Abstract
Accumulation of inflammatory microglia in Alzheimer's senile plaques is a hallmark of the innate response to β-amyloid fibrils and can initiate and propagate neurodegeneration characteristic of Alzheimer's disease (AD). The molecular mechanism whereby fibrillar β-amyloid activates the inflammatory response has not been elucidated. CD36, a class B scavenger receptor, is expressed on microglia in normal and AD brains and binds to β-amyloid fibrils in vitro. We report here that microglia and macrophages, isolated from CD36 null mice, had marked reductions in fibrillar β-amyloid–induced secretion of cytokines, chemokines, and reactive oxygen species. Intraperitoneal and stereotaxic intracerebral injection of fibrillar β-amyloid in CD36 null mice induced significantly less macrophage and microglial recruitment into the peritoneum and brain, respectively, than in wild-type mice. Our data reveal that CD36, a major pattern recognition receptor, mediates microglial and macrophage response to β-amyloid, and imply that CD36 plays a key role in the proinflammatory events associated with AD.
Keywords: microglia, scavenger receptor, Alzheimer's disease, chemokine, reactive oxygen species
Introduction
The senile plaque is a key pathological feature of Alzheimer's disease (AD)*and is composed of insoluble β-amyloid fibril deposits (fAβ), activated microglia, astrocytes, and degenerating neurons. Data from humans with AD and animal models of AD indicate that accumulation of inflammatory microglia at sites of fAβ deposition contributes significantly to neuronal degeneration (1–8). fAβ itself is believed to initiate the accumulation and activation of microglia (5–7). Innate immune recognition of fAβ is therefore a key event that initiates the inflammatory cascade at sites of fAβ deposition that contributes to the pathogenesis of AD. The molecular mechanism responsible for fAβ's proinflammatory effects on microglia, however, is not known.
Microglia are macrophage lineage immune cells of the brain that express pattern recognition receptors, which are thought to mediate their innate interactions with foreign or modified host proteins. These receptors include the class A scavenger receptor (SR-A; 9, 10), expressed in the central nervous system exclusively on microglial cells (7, 11, 12), and the class B scavenger receptor type 2, or CD36, which is expressed on microglia and endothelial cells in the central nervous system (13). SR-A promotes microglial endocytosis of fAβ but is not required for fAβ-induced microglial activation (10, 14). CD36 also binds fAβ and recent data from our lab suggests that it may play a role in fAβ activation of microglial cells (13).
In this study, we use macrophages and microglia from CD36-deficient mice to demonstrate that CD36 is required for fAβ-induced activation of these cells to produce reactive oxygen species (ROS), TNFα, IL-1β, and several chemokines that induce microglial chemotaxis. We also show that the absence of CD36 significantly decreased the recruitment of microglia and macrophages to sites of fAβ deposition in vivo. These data identify CD36 as a mediator of the innate microglial response to fAβ and suggest that this pattern recognition receptor plays an important role in initiating the inflammatory response in AD.
Materials and Methods
Mice.
CD36−/− mice were generated by targeted gene disruption in murine embryonic stem cells by homologous recombination (15). CD36−/− and CD36+/+ littermate controls from the mating of heterozygous CD36−/+ mice in a mixed C57BL/6J and 129/SvEv background were used for the in vitro experiments. CD36−/− mice backcrossed into C57BL/6J for five generations and CD36+/+ C57BL/6J mice littermates or C57BL/6J purchased from The Jackson Laboratory were used for the in vivo experiments. 5–6-mo-old mice were used for intraperitoneal and intracerebral injection experiments.
Isolation of Primary Macrophage and Microglial Culture.
Thioglycollate-elicited peritoneal macrophages were harvested from 8–12-wk-old mice as previously described (16). Primary neonatal microglia were prepared from mixed brain cultures as previously described (9, 13). Adult mouse microglia were prepared from 5–6-mo-old CD36−/− and CD36+/+ mice as previously described (17). In brief, mouse brains were cut into small pieces (1–2 mm), vigorously triturated to obtain single cell suspensions, and then passed through a percoll gradient to separate cells from debris. Microglia were then separated from other brain cells by differential adhesion to tissue culture–treated plastic. Cells were confirmed to be >95% microglia or macrophages by staining with F4/80 and anti-CR3 antibodies and uptake of DiI AcLDL.
Preparation of Fibrillar β-Amyloid.
β-Amyloid 1–42 peptide and control peptide with identical amino acid composition but reverse sequence (revAβ) were obtained from American Peptide Company or Biosource International. Aβ 1−42 fibrils (fAβ) were generated and confirmed to be fibrils by several methods including thioflavin S staining, transmission electron microscopy, and SDS-PAGE as previously described (13).
Measurement of ROS Production and Adhesion Assay.
ROS production was measured by nitro blue tetrazolium (NBT) reduction assay (13). In brief, 105 peritoneal macrophages were plated on 6-mm2 multispot glass slides and stimulated with HBSS containing 1 mg/ml BSA alone, HBSS/BSA plus 10 μg fAβ (1.67 μg/mm2), or 1 mg/ml zymosan for 10 min at 37°C. 50 μl of 1 mg/ml NBT was added to each spot and cells were incubated at 37°C for 1 h. ROS production correlates with formation of a dark blue colored insoluble formazan deposit, which was quantified by microscope video capture and Scion image analysis software. Adhesion assay was performed on multispot slides coated with collagen IV (CIV) and fAβ as previously described in detail (9, 18).
