Abstract
Zymosan is a β-glucan– and mannan-rich particle that is widely used as a cellular activator for examining the numerous responses effected by phagocytes. The macrophage mannose receptor (MR) and complement receptor 3 (CR3) have historically been considered the major macrophage lectins involved in the nonopsonic recognition of these yeast-derived particles. Using specific carbohydrate inhibitors, we show that a β-glucan receptor, but not the MR, is a predominant receptor involved in this process. Furthermore, nonopsonic zymosan binding was unaffected by genetic CD11b deficiency or a blocking monoclonal antibody (mAb) against CR3, demonstrating that CR3 was not the β-glucan receptor mediating this activity. To address the role of the recently described β-glucan receptor, Dectin-1, we generated a novel anti–Dectin-1 mAb, 2A11. Using this mAb, we show here that Dectin-1 was almost exclusively responsible for the β-glucan–dependent, nonopsonic recognition of zymosan by primary macro-phages. These findings define Dectin-1 as the leukocyte β-glucan receptor, first described over 50 years ago, and resolves the long-standing controversy regarding the identity of this important molecule. Furthermore, these results identify Dectin-1 as a new target for examining the immunomodulatory properties of β-glucans for therapeutic drug design.
Keywords: lectin, macrophage, receptor, immunology, glucans
Introduction
The ability of zymosan particles to stimulate cells of the reticuloendothelial system was noted almost 50 yr ago (1) and has led to their wide use in the investigation of many phagocyte responses. Zymosan is a yeast-derived particle composed principally of polysaccharides, of which β-glucan, the active component mediating the cellular effects (2), and mannan are the major constituents (3). In vivo administration of zymosan, or purified soluble β-glucans, has a number of desirable effects on immune function, including the ability to confer resistance to tumors and various infections, prompting interest in the development of β-glucan–based therapeutics (4, 5). Despite the significant therapeutic implications, the molecular mechanism through which these effects are mediated is not known.
Early studies, using carbohydrate inhibitors to block various leukocyte receptors, suggested that the cellular recognition of unopsonized zymosan is mediated by the mannose receptor and a β-glucan receptor (6–8). The identity of the β-glucan receptor, which has been defined as a β-glucan inhibitable receptor for particulate activators of the alternative complement pathway (6), is controversial. The ability of CR3 to recognize β-glucans led to the proposal that this receptor is the major β-glucan receptor on leukocytes and that it mediates all the immunomodulatory effects of these carbohydrates, including the β-glucan–dependent binding of zymosan (4, 9–11). Conflicting evidence, however, indicated that another receptor(s) mediates this activity (6, 12–14), and although we and others have identified additional receptors capable of recognizing β-glucans (15–17), their role in primary cells is unclear.
The main challenge in defining the contribution of these various receptors to the recognition of β-glucans has been the lack of receptor-specific reagents. Here, using novel and specific reagents, we have defined the receptors involved in the nonopsonic recognition of zymosan and soluble β-glucans in primary macrophages. We have shown that neither the MR nor CR3 are significantly involved, rather we demonstrate that the recently described Dectin-1 (17, 18) plays a major role in this process. These studies suggest that Dectin-1 is the leukocyte β-glucan receptor, the identity of which has remained elusive since its first description over five decades ago.
Materials and Methods
Cells.
Thioglycollate (Tg)- or Biogel-elicited peritoneal and bone marrow–derived macrophages (BMDMs) were isolated from C57BL/6 mice by standard procedures and cultured overnight in 24-well plates. Animals were kept and handled according to institutional guidelines. C57BL/6 CD11b−/− mice, generated as described previously (19), were a gift from Dr. G. Hagger (Glaxo-SmithKline, Stevenage, UK). Cells were maintained in RPMI with 10% heat-inactivated FCS, 50 IU/ml penicillin G, 50 μg/ml streptomycin, and 2 mM glutamine (RPMI-medium); except for BMDMs, which were cultured in RPMI-medium supplemented with 15% (vol/vol) L-cell conditioned medium, as a source of M-CSF (20). BMDMs were used 5 to 7 d after isolation and culture.
Generation of mAbs against Dectin-1.
