells were mixed with CD4+ T cells from TCR Tg mice that were stimulated with specific peptide and APC under conditions where the activated suppressor cells were not restimulated in the assay culture (unpublished data). In some experiments, antibodies directed at all TGF-β isoforms or antibodies directed at latent TGF-β1 were used with identical results (unpublished data). Similar results were obtained using limiting numbers of activated suppressors in order to maximize the capacity of high dose anti–TGF-β1 to reverse suppression (unpublished data).
Figure 1.
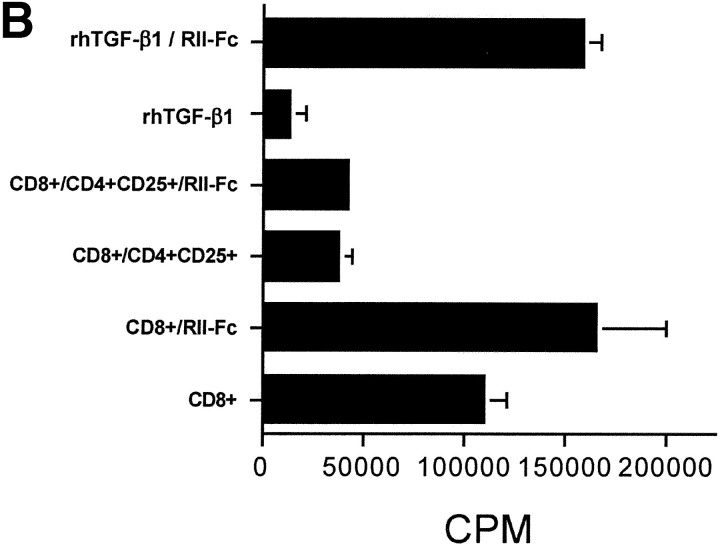
TGF-β1 blockade does not reverse CD4+CD25+-mediated suppression of T cell proliferation. (A) WT CD4+ T cells (5 × 104) were stimulated with irradiated T cell–depleted spleen cells (2 × 105) either alone or with freshly isolated or activated (•) CD4+CD25+ T cells (1:2 suppressor/responder ratio) in the presence of titrated doses of anti–TGF-β1. Results from a representative experiment of three are shown. (B) OT-I Tg CD8+ T cells were stimulated with H-2Kb/OVA257–264 soluble tetramer (0.1 μg/ml) either alone or with activated CD4+CD25+ T cells (1:2 suppressor/responder ratio) in the presence or absence of soluble TGF-βRII-Fc (10 μg/ml). In some instances, rhTGF-β1 was added as a control (5 ng/ml).
Activated CD4+CD25+-mediate suppression of antigen-specific CD8+ T cell activation in an APC-free manner when the CD8+ T cells are stimulated with peptide-MHC class I tetramers (9). To further evaluate the potential role of TGF-β1 in the suppression of T cell activation, we stimulated CD8+ T cells from OT-I mice with soluble H-2Kb/OVA257–264 tetramers, in the presence of activated CD4+CD25+ T cells, and either in the presence or absence of saturating doses of sTGF-βRII-Fc, a natural competitor of TGF-β1. The sTGF-βRII-Fc reagent is capable of binding various isoforms, including TGF-β1, with sufficient affinity to act as a potent inhibitor of TGF-β1 activity. When OT-I Tg CD8+ T cells were stimulated with tetramer in the presence of activated CD4+CD25+ T cells, marked suppression of proliferation was observed at a suppressor/responder ratio of 1:2 (>65%) (Fig. 1 B). Interestingly, suppression was not abrogated in the presence of a concentration of sTGF-βRII-Fc (10 μg/ml) that was capable of inhibiting the suppressive effects of rTGF-β1 (5 ng/ml) on CD8+ T cell proliferation (Fig. 1 B). In summary, in vitro blockade of TGF-β1 by means of neutralizing antibodies or sTGF-βRII-Fc had no effect on suppression mediated by resting or activated CD4+CD25 T cells in APC-dependent and -independent systems.
CD4+ T Cells from Smad3−/− or DNRIITg Mice Are Susceptible To Suppression Mediated by CD4+CD25+ T Cells while Resistant to the Antiproliferative Effects of TGF-β1.