Monocyte Chemoattractant Protein 1 (MCP-1) ELISA and Chemotaxis Assay.
Cells were incubated on 6-mm2 multispot slides (105 cells/spot) overnight in RPMI containing 10% FCS at 37°C. Cells were transferred to RPMI containing 1% FCS for 2 h and then stimulated with 10 μg fAβ, revAβ, or 100 ng/ml of bacterial LPS (Sigma-Aldrich) for 24 h. MCP-1 in supernatants was measured by ELISA (R&D Systems). Chemotaxis assays were performed using the Neuroprobe chemotaxis instrument (Neuroprobe). In brief, 31 μl cell supernatant or medium containing MCP-1 (PeproTech) were placed in the lower compartment of the chemotaxis chamber, and 50 μl of 106 cells/ml (in RPMI containing 1% FCS) were placed in the upper compartment. Cells were allowed to migrate for 3 h at 37°C and the number of cells migrating into the lower chamber was counted using an inverted light microscope. To test the effect of neutralizing anti–MCP-1 antibodies on chemotaxis, cell supernatant or medium containing 250 ng/ml MCP-1 were incubated with 10 μg/ml goat IgG anti–mouse MCP-1 or control goat IgG (R&D Systems) for 30 min before initiating chemotaxis. Each data point is the result of at least triplicate measurements and each experiment was repeated between three and eight times.
Quantitative Real-Time PCR (Q-PCR).
2 μg total RNA from each sample was reverse transcribed using multiscribe reverse transcriptase (Applied Biosystems). Oligonucleotide primers were designed using Primer Express software 1.0 (Applied Biosystems). A series of standards was prepared by performing 10-fold serial dilutions of full-length cDNAs in the range of 20 million copies to 2 copies per PCR reaction. Subsequent analysis was performed on the data output from the MX4000™ software (Stratagene) using Microsoft Excel XP. Relative quantification of mRNA expression was calculated by the comparative cycle method described by the manufacturer (Stratagene).
Immunofluorescence Staining of Cultured Microglia and Macrophages.
Microglia and macrophages adherent to multispot slides were fixed in ice cold methanol and stained with 2 μg/ml mAb to mouse CD36 (Cascade Biologics, Inc.), followed by 1 μg/ml FITC-labeled secondary antibody (Sigma-Aldrich). Stained cells were visualized by fluorescence microscopy and digitally photographed.
Intraperitoneal Injection of fAβ.
CD36+/+ or CD36−/− mice were injected with 1 ml PBS containing 1 mg BSA (carrier) and 100 μg fAβ or 1 mg zymosan. 24 h later, peritoneal cells were collected by lavage with 10 ml ice cold PBS and counted. 1 ml of the peritoneal lavage was then plated on a glass slide using Cytospin3 (Shandon), stained with Hema 3 staining kit (Fisher Scientific), and 500 cells from each mouse were counted.
Stereotaxic Brain Microinjection.
5–6-mo-old adult mice were anesthetized with intraperitoneal injection of 0.1 ml saline containing 2.5 mg ketamine and 0.5 mg xylazine. After mice were immobilized in a Kopf stereotaxic apparatus, a linear skin incision was made over the bregma, and a 1-mm burr hole was drilled in the skull 1 mm anterior to and 2 mm lateral to the bregma on both sides. 2 μl fAβ containing 1 mg/ml solution was inoculated on the right side using a 5-μl Hamilton syringe. 2 μl nonfibrillar revAβ was inoculated at the same depth and stereotaxic coordinates on the left side of the brain, providing a control inoculation in the same animal. 48 h later, the mice were anesthetized and perfused by an intracardiac infusion of ice cold PBS containing 4% neutral paraformaldehyde (PFA-PBS) in the left ventricle. The brains were removed, placed in 4% PFA-PBS overnight at 4°C, and then transferred to 30% sucrose in PBS and stored at 4°C until sectioning.
Immunohistochemistry.
20-μm frozen sections from CD36+/+ and CD36−/− mice were stained for 1 h at 25°C with 1 nM 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Molecular Probes) and 10 μg/ml FITC-labeled F4/80 monoclonal antibody (Serotec). The sections were washed four times in PBS, and 50 μl Vectashield (Vector Laboratories) was added per slide before adding the coverslips. For quantitation of the number of recruited microglia to sites of microinjection, five serial sections from each site of injection were visualized by fluorescence microscopy (magnifications, ×4 and ×40) and digitally photographed to record the FITC and DAPI staining. DAPI and FITC staining were then overlaid for each section using Adobe Photoshop (Adobe Systems). The fluorescence intensity of FITC-labeled microglia at each microinjection site was then quantified using Scion's image analysis software and used as a measure of microglial number.
Statistical Analysis.
Statistical analysis was performed using one-way ANOVA provided in the “Microcal Origin 5” graphics and statistics software. P-values were <0.005 where considered significant.
