Abstract
It has been recently demonstrated that regulatory CD4+CD25+ CD45RO+ T cells are present in the peripheral blood of healthy adults and exert regulatory function similar to their rodent counterparts. It remains difficult to understand how the small fraction of these T cells that regulate via direct cell-to-cell contact and not via secretion of immunosuppressive cytokines could mediate strong immune suppression. Here we show that human CD4+CD25+ T cells induce long-lasting anergy and production of interleukin (IL)-10 in CD4+CD25− T cells. These anergized CD4+CD25− T cells then suppress proliferation of syngenic CD4+ T cells via IL-10 but independent of direct cell contact, similar to the so-called type 1 regulatory T (Tr1) cells. This ‘catalytic’ function of CD4+CD25+ T cells to induce Tr1-like cells helps to explain their central role for the maintenance of immune homeostasis.
Keywords: regulatory T cells, human, immune tolerance, anergy, IL-10
Introduction
There is now compelling evidence that CD4+ T cells specialized for the suppression of immune responses play a critical role in immune regulation. It has been convincingly demonstrated in rodents, that cells with this function are enriched within the CD4+CD25+ subset (1–3). Recent studies demonstrate that CD4+CD25+ T cells are also relevant as an immune regulator in humans (4–7). It was shown that CD4+CD25+ T cells similar to their rodent counterpart constitute a small fraction of CD4+ T cells (average 6%). They are naturally anergic to mitogenic stimuli, inhibit the proliferation of CD4+ and CD8+ T cells after stimulation via their TCR, and do so in a cytokine-independent yet cell contact-dependent manner (4–7).
Progress has been made to elucidate the mechanisms by which CD4+CD25+ T cells exert their regulatory function. It has been suggested that CD4+CD25+ T cells bind TGF-β on their cell surface and thereby mediate contact-dependent suppression of other T cells (8). Two groups have described the increased expression of glucocorticoid-induced TNF receptor (GITR) on CD4+CD25+ T cells compared with resting CD4+CD25− T cells and they show, that anti-GITR Abs abrogate CD4+CD25+-mediated suppression (9, 10).
Very little is still known about requirements for the development and physiological regulation of CD4+CD25+ T cell function. Survival and/or expansion of CD4+CD25+ T cells in the periphery seems to be dependent on IL-2 and costimulatory molecules, as mice lacking these components show major deficiencies in CD4+CD25+ T cells (11–13). It is difficult to understand how CD4+CD25+ T cells exert their suppressive function in vivo as they constitute only 6% of CD4+ T cells and need direct cell contact and activation via their TCR to suppress other T cells. In in vitro experiments usually rather high ratios of CD4+CD25+ T cells have to be employed to potently suppress proliferation of CD4+CD25− T cells. These are conditions that probably would not occur in vivo as CD4+CD25+ T cells, even if antigen-specifically activated do not expand and proliferate well because of their anergic state.
It has been shown before that anergized T cells can mediate regulatory function on other T cells (14). In this report we show that CD4+CD25+ T cells do not only anergize other CD4+ T cells but that they also induce high level production of IL-10 in the cells they suppress. The resulting IL-10–producing anergized T cells are then able to suppress T cell proliferation in an IL-10–dependent fashion. These findings give insight into the mechanisms used by CD4+CD25+ T cells to execute their important in vivo function.
Materials and Methods
Culture Medium.
RPMI 1640 (Bio Whitaker) supplemented with 1% heat-inactivated autologous plasma, 20 μg/ml gentamicin (Merck), and 2 mM glutamine (Bio Whitaker) was used for the generation of dendritic cells (DCs), X-VIVO-20 (Bio Whitaker) supplemented with 1% heat-inactivated single donor human serum, 20 μg/ml gentamicin (Merck), and 2 mM glutamine (Bio Whitaker) for T cell culture.
Cytokines.
All cytokines used in this study were recombinant human proteins. Final concentrations were: 1,000 U/ml GM-CSF (Leukomax™; Novartis); 800 U/ml IL-4 (Sandoz); and IL-2 (Proleukin; Chiron Corp.) were used at the concentrations indicated; for DC maturation we used a cocktail consisting of 2 ng/ml IL-1β (Sigma-Aldrich), 1,000 U/ml IL-6 (Sandoz), 10 ng/ml TNF-α (Bender), and 1 μg/ml PGE2 (Sigma-Aldrich).
Abs.