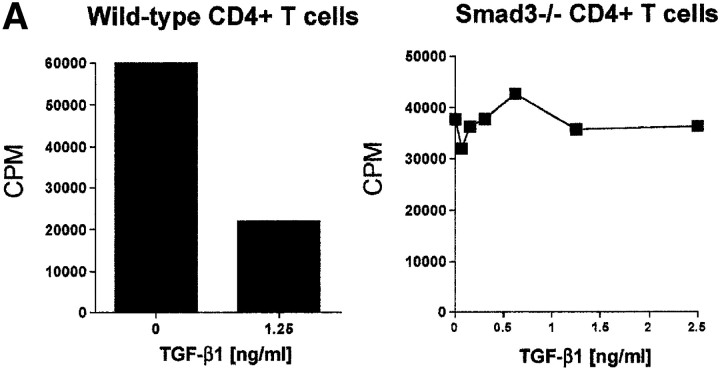
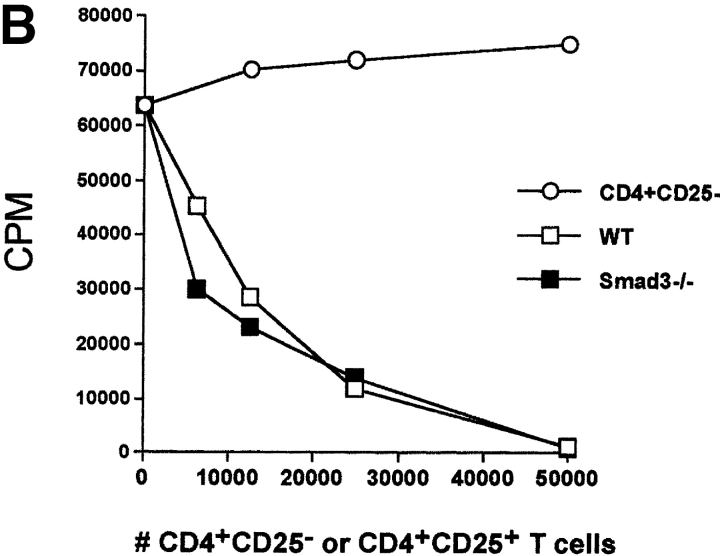
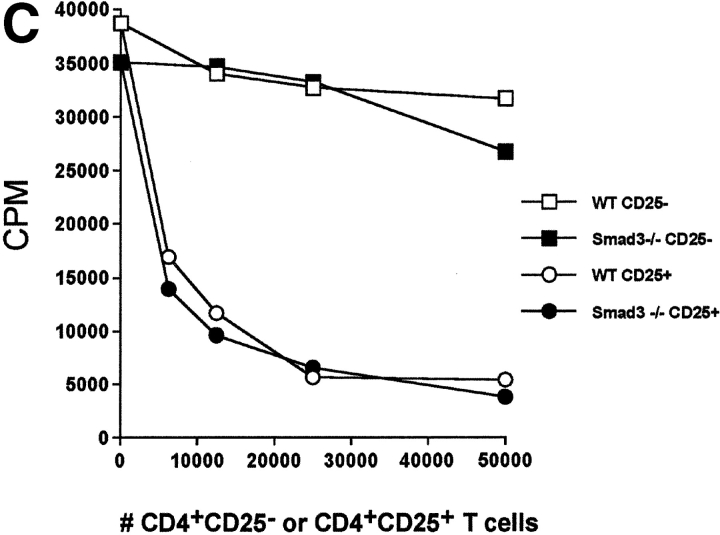
In an attempt to further evaluate the effector role of TGF-β1 in CD4+CD25+-mediated suppression, we made use of genetic models characterized by TGF-β1 unresponsiveness in T cells. Targeted disruption of the Smad3 gene resulted in the complete loss of sensitivity to TGF-β1 growth inhibition in T cells (18). More importantly, the ability of TGF-β1 to suppress T cell proliferation and IL-2 gene transcription depends on the successful activation of Smad3 pathway (35). If CD4+CD25+-mediated suppression requires TGF-β1, then CD4+CD25+ T cells would not suppress CD4+ T cells from Smad3−/− mice. When stimulated with soluble anti-CD3, CD4+ T cells from Smad3−/− mice were completely resistant to the antiproliferative effects of exogenous rhTGF-β1 (Fig. 2 A), while the proliferation of WT CD4+ T cells was strongly inhibited (Fig. 2 A). More importantly, CD4+ T cells from Smad3−/− mice were as susceptible to suppression mediated by CD4+CD25+ T cells from WT mice as CD4+CD25− T cells from WT mice (Fig. 2 B). These results suggest that CD4+CD25+-mediated suppression does not occur via a Smad3-dependent TGF-β signaling pathway in target CD4+ T cells. Smad3-dependent signaling in CD4+CD25+ T cells was also not an essential requirement for suppressor function as CD4+CD25+ T cells from Smad3−/− were as efficient in mediating suppression as CD4+CD25− T cells from WT mice (Fig. 2 C).
Figure 2.
CD4+ T cells from TGF-β1–insensitive Smad3−/− mice are susceptible to suppression mediated by CD4+CD25+ T cells. (A) WT or Smad3−/− CD4+ T cells (5 × 104) were stimulated with anti-CD3 (0.5 μg/ml) and irradiated, T cell–depleted spleen cells (2 × 105) in the presence or absence of exogenous rhTGF-β1. (B) WT or Smad3−/− CD4+ T cells (5 × 104) were stimulated as described in A in the presence of titrated numbers of either activated CD4+CD25+ or CD4+CD25− T cells. (C) Smad3-dependent signaling in CD4+CD25+ T cells is not required for suppressor effector function. WT CD4+ T cells were stimulated as described in A and in the presence of either freshly isolated CD4+CD25+ or CD4+CD25− T cells from WT or Smad3−/− mice. The final purity of sorted suppressors was >98%.