Results
Microglia Stimulated with fAβ Secrete Mononuclear Phagocyte Chemoattractants.
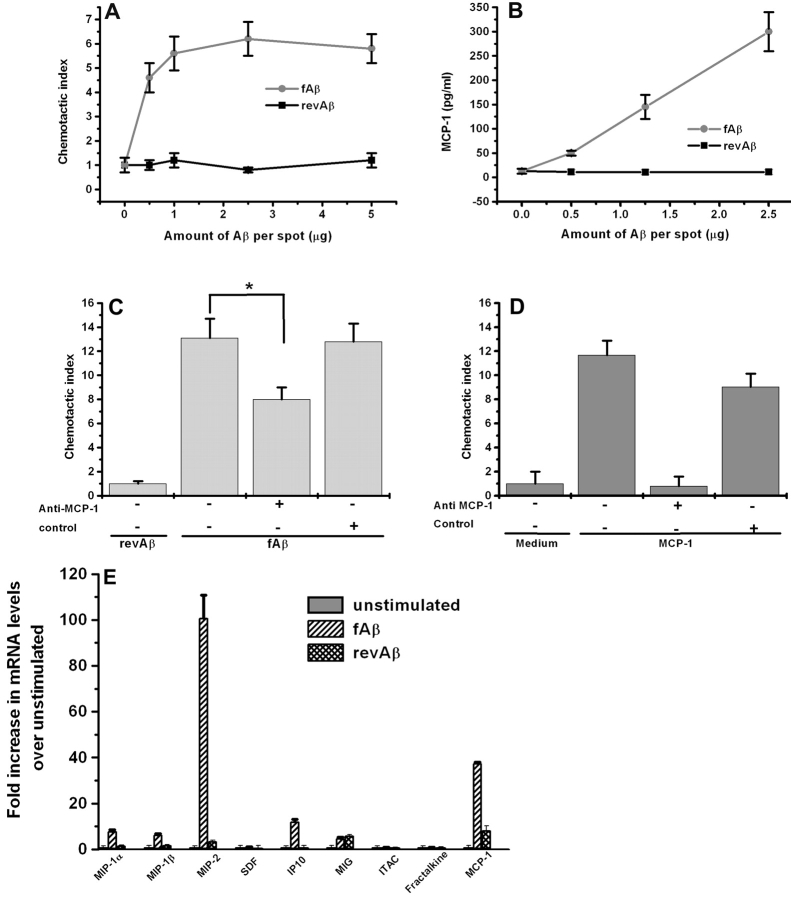
To test whether fAβ-stimulated microglia secrete mononuclear phagocyte chemoattractants, primary mouse microglia were allowed to adhere to sterile multispot glass slides in the presence of increasing concentrations of fAβ. After 24 h, the supernatants were assayed for chemotactic activity using the human monocyte cell line THP-1 in a transwell chemotaxis assay. The chemotactic activity secreted into the supernatant of microglial cell cultures reached a plateau at 2.5 μg/spot with a chemotactic index of 6 (Fig. 1 A). Control nonfibrillar Aβ 42-1 (revAβ) did not induce microglia to secrete chemotactic activity for THP-1 cells (Fig. 1 A). Medium containing fAβ alone in the lower compartment (i.e., in the absence of any previous interaction with microglia) was not chemotactic for THP-1 cells (unpublished data). These data demonstrate that fAβ activates microglia to secrete monocyte chemoattractants.
Figure 1.
fAβ stimulates primary mouse microglia to produce monocyte chemotactic activity and up-regulate chemokine mRNAs. (A) Primary mouse microglia were cultured on multispot slides and stimulated with the indicated concentrations of fAβ and revAβ. Chemotaxis of THP-1 monocytic cells was assayed using the Neuroprobe transwell assay. Chemotactic index is the number of THP-1 cells that migrated in response to supernatant from stimulated microglia/number of cells that migrated in response to supernatant from unstimulated microglia. No significant chemotactic activity for THP-1 cells was induced in response to the revAβ (n = 8). (B) Dose response curve for MCP-1 production measured by ELISA in the supernatant of mouse microglia stimulated with increasing concentrations of fAβ (n = 3). (C) 10 μg/ml anti–MCP-1 neutralizing antibody partially inhibited monocyte chemotactic activity produced in the supernatant of primary mouse microglia stimulated with 10 μg/spot fAβ (n = 3). Isotype-matched antibodies had no effect. (D) 10 μg/ml anti–MCP-1 neutralizing antibody totally inhibited MCP-1–induced monocyte chemotactic activity (n = 3). (E) Microglia were stimulated with 10 μg/spot fAβ for 24 h and RNA was harvested for Q-PCR measurement of the indicated chemokines (n = 2). Each data point is the average ± SEM of triplicate measurements from the indicated number of experiments (n). *, P < 0.005.
fAβ-stimulated Microglia Secrete MCP-1.
Having established that fAβ-stimulated microglia secrete monocyte cell chemoattractants, we proceeded to identify these chemoattractants. Because MCP-1/CCL2 is a potent monocyte chemoattractant found in senile plaques (19) and is produced by fAβ-stimulated monocytes and a microglial cell line (20), we speculated that it is also produced by fAβ-stimulated microglia. We measured MCP-1 levels in supernatants of fAβ-stimulated primary mouse microglia using an ELISA and found that fAβ-stimulated microglia secreted MCP-1 into the medium in a dose-dependent fashion (Fig. 1 B).