For immunostaining PE- and FITC-conjugated Abs (all from BD PharMingen) against CD3 (UCHT 1), CD4 (RPA-T4), CD5 (UCHT 2), CD8 (RPA-T8), CD14 (M5E2), CD19 (HIB 19), CD25 (M-A251), CD28 (CD28.2), CD45 RA (HI 100), CD45 RO (UCHL 1), CD56 (B159), CD62L (DREG-56), CD80 (L307.4), CD83 HB15e), CD86 (FUN-1), CD95 (DX 2), CD95L (G247–4),CD122 (MiK-β2), CD152 (BNI3.1), CD154 (TRAP 1), HLA-DR (G46–6), and respective mouse and rat isotype controls were employed. Abs used for intracellular cytokine staining were FITC- and PE-conjugated anti–IL-2 (MQ1–17H12), anti–IL-4 (8D4–8), anti–IL-10 (JES3–19F1), and anti–IFN-γ (4S.B3; all from BD PharMingen). Unconjugated anti–IL-10 (JES3–19F1) (BD PharMingen) was used for neutralization experiments, anti-CD3 (UCHT1) and anti-CD28 (CD28.2) for polyclonal activation of T cells.
Cytokine Assays.
T cells were stimulated with allogeneic DCs or with platebound anti-CD3 (10 μg/ml) plus soluble anti-CD28 (10 μg/ml) in X-VIVO-20 plus 1% serum. Cytokine analysis was performed at different time points by analysis of supernatants with commercially available ELISA kits for human IL-10, IFN-α (Biosource International), and TGF-β (BD PharMingen). IL-2, IL-4, IL-5, IFN-γ, and TNF-α were measured by a cytometric bead array (Th1/Th2 Cytokine CBA 1; BD PharMingen) according to the manufacturer's instructions. For analysis of intracellular cytokine production T cells were either stimulated with 20 ng/ml PMA and 500 μg/ml Ca2+ ionophore A23187 (both from Sigma-Aldrich) for 6 h or with platebound anti-CD3 and soluble anti-CD28 Ab for 6 h. 2 μM monensin (Sigma-Aldrich) was added for the last 5 h of culture. Cells were collected, washed, fixed, and saponine permeabilized (fix/perm solution; BD PharMingen) and stained with cytokine specific Ab or isotype.
Cell Isolation and DC Generation.
DCs were generated from buffy coats or leukapheresis products (both obtained from the Dept. of Transfusion Medicine, University of Erlangen, Erlangen, Germany, from healthy donors after informed consent was given) as described previously (15, 16). In brief, PBMCs were isolated by Ficoll density gradient centrifugation. Monocytes were isolated by plastic adherence and cultured in RPMI 1640 medium, supplemented with IL-4 and GM-CSF. At day 6 a maturation cocktail (IL-1β, IL-6, PGE2, and TNF-α) was added. At day 7 nonadherent cells were harvested and constituted mature DCs that were >90% double positive for costimulatory molecules (CD80, CD86) and CD83.
CD4+ T cells were isolated from PBMCs with a negative CD4+ T cell isolation kit (Miltenyi Biotech). CD4+CD25+ T cells were isolated from the pure, untouched CD4+ T cells using CD25 microbeads (Miltenyi Biotech). Purity was assessed by FACS®.
Flow Cytometric Analysis.
For immunofluorescence staining cells were washed and stained for 20 min at 4°C with optimal dilution of each Ab. Cells were washed again and analyzed by flow cytometry (FACScan™ and CELLQuest™ software; Becton Dickinson). For analysis of intracellular CD152 cells were stained for CD4 expression, fixed, and saponine permeabilized (fix/perm solution; BD PharMingen) and stained with CD152-specific Ab or isotype.
Fixation of CD4+CD25+ and CD4+CD25− T Cells.
For fixation experiments CD4+CD25+ and CD4+CD25− T cells were isolated and divided into three fractions. One part of each was activated with platebound anti-CD3 and soluble anti-CD28 overnight. Next day the stimulated parts and one resting part were fixated with 2% paraformaldehyde for 1 h at 4°C. Thereafter, fixated cells were washed extensively and used in regulation assays together with the untreated fraction.
Induction of Anergized T Cells.
To induce anergized CD4+ CD25− T cells with CD4+CD25+ T cells, both populations were isolated as described. They were either used directly or fixated as described above. 5 × 105 of CD4+CD25+ and CD4+CD25− T cells were cultivated either with platebound anti-CD3 and soluble anti-CD28 (10 μg/ml each) or with allogeneic mature DCs (5 × 104 cells) for 48 h in 48-well plates. Thereafter, cells were harvested, washed, and used in proliferation experiments.
Proliferation Assays.
To assess proliferation of the differently cultured CD4+ subtypes 105 sorted T cells were incubated in X-VIVO-20 with 5 × 103 DCs in 96-well, U-bottomed plates or 10 μg/ml of platebound anti-CD3 plus 10 μg/ml soluble anti-CD28 in 96-well, flat-bottomed plates. For assessment of regulatory properties 105 resting CD4+CD25− autologous T cells were cultured with 5 × 103 DCs or platebound anti-CD3 and soluble anti-CD28 in 96-well, U-bottomed plates. Purified CD4+CD25+ and CD4+CD25−T cells, anergized CD4+CD25− T cells or fixated CD4+CD25+ and CD4+CD25− T cells were added usually at a 1:1 ratio if not indicated differently. After 4–5 d of culture 3[H]Tdr (37 KBq/well) was added for additional 16 h. Proliferation was measured using a liquid scintillation counter.