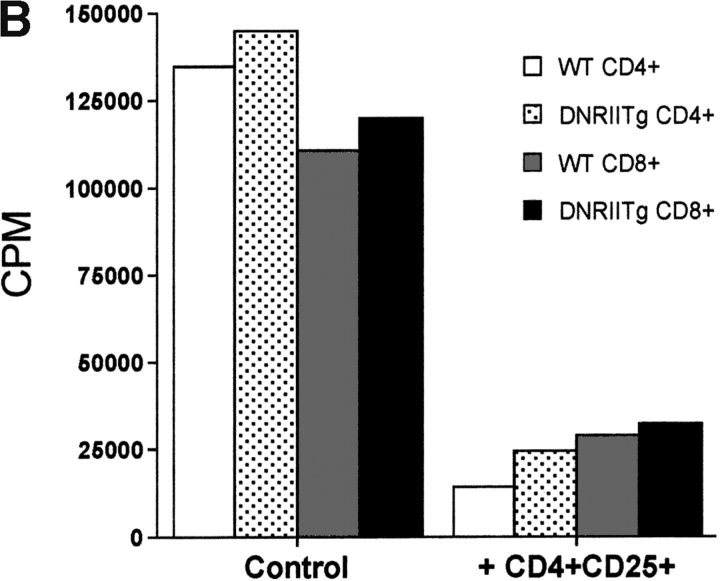
To rule out the possibility that CD4+CD25+ T cells may exert a TGF-β1–dependent effect, which does not require signaling via Smad3, we also tested the susceptibility of CD4+ T cells from a transgenic mouse that overexpresses a dominant-negative form of the TGF-β type II receptor in T cells (17). Both CD4+ and CD8+ T cells from DNRIITg mice were markedly resistant to the antiproliferative effects of exogenous rhTGF-β1 (Fig. 3 A), while both populations remained susceptible to CD4+CD25+-mediated suppression (Fig. 3 B). Taken together, these results strongly suggest that CD4+CD25+-mediated suppression can occur without functional TGF-β1–induced signaling in responding T cells.
Figure 3.
CD4+CD25+ T cells suppress T cells from DNRIITg mice. (A) T cells from WT or DNRIITg mice (5 × 104 cells) were stimulated with anti-CD3 (0.5 μg/ml) and irradiated, T cell–depleted spleen cells (2 × 105) in the presence or absence of exogenous rhTGF-β1. (B) CD4+ or CD8+ T cells (5 × 104) from WT or DNRIITg mice were stimulated as described in A in the presence or absence of either activated CD4+CD25+ or CD4+CD25− T cells.
CD4+CD25+ T Cells from TGF-β1−/− Mice Function Normally.
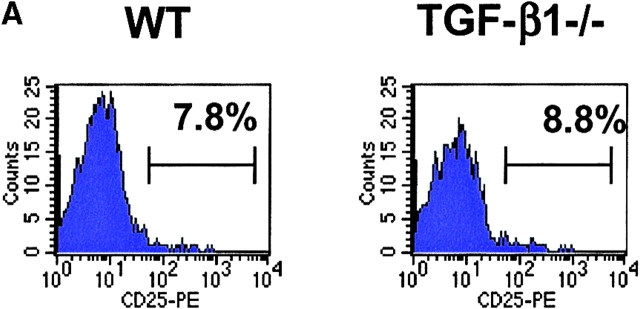
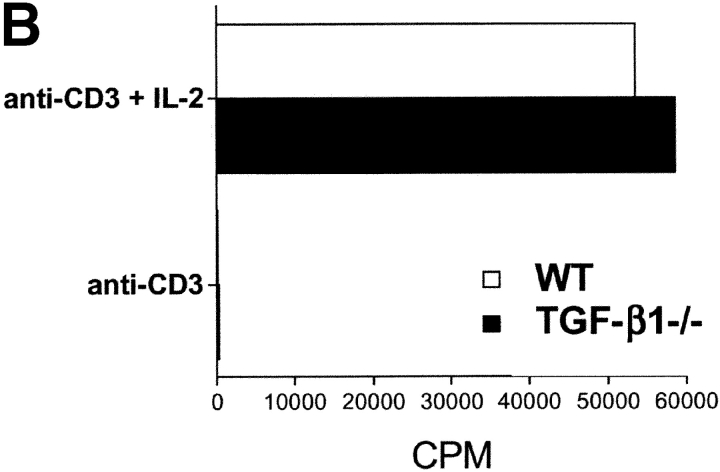
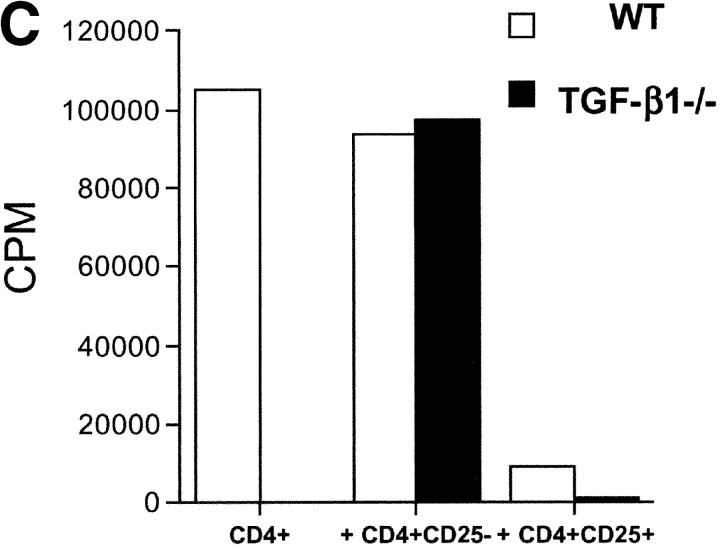
The most direct approach for the assessment of TGF-β1 function in CD4+CD25+-mediated suppression is the evaluation of the suppressor function of CD4+CD25+ T cells from TGF-β1−/− mice. As these mice rapidly develop an autoimmune phenotype by 3–4 wk of age (13), we isolated CD4+CD25+ T cells from 5–7-d old mice. At this age, the mice were phenotypically normal and demonstrated no visible sign of disease. Furthermore, analysis of activation marker expression on total CD4+ and CD8+ T cells, as well as CD4+CD25+ T cells from these mice revealed expression patterns indistinguishable from those seen in control WT littermates (unpublished data). Moreover, the frequency of CD4+CD25+ T cells was comparable between WT and TGF-β1−/− neonatal mice, comprising ∼8% of total CD4+ T cells (Fig. 4 A). CD4+CD25+ T cells from TGF-β1−/− mice resembled CD4+CD25+ T cells from WT mice as they did not proliferate spontaneously (unpublished data), were nonresponsive when stimulated