The mAb, 2A11, specific for Dectin-1, was generated by immunization of Fischer rats with NIH3T3 cells transduced with full-length Dectin-1 (17) and subsequent boosting with soluble recombinant, hemagglutinin (HA)-tagged, Dectin-1. Recombinant Dectin-1 was harvested from supernatants of the human 293T fibroblast cell line transfected with pcDNA3.1 (Invitrogen) encoding an NH2-terminal leader and HA-tag sequence fused to the extracellular portion of Dectin-1 (amino acids 66 to 244). Splenic B cells from immunized rats were then fused with the Y3 rat myeloma cell line (21), according to standard protocols. Hybridoma supernatants were initially screened by ELISA against the soluble recombinant form of Dectin-1. The mAb 2A11 (IgG2b) was subsequently selected based on its ability to recognize unfixed and unpermeabilized cells transduced with Dectin-1, hence detecting an extracellular epitope. Other antibodies used in this study were 5C6 (anti-CR3; reference 22) and a rat IgG2b isotype control.
Flow Cytometry and Immunoprecipitation.
Flow cytometry was performed according to conventional protocols. Cells were examined by three-color FACS® analysis using biotin-conjugated 2A11, phycoerythrin-conjugated F4/80 (Serotec) and FITC-conjugated 5C6. Biotin- or FITC-conjugated rat IgG2b were used as isotype controls. Allophycocyanin-conjugated streptavidin (BD Biosciences) was used to detect the biotin conjugates.
For immunoprecipitation, soluble recombinant HA-tagged Dectin-1 was incubated with 2A11 or rat IgG2b isotype control and then captured with sheep anti–rat IgG magnetic beads (Dynal). Western blotting, after SDS-PAGE, was performed according to standard protocols. The mouse mAb HA11 (anti-HA; Covance) and donkey anti–mouse IgG horseradish peroxidase conjugate (Jackson ImmunoResearch Laboratories) were used to detect the HA-tagged protein using the ECL chemiluminescence substrate (Amersham Biosciences).
Fluorescent Zymosan Binding Assays.
The fluorescence-based binding assays using FITC-labeled zymosan were performed as described (17, 23), except that in all experiments the cells were maintained at 4°C, to prevent the local release of opsonins, including complement (24, 25). In brief, macrophages were plated at 2.5 × 105 cells/well in 24-well plates in RPMI-medium overnight. The cells were cooled to 4°C and washed three times with prechilled culture medium. Zymosan-FITC (Molecular Probes) was added to the cells at a ratio of 25 particles/cell for 1 h on ice. After incubation with the carbohydrate or antibody inhibitors, described below, unbound zymosan was removed by extensive washing with medium and cells were then lysed with 3% Triton X-100. FITC in lysates was quantified using a Titretek Fluoroskan II (Labsystems Group Ltd.). Unless otherwise stated the amount of fluorescence was normalized to the uninhibited control and expressed at percent relative fluorescence. All experiments were repeated at least three times.
To obtain opsonized particles, FITC-labeled zymosan was incubated with neat normal mouse serum for 30 min at 37°C and then washed extensively in RPMI-medium before use. Binding of opsonized FITC-labeled zymosan to cells was performed as for the unopsonized zymosan, described above.
Carbohydrate and Antibody Inhibition Experiments.
All carbohydrates were obtained from Sigma-Aldrich, except for glucan phosphate, a structurally defined and biologically active glucan, which was prepared as described (26). To perform inhibition experiments, carbohydrates (100 μg/ml) were added to washed cells for 20 min before the addition of FITC-labeled zymosan.
The antibody inhibition experiments were performed similarly, except that the cells were incubated with the mAbs in PBS for 1 h, and then washed extensively before the determination of zymosan binding.
To determine the role of Dectin-1 in the direct recognition of soluble β-glucans, carbohydrates were added to the cells at 100 μg/ml for 1 h at 4°C, after which the cells were washed and stained in situ. The cells were then detached using a cell scraper and analyzed by flow cytometry, as described above. To internalize the receptor, the washed cells were warmed at 37°C for 7 min and then immediately returned to 4°C.
Results and Discussion
The Recognition of Unopsonized Zymosan Is Predominantly Mediated by a β-Glucan Receptor.