We proceeded to determine what percentage of the monocyte chemotactic activity secreted by fAβ-stimulated primary microglia was due to MCP-1. Neutralizing antibody to MCP-1 blocked ∼40% of the THP-1 cell chemotactic activity found in supernatants of microglia stimulated with 10 μg/spot fAβ (Fig. 1 C). Control antibodies had no effect on THP-1 migration to the identical supernatant. In control experiments, the same neutralizing anti–MCP-1 antibody totally blocked migration of THP-1 cells in response to 250 ng/ml MCP-1, confirming the effectiveness of these antibodies (Fig. 1 D).
fAβ Stimulation Up-regulates Several Microglial Chemokine mRNAs.
Because MCP-1 accounted for only 40% of the THP-1 cell chemotactic activity found in supernatants of fAβ- stimulated microglia, we tested whether additional chemokines are also up-regulated in these cells. Using Q-PCR, we found that in addition to MCP-1, fAβ stimulation up-regulates several microglial chemokine mRNA including macrophage inflammatory protein (MIP)-1α/CCL3, MIP-1β/CCL4, and MIP-2/CXCL2, which have also been described as microglial chemoattractants (Fig. 1 E). As expected, microglia incubated with equal amounts of control revAβ did not up-regulate their chemokine mRNA levels significantly (Fig. 1 E). Not all chemokines were induced by fAβ stimulation of microglia as can be seen by the lack of stromal cell–derived factor-1 (SDF-1)/CXCL12, interferon-inducible T cell α chemoattractant (I-TAC)/CXCL11, and fractalkine/CX3CL1 induction. These data indicate that several chemokines, in addition to MCP-1, are likely to play roles in the recruitment of microglia to senile plaques in AD.
CD36 Is Required for fAβ-induced Monocyte Chemoattractant Production by Microglia.
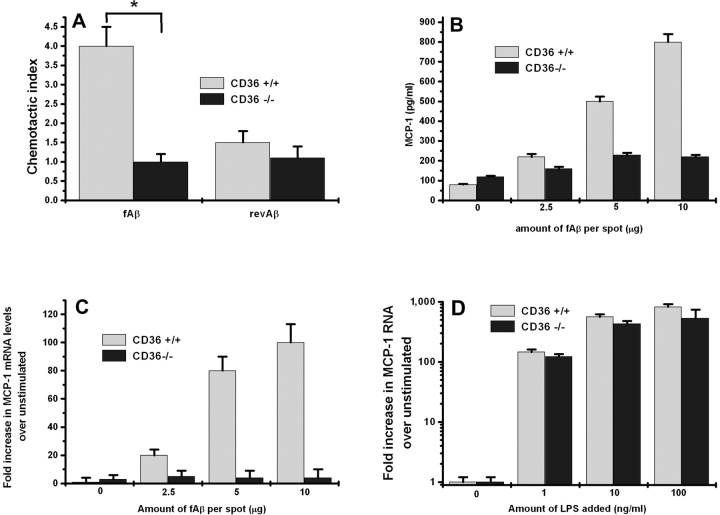
To determine whether CD36 is required for production of THP-1 chemotactic activity by mouse microglia, we used primary mouse microglia isolated from CD36 null mice (CD36−/−) and CD36+/+ wild-type littermate controls. Supernatants collected from CD36−/ − microglia stimulated with 10 μg/spot fAβ produced no chemotactic activity for THP-1 cells, whereas similarly treated CD36+/+ microglia produced significant THP-1 chemotactic activity (Fig. 2 A). CD36−/− and CD36+/+ microglia did not respond to the control revAβ peptide but produced equal amounts of chemotactic activity when stimulated with bacterial LPS, confirming that the machinery necessary to produce chemoattractants is intact in CD36−/− microglia. These data demonstrate an essential role for CD36 in fAβ activation of microglia to produce monocyte chemoattractants.
Figure 2.
fAβ-stimulated chemoattractant production in microglia and macrophages is CD36 dependent. (A) Primary mouse microglia from CD36−/− mice were cultured on multispot slides and stimulated with the indicated concentrations of fAβ and revAβ for 24 h, and the supernatant was collected and assayed for THP-1 chemotactic activity. CD36−/− microglia produce significantly less chemotactic activity for THP-1 cells when stimulated with fAβ compared with CD36+/+ microglia (n = 7). (B) Thioglycollate-elicited peritoneal macrophages from CD36−/− and CD36+/+ mice cultured on multispot slides were incubated with the indicated concentrations of fAβ for 24 h. MCP-1 released into their supernatant was measured by ELISA. (C) MCP-1 mRNA levels in CD36−/− and CD36+/+ macrophages stimulated as described above with the indicated concentrations of fAβ (n = 3). (D) MCP-1 mRNA levels in CD36−/− and CD36+/+ macrophages stimulated with 1, 10, or 100 ng/ml LPS (n = 3). Each data point is the average ± SEM of triplicate measurements from the indicated number of experiments (n). *, P < 0.003.