via their TCR, but proliferated vigorously in the presence of anti-CD3 and exogenous IL-2 (Fig. 4 B). This was strongly suggestive that the CD4+CD25+ T cells present in neonatal TGF-β1−/− were not derived from a preactivated effector T cell pool. More importantly, CD4+CD25+ T cells from TGF-β1−/− neonatal mice were as efficient as CD4+CD25+ T cells from WT mice in mediating suppression of the response of WT CD4+ T cells to anti-CD3 stimulation (Fig. 4 C).
Figure 4.
CD4+CD25+ regulatory T cell activity is operative in TGF-β1−/− mice. (A) The frequency of CD4+CD25+ T cells is comparable in WT and TGF-β1−/− neonatal mice. (B) WT or TGF-β1−/− CD4+ CD25+ or CD4+CD25− T cells (5 × 104) were stimulated with anti-CD3 (0.5 μg/ml) and irradiated, T cell–depleted spleen cells (2 × 105) in the presence or absence of exogenous IL-2 (5 ng/ml). (C) WT CD4+ T cells were stimulated as described in B in the presence of either freshly isolated CD4+CD25+ or CD4+ CD25− T cells from WT or TGF-β1−/− neonatal mice.
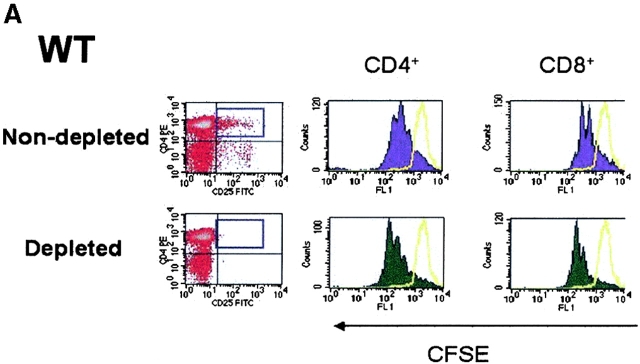
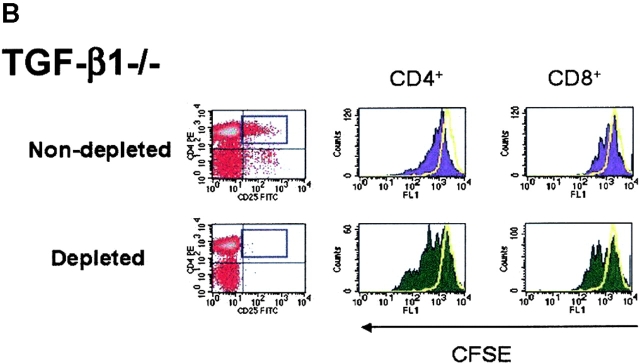
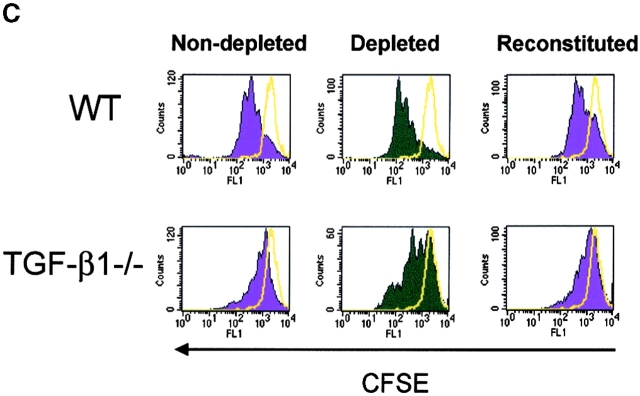
As TCR-induced proliferation of CD4+ T cells from normal mice is enhanced in the absence of CD4+CD25+ T cells in vitro (4, 36), we next evaluated whether depletion of CD4+CD25+ T cells from lymphoid populations derived from TGF-β−/− mice also resulted in enhanced proliferation. Therefore, we first depleted CD25+ T cells from either WT (Fig. 5 A) or TGF-β1−/− lymphoid cells from neonatal mice (Fig. 5 B), stimulated the depleted population with soluble anti-CD3, and examined the frequency of dividing cells based on CFSE dilution by flow cytometry. As shown in Fig. 5 A, the number of cell divisions and the frequency of dividing CD4+ and CD8+ T cells increases in an identical fashion when CD25+ T cells are removed from WT and TGF-β1−/− lymphoid cells. In addition, when CD25-depleted TGF-β1−/− populations were reconstituted with nondepleted TGF-β1−/− cells, the proliferative response was similar to that seen in nondepleted controls (Fig. 5 C). This result clearly suggests that CD25+ T cell regulatory activity is normal in neonatal TGF-β1−/− lymphoid tissues and that solely a deficiency of CD4+CD25+ T cells does not establish autoimmunity in TGF-β1−/− mice.