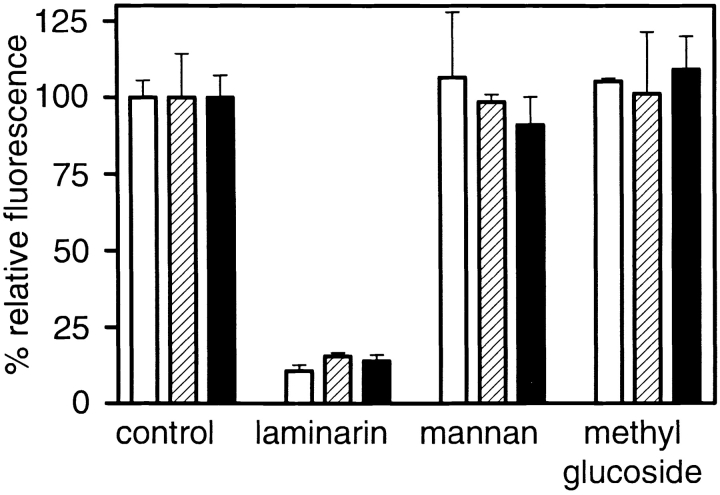
To investigate the role of various receptors in recognition of zymosan, we examined the ability of receptor-specific carbohydrates to inhibit the binding of FITC-labeled zymosan to a variety of primary macrophages in the absence of complement. Laminarin, a soluble fungal derived β-glucan widely used to define leukocyte β-glucan receptor activity (6, 8, 10), exerted a strong inhibitory effect on the recognition of unopsonized zymosan (Fig. 1) . This is in accordance with early studies, which demonstrated a role for a β-glucan receptor in the nonopsonic recognition of zymosan (6, 14). In contrast, the addition of mannan, also previously shown to inhibit the recognition of unopsonized zymosan (7, 8), had no effect. Our results support other studies, which indicated that inhibition of zymosan binding by mannan was a result of contamination of the yeast mannan preparations with β-glucans (6, 14). This suggests that the mannose receptor does not play a significant role in this process. Surprisingly, methyl glucoside, an inhibitor of the CD11b lectin binding domain (10), had no effect, even at 40-fold molar excess compared with laminarin. This lack of inhibition was not due to the experimental conditions, as the ability of the lectin domain of CR3 to bind carbohydrate ligands at 4°C has been previously shown (10). This suggested that CR3 may not be involved in the recognition of unopsonized zymosan.
Figure 1.
A β-glucan receptor is a major receptor for unopsonized zymosan. The β-glucan, laminarin, specifically inhibits the recognition of unopsonized FITC-labeled zymosan by BMDM (white bars), Tg- (striped bars), and Biogel-elicited macrophages (black bars). The addition of carbohydrates which block the mannose receptor (mannan) and the lectin domain of CR3 (methyl glucoside) had no effect on zymosan binding. The data shown have been normalized to the uninhibited control and expressed as percent relative fluorescence.
CR3 Is Not Involved in the Nonopsonic Recognition of Zymosan by Macrophages.
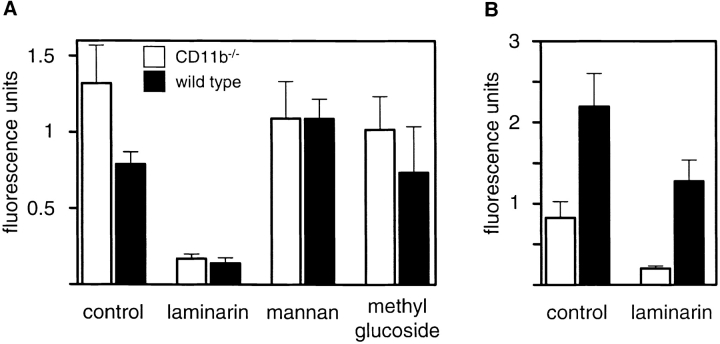
To confirm the lack of involvement of CR3, we next examined the ability of macrophages from CD11b−/− mice, which lack functional CR3 (19), to recognize zymosan. CR3-deficient and wild-type BMDMs bound unopsonized zymosan equally well, and in a β-glucan–dependent fashion (Fig. 2 A). As before, binding of unopsonized zymosan was not inhibited by mannan or methyl glucoside.
Figure 2.