CD36 Is Required for fAβ-induced MCP-1 Protein Secretion and mRNA Induction.
To test whether CD36 is involved in fAβ-induced MCP-1 secretion, peritoneal macrophages isolated from CD36−/− mice were stimulated with fAβ and the supernatant was analyzed for MCP-1 protein by ELISA. MCP-1 protein was undetectable in the culture supernatants of CD36−/− macrophages stimulated with increasing concentrations of fAβ for 24 h. In contrast, CD36+/+ macrophages produced a dose-dependent increase in MCP-1 protein secretion accumulating ∼800 pg/ml in the medium after 24 h of stimulation with fAβ at 10 μg/spot (Fig. 2 B). CD36−/− and CD36+/+ macrophages produced equal amounts of MCP-1 when stimulated with bacterial LPS (unpublished data).
To determine whether CD36 is necessary for MCP-1 mRNA accumulation in response to fAβ, RNA was isolated from CD36−/− and CD36+/+ peritoneal macrophages stimulated for 24 h with increasing concentrations of fAβ. MCP-1 mRNA levels were determined using Q-PCR. MCP-1 mRNA was not induced in CD36−/− macrophages after 24 h of stimulation with a dose range of fAβ. In contrast, fAβ induced a dose-dependent increase in MCP-1 mRNA accumulation in CD36+/+ macrophages, reaching 100-fold induction over unstimulated CD36+/+ macrophages after 24 h of fAβ stimulation (10 μg/spot; Fig. 2 C). CD36−/− and CD36+/+ macrophages showed comparable increases in their MCP-1 mRNA levels when stimulated with increasing concentrations of bacterial LPS (Fig. 2 D). These data demonstrate that fAβ induces MCP-1 protein secretion and mRNA accumulation in peritoneal macrophages in a CD36-dependent manner, and that at a wide range of LPS concentrations, including doses of LPS producing the same fold stimulation as fAβ, CD36 was not necessary for LPS-induced up-regulation of MCP-1 mRNA.
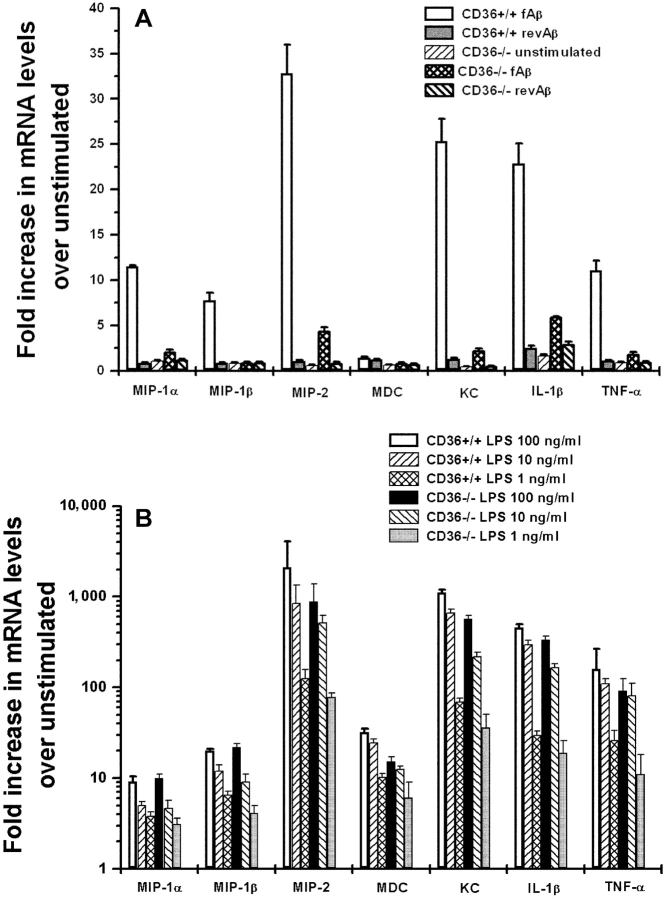
CD36 Is Required for fAβ Induction of TNFα, IL-1β, and Several Chemokine mRNAs.
Proinflammatory cytokines such as TNFα and IL-1β are up-regulated in the brains of AD patients and transgenic mice with AD-like pathology (21–23) and secreted by fAβ-stimulated microglia and macrophages in culture (24, 25). To determine whether CD36 is required for up-regulation of TNFα and IL-1β mRNA levels after fAβ stimulation, we used Q-PCR and CD36+/+ and CD36−/− macrophages. CD36+/+ macrophages markedly up-regulated mRNA for TNFα and IL-1β in response fAβ (12- and 24-fold, respectively; Fig. 3 A). In marked contrast, CD36−/− macrophages had a minimal response to fAβ stimulation and had significantly less TNFα and IL-1β mRNA (Fig. 3 A). Interestingly, as observed with microglia (Fig. 1 E), fAβ also induced the accumulation of mRNA for additional chemokines such as MIP-1α, MIP-1β, MIP-2, and KC/CXCL1 in macrophages (Fig. 3 A). These chemokines were also induced in a CD36-dependent manner. Not all chemokines were induced by fAβ stimulation of macrophages as can be seen by the lack of monocyte-derived chemokine (MDC)/CCL22 induction (Fig. 3 A). The accumulation of mRNA encoding these cytokines and chemokines was equivalent in CD36−/− and CD36+/+ macrophages stimulated with increasing concentrations of bacterial LPS, indicating that the absence of CD36 did not result in a general loss in cytokine or chemokine responsiveness (Fig. 3 B). These data demonstrate that CD36 plays an important role in the induction of proinflammatory cytokines and chemokines in response to fAβ stimulation. These data also show that at a wide range of LPS concentrations, including doses of LPS producing similar fold stimulation as fAβ, CD36 was not necessary for LPS-induced up-regulation of these proinflammatory cytokines and chemokines, confirming that the machinery to produce these proinflammatory molecules is intact in CD36−/− mice. In addition, these data show that fAβ stimulation induces similar chemokine responses in microglia and macrophages.