Figure 5.
In vitro depletion of CD4+CD25+ regulatory T cell activity in TGF-β1−/− mice enhances T cell proliferation. CD25-depleted or nondepleted splenocytes/lymph node cells from either WT (A) or TGF-β1−/− (B) neonatal mice were labeled with CFSE and stimulated with anti-CD3 (0.5 μg/ml) and irradiated, T cell–depleted spleen cells (2 × 105) for a period of 48 h. Cells were then analyzed by flow cytometry for CFSE division status relative to unstimulated T cells (solid yellow line). All histograms were gated on CD4+ or CD8+ T cells. (C) CD25-depleted WT or TGF-β1−/− cells were reconstituted with the respective nondepleted cell preparation, stimulated with soluble anti-CD3, and then examined the frequency of dividing cells based on CFSE dilution by flow cytometry. Cells were gated on CD4+ T cells.
Anti–TGF-β Does Not Abrogate CD4+CD25+-mediated Suppression of AIG.
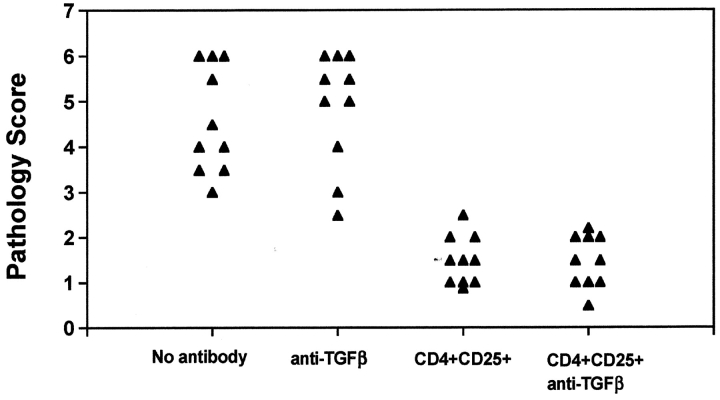
To evaluate the potential involvement of TGF-β1 in CD4+CD25+-mediated suppression of autoimmunity in vivo, we transferred CD4+CD25− T cells to nu/nu recipients. After 6–8 wk, all animals manifested pathologic changes consistent with severe AIG (Fig. 6) . When spleen cells from normal BALB/c donors containing CD4+CD25+ T cells were cotransferred with the CD4+CD25− T cells, marked suppression of disease was observed. Anti–TGF-β1 did not enhance disease severity when given to recipients of CD4+CD25− effectors, nor did it reverse the suppression mediated by the CD4+ CD25+ population.
Figure 6.
Anti–TGF-β does not reverse suppression of AIG mediated by CD4+CD25+ T cells. Nu/nu mice (6–8 wk) were injected with splenocytes depleted of CD25+ cells (20 × 106 cells), as described in the Materials and Methods. Simultaneously, some nu/nu mice were coinjected with CD4+CD25+ T cells (2 × 106 cells). Anti–TGF-β (2 mg) was injected on days –1 and 1 of cell transfer and weekly thereafter for the duration of the experiment. Stomachs were harvested 6 wk after transfer and H&E stains of formalin-fixed, paraffin-embedded sections were analyzed for gastric pathology.
Discussion
Over the past 10 y a number of regulatory CD4+ T cell populations capable of inhibiting immune responses both in vivo and in vitro have been described previously (2, 4, 37, 38). Some of the immunosuppressive effects of these regulatory T cells appear to be mediated by secretion of suppressor cytokines such as IL-4, IL-10, and TGF-β1. CD4+CD25+ T cells in mouse and man represent a population of naturally occurring professional suppressor T cells whose inhibitory effects have been studied by a number of groups. It is widely accepted that this population of suppressor cells mediates its function in vitro by a direct cell contact–dependent mechanism that is independent of the above-mentioned secreted suppressor cytokines (5, 39). Nakamura et al. (30) have recently offered an alternative hypothesis by demonstrating that activated CD4+CD25+ T cells secrete latent TGF-β1 that is subsequently bound to their surface. Membrane-bound latent TGF-β1 is then delivered to responder CD4+CD25− T cells on whose surface latent TGF-β1 would be activated and bound to TGF-βRII with resultant delivery of a suppressive signal for T cell activation. In light of the critical role of TGF-β1 in regulating T cell responses (40), the major goal of the present study was to reexplore the potential involvement of TGF-β1 in suppressor T cell effector function. To achieve this, we have made use of several genetic model systems in which CD4+CD25+ T cell suppressor function can be assessed in vitro in the complete absence of suppressor-derived TGF-β1 production and target T cell responsiveness to TGF-β1. Collectively, our studies demonstrate that CD4+CD25+-mediated T cell suppression can occur independently of TGF-β1 production and responsiveness in vitro and for suppression of AIG in vivo.