CR3 is not a receptor for unopsonized zymosan on macrophages. (A) The β-glucan dependent recognition of unopsonized zymosan is unaffected in CR3-deficient (CD11b−/−) BMDM. (B) CR3 deficiency does affect the binding of serum opsonized FITC-labeled zymosan, although recognition of opsonized zymosan in both types of macrophage retains a β-glucan–dependent component. The data are shown as the directly measured fluorescence units and have not been normalized, so as to allow a direct comparison between the macro-phages. Note the difference in scale between the panels (A and B). The data shown are the average of triplicates and error bars indicate the standard deviation.
Opsonized zymosan showed markedly enhanced binding to the wild-type cells but not to CD11b−/ −cells, as expected, consistent with the absence of CR3 from these cells (Fig. 2 B). Furthermore, laminarin was still able to inhibit the binding of opsonized particles to wild-type cells partially. This suggests that β-glucan recognition can also contribute to the binding of opsonized yeast particles, explaining previous observations reporting the lack of specificity of C3-opsonized zymosan for CR3 in macrophages (27). These results therefore demonstrate that a macrophage β-glucan receptor, distinct from CR3, was a major receptor involved in the nonopsonic recognition of zymosan and that this receptor also contributed to the recognition of opsonized zymosan particles.
Dectin-1 Is Expressed on the Surface of Macrophages.
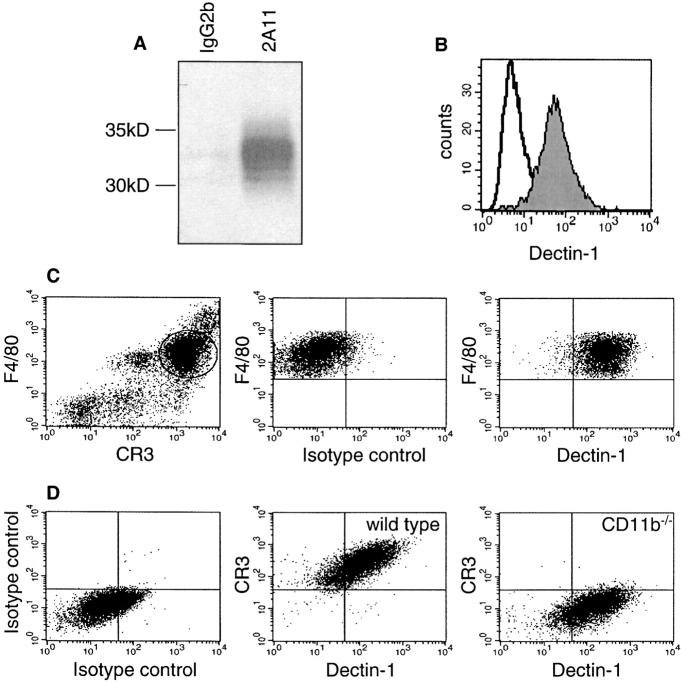
We recently identified Dectin-1 as a receptor which could bind unopsonized zymosan in transduced murine fibroblasts through a binding site which was distinct from that for T cell recognition (17, 18, 23). Although Dectin-1 was initially suggested to be a dendritic cell–specific receptor (18), we have previously shown that Dectin-1 mRNA was expressed in other cell types, including primary macrophages and PMN (17, 23). To explore the role of this receptor in primary macrophages, we generated a novel Dectin-1–specific monoclonal antibody, 2A11 (IgG2b), as described in Materials and Methods. The specificity of this mAb was confirmed by its ability to immunoprecipitate soluble recombinant HA-tagged Dectin-1 (Fig. 3 A). Furthermore, 2A11 specifically stained the surface of live Dectin-1 transduced NIH3T3 fibroblasts, indicating that it recognized an extracellular epitope (Fig. 3 B).
Figure 3.
The mAb 2A11 recognizes Dectin-1 and detects its expression on the surface of primary macrophages. (A) The mAb 2A11, but not an IgG2b control mAb, recognizes Dectin-1, and can specifically immunoprecipitate soluble recombinant hemagglutinin (HA)-tagged Dectin-1. (B) The mAb 2A11 stains Dectin-1 transductants (shaded histogram), but not nontransduced cells (unshaded histogram), by flow cytometry. (C) 2A11 detects Dectin-1 expression on the surface of freshly isolated F4/80+CR3+ Tg macrophages. The gate shown in the left panel denotes the population examined in the right panels. (D) Dectin-1 is expressed at a similar level on the surface of both wild-type and CD11b−/− bone marrow–derived macrophages.