Figure 3.
fAβ up-regulates chemokine and cytokine mRNA in CD36+/+ but not in CD36−/− macrophages. Peritoneal macrophages from CD36−/− and CD36+/+ mice cultured on multispot slides were incubated with 10 μg/spot of fAβ or revAβ (A), or 1, 10, or 100 ng/ml LPS (B) for 24 h. mRNA was harvested for Q-PCR measurement of the indicated chemokines and cytokines. Each data point is the average ± SEM of two independent experiments each performed in duplicate.
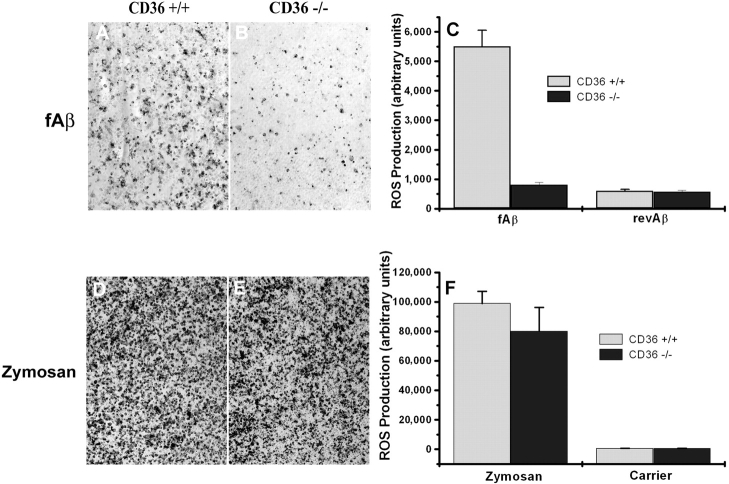
CD36 Is Required for fAβ-induced ROS Production by Macrophages.
Binding of microglia to fAβ leads to ROS production (9, 13). Previously, we found that antibodies to CD36 inhibited ∼50% of the ROS produced by microglia stimulated with fAβ (13). Therefore, we used CD36−/− macrophages to extend these findings and found that CD36−/− macrophages produced essentially no ROS when stimulated with fAβ compared with the robust production of ROS by similarly treated CD36+/+ macrophages (Fig. 4, A and B) . CD36−/− and CD36+/+ macrophages produced essentially no ROS when stimulated with revAβ (Fig. 4 C) and produced equal amounts of ROS when stimulated with zymosan (Fig. 4, D–F). These data demonstrate that CD36 is required for fAβ induction of ROS production by macrophages and suggest that antibodies to CD36 are not completely effective at inhibiting CD36 function.
Figure 4.
fAβ-stimulated but not zymosan-stimulated ROS production is CD36 dependent. Macrophages from CD36+/+ and CD36−/− mice were cultured on multispot slides and incubated with 10 μg/spot fAβ, revAβ, 1 mg/ml zymosan, or carrier alone for 1 h. ROS production was assayed by the NBT reduction method, which produces visible dark blue formazan deposits. A representative field is shown (original magnification, ×10). The amount of deposited formazan (dark areas) was quantified using digital video microscopy and Scion image analysis software as arbitrary units per 105 cells. (A–C) fAβ-stimulated macrophages. (D–F) Zymosan-stimulated macrophages. Each data point is the average ± SEM of 10 measurements per sample from 3 independent experiments.
CD36 Is Not Necessary for Adhesion of Microglia and Macrophages to fAβ.