We have been unable to confirm the observations of Nakamura et al. (30) that suppression of anti-CD3 mediated activation by freshly explanted CD4+CD25+ could be reversed by high concentrations of anti–TGF-β1. More importantly, we did not observe any effects of either anti–TGF-β1 antibodies or sTGF-βRII-Fc on the immunosuppressive effects of activated CD4+CD25+ T cells. We have previously shown that the latter population has enhanced suppressor function and also mediates antigen nonspecific suppression in the absence of reengagement of their TCR. Activated CD4+CD25+ T cells were shown by Nakamura et al. (30) to be highly reactive with an anti–rhTGF-β1 polyclonal chicken antibody as measured by flow cytometry and therefore should have been readily susceptible to inhibition by anti–TGF-β1. We also have been unable to consistently observe significant differences in the expression of cell surface TGF-β1 on activated CD4+CD25+ T cells when compared with similarly activated CD4+CD25− T cells. Both activated populations stained very weakly although in some studies the activated CD25+ population was marginally brighter (unpublished data). It should also be pointed out that cell surface TGF-β1 could only be detected with a polyclonal chicken anti-rhTGF-β1 serum and studies demonstrating the specificity of the staining by blocking with rhTGF-β1 were not reported. The reasons for the discrepancy observed between these two studies are unknown but may reflect technical differences in experimental protocols.
It is always difficult to determine the mechanism of reversal of inhibition by adding a neutralizing antibody to a complex mixture of cells that are both capable of producing and responding to the target cytokine. As an alternative approach, we used CD4+CD25+ T cells that could not produce TGF-β1 and CD4+CD25− T cells that could not respond to TGF-β1-induced growth arrest. Activation of CD4+CD25− T cells from the Smad3−/− and DNRIITg mice was completely resistant to the immunosuppressive effects of TGF-β1 yet T cells from each mouse model were readily suppressed by CD4+CD25+ T cells from WT mice. We were also able to purify CD4+CD25+ T cells from TGF-β1−/− mice and demonstrate that they phenotypically and functionally resembled CD4+CD25+ T cells from WT mice. Importantly, they were as suppressive as WT CD4+CD25+ T cells when mixed with CD4+ CD25− T cells from WT mice. As the autoimmune syndrome seen in TGF-β1−/− mice is much more severe than that seen in d3Tx mice that lack CD4+CD25+ T cells, it is likely that CD4+CD25+-mediated suppression, although operative in young TGF-β1−/− mice, is overwhelmed by other manifestations of a global deficiency in TGF-β1. Collectively, these studies rule out the possibility that production of, or responsiveness to, TGF-β1 is absolutely required for CD4+CD25+-mediated suppression in vitro.
Interpretation of the effects of anticytokine reagents on the reversal of regulatory T cell mediated suppression of autoimmune disease in vivo is also difficult. It has previously been reported that suppression of IBD induced by transfer of CD4+CD45RBhigh T cells into SCID recipients by cotransfer of regulatory CD4+CD45RBlow T cells can be reversed by treatment of the recipient animals with anti–TGF-β1 (20). CD4+CD45RClow T cell–mediated suppression of thyroiditis in the rat was also reversed by anti–TGF-β (21). Although both murine CD4+CD45RBlow T cells and rat CD4+CD45RClow are highly enriched in CD4+CD25+ T cells, they also contain CD4+CD25− cells that are derived from the memory/effector cell pool. The cellular source(s) of the TGF-β1 that mediates inhibition of autoimmune disease in these models is unknown and it may be produced by members of the regulatory population or even by host lymphoid or nonlymphoid cells, such as gut epithelium. In the present report, suppression of AIG induced by CD4+CD25− T cells by cotransfer of CD4+CD25+ T cells was not reversed by an anti–TGF-β treatment protocol identical to that used to reverse suppression of IBD. AIG also differs from IBD in that CD4+CD25+ T cells from IL-10−/− mice are capable of mediating suppression (41). One major difference between IBD and AIG is that intestinal bacteria play a major role in the induction of disease (42) in the former, while AIG can be readily induced in germ-free mice (43). The involvement of IL-10 and TGF-β1 in suppression of IBD does not rule out that cell contact mediated inhibition of effector function is also operative. IL-10 and TGF-β1 may first be required to suppress the strong bacteria-driven inflammatory response before the cell contact–mediated pathway can function.