As the epitope recognized by the mAb 2A11 was sensitive to fixation (unpublished data), we examined the expression of Dectin-1 on the surface of live primary macrophages by flow cytometry. Consistent with our previous data (17, 23), we detected Dectin-1 protein on the surface of freshly isolated Tg-elicited peritoneal (Fig. 3 C), Biogel-elicited peritoneal (unpublished data), and on BMDM (Fig. 3 D). Dectin-1 was found to be expressed on the surface of all macrophage populations tested as well as on monocytes, dendritic cells, and neutrophils (unpublished data), demonstrating that Dectin-1 is not restricted to cells of the dendritic cell lineage. Similar levels of Dectin-1 were also observed on the surface of macrophages from CR3-deficient and wild-type mice (Fig. 3 D). This observation is compatible with a role for Dectin-1 in the nonopsonic, β-glucan–dependent, recognition of zymosan by CD11b-deficient cells, see below.
Dectin-1 Plays a Major Role in the β-Glucan–dependent Recognition of Zymosan by Macrophages.
Having shown that nonopsonic zymosan recognition by primary macrophages was mediated by a β-glucan inhibitable receptor, which was different to CR3, we next examined the role of Dectin-1 in this process. We observed that the mAb, 2A11, inhibited the binding of unopsonized zymosan to a level comparable with the inhibition obtained with the exogenous β-glucans, glucan phosphate or laminarin, indicating that this mAb bound at or near the β-glucan binding site (Fig. 4 A). 2A11 could also inhibit the binding of zymosan to NIH3T3 transductants expressing Dectin-1 (unpublished data). The mAb, 5C6 (22), which blocks the lectin activity of CR3 (9), had no effect on zymosan binding, supporting our previous observations, described above. Similar results were obtained when macrophages were plated on dishes coated with receptor specific antibodies, a process shown to sequester receptors from the upper, ligand-interacting, cell surface (28; and unpublished data).
Figure 4.
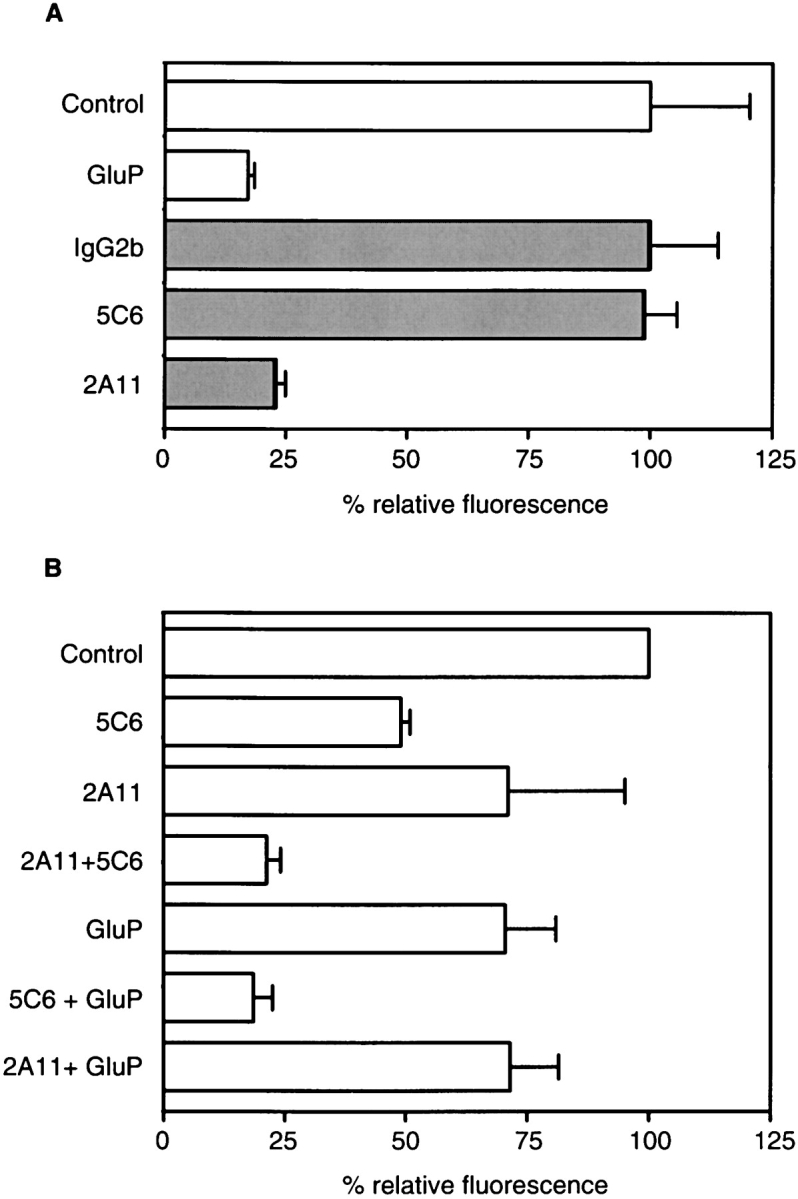
Dectin-1 is a major macrophage receptor for unopsonized zymosan. (A) 2A11 (anti-Dectin-1), but not 5C6 (anti-CR3), specifically inhibits the recognition of unopsonized FITC-labeled zymosan by Tg macrophages. The level of inhibition with 2A11 is similar to that obtained with the exogenous β-glucan, glucan phosphate (GluP), or laminarin (unpublished data). Samples have been normalized to their respective controls, indicated by similar shading, and the data shown are from a representative