Abstract
Immunoglobulin (Ig)E-mediated activation of mast cells has long been thought to occur only when FcεRI receptor-bound IgE is cross-linked via multivalent antigens. However, recent studies have raised the possibility that mast cells may be activated by the binding of IgE to the FcεRI receptor in the absence of antigen. Here we demonstrate that IgE binding without antigen induces the expression of histidine decarboxylase (HDC) in mouse interleukin (IL)-3–dependent bone marrow–derived mast cells (BMMCs). The induction of HDC by the binding of IgE was found to require an influx of extracellular calcium ions, which was attenuated by pretreatment with U73122, a phospholipase C inhibitor. Furthermore, the increase in HDC activity upon sensitization with IgE was completely suppressed by pretreatment of BMMCs with protein kinase C inhibitors, such as H7, staurosporine, and Gö6976. In addition, immediate activation of the tyrosine kinase Lyn was not detectable upon treatment with IgE. These results suggest that the binding of IgE to its receptor in the absence of antigen results in de novo synthesis of HDC in BMMCs through a signaling pathway distinct to that operating during antigen-stimulated FcεRI activation.
Keywords: histidine decarboxylase, enzyme induction, protein kinase C, calcium signaling, IgE receptors
Introduction
Mast cells are found in various tissues throughout the body and trigger allergic and inflammatory responses by releasing a wide variety of mediators, such as histamine, arachidonic acid metabolites, and neutral proteases (1, 2). Activation and signaling of FcεRI, the high-affinity receptor for IgE, has long been thought to occur only when receptor-bound IgE is cross-linked via multivalent antigens (1–4). However, recently reported evidence has raised the possibility that higher concentrations of IgE can trigger a number of signaling pathways in mast cells and circulating basophils, even in the absence of antigens. Treatment of cells with IgE in the absence of antigen was found to enhance the expression of FcεRI by various mast cell populations in vitro and in mouse peritoneal cells in vivo (5–8). It has been demonstrated that IL-3–dependent bone marrow–derived mast cells (BMMCs)* respond with an increase in cytosolic Ca2+ levels when treated with IgE alone (9) and that monomeric IgE stimulates multiple phosphorylation events in mouse BMMCs, leading to potent cytokine production and enhanced survival (10, 11). These observations suggest that monomeric IgE is involved in the activation of mast cells, which is distinct from induction via antigen stimulation.
The above studies have indicated that monomeric IgE in the absence of antigen may not be able to stimulate the degranulation and release of histamine from mast cells. However, the regulation of histamine synthesis by monomeric IgE remains to be clarified. We previously purified l-histidine decarboxylase (HDC), the rate-limiting enzyme of histamine synthesis in mammals, cloned its cDNA from a mouse mastocytoma cell line, and prepared a specific antibody for HDC, in order to elucidate the mechanism regulating histamine synthesis (12, 13). As previous reports have demonstrated that atopic patients, who possess extremely high concentrations of serum IgE, show higher serum histamine concentrations than normal individuals (14, 15), we hypothesized that monomeric IgE may be involved in the up-regulation of HDC. The purpose of our present study was to investigate the effects of monomeric IgE on histamine synthesis and to identify the signaling pathway involved in this histamine synthesis.
Materials and Methods
Mice.
Specific pathogen-free, 8-wk-old female Balb/c mice were obtained from Japan SLC.
Materials.
The anti-HDC antibody was prepared as described previously (16). The following materials were commercially obtained from the sources indicated: an anti-DNP mouse monoclonal IgE (SPE-7) and DNP-conjugated human serum albumin from Sigma-Aldrich, an anti-DNP IgG (HDP-1) from Oxford Biomedical Research, an anti-trinitrophenyl IgE and an anti–mouse FcγRIIB/III (2.4G2) antibody from BD Biosciences, mouse whole IgG molecule from CHEMICON International Inc. [α-32P]dCTP (3,000 Ci/mmol), and [γ-32P]ATP (3,000 Ci/mmol) from Du Pont-New England Nuclear, a horseradish peroxidase–conjugated anti–rabbit IgG antibody from Dako, an ECL kit from Amersham Biosciences, Fura-2/AM from Dojindo Laboratories, an agarose-conjugated anti-Lyn antibody, and an anti-Lyn mouse monoclonal antibody from Santa Cruz Biotechnology, Inc. All other chemicals were commercial products of reagent grade.