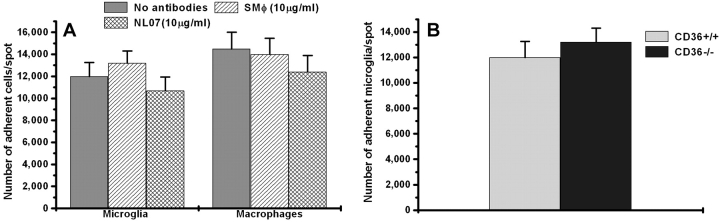
We have recently shown that cells transfected with CD36 gain the ability to adhere to multispot glass slides coated with CIV and fAβ, and that two anti-CD36 inhibitory antibodies, SMφ and NL07, blocked this adhesion (13). To determine if CD36 is necessary for adhesion of microglia and macrophages to fAβ-coated surfaces, we performed the same adhesion assay in the presence of antibodies to CD36 using microglia and macrophages isolated from wild-type mice. As expected and previously reported (13), microglia adhere avidly to multispot slides coated with CIV and fAβ but not to surfaces coated with CIV alone (unpublished data). Anti-CD36 antibodies had no effect on adhesion of wild-type microglia to surfaces coated with CIV and fAβ (Fig. 5 A), even though these same antibodies inhibited adhesion of CD36 transfected cells to surfaces coated with CIV and fAβ (13). We also repeated this adhesion experiment using CD36−/− microglia. CD36+/+ and CD36−/− microglia bound comparably well to fAβ-coated surfaces (11,995 ± 900 vs. 14,450 ± 1,575 cells/spot, respectively; Fig. 5 B). These data indicate that although CD36 is capable of binding fAβ, this interaction is not required for microglial adhesion to a fAβ-coated substrate. Although other receptors mediate this adhesion response, our data demonstrate that they are unable to compensate for the loss of CD36 in the fAβ-stimulated signaling activation pathway.
Figure 5.
Microglia and macrophage adhesion to fAβ-coated surfaces is CD36 independent. (A) 25 × 103 primary mouse microglia macrophages were allowed to adhere to multispot slides coated with CIV and 1 μg/spot fAβ for 1 h in the presence of the indicated anti-CD36 mAb (10 μg/ml). Nonadherent cells were washed away and the number of adherent cells was counted. (B) The same experiment was repeated using microglia from CD36+/+ and CD36−/− mice. Each data point is the average ± SEM of triplicate measurements from three independent experiments.
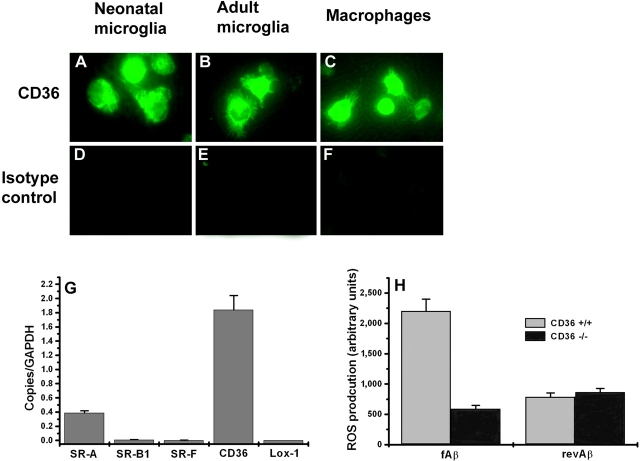
CD36 Is Expressed on Adult Mouse Microglia in Culture and Mediates fAβ-induced ROS Production.
Microglia in AD brains and human fetal microglia express CD36 (13). We confirmed that adult mouse microglia also express CD36. Neonatal microglia, adult macrophages, and adult microglia stain with mAb specific for CD36 but not with a control isotype matched mAb (Fig. 6, A–F) . Using Q-PCR we also found that CD36 and SR-A are expressed at the mRNA level. Interestingly, mRNA levels for other scavenger receptors, including SRB-1, SR-F, and Lox-1, were negligible in unstimulated adult mouse microglia (Fig. 6 G). To determine whether CD36 expressed on adult mouse microglia plays a similar role to CD36 expressed on neonatal microglia and adult macrophages in mediating the response to fAβ, we measured ROS production in adult mouse microglia isolated from CD36+/+ and CD36−/− mice stimulated with fAβ or revAβ. As expected, adult CD36−/− microglia produced essentially no ROS when stimulated with fAβ compared with the robust production of ROS by similarly treated adult CD36+/+ microglia (Fig. 6 H). These data confirm that CD36 plays a key role in fAβ-induced ROS production in adult mouse microglia and show that adult mouse microglia behave similarly to neonatal mouse microglia and adult macrophages in terms of their responses to fAβ.
Figure 6.
CD36 is expressed on adult mouse microglia and mediates fAβ-induced ROS production. (A–F) Neonatal mouse microglia (A and D), adult mouse microglia (B and E), or peritoneal mouse macrophages (C and F) were stained with mAb to mouse CD36 or isotype-matched control followed by FITC-labeled secondary antibody, visualized by fluorescence microscopy, and digitally photographed. (G) mRNA from adult mouse microglia was harvested for Q-PCR using probes specific for SR-A, SR-B1, SR-F, CD36, and Lox-1. (H) Adult microglia from CD36+/+ and CD36−/− mice were cultured on multispot slides and incubated with 10 μg/spot fAβ or revAβ for 1 h. ROS production was assayed by the NBT reduction method, which produces visible dark blue formazan deposits. The amount of deposited formazan was quantified using digital video microscopy and Scion image analysis software.
Intraperitoneal Injection of fAβ Leads to a CD36-dependent Inflammatory Response.