Taken together, our studies do not support the view that secreted or cell surface–associated TGF-β1 contribute significantly to the in vitro suppressive functions of CD4+CD25+ T cells. Although CD4+CD25+ T cells from Smad3−/− mice, which cannot respond to TGF-β1-induced growth inhibition, appeared to function as efficiently as CD4+CD25+ T cells from WT mice, it remains possible that TGF-β1 could play a role in the induction of CD4+CD25+ suppressor activity. Such a stimulatory effect on CD4+CD25+ suppressor activity would function in a Smad3-independent fashion. Resting CD4+CD25+ T cells selectively express high levels of mRNA for Tgif, a transcriptional corepressor that suppresses TGF-β1–induced transcription (44, 45). CD4+CD25+ suppressor T cells may therefore use unique signaling pathways to resist the traditional downregulatory effects of TGF-β1 and somehow permit TGF-β1 stimulatory effects. Lastly, a number of studies have suggested that TGF-β1 may play a critical role in the development of suppressor function in CD4+CD25− T cells. Yamagiwa et al. (46) have demonstrated culture of human CD4+CD45RA+RO− T cells in the presence of allogeneic stimulators and TGF-β1 resulted in the development of a population of potent suppressors that inhibit the induction of CD8+ cytotoxic effector cells. Although the induction of suppressor cells from CD45RA+ population also required the presence of a very small number of (<1%) of CD4+CD25+ T cells, it is highly unlikely that the suppressors generated in these cultures were derived from this small number of precursors. Zeller et al. (47) generated a population of murine alloantigen-specific suppressor T cells by coculture of CD4+ responder cells with alloantigen-bearing stimulators in the presence of both IL-10 and TGF-β1 T cells primed with alloantigen under these conditions readily suppressed both primary and secondary MLR cultures and were also markedly impaired in their ability to induce GVHD responses upon adoptive transfer to MHC class II disparate recipients. Although the relationship of these suppressor T cells to CD4+CD25+ T cells remains unclear, the recent studies of Barrat et al. (48) clearly demonstrate that potent IL-10–producing suppressor T cells can be generated by culture of CD4+CD25− T cells with antigen and the combination of vitamin D3 and dexamethasone. Thus, while most studies have focused on the role of TGF-β1 as an effector suppressor cytokine, TGF-β1 may also play an equally important role in the induction and maintenance of suppressor T cells from CD4+CD25− T precursor cells both in vitro and in vivo. Nonetheless, our current study demonstrates for the first time that CD4+CD25+ regulatory T cells can mediate contact-dependent and antigen-nonspecific suppression in the complete absence of suppressor-derived TGF-β1 production and functional TGF-β1 responsiveness by target T cells.
Footnotes
Abbreviations used in this paper: AIG, autoimmune gastritis; DNRIITg, dominant-negative TGF-β type RII transgenic; IBD, inflammatory bowel disease; WT, wild-type.
References
- 1.Bach, J.F. 1995. Organ-specific autoimmunity. Immunol. Today. 16:353–355. [DOI] [PubMed] [Google Scholar]
- 2.King, C., and N. Sarvetnick. 1997. Organ-specific autoimmunity. Curr. Opin. Immunol. 9:863–871. [DOI] [PubMed] [Google Scholar]
- 3.Moller, G. 1996. Dominant immunological tolerance. Immunol. Rev. 149:1–17. [Google Scholar]
- 4.Shevach, E.M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423–449. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. [DOI] [PubMed] [Google Scholar]
- 6.Shevach, E.M. 2000. Suppressor T cells: rebirth, function and homeostasis. Curr. Biol. 10:R572–R575. [DOI] [PubMed] [Google Scholar]
- 7.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 8.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccirillo, C.A., and E.M. Shevach. 2001. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 167:1137–1140. [DOI] [PubMed] [Google Scholar]
- 10.Suri-Payer, E., and H. Cantor. 2001. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J. Autoimmun. 16:115–123. [DOI] [PubMed] [Google Scholar]
- 11.Dang, H., A.G. Geiser, J.J. Letterio, T. Nakabayashi, L. Kong, G. Fernandes, and N. Talal. 1995. SLE-like autoantibodies and Sjogren's syndrome-like lymphoproliferation in TGF-β knockout mice. J. Immunol. 155:3205–3212. [PubMed] [Google Scholar]
- 12.Geiser, A.G., J.J. Letterio, A.B. Kulkarni, S. Karlsson, A.B. Roberts, and M.B. Sporn. 1993. Transforming growth factor β 1 (TGF-β1) controls expression of major histocompatibility genes in the post-natal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-β1 null mouse phenotype. Proc. Natl. Acad. Sci. USA. 90:9944–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christ, M., N.L. McCartney-Francis, A.B. Kulkarni, J.M. Ward, D.E. Mizel, C.L. Mackall, R.E. Gress, K.L. Hines, H. Tian, and S. Karlsson. 1994. Immune dysregulation in TGF-β1-deficient mice. J. Immunol. 153:1936–1946. [PubMed] [Google Scholar]
- 14.Letterio, J.J. 2000. Murine models define the role of TGF-β as a master regulator of immune cell function. Cytokine & Growth Factor Rev. 11:81–87. [DOI] [PubMed] [Google Scholar]
- 15.Letterio, J.J., and A.B. Kulkarni. 1997. The transforming growth factor-b1 knockout mouse. Cytokine Knockout Mice. S. Durum and K. Muegge, editors. Humana, Totow, NJ. 369–400.