experiment and are the average of triplicates with error bars indicating the standard deviation. (B) Both 2A11 and 5C6 can inhibit the binding of opsonized FITC-labeled zymosan, although the absolute values vary depending on the level of opsonization. The simultaneous addition of both antibodies, however, always had an additive inhibitory effect. To demonstrate the variation in the level of opsonization and its effects on the inhibition obtained with the antibodies, the data shown are the average of the means of triplicates from three independent experiments, and the error bars indicate the standard error of the mean.
We also examined the effects of these antibodies on the recognition of opsonized zymosan by macrophages (Fig. 4 B). Although we found that the individual levels of inhibition by 2A11 and 5C6 depended on the degree of opsonization, the simultaneous addition of both antibodies consistently had a additive effect in inhibiting the binding of opsonized zymosan. The levels of inhibition obtained with 5C6 were, however, similar to those previously obtained with other anti-CR3 antibodies (9). Furthermore, the inhibition obtained with 2A11 always correlated with that obtained with exogenously added β-glucans, indicating that Dectin-1 was mediating the β-glucan–dependent recognition of opsonized zymosan, as discussed above. Thus, we conclude that Dectin-1 is a major receptor for unopsonized zymosan on macrophages, and that it can also contribute to the recognition of opsonized zymosan particles.
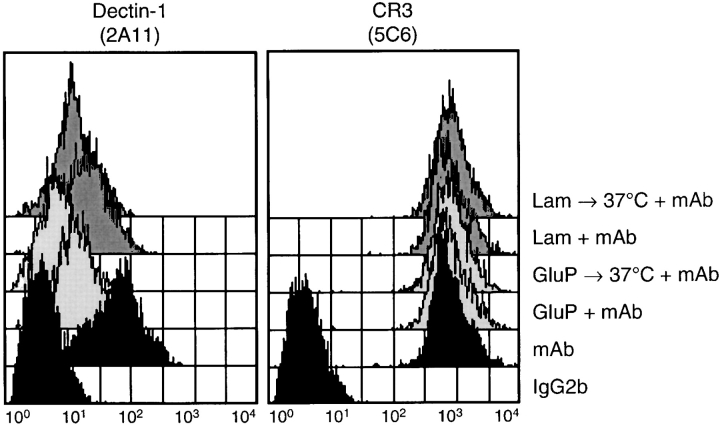
As Dectin-1 acts as a pattern recognition receptor for a variety of β-1,3 and/or β-1,6 glucan linked carbohydrates in transduced murine fibroblasts (17), we extended our observations to determine if binding of soluble β-glucans to primary cells was also mediated by this receptor. The soluble β-glucans, glucan phosphate and laminarin, shown to be the best β-glucan inhibitors of Dectin-1 binding to zymosan (17, 23), masked the 2A11 epitope on macrophages (Fig. 5) . This indicates that Dectin-1 was involved in the recognition of these polysaccharides. Warming the cells briefly further reduced the detection of this receptor (Fig. 5), consistent with the observation that β-glucans are internalized after receptor binding (26). No change in the level of surface expressed CR3 was observed, even after warming (Fig. 5). Taken together, these results suggest that Dectin-1 is a major receptor for both unopsonized-particulate and soluble β-glucans on macrophages.