Transwell Experiments.
Transwell experiments were performed in 24-well plates. 106 CD4+CD25− T cells were stimulated with 5 × 104 DCs. In addition, 106 CD4+CD25+, CD4+CD25−, and anergized CD4+CD25− T cells were either added directly to the culture or were placed in transwell chambers (Millicell, 0.4 μm; Millipore). After 5 d of coculture T cells were transferred to 96-well plates (105 cells per well) in triplicates. Proliferation was measured after 16-h pulse with 3[H]Tdr using a liquid scintillation counter.
CFSE Labeling and Sorting.
CD4+CD25+ and CD4+CD25− T cells were labeled with 0.5 μM of CFSE (Molecular Probes) for 15 min at 37°C. Reaction was stopped with ice-cold PBS buffer and cells were washed extensively. 5 × 105 CD4+CD25+ CFSE-labeled T cells were then cultured with 5 × 105 unlabeled CD4+CD25− T cells (and vice versa) with platebound anti-CD3 and soluble anti-CD28 in 48-well plates. Proliferation was controlled by FACS® for different time points. After 48 h cells were harvested and sorted on a FACSVantage™ (Becton Dickinson). Sorted cells were used for further regulation assays.
Results
Coculture of CD4+CD25+ and CD4+CD25− T Cells Yields Low Proliferating, IL-10–producing T Cells.
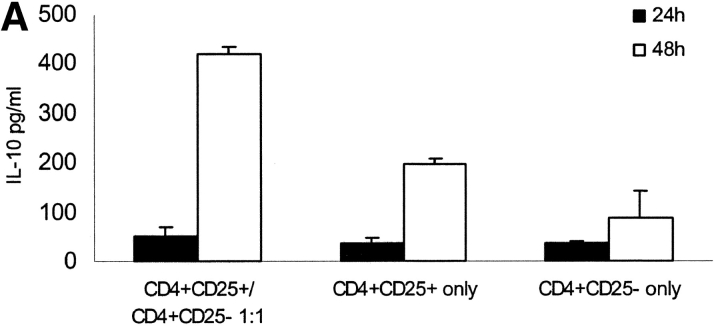
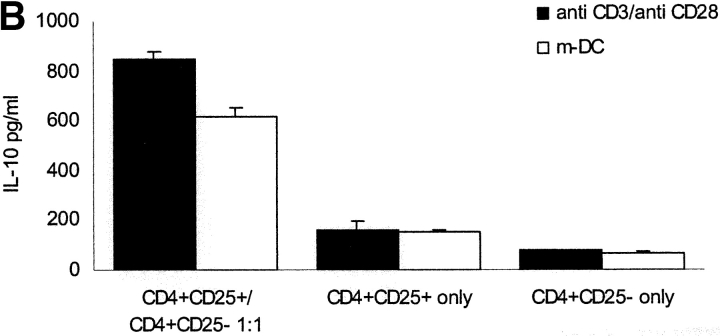
CD4+ CD25+ and CD4+CD25− subpopulations were separated by MACS® from healthy donors. Separately or mixed at a 1:1 ratio the cells were stimulated polyclonally with platebound anti-CD3 and soluble anti-CD28 or with mature allogeneic DCs. As shown before coculture of CD4+CD25+ and CD4+CD25− T cells results in a constantly low-proliferating T cell population (unpublished data). We analyzed the supernatant of this coculture after various time points for different cytokines and found a high level of IL-10 production peaking 48 h after onset of culture (Fig. 1 A). As shown before CD4+CD25+ T cells alone also produce sizeable amounts of IL-10 (∼200 pg/ml). One might speculate that IL-10 production was only attributable to CD4+ CD25+ T cells, but IL-10 production in the coculture was 2–4 times higher than production of CD4+CD25+ T cells alone (Fig. 2, A and B) . Intracellular FACS® revealed that the number of IL-10–producing cells more than doubled (Fig. 1 C). High IL-10 production after 48 h of coculture was observed regardless if polyclonal activation (platebound anti-CD3 and soluble anti-CD28) or allogeneic mature DCs were used (Fig. 1 B). In none of the cultures increased production of TGF-β or Inf-α could be observed (unpublished data). CD4+CD25− T cells alone did not produce significant amounts of IL-10 (Fig. 1, A–C).
Figure 1.