- 16.Gorelik, L., and R.A. Flavell. 2000. Abrogation of TGF-β signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181. [DOI] [PubMed] [Google Scholar]
- 17.Lucas, P.J., S.J. Kim, S.J. Melby, and R.E. Gress. 2000. Disruption of T cell hemostasis in mice expressing a T cell-specific dominant negative transforming growth factor β II receptor. J. Exp. Med. 191:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, X., J.J. Letterio, R.J. Lechleider, L. Chen, R. Hayman, H. Gu, A.B. Roberts, and C. Deng. 1999. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 18:1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelick, L., and R.A. Flavell. 2002. Transforming growth factor-β in T cell biology. Nat. Rev. Immunol. 2:46–53. [DOI] [PubMed] [Google Scholar]
- 20.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddon, B., and D. Mason. 1999. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor β and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+ CD45RC− cells and CD4+CD8− thymocytes. J. Exp. Med. 189:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Read, S., S. Mauze, C. Asseman, A. Bean, R. Coffman, and F. Powrie. 1998. CD38+ CD45RBlow CD4+ T cells: a population of T cells with immune regulatory activities in vitro. Eur. J. Immunol. 28:3435–3447. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 24.Stephens, L.A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247–1254. [DOI] [PubMed] [Google Scholar]
- 25.Taams, L.S., J. Smith, M.H. Rustin, M. Salmon, L.W. Poulter, and A.N. Akbar. 2001. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122–1131. [DOI] [PubMed] [Google Scholar]
- 26.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng, W.F., P.J. Duggan, F. Ponchel, G. Matarese, G. Lombardi, A.D. Edwards, J.D. Isaacs, and R.I. Lechler. 2001. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 98:2736–2744. [DOI] [PubMed] [Google Scholar]
- 29.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni, A.B., C.G. Huh, D. Becker, A. Geiser, M. Lyght, K.C. Flanders, A.B. Roberts, M.B. Sporn, J.M. Ward, and S. Karlsson. 1993. Transforming growth factor β 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA. 90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coligan, J. 1999. Current Protocols in Immunology. J. Coligan, editor. John Wiley & Sons, Inc., New York. 4–9.
- 33.McHugh, R.S., E.M. Shevach, D.H. Margulies, and K. Natarajan. 2001. A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur. J. Immunol. 31:2094–2103. [DOI] [PubMed] [Google Scholar]
- 34.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 35.Datto, M.B., J.P. Frederick, L. Pan, A.J. Borton, Y. Zhuang, and X.F. Wang. 1999. Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol. Cell. Biol. 19:2495–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suri-Payer, E., A. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 37.Fowell, D., F. Powrie, A. Saoudi, B. Seddon, V. Heath, and D. Mason. 1995. The role of subsets of CD4+ T cells in autoimmunity. Ciba Found. Symp. 195:173–182. [DOI] [PubMed] [Google Scholar]
- 38.Katz, J.D., C. Benoist, and D. Mathis. 1995. T-helper cell subsets in insulin-dependent diabetes. Science. 268:1185–1188. [DOI] [PubMed] [Google Scholar]
- 39.Shevach, E.M., R.S. McHugh, C.A. Piccirillo, and A.M. Thornton. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 182:58–67. [DOI] [PubMed] [Google Scholar]
- 40.Prud'homme, G.J., and C.A. Piccirillo. 2000. The inhibitory effects of transforming growth factor-β-1 (TGF-β1) in autoimmune diseases. J. Autoimmun. 14:23–42. [DOI] [PubMed] [Google Scholar]
- 41.Suri-Payer, E., and H. Cantor. 2001. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+ CD25+ T cells. J. Autoimmun. 16:115–123. [DOI] [PubMed] [Google Scholar]
- 42.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. [DOI] [PubMed] [Google Scholar]
- 43.Groux, H., and F. Powrie. 1999. Regulatory T cells and inflammatory bowel disease. Immunol. Today. 20:442–446. [DOI] [PubMed] [Google Scholar]
- 44.McHugh, R.S., M.J. Whitters, C.A. Piccirillo, D.A. Young, E.M. Shevach, M. Collins, and M.C. Byrne. 2002. CD4+ CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323. [DOI] [PubMed] [Google Scholar]
- 45.Gavin, M.A., S.R. Clarke, E. Negrou, A. Gallegos, and A. Rudensky. 2001. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 3:33–41. [DOI] [PubMed] [Google Scholar]
- 46.Yamagiwa, S., J.D. Gray, S. Hashimoto, and D.A. Horwitz. 2001. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166:7282–7289. [DOI] [PubMed] [Google Scholar]
- 47.Zeller, J.C., A. Panoskaltsis-Mortari, W.J. Murphy, F.W. Ruscetti, S. Narula, M.G. Roncarolo, and B.R. Blazar. 1999. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-β. J. Immunol. 163:3684–3691. [PubMed] [Google Scholar]
- 48.Barrat, F.J., D.J. Cua, A. Boonstra, D.F. Richards, C. Crain, H.F. Savelkoul, R. de Waal-Malefyt, R.L. Coffman, C.M. Hawrylowicz, and A. O'Garra. 2002. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]