Figure 5.
Soluble β-glucans block the ability of 2A11 to detect Dectin-1 on Tg-elicited peritoneal macrophages. Flow cytometric analysis of the surface levels of Dectin-1 and CR3 before and after the addition of glucan phosphate (GluP) or laminarin (Lam), with or without brief warming at 37°C to induce receptor internalization. Cells were stained with isotype-control (IgG2b) or receptor-specific mAbs, in the presence and absence of the exogenous glucans, as indicated above the panels. No difference in 2A11 staining was observed when the cells were warmed without glucan phosphate or laminarin (unpublished data).
Our results establish a predominant role for Dectin-1 in the innate recognition of β-glucans. We have demonstrated that the β-glucan receptor, Dectin-1, is expressed on primary macrophages and that it is a major receptor for unopsonized zymosan. These conclusions, which contradict previous established dogma, are strengthened by the use in our experiments of structurally defined, pure β-glucans, receptor-specific reagents, and the care taken in controlling for the contribution of opsonic factors released from the cells under study (24, 25).
The identification of Dectin-1 as a major leukocyte β-glucan receptor defines the fundamental mechanism of β-glucan recognition by macrophages that has remained elusive since its first description over five decades ago. As soluble β-glucans, which can be detected in the plasma of patients with invasive fungal infections (29), do not have the capacity to activate complement (30), the nonopsonic receptor Dectin-1 could play a central role in the innate recognition of these carbohydrate polymers and may be a therapeutic target for novel drug design.
Acknowledgments
This work was supported by the Wellcome Trust, Arthritis Research Campaign, Yamanouchi Research Institute, and the Medical Research Council, UK.
G.D. Brown and P.R. Taylor contributed equally to this work.
References
- 1.Benacerraf, B., and M.M. Sebestyen. 1957. Effect of bacterial endotoxins on the reticuloendothelial system. Fed. Proc. 16:860–867. [PubMed] [Google Scholar]
- 2.Riggi, S.J., and N.R. Di Luzio. 1961. Identification of a reticuloendothelial stimulating agent in zymosan. Am. J. Physiol. 200:297–300. [DOI] [PubMed] [Google Scholar]
- 3.Di Carlo, F.J., and J.V. Fiore. 1958. On the composition of zymosan. Science. 127:756–757. [DOI] [PubMed] [Google Scholar]
- 4.Ross, G.D., V. Vetvicka, J. Yan, Y. Xia, and J. Vetvickova. 1999. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 42:61–74. [DOI] [PubMed] [Google Scholar]
- 5.Williams, D.L. 1997. Overview of (1,3)-beta-D-glucan immunobiology. Mediators Inflamm. 6:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czop, J.K., and K.F. Austen. 1985. A beta-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J. Immunol. 134:2588–2593. [PubMed] [Google Scholar]
- 7.Sung, S.S., R.S. Nelson, and S.C. Silverstein. 1983. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J. Cell Biol. 96:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giaimis, J., Y. Lombard, P. Fonteneau, C.D. Muller, R. Levy, M. Makaya-Kumba, J. Lazdins, and P. Poindron. 1993. Both mannose and beta-glucan receptors are involved in phagocytosis of unopsonized, heat-killed Saccharomyces cerevisiae by murine macrophages. J. Leukoc. Biol. 54:564–571. [DOI] [PubMed] [Google Scholar]
- 9.Xia, Y., V. Vetvicka, J. Yan, M. Hanikyrova, T. Mayadas, and G.D. Ross. 1999. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 162:2281–2290. [PubMed] [Google Scholar]
- 10.Thornton, B.P., V. Vetvicka, M. Pitman, R.C. Goldman, and G.D. Ross. 1996. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156:1235–1246. [PubMed] [Google Scholar]
- 11.Ross, G.D. 2000. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit. Rev. Immunol. 20:197–222. [PubMed] [Google Scholar]
- 12.Van Strijp, J.A., D.G. Russell, E. Tuomanen, E.J. Brown, and S.D. Wright. 1993. Ligand specificity of purified complement receptor type three (CD11b/CD18, alpha m beta 2, Mac-1). Indirect effects of an Arg-Gly-Asp (RGD) sequence. J. Immunol. 151:3324–3336. [PubMed] [Google Scholar]