Coculture of CD4+CD25+ and CD4+CD25− T cells results in high level IL-10 production. CD4+CD25+ and CD4+CD25− T cells were MACS® sorted from PBMCs of healthy individuals. These cells were either cultured alone or at a 1:1 ratio and activated with platebound anti-CD3 and soluble anti-CD28 (10 μg/ml, respectively). (A) After various time points supernatants were analyzed for cytokine production by ELISA. IL-10 production peaked 48 h after onset of culture and was markedly higher in the coculture of CD4+CD25+ and CD4+CD25− T cells than in the cultures of each of the cell types alone. A representative out of five independent standardized experiments is shown. No elevated levels of INF-α or TGF-β could be measured (data not shown). (B) The different T cell populations were also activated with mature allogeneic DCs (DC/T cell ratio 1:20) compared with anti-CD3 and anti-CD28 (10 μg/ml, respectively). Cytokines were measured 48 h after onset of culture. Results were similar in five independent experiments. (C) For the last 6 h of activation with anti-CD3 and anti-CD28 2 μM monensin was added to the cultures. Staining of CD4 surface expression was performed. Cells were washed, fixed, permeabilized, and stained for intracellular IL-10 using PE-conjugated specific Abs. One of five independent experiments is shown.
Figure 2.
Activated fixed CD4+CD25+ T cells show similar regulatory potential as viable CD4+CD25+ T cells and can induce IL-10 production in CD4+CD25− T cells. (A) CD4+ T cell subpopulations were sorted by MACS® CD4+CD25+ T cells were divided into three fractions. One part was activated with platebound anti-CD3 (10 μg/ml) and soluble anti-CD28 (10 μg/ml) overnight and fixed the next day with paraformaldehyde 2% (activated-fixed). The third part was fixed with paraformaldehyde without activation (resting-fixed) and the second part was left untreated (viable). Each fraction was mixed with syngeneic CD4+CD25− T cells at a 1:1 ratio (105 T cells per 96 well) and stimulated with platebound anti-CD3 (10 μg/ml) and soluble-anti-CD28 (10 μg/ml). Proliferation was determined by 3[H]Tdr incorporation after 5 d. Results are representative of five independent experiments, shown as mean cpm of triplicate cultures. Similar results were observed when T cells were stimulated with mature allogeneic DCs (DC/T cell ratio of 1:20; data not shown) (B) CD4+CD25+ and CD4+CD25− T cells were either cultured alone or CD4+CD25− T cells were mixed at a 1:1 ratio with activated-fixed, resting-fixed or viable CD4+CD25+ T cells. T cells were stimulated with mature allogeneic DCs at the same ratio as in A. In a parallel transwell approach CD4+CD25+ T cells were stimulated with allogeneic DCs (DC/T ratio 1:20) in a transwell chamber, and CD4+CD25− T cells were placed in the well together with allogeneic DCs again at a DC/T ratio of 1:20. IL-10 production was measured by ELISA 48 h after onset of culture. Results were similar in five independent experiments.
Activated, Paraformaledhyde-fixed CD4+CD25+ T Cells Show Similar Regulatory Capacity as Viable Cells.
It is known that CD4+CD25+ T cells exert their regulatory function in a cell contact–dependent yet cytokine-independent manner. To further analyze their regulatory function, isolated CD4+CD25+ T cells were divided into three parts. One was activated overnight polyclonally with platebound anti-CD3 and soluble anti-CD28 and fixed thereafter with paraformaldehyde (“activated-fixed”), the second part was fixed with paraformaldehyde without prior activation (“resting-fixed”), and the third part was left untreated (“viable”). After this procedure the three differently treated fractions of CD4+CD25+ T cells were used in regulation assays with syngenic CD4+CD25− T cells. As shown in Fig. 2 A activated-fixed CD4+CD25+ T cells had a similar regulatory capacity as their normal viable counterpart. This is in sharp contrast to resting-fixed CD4+CD25+ T cells that do not show any regulatory function at all. Activated-fixed and viable CD4+CD25+ T cells almost completely suppressed proliferation of CD4+CD25− T cells when a 1:1 ratio was used. This underlines and extends prior findings on the regulatory function, demonstrating that surface molecules induced after activation of CD4+CD25+ are responsible for the regulatory capacity of these cells.
CD4+CD25+ Regulatory T Cells Induce IL-10 Production in Anergized CD4+CD25− T Cells in a Cell Contact–dependent Manner.
In further experiments we used the above mentioned findings to analyze the requirements for induction of IL-10–producing anergized CD4+CD25− T cells. CD4+CD25+ and CD4+CD25− T cells were either cultured alone or at a 1:1 ratio with normal viable CD4+CD25+ T cells, activated-fixed CD4+CD25+ T cells, resting-fixed CD4+CD25+ T cells, or in a transwell setting. IL-10 production was measured 48 h after onset of culture.
As shown in Fig. 2 B a high level IL-10 production was achieved in coculture either with viable CD4+CD25+ or activated-fixed CD4+CD25+ T cells. In transwell experiments IL-10 production similar to CD4+CD25+ T cells alone was observed and CD4+CD25− T cells alone produced negligible amounts of IL-10.