Preparation of BMMCs.
BMMCs were prepared as described previously with minor modifications (17). Cells were cultured in the complete RPMI containing 50% WEHI-3–conditioned medium as a source of IL-3 and 10% fetal bovine serum at 37°C in a fully humidified 5% CO2 atmosphere. After 4 wk of culture, >95% of the viable cells were confirmed to be immature mast cells, as assessed by staining with acidic Toluidine blue. A linear increase in the surface expression of FcεRI was observed upon flow cytometric analyses in the presence of 3 μg/ml anti-DNP IgE (data not shown), which is consistent with previous studies (7, 8).
HDC Assay.
Cells were rinsed with PBS followed by centrifugation and the cell pellet was lysed (107 cells/ml) with 10 mM HEPES–NaOH, pH 7.3, containing 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and a protease inhibitor mixture (0.2 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.1 mM benzamidine, 1 μg/ml pepstatin A, and 10 μg/ml E-64) on ice for 30 min. The cells were centrifuged at 100,000 g for 1 h at 4°C and the supernatant was used for the measurement of HDC activity as described previously (18).
Northern Blot Analyses.
Total RNA was extracted from cells using ISOGEN (Nippon Gene), according to the manufacturer's instructions. Total RNA (3 μg) obtained was electrophoretically separated on a 1.5% agarose/formaldehyde gel. After electrophoresis, the RNA was transferred onto a Biodyne A membrane (Pall) in 20× SSC (1× SSC is composed of 0.15 M NaCl and 0.015 M sodium citrate) by capillary blotting. [32P]-Labeled specific cDNA probes were synthesized in the presence of [α-32P]dCTP and hybridized onto the filter in hybridizing solution (6× SSC, 5× Denhardt's solution, 0.5% SDS, and 100 μg/ml salmon sperm DNA) at 68°C overnight. The filter was rinsed twice in 2× SSC at room temperature and twice in 2× SSC containing 1% SDS at 60°C. The filter was then analyzed using a Fujix BAS 2000 Bio-Imaging Analyzer.
Immunoblot Analyses.
Cells were homogenized in 50 mM HEPES–NaOH, pH 7.3, containing 1 mM dithiothreitol, 1% Triton X-100, and the protease inhibitor mixture, and centrifuged at 15,000 g for 30 min at 4°C. The resultant supernatant (50 μg protein/lane) was subjected to SDS-PAGE (10% slab gel), and the separated proteins were transferred electrophoretically onto a PVDF membrane (Millipore). Immunoblot analysis was performed as described previously (18). An anti-HDC antibody (1:500) was used as the primary antibody, and a horseradish peroxidase–conjugated anti–rabbit IgG antibody (1:3,000) was used as the secondary antibody. The membranes were stained using an ECL kit according to the manufacturer's instructions.
Cell Culture Under Ca2+-free Conditions.
Cells were washed twice in PIPES buffer (25 mM PIPES, pH 7.4, containing 125 mM NaCl, 2.7 mM KCl, 5.6 mM glucose, 1 mM CaCl2, and 0.1% bovine serum albumin), or in Ca2+-free PIPES buffer. The cells were then incubated in buffer with or without Ca2+ in the presence or absence of 3 μg/ml IgE for 90 min at 37°C. The cells were harvested and Northern blot analyses were performed as described above.
Measurement of Cytosolic Ca2+ Concentrations.
Cells were loaded with 2 μM Fura-2/AM in modified Tyrode's buffer (130 mM NaCl containing 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 10 mM HEPES, NaOH, pH 7.3, and 0.1% bovine serum albumin) for 45 min at room temperature and then washed in modified Tyrode's buffer. For Ca2+ free conditions, the buffer was replaced with Ca2+ free modified Tyrode's buffer containing 0.3 mM EGTA. Fluorescent intensities were measured, at an excitation wavelength of 340 or 380 nm and an emission wavelength of 510 nm, with a fluorescence spectrometer (CAF-100; Jasco) as described previously (19).
Treatment with Various Kinase Inhibitors.