- 13.Elstad, M.R., C.J. Parker, F.S. Cowley, L.A. Wilcox, T.M. McIntyre, S.M. Prescott, and G.A. Zimmerman. 1994. CD11b/CD18 integrin and a beta-glucan receptor act in concert to induce the synthesis of platelet-activating factor by monocytes. J. Immunol. 152:220–230. [PubMed] [Google Scholar]
- 14.Goldman, R. 1988. Characteristics of the beta-glucan receptor of murine macrophages. Exp. Cell Res. 174:481–490. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman, J.W., J. Lindermuth, P.A. Fish, G.P. Palace, T.T. Stevenson, and D.E. DeMong. 1998. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 273:22014–22020. [DOI] [PubMed] [Google Scholar]
- 16.Pearson, A., A. Lux, and M. Krieger. 1995. Expression cloning of dSR-CI, a class C macrophage-specific scavenger receptor from Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 92:4056–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown, G.D., and S. Gordon. 2001. Immune recognition: A new receptor for beta-glucans. Nature. 413:36–37. [DOI] [PubMed] [Google Scholar]
- 18.Ariizumi, K., G.L. Shen, S. Shikano, S. Xu, R. Ritter III, T. Kumamoto, D. Edelbaum, A. Morita, P.R. Bergstresser, and A. Takashima. 2000. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275:20157–20167. [DOI] [PubMed] [Google Scholar]
- 19.Melo, M.D., I.R. Catchpole, G. Haggar, and R.W. Stokes. 2000. Utilization of CD11b knockout mice to characterize the role of complement receptor 3 (CR3, CD11b/CD18) in the growth of Mycobacterium tuberculosis in macrophages. Cell. Immunol. 205:13–23. [DOI] [PubMed] [Google Scholar]
- 20.Hume, D.A., and S. Gordon. 1983. Optimal conditions for proliferation of bone marrow-derived mouse macrophages in culture: the roles of CSF-1, serum, Ca2+, and adherence. J. Cell. Physiol. 117:189–194. [DOI] [PubMed] [Google Scholar]
- 21.Galfre, G., C. Milstein, and B. Wright. 1979. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 277:131–133. [DOI] [PubMed] [Google Scholar]
- 22.Rosen, H., and S. Gordon. 1987. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J. Exp. Med. 166:1685–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willment, J.A., S. Gordon, and G.D. Brown. 2001. Characterisation of the human beta-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276:43818–43823. [DOI] [PubMed] [Google Scholar]
- 24.Ezekowitz, R.A., R.B. Sim, G.G. MacPherson, and S. Gordon. 1985. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro. Role of type 3 complement receptors and macrophage-derived complement. J. Clin. Invest. 76:2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ezekowitz, R.A., R.B. Sim, M. Hill, and S. Gordon. 1984. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J. Exp. Med. 159:244–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller, A., P.J. Rice, H.E. Ensley, P.S. Coogan, J.H. Kalbfleish, J.L. Kelley, E.J. Love, C.A. Portera, T. Ha, I.W. Browder, and D.L. Williams. 1996. Receptor binding and internalization of a water-soluble (1→3)-beta-D- glucan biologic response modifier in two monocyte/macrophage cell lines. J. Immunol. 156:3418–3425. [PubMed] [Google Scholar]
- 27.Egwang, T.G., J. Gauldie, and A.D. Befus. 1983. Lack of specificity of the C3-opsonized zymosan reagent for the assay of membrane complement receptors. J. Immunol. Methods. 61:253–257. [DOI] [PubMed] [Google Scholar]
- 28.Michl, J., M.M. Pieczonka, J.C. Unkeless, and S.C. Silverstein. 1979. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J. Exp. Med. 150:607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiss, E., T. Obayashi, K. Orle, M. Yoshida, and R.M. Zancope-Oliveira. 2000. Non-culture based diagnostic tests for mycotic infections. Med. Mycol. 38:147–159. [PubMed] [Google Scholar]
- 30.Czop, J.K., and K.F. Austen. 1985. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte beta-glucan receptor. J. Immunol. 135:3388–3393. [PubMed] [Google Scholar]