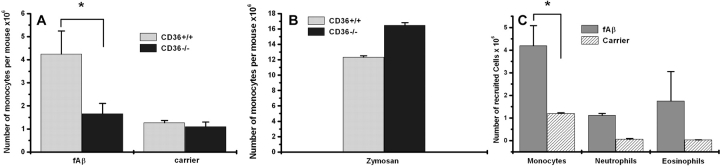
Intraperitoneal injection of proinflammatory stimuli is an established model for studying recruitment of leukocytes in vivo (16). To begin to explore the role of CD36 in fAβ-stimulated inflammatory responses in vivo, we injected 100 μg fAβ into the peritoneum of CD36+/+ and CD36−/− mice. 24 h later, the cellular content of the peritoneal cavity was recovered by peritoneal lavage and analyzed. Intraperitoneal injection of fAβ led to a fourfold increase in the number of monocytes recruited into the peritoneum of CD36+/+ mice compared with injection of carrier alone (Fig. 7 A). Monocytes/macrophages constituted ∼60% of the inflammatory cells recruited to the peritoneum with the remaining cells being ∼20% neutrophils and 20% eosinophils as determined by microscopic analysis of stained cytospin slides (Fig. 7 C). Intraperitoneal injection of fAβ into CD36−/− mice failed to induce the recruitment of leukocytes into the peritoneum (Fig. 7 A). As a control for the ability of CD36−/− macrophages to migrate into the peritoneum in response to a non-CD36–dependent inflammatory stimulus, we found that intraperitoneal zymosan induced equivalent numbers of mononuclear cell recruitment into the peritoneum of CD36+/+ and CD36−/− mice (Fig. 7 B). We confirmed this in vivo control in vitro by demonstrating that macrophages from CD36−/− and CD36+/+ mice chemotaxed equally to MCP-1 (unpublished data).
Figure 7.
fAβ-induced recruitment of monocytes into the peritoneum is CD36 dependent. (A) CD36+/+ or CD36−/− mice were injected intraperitoneally with 100 μg fAβ. 24 h later, the peritoneal cells were harvested by lavage and the number of total cells counted was and differential cell counts were performed on 500 Heme3-stained cells per mouse (C). fAβ lead to recruitment of monocytes in CD36+/+ (n = 10) but not CD36−/− (n = 8) mice. Injection of carrier alone (PBS with 1 mg/ml BSA) did not induce significant monocyte recruitment. (B) CD36−/− and CD36+/+ mice injected with 1 mg zymosan recruited equivalent amounts of monocytes after 24 h. Each data point is the average ± SEM of results from the indicated number of mice (n) per genotype. *, P < 0.005.
Microglial Recruitment to Intracerebral Sites of fAβ Deposition Is CD36 Dependent.
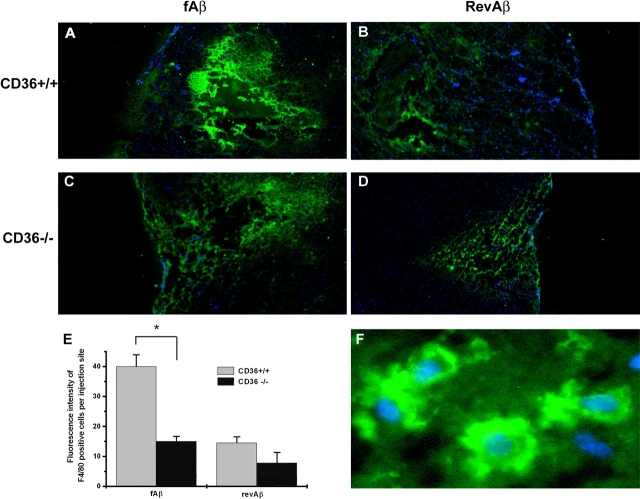
Stereotaxic intracerebral injection of fAβ in aged primates induces a strong microglial reaction and neurotoxicity (5), and has been used as a model for studying the effects of fAβ in vivo and for microglial function in AD (5, 6). To determine the role of CD36 in the recruitment of microglia to sites of intracerebral fAβ deposition, we stereotaxically microinjected 2 μg fAβ in the cortex of 5–6-mo-old CD36−/− and CD36+/+ mice. As a control, we injected 2 μg nonfibrillar revAβ in the cortex of the opposite hemisphere. 48 h later, the mice were killed and serial brain sections were analyzed for the number of microglia recruited to the site of microinjection using immunofluorescent staining with F4/80, a mAb that binds a well-established microglial and macrophage cell surface marker (7). Nuclei were visualized by costaining with the nucleic acid stain DAPI. Stereotaxic microinjection of fAβ led to significant microglial cell recruitment in CD36+/+ mice quantified by measuring fluorescence intensity of F4/80+ cells per site of injection per brain section (40 ± 3.9 fluorescence units; Fig. 8, A and E) . As a control, microinjection of an equal amount of revAβ induced significantly less microglial cell recruitment in CD36+/+ mice (14.5 ± 2 fluorescence units; Fig. 8, B and E). In contrast to CD36+/+ mice, injection of fAβ into CD36−/− mice induced significantly less microglial cell recruitment, and no significant difference was observed between fAβ and revAβ in the CD36−/− mice (15 ± 1.7 vs. 7.8 ± 3.5, respectively). These data demonstrate for the first time that CD36 is required for the recruitment of microglia to sites of