BMMCs were treated for the indicated periods with various kinase inhibitors at the concentrations indicated, before the addition of IgE. Protein kinase C (PKC) inhibitors: Staurosporine (10 min, 1 μM), H7 (30 min, 0.1 mM), chelerythrine chloride (60 min, 10 μM), Gö6976 (60 min, 10 μM), PKC inhibitors 19–27, myristoylated peptide (60 min, 0.1 mM), Ro-32–0432 (60 min, 1 μM), and bisindolylmaleimide (25 min, 1 μM); tyrosine kinase inhibitors: herbimycin A (30 min, 1.5 μM), genistein (30 min, 0.1 mM), PP2 (10 min, 10 μM), and PP3, an inactive analogue of PP2 (10 min, 10 μM); other inhibitors: H89 (protein kinase a [PKA], 30 min, 10 μM), PD98059 (mitogen-activated protein kinase [MAPK]/ERK kinase [MEK], 30 min, 50 μM), SB203580 (p38, 30 min, 10 μM), LY294002 (phosphoinositide 3 [PI3]-kinase, 30 min, 50 μM), wortmannin (PI3-kinase, 15 min, 0.1 μM), and W7 (calmodulin, 30 min, 10 μM).
Immunoprecipitation and In Vitro Kinase Assay for Lyn.
Cells were incubated in the presence or absence of 3 μg/ml anti-DNP IgE for 5 min. In the experiment of antigen stimulation, cells were incubated with 1 μg/ml anti-DNP IgE for 12 h and then stimulated by the addition of antigens (30, 100, and 300 ng/ml DNP-human serum albumin) for 5 min. Immunoprecipitation with an agarose-conjugated anti-Lyn antibody (20 μg/ml) was performed as described previously (18) in the presence of 1 mM sodium vanadate. The resultant precipitate was washed four times with the RIPA buffer followed by two times of washing with 20 mM HEPES-NaOH, pH 8.0, containing 150 mM NaCl, 10 mM magnesium acetate, and 20 mM MnCl2 (kinase assay buffer). The precipitate was incubated in kinase assay buffer containing 10 μM ATP in the presence of 10 μCi [γ-32P]ATP for 5 min at 30°C. Immunoblot analyses were also performed as described above using an anti-Lyn mouse monoclonal antibody (1:200) to confirm an equal amount of precipitated Lyn.
Results
Induction of Histamine Synthesis by IgE in the Absence of Antigen.
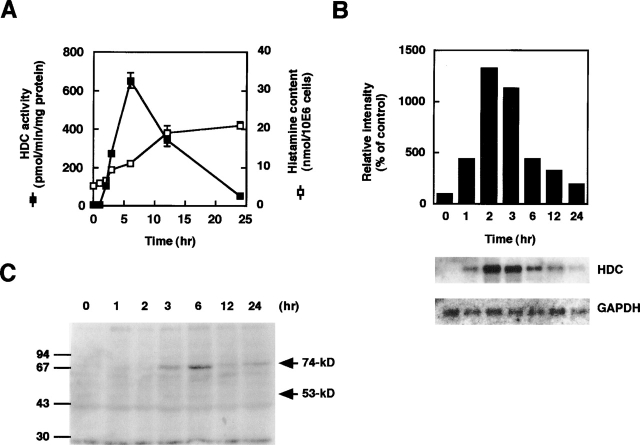
We found significant increases in the HDC activity of BMMCs upon sensitization with IgE. Among the various types of IgG and IgE, only anti-DNP IgE drastically induced HDC activity in BMMCs in the absence of antigen 6 h after stimulation (Table I). Both the anti-DNP IgG and the polyclonal IgG mixture were unable to increase the HDC activity of BMMCs. IgE has been shown to bind to the Fc receptors for IgG (FcγRII and FcγRIII), in addition to FcεRI in mouse BMMCs (20), and IgE-mediated systemic anaphylaxis has been shown to be enhanced in FcγRII-deficient mice whereas being attenuated in FcγRIII-deficient mice (21). Blockage of these FcγRs with the 2.4G2 antibody has no effect on the increase in HDC activity induced by IgE. The HDC activity of BMMCs increased dose dependently by anti-DNP IgE treatment, and plateau levels were reached at >3 μg/ml anti-DNP IgE (data not shown). Northern blot analyses revealed that this induction occurs in the transcriptional level and a >10-fold increase was observed by the addition of 3 μg/ml anti-DNP IgE (Fig. 1 B). This induction of HDC in BMMCs was found to be transient. A significant increase in the HDC activity was first observed 2 h after the addition of anti-DNP IgE, and a maximal level of HDC activity was obtained 6 h after the addition of anti-DNP IgE (Fig. 1 A). A significant increase in HDC mRNA expression was observed 1 h after stimulation with anti-DNP IgE (Fig. 1 B). Cellular histamine content significantly increased at a later phase (12 h) with an approximately fourfold increase in histamine content being detected 24 h after the addition of anti-DNP IgE (Fig. 1 A). Immunoblot analyses using an anti-HDC antibody revealed that the dominant form of HDC induced in the BMMCs was the 74-kD form (Fig. 1 C). The 53-kD form of HDC, which we previously reported to be found in a rat mast cell line (18), was hardly detectable in the BMMCs both in the presence and absence of anti-DNP IgE (Fig. 1 C).
Table I.
Increase in HDC Activity Induced by IgE or Various Activators of the Ca2+–PKC Pathway
| HDC activity | |
|---|---|
| pmol/min/mg protein | |
| None | 3.13 ± 1.68 |
| Anti-DNP IgE | 652 ± 54.8a |
| Anti-DNP IgG | 5.80 ± 1.95 |
| Polyclonal IgG | 1.43 ± 1.00 |
| Anti-DNP IgE + 2.4G2 | 626 ± 46.2a |
| IgE/antigen | 389 ± 10.3a , b |
| Thapsigargin | 618 ± 162a |
| TPA | 96.3 ± 23.3a |
| A23187 | 1,240 ± 118a |
BMMCs were cultured in the presence of an anti-DNP IgE (3 μg/ml), an anti-DNP IgG (3 μg/ml), purified polyclonal IgG (3 μg/ml), thapsigargin (100 nM), TPA (10 nM), and A23187 (0.3 μM) for 6 h at 37°C. Blocking of FcγRII and FcγRIII was performed by pretreatment with the 2.4G2 antibody (10 μg/ml) for 10 min. In the experiment of antigen stimulation (IgE/antigen), BMMCs were incubated in the presence of 1 μg/ml anti-DNP IgE for 24 h at 37°C, washed, and then incubated with 30 ng/ml DNP-human serum albumin for 6 h. The concentration of each reagent was optimized in preliminary experiments to obtain its maximal effect. The values obtained are represented as the means ± SEM (n = 3).
P < 0.01 is regarded as significant by the Student's t test (vs. None).
P < 0.05 is regarded as significant by the Student's t test (vs. Anti-DNP IgE).
Figure 1.
Time course of HDC activity and mRNA accumulation induced by IgE. BMMCs were cultured in the presence of 3 μg/ml anti-DNP IgE for the periods indicated. (A) HDC activity (closed boxes) and histamine content (open boxes) of the cells are demonstrated. The values are represented as the means ± SEM (n = 3). (B) Transient increase in HDC mRNA accumulation is represented. The relative intensities of the bands hybridized with the probe for HDC were normalized by those with the probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). This is a representative figure of three independent experiments showing similar results. (C) The result of immunoblot analyses using an anti-HDC antibody is demonstrated. The arrows indicate the position of the 74-kD form (74-kD) and 53-kD form (53-kD) of HDC. Note that the 53-kD form is undetectable.
Requirement of Extracellular Ca2+ Influx for the Induction of HDC mRNA.
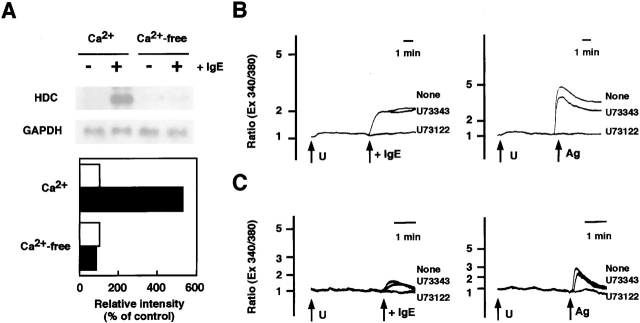
As monomeric IgE has been found to increase cytoplasmic Ca2+ levels in BMMCs (9), the effect of extracellular Ca2+ on the induction of HDC mRNA by anti-DNP IgE was investigated. Induction of HDC mRNA by anti-DNP IgE was completely suppressed by the depletion of extracellular Ca2+ (Fig. 2 A). The addition of anti-DNP IgE caused an increase in cytosolic Ca2+, which was completely suppressed by pretreatment with a phospholipase c (PLC) inhibitor, U73122 (Fig. 2 B). The increase in cytosolic Ca2+ induced by anti-DNP IgE was sustained for a longer period compared with that induced by antigen stimulation. U73343, an inactive analogue of U73122, was unable to suppress the increase in cytosolic Ca2+ induced by both the addition of anti-DNP-IgE and antigen stimulation. A slight increase in cytoplasmic Ca2+ by anti-DNP IgE was also observed in the absence of extracellular Ca2+, which was also attenuated by pretreatment with U73122 (Fig. 2 C).
Figure 2.
Requirement of extracellular Ca2+ for the induction of HDC by IgE. (A) BMMCs were incubated in PIPES buffer with (Ca2+) or without Ca2+ (Ca2+-free) for 90 min in the presence (black bars) or absence (white bars) of 3 μg/ml anti-DNP IgE. The cells were then harvested and Northern blot analyses were performed. The relative intensities of the hybridized bands are demonstrated as described in the legend to Fig. 1. This is a representative figure of three independent experiments showing similar results. (B and C) Ca2+ influx induced by anti-DNP IgE was compared with that induced by antigen stimulation. BMMCs were loaded with 2 μM Fura-2/AM in modified Tyrode's buffer in the presence (B) or absence (C) of Ca2+ as described in Materials and Methods. U73122 or U73343 (3 μM) was added (U) 3 min (C) or 7 min (B) before the stimulation. (Left panels) Stimulation with anti-DNP IgE (10 μg/ml) added to the culture. (Right panels) Stimulation with antigen (Ag, DNP-human serum albumin, 30 ng/ml) after 12 h of sensitization with anti-DNP IgE (1 μg/ml).
Effects of 12-O-Tetradecanoyl Phorbol 13-Acetate, Thapsigargin, and A23187 on the Induction of HDC.
The effects of various activators of the Ca2+-PKC pathway on the induction of HDC activity were investigated. A phorbol ester, 12-O-tetradecanoyl phorbol 13-acetate (TPA), which is known to be an activator of PKC, slightly but significantly induced HDC activity in the BMMCs, whereas thapsigargin, an inhibitor of the Ca2+-ATPase in the endoplasmic reticulum, and A23187, a calcium ionophore, drastically increased HDC activity in the BMMCs, to levels comparable to those induced by anti-DNP IgE (Table I). Cross-linking of the FcεRI with multivalent antigens also caused the induction of HDC, and this inducible effect was smaller than that induced by IgE alone (Table I). The accumulation of HDC mRNA in the cells activated by antigen stimulation was also observed but was smaller than that observed in cells treated with anti-DNP IgE alone (data not shown).
Effects of Various Kinase Inhibitors on HDC Expression Induced by IgE.
The effects of an array of kinase inhibitors on the induction of HDC were investigated. PKC inhibitors, such as staurosporine, H7, and Gö6976, significantly suppressed the increase of HDC activity and mRNA expression whereas the other PKC inhibitors did not (Fig. 3, A and C) . Chelerythrine moderately suppressed the increase in HDC activity but not the increase in mRNA expression. Inhibitors of PKA (H89), MEK (PD98059), p38 (SB203580), calmodulin (W7), and PI3-kinase (LY294002, and wortmannin) were all ineffective in suppressing the induction of HDC activity (Fig. 3, A and B). The Src family protein tyrosine kinase inhibitors, herbimycin A and PP2, had a modest inhibitory effect on the induction of HDC (Fig. 3, A and D). All the other inhibitors tested were ineffective in inhibiting the induction of HDC.
Figure 3.
Effects of various kinase inhibitors on the induction of HDC by IgE. BMMCs were pretreated with various kinase inhibitors as described in Materials and Methods. The cells were further incubated in the presence or absence of 3 μg/ml anti-DNP IgE for 3 h (for Northern blot) or 6 h (for HDC activity). (Top panels) HDC activity of the cells is presented. The values are represented as the means ± SEM (n = 3). (Bottom panels) HDC mRNA accumulation in the cells were measured by Northern blot analyses. The relative intensities of the hybridized bands are demonstrated as described in the legend to Fig. 1. This is a representative figure of three independent experiments showing similar results.
Absence of Lyn Activation.
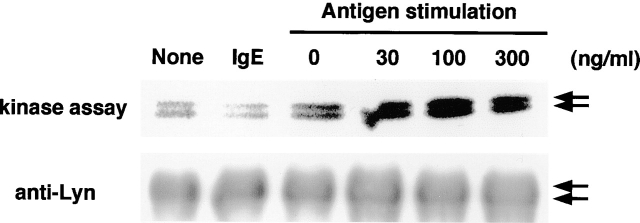
As the protein tyrosine kinase Lyn has been found to play critical roles in the early events induced by the cross-linking of the FcεRI (22), in vitro kinase assay for Lyn was performed in BMMCs. A significant increase in [32P] incorporation was observed in the bands, of which molecular mass was 53 and 56 kD, concentration dependently by antigen stimulation, whereas no changes were seen upon treatment with anti-DNP IgE alone (Fig. 4) . Immunoblot analyses using an anti-Lyn antibody indicated that each immunoprecipitate contained the same amounts of Lyn.
Figure 4.
Absence of enzymatic activation of Lyn upon treatment with IgE. Enzymatic activation of Lyn following IgE sensitization was measured. BMMCs were incubated with anti-DNP IgE (IgE, 3 μg/ml) for 5 min. In the experiment of antigen stimulation, cells were sensitized with anti-DNP IgE (1 μg/ml) for 12 h and then incubated with DNP-human serum albumin (0, 30, 100, 300 ng/ml) for 5 min. In vitro kinase assay using [γ-32P]ATP was performed as described in Materials and Methods. Immunoblot analyses were also performed using an anti-Lyn antibody (1:200) to confirm an equal amount of precipitated Lyn. The arrows indicate Lyn tyrosine kinase, which is detected as two bands with molecular mass of 53 and 56 kD.
Discussion
We have demonstrated that IgE is able to induce histamine synthesis in mouse BMMCs even in the absence of antigen. Recent research has clearly demonstrated that monomeric IgE activates mouse BMMCs exclusively via their FcεRI (10, 11). We performed this study using the same commercial IgE preparation (SPE-7; Sigma-Aldrich) as these previous studies. Stimulation of FcγRII and FcγRIII by anti-DNP IgG or by polyclonal IgG caused no significant changes in HDC activity. Furthermore, blocking of these FcγRs by the 2.4G2 antibody did not alter the activation of HDC by IgE, indicating that FcγRII and FcγRIII may not be involved in the induction of HDC. Intracellular histamine content was significantly increased 12 h after the addition of IgE. As prolonged treatment of BMMC with monomeric IgE has been reported to enhance the degree of degranulation by antigen stimulation (8), the increase in the amount of stored histamine by monomeric IgE in our study indicates that prolonged sensitization of mast cells with IgE may lead to an enhanced release of histamine. Regarding HDC protein expression in BMMCs, the 74-kD precursor form of HDC was the dominant form detected. We previously demonstrated that the 74-kD form of HDC is present in the cytosol as an enzymatically active form (18) and is degraded via the ubiquitin-proteasome pathway (23). In BMMCs treated with IgE, the rapid disappearance of the 74-kD form may be due to its rapid degradation via the ubiquitin–proteasome system.
Our results showing the lack of an increase in HDC expression in the absence of extracellular Ca2+ indicate that the induction of HDC requires the influx of extracellular Ca2+. Indeed, addition of IgE to BMMCs caused a significant increase in cytoplasmic Ca2+. Although such a sustained increase of cytosolic Ca2+ has previously been reported (9), it has been unclear as to which signaling pathway is involved in this Ca2+ increase. In our experiments, a PLC inhibitor, U73122, completely inhibited the increase in cytosolic Ca2+, indicating that PLC may be activated upon stimulation with IgE even in the absence of antigen. Although a slight increase in cytoplasmic Ca2+ by IgE was observed in the absence of extracellular Ca2+, it is likely that the sustained influx of extracellular Ca2+ is essential for the induction of HDC by monomeric IgE. Recently, it has been reported that thapsigargin induces HDC in a mouse macrophage cell line, via the activation of extracellular signal–regulated kinase (ERK; reference 24). In this study, we also confirmed that A23187, TPA, and thapsigargin induce HDC activity in BMMCs. TPA was able to induce HDC activity, but the degree of induction was much lower than that induced by IgE. These observations suggest that the influx of extracellular Ca2+ may play a crucial role in the induction of HDC in addition to activation of PKC. Evaluation of the effects of various PKC inhibitors on the induction of HDC by IgE suggests that this induction may be mediated by Ca2+-dependent PKC, as Gö6976 has been reported to be a selective inhibitor for Ca2+-dependent PKC isozymes (25). However, as the other PKC inhibitors, such as bisindolylmaleimide and Ro-32–0432, were found to be ineffective, we cannot exclude the possibility that another PKC-like kinase is involved in the induction of HDC.
IL-6 production induced by monomeric IgE has been found to be inhibited by a PI-3 kinase inhibitor, LY294002, a MEK inhibitor, PD98059, and a p38 inhibitor, SB203580 (10). Although we have also observed these inhibitory effects on the IL-6 production under similar conditions to the previous report (3 μg/ml IgE, 3 h, control; 5.47 ± 0.634, + LY294002; 0 ± 0, + PD98059; 1.06 ± 0.164, + SB203580; 1.40 ± 0.193 ng/106 cells, n = 6), HDC induction by IgE was not suppressed by these inhibitors. These results indicate that signaling pathways involved in the induction of HDC and IL-6 are different, although both genes are induced by monomeric IgE in BMMCs. Induction of histamine synthesis in BMMCs was also found to be stimulated by antigen stimulation, as well as IL-6 production is. It remains to be clarified as to why histamine synthesis and IL-6 production is induced via IgE both in the presence and absence of antigen. One possible explanation is that the two pathways of HDC induction may occur in a different context of mast cell differentiation.
The partial suppression of HDC induction by herbimycin A and by PP2 indicates the involvement of Src family tyrosine kinases including Lyn, although stimulation with IgE did not augment the enzymatic activity of Lyn. This observation suggests that Src family tyrosine kinase other than Lyn may be involved in the induction of HDC. The inhibitory effect of a PLC inhibitor, U73122, indicates that PLC may be involved in the increase in cytosolic Ca2+ by IgE, although the signaling pathway responsible for the activation of PLC remains unknown. Further analyses on the pathways downstream of FcεRI activation, including the identification of the PKC isozyme activated by monomeric IgE, should be required.
In summary, we have demonstrated that monomeric IgE can induce histamine synthesis in BMMCs in the absence of its antigen. This induction requires the influx of extracellular Ca2+ and may be mediated by PKC.
Acknowledgments
We thank Ms A. Popiel for her help in preparation of the manuscript.
This study was supported by grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
Footnotes
Abbreviations used in this paper: BMMC, IL-3-dependent bone marrow–derived mast cell; HDC, l-histidine decarboxylase; MEK, MAPK/ERK kinase; PI3-kinase, phosphoinositide 3-kinase; PLC, phospholipase C; PKC, protein kinase C; TPA, 12-O-tetradecanoyl phorbol 13-acetate.
References
- 1.Metcalfe, D.D., D. Baram, and Y.A. Mekori. 1997. Mast cells. Physiol. Rev. 77:1033–1079. [DOI] [PubMed] [Google Scholar]
- 2.Costa, J.J., P.F. Weller, and S.J. Galli. 1997. The cells of the allergic response. Mast cells, basophils, and eosinophils. JAMA. 278:1815–1822. [PubMed] [Google Scholar]
- 3.Mekori, Y.A., and D.D. Metcalfe. 2000. Mast cells in innate immunity. Immunol. Rev. 173:131–140. [DOI] [PubMed] [Google Scholar]
- 4.Kinet, J.-P. 1999. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu. Rev. Immunol. 17:931–972. [DOI] [PubMed] [Google Scholar]
- 5.Furuichi, K., J. Rivera, and C. Isersky. 1985. The receptor for immunoglobulin E on rat basophilic leukemia cells: effect of ligand binding on receptor expression. Proc. Natl. Acad. Sci. USA. 82:1522–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quarto, R., J.-P. Kinet, and H. Metzger. 1985. Coordinate synthesis and degradation of the alpha-, beta- and gamma-subunit of the receptor for immunoglobulin E. Mol. Immunol. 22:1045–1051. [DOI] [PubMed] [Google Scholar]
- 7.Hsu, C., and D. MacGlashan, Jr. 1996. IgE antibody up-regulates high affinity IgE binding on murine bone marrow derived mast cells. Immunol. Lett. 52:129–134. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi, M., C.S. Lantz, H.C. Oettgen, I.M. Katona, T. Fleming, K. Yano, I. Miyajima, J.-P. Kinet, and S.J. Galli. 1997. IgE enhances mouse mast cell FcεRI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J. Exp. Med. 185:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber, M., C.D. Helgason, J.E. Damen, L. Liu, R.K. Humphries, and G. Krystal. 1998. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. USA. 95:11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalesnikoff, J., M. Huber, V. Lam, J.E. Damen, J. Zhang, R.P. Shiraganian, and G. Krystal. 2001. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 14:801–811. [DOI] [PubMed] [Google Scholar]
- 11.Asai, K., J. Kitaura, Y. Kawakami, N. Yamagata, M. Tsai, D.P. Carbone, F. Liu, S.J. Galli, and T. Kawakami. 2001. Regulation of mast cell survival by IgE. Immunity. 14:791–800. [DOI] [PubMed] [Google Scholar]
- 12.Ohmori, E., T. Fukui, N. Imanishi, K. Yatsunami, and A. Ichikawa. 1990. Purification and characterization of L-histidine decarboxylase from mouse mastocytoma P-815 cells. J. Biochem. 107:834–839. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto, J., K. Yatsunami, E. Ohmori, Y. Sugimoto, T. Fukui, T. Katayama, and A. Ichikawa. 1990. cDNA-derived amino acid sequence of L-histidine decarboxylase from mouse mastocytoma p-815 cells. FEBS Lett. 276:214–218. [DOI] [PubMed] [Google Scholar]
- 14.Ring, J., T. Senter, R.C. Cornell, C.M. Arroyave, and E.M. Tan. 1979. Plasma complement and histamine changes in atopic dermatitis. Br. J. Dermatol. 100:521–530. [DOI] [PubMed] [Google Scholar]
- 15.Ring, J. 1983. Plasma histamine concentrations in atopic eczema. Clin. Allergy. 13:545–552. [DOI] [PubMed] [Google Scholar]
- 16.Asahara, M., S. Musiake, S. Shimada, H. Fukui, Y. Kinoshita, C. Kawanami, T. Watanabe, S. Tanaka, A. Ichikawa, Y. Uchiyama, et al. 1996. Reg gene expression is increased in rat gastric enterochromaffin-like cells following water immersion stress. Gastroenterology. 111:45–55. [DOI] [PubMed] [Google Scholar]
- 17.Rottem, M., J.P. Goff, J.P. Albert, and D.D. Metcalfe. 1993. The effects of stem cell factor on the ultrastructure of FcεRI+ cells developing in IL-3-dependent murine bone marrow-derived cell cultures. J. Immunol. 151:4950–4963. [PubMed] [Google Scholar]
- 18.Tanaka, S., K. Nemoto, E. Yamamura, and A. Ichikawa. 1998. Intracellular localization of the 74- and 53-kDa forms of L-histidine decarboxylase in a rat basophilic/mast cell line, RBL-2H3. J. Biol. Chem. 273:8177–8182. [DOI] [PubMed] [Google Scholar]
- 19.Grynkiewicz, G., M. Penie, and R.Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450. [PubMed] [Google Scholar]
- 20.Takizawa, F., M. Adamczewski, and J.-P. Kinet. 1992. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as FcγRII and FcγRIII. J. Exp. Med. 176:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ujike, A., Y. Ishikawa, M. Ono, T. Yuasa, T. Yoshino, M. Fukumoto, J.V. Ravetch, and T. Takai. 1999. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J. Exp. Med. 189:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner, H., and J.-P. Kinet. 1999. Signaling through the high-affinity IgE receptor FcεRI. Nature. 402:B24–B30. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka, S., K. Nemoto, E. Yamamura, S. Ohmura, and A. Ichikawa. 1997. Degradation of the 74 kDa form of L-histidine decarboxylase via the ubiquitin-proteasome pathway in a rat basophilic/mast cell line (RBL-2H3). FEBS Lett. 417:203–207. [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi, M., N. Hirasawa, Y. Kobayashi, S. Oikawa, A. Murakami, and K. Ohuchi. 2000. Participation of mitogen-activated protein kinase in thapsigargin- and TPA-induced histamine production in murine macrophage RAW264.7 cells. Br. J. Pharmacol. 129:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martiny-Baron, G., M.G. Kazanietz, H. Mischak, P.M. Blumberg, G. Kochs, H. Hug, D. Marme, and C. Schachtele. 1993. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268:9194–9197. [PubMed] [Google Scholar]