CD4+CD25− T Cells Anergized by CD4+CD25+ T Cells Suppress Activation of Syngenic CD4+ T Cells in an IL-10–dependent Manner.
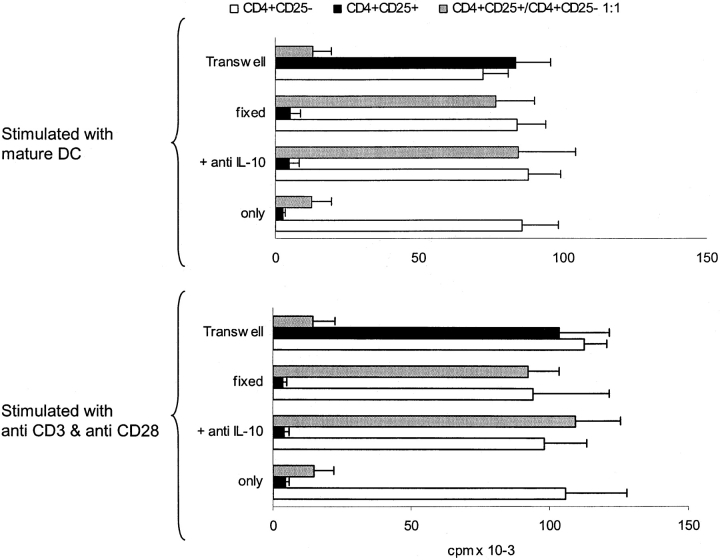
IL-10 is known as a cytokine with potent immunosuppressive function. Therefore, it was tempting to speculate that the high IL-10 production of anergized CD4+CD25− T cells would lead to secondary suppression of other T cells. In the next set of experiments we sought to investigate this matter. As it is known that IL-10 can act indirectly on T cells via influence on APC, we choose a polyclonal, cell-free T cell stimulus (platebound anti-CD3 and soluble anti-CD28) and mature allogeneic DCs, because they are known as the most powerful APC and largely resistant to IL-10 (17). CD4+CD25+ and CD4+ CD25− T cells alone or at a 1:1 ratio were stimulated with allogeneic DCs (Fig.3 , top panel) or with bound anti-CD3 and soluble anti-CD28 (Fig. 3, bottom panel) for 48 h. Thereafter, cells were either fixed with paraformaldehyde or used viable. As expected activated CD4+CD25+ T cells induced strong suppression of CD4+ proliferation and at a 1:1 ratio almost abolished it, whereas activated CD4+CD25− T cells did not alter proliferation of syngenic CD4+ T cells. When the cocultured CD4+CD25+ and CD4+CD25−T cells were used in regulation assays, they mediated a strong inhibition of CD4+ T cell proliferation (Fig. 3). This phenomenon was seen regardless of the stimulus used (Fig. 3, top and bottom panel). We further added anti–IL-10 Ab to the regulation assay or performed it in a transwell setting. As shown before, anti–IL-10 did not alter suppressive function of pure CD4+CD25+ T cells, whereas in a transwell setting CD4+CD25+ T cells could not mediate suppression. The opposite was true for regulation by anergized CD4+CD25− T cells. Addition of IL-10 Abs almost completely abolished inhibition, whereas a transwell setting did not markedly change the regulatory function of these cells, suggesting that inhibition is mediated dominantly by secretion of IL-10 (Fig. 3). Similar effects were seen with polyclonally or allogeneic stimulated cells (Fig. 3, top and bottom panel).
Figure 3.
CD4+CD25− T cells anergized by CD4+CD25+ T cells suppress proliferation of CD4+ T cells in a IL-10–dependent manner. MACS® sorted CD4+CD25+ and CD4+CD25− T cells were either cultured alone or mixed at a 1:1 ratio (2 × 106 T cells per 24 well) and stimulated with mature allogeneic DCs (DC/T cell ratio 1:20) or immobilized anti-CD3/soluble anti-CD28. After 48 h of culture cells were harvested and one fraction of each population was fixed with paraformaldehyde for 1 h. Viable and fixed cells were cocultured with syngeneic resting CD4+CD25− T cells at a 1:1 ratio (105 T cells per 96 well) and stimulated as before with immobilized anti-CD3/soluble anti-CD28 (bottom panel) or mature allogeneic DCs (top panel) in the presence or absence of 10 μg/ml anti–IL-10 Abs. In a parallel transwell approach the three different T cell populations were placed in a transwell chamber and resting CD4+CD25− T cells were stimulated with DCs (DC/T cell ratio 1:20; top panel) or platebound anti-CD3/soluble anti-CD28 (bottom panel) in the well. Proliferation after 5 d was determined by 3[HT]Tdr incorporation. One out of four independent experiments is shown.
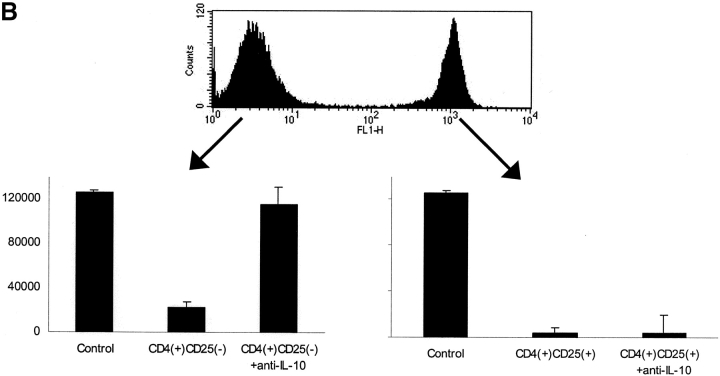
To further exclude that the observed effects are mediated by CD4+CD25+ T cell directly we performed CFSE labeling and FACS® sorting experiments. CD4+CD25− T cells were labeled with CFSE and then mixed with CD4+CD25+ unlabeled T cells at a 1:1 ratio. This mixture was stimulated with immobilized anti-CD3/soluble anti-CD28 for 48 h. Thereafter, cells were sorted by FACS® and used in regulation assays with syngenic CD4+CD25− T cells. As shown in Fig. 4 regulation was mediated by both the labeled and unlabeled fractions, which was abolished by addition of anti–IL-10 in the case of unlabeled (anergized CD4+CD25− T cells). Not surprisingly activated CD4+ CD25+ T cells showed inhibition of T cells proliferation themselves. This could not be abolished by anti–IL-10, clearly demonstrating that anergized CD4+CD25− T cells mediate suppression via IL-10 which is distinct from the mechanisms used by CD4+CD25+ T cells.
Figure 4.
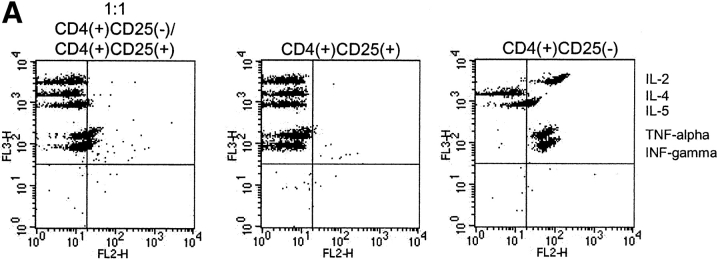
(A) Anergized CD4+CD25− T cells predominantly secrete IL-10. CD4+CD25+ and CD4+CD25− T cells were isolated as described and stimulated alone or at a 1:1 ratio with anti-CD3/anti-CD28. 48 h after stimulation supernatant was harvested and analyzed by a cytometric bead array for IL-2, IL-4, IL-5, TNF-α, and INF-γ. Results were similar in five independent experiments. (B) Before mixing CD4+CD25− and CD4+CD25+ T cells at a 1:1 ratio, CD4+CD25+ T cells were labeled with 0.5 μM CFSE for 15 min. Cells were then mixed and stimulated with immobilized anti-CD3/soluble anti-CD28. After 48 h cells were harvested and sorted on a FACSVantage™. The positive and the negative fraction were then cocultured with syngeneic resting CD4+CD25− T cells (105 T cells per 96 well). Proliferation was measured after 5 d by 3[HT]Tdr incorporation. One out of five independent experiments is shown.
Anergized CD4+CD25− T Cells Predominantly Produce IL-10.
To analyze the cytokine secretion pattern of anergized CD4+CD25− T cells, CD4+CD25+ and CD4+ CD25− T cells were sorted and stimulated alone or at a 1:1 mixture as described before. After 48 h of culture supernatants were analyzed for the cytokines IL-2, IL-4, IL-5, TNF-α, and INF-γ by a cytometric bead array, which allows multiparameter analysis in a single sample. As shown in Fig. 4 anergized CD4+CD25− T cells similar to CD4+CD25+ T cells do only produce very low levels of TNF-α and INF-γ and no IL-2, IL-4, or IL-5. CD4+CD25− T cells on the other hand produce high levels of IL-2, TNF-α, and INF-γ and low to moderate levels of IL-4 and IL-5, resembling a Th1 phenotype.
Surface phenotyping with the Abs mentioned in material and method did not reveal striking differences between activated CD4+CD25+, activated CD4+CD25−, and cocultured CD4+CD25+/CD4+CD25− T cells after 48 h of activation (unpublished data).
Discussion
The important in vivo function of CD4+CD25+ regulatory T cells has been thoroughly demonstrated in rodents (1–3). Lately, we and others have shown that a similar population of regulatory T cells also exists in humans. These findings have been confirmed and extended by several groups up to now (4–8, 18–22). Still numerous characteristics of CD4+CD25+ T cells need to be explained. One important question that has to be answered is how CD4+CD25+ T cells execute their important function in vivo, as they only constitute a small population of peripheral CD4+ T cells (average 6%), that need direct cell contact as well as stimulation via the TCR to suppress unwanted T cell activation. In vitro studies usually employ high ratios of CD4+CD25+/CD4+CD25− T cells, a situation that is hard to imagine at an inflammatory site in vivo.
As described, coculture of CD4+CD25+ and CD4+ CD25− T cell leads to marked reduction of T cell proliferation (6). This effect is stable for several days (unpublished data). Although CD4+CD25+ T cells produce sizeable quantities of IL-10 this cytokine does not seem to be responsible for the regulatory effects (6). We analyzed the supernatant of CD4+CD25+ and CD4+CD25− cocultures and found that high levels of IL-10 are produced, peaking after 48 h. IL-10 levels in the coculture were markedly higher than IL-10 produced by CD4+CD25+ T cells alone, suggesting that it was not only attributable to CD4+CD25+ T cells. This was further confirmed by intracellular FACS® analysis. IL-10 is known to inhibit cytokine production from T cells (23) and exert antiinflammatory and suppressive effects on most haematopoietic cells. It is also involved in the induction of peripheral tolerance via effects on T cell–mediated responses (23). IL-10 indirectly suppresses T cell responses by potently inhibiting the antigen-presenting capacity of APC, including DCs (24), Langerhans cells, and macrophages (25). In addition, IL-10 directly regulates T cells by inhibiting their ability to produce IL-2, TNF-α (26), IL-5 (27), and to proliferate (28).
It was important to rule out the effects seen were not only due to CD4+CD25+ T cells in the coculture. In a set of pilot experiments we could show that CD4+CD25+ T cells when paraformaldehyde-fixed after polyclonal activation have similar regulatory properties as viable CD4+CD25+ T cells. In coculture experiments, using activated-fixed and viable CD4+CD25+ T cells together with CD4+CD25− T cells, it turned out that IL-10 production remained high, even if activated-fixed CD4+CD25+ T cells were used. This showed that IL-10 production was not attributable to increased production by CD4+CD25+ but due to the anergized CD4+CD25− T cells. In a parallel transwell approach we showed that direct cell contact between CD4+CD25+ and CD4+CD25− T cells is necessary to prime CD4+CD25− T cells to become IL-10 producers.
Further experiments were performed to analyze which effect this high level of IL-10 might have on T cell proliferation. Indeed it was shown that proliferation of syngenic CD4+ T cells could be markedly decreased by anergized CD4+CD25− T cells. Addition of anti–IL-10 abolished the suppressive effects of anergized CD4+CD25− T cells, while a transwell setting permitting the free exchange of soluble factors, but no cell contact, did not change suppression. Furthermore, we CSFE-labeled CD4+CD25+ T cells and separated them from CD4+CD25− T cells after 48 h of coculture by FACS® sorting. Both populations strongly inhibited CD4+ T cell proliferation which was almost abolished in the unlabeled e.g., CD4+CD25− fraction by the addition of anti–IL-10, demonstrating that IL-10 indeed is crucial for the suppressive function of anergized CD4+CD25− T cells. This is not surprising, as other reports have shown that activation of human CD4+ T cells in the presence of IL-10 results in a state of functional unresponsiveness without death, termed anergy (19). CD4+ T cells with low proliferative capacity that are generated in the presence of IL-10 have been termed Tr1. The cells that are generated in the presence of CD4+CD25+ T cells show some characteristics resembling Tr1 cells, especially their low proliferative capacity and the high level production of IL-10. But in some instances they differ, as Tr1 are also defined by their ability to produce TGF-β and anergized CD4+CD25− T cells did not produce significant amounts of TGF-β at least by the assay used. Further on we clearly demonstrate that cell contact between CD4+CD25+ and CD4+CD25− T cells and not IL-10 is crucial for the induction phase of inhibitory, anergized, IL-10–producing, CD4+CD25− T cells. But as coculture of anergized CD4+CD25− with syngenic resting CD4+CD25− T cells results in anergic, IL-10–releasing CD4+CD25− T cells this IL-10 production may then also contribute to the generation of Tr1-like cells as described for Tr1 cells.
To distinguish between indirect effects via APC modulation and direct effect on T cells we used as a stimulus not only allogeneic DCs but also immobilized anti-CD3/soluble anti-CD28 as a cell-free T cell stimulation system. As the effects seen were independent of the stimuli used, a direct effect on T cells is most likely.
The data presented here may serve as an explanation of how CD4+CD25+ T cells fulfill their important in vivo function. At sites of inflammation if activated they would anergize CD4+ T cells in their close environment in an antigen-unspecific bystander effect fashion (29). Our findings suggest, however, that anergized CD4+ T cells (including pathogenic ones) in turn will produce high levels of IL-10, thereby creating an immunosuppressive environment either by indirect effect via influence on APC (30) or via direct effects on other T cells thereby effectively abrogating unwanted T cell activation.
Acknowledgments
This work was supported by grant 01.10.29.1 from the ELAN-Fonds fuer Forschung und Lehre of the Friedrich-Alexander-University Erlangen Nuremberg and in part by the Fifth (European Community) Framework Program (1998–2002) “Quality of Life Management of Living Resources,” contract QLK2-200-00470, by the Bundesministerium für Bildung und Forschung grant 01GE9911/7, Forschungsverbünde zur somatischen Gentherapie, and by the German Science Foundation (SFB 263, C13).
References
- 1.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 3.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor, P.A., R.J. Noelle, and B.R. Blazar. 2001. CD4+ CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142. [DOI] [PubMed] [Google Scholar]
- 10.McHugh, R.S., M.J. Whitters, C.A. Piccirillo, D.A. Young, E.M. Shevach, M. Collins, and M.C. Byrne. 2002. CD4+ CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323. [DOI] [PubMed] [Google Scholar]
- 11.Papiernik, M., M.L. de Moraes, C. Pontoux, F. Vasseur, and C. Penit. 1998. Regulatory CD4 T cells: expression of IL-2R α chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371–378. [DOI] [PubMed] [Google Scholar]
- 12.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 13.Kumanogoh, A., X. Wang, I. Lee, C. Watanabe, M. Kamanaka, W. Shi, K. Yoshida, T. Sato, S. Habu, M. Itoh, N. Sakaguchi, S. Sakaguchi, and H. Kikutani. 2001. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J. Immunol. 166:353–360. [DOI] [PubMed] [Google Scholar]
- 14.Jooss, K., B. Gjata, O. Danos, H. von Boehmer, and A. Sarukhan. 2001. Regulatory function of in vivo anergized CD4+ T cells. Proc. Natl. Acad. Sci. USA. 98:8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood: an improved method with special regard to clinical applicability. J. Immunol. Methods 196:137–151. [DOI] [PubMed] [Google Scholar]
- 16.Thurner, B., C. Roder, D. Dieckmann, M. Heuer, M. Kruse, A. Glaser, P. Keikavoussi, E. Kampgen, A. Bender, and G. Schuler. 1999. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223:1–15. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 18.Ng, W.F., P.J. Duggan, F. Ponchel, G. Matarese, G. Lombardi, A.D. Edwards, J.D. Isaacs, and R.I. Lechler. 2001. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 98:2736–2744. [DOI] [PubMed] [Google Scholar]
- 19.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagiwa, S., J.D. Gray, S. Hashimoto, and D.A. Horwitz. 2001. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166:7282–7289. [DOI] [PubMed] [Google Scholar]
- 21.Stephens, L.A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247–1254. [DOI] [PubMed] [Google Scholar]
- 22.Taams, L.S., J. Smith, M.H. Rustin, M. Salmon, L.W. Poulter, and A.N. Akbar. 2001. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122–1131. [DOI] [PubMed] [Google Scholar]
- 23.Moore, K.W., P. Vieira, D.F. Fiorentino, M.L. Trounstine, T.A. Khan, and T.R. Mosmann. 1990. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 248:1230–1234. [DOI] [PubMed] [Google Scholar]
- 24.Steinbrink, K., M. Wolfl, H. Jonuleit, J. Knop, and A.H. Enk. 1997. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 159:4772–4780. [PubMed] [Google Scholar]
- 25.Romagnoli, P., D. Hudrisier, and J.P. van Meerwijk. 2002. Preferential recognition of self antigens despite normal thymic deletion of CD4+CD25+ regulatory T cells. J. Immunol. 168:1644–1648. [DOI] [PubMed] [Google Scholar]
- 26.de Waal, M.R., H. Yssel, and J.E. de Vries. 1993. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 150:4754–4765. [PubMed] [Google Scholar]
- 27.Schandene, L., C. Alonso-Vega, F. Willems, C. Gerard, A. Delvaux, T. Velu, R. Devos, M. de Boer, and M. Goldman. 1994. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J. Immunol. 152:4368–4374. [PubMed] [Google Scholar]
- 28.Bejarano, M.T., M.R. de Waal, J.S. Abrams, M. Bigler, R. Bacchetta, J.E. de Vries, and M.G. Roncarolo. 1992. Interleukin 10 inhibits allogeneic proliferative and cytotoxic T cell responses generated in primary mixed lymphocyte cultures. Int. Immunol. 4:1389–1397. [DOI] [PubMed] [Google Scholar]
- 29.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrink, K., H. Jonuleit, G. Muller, G. Schuler, J. Knop, and A.H. Enk. 1999. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood. 93:1634–1642. [PubMed] [Google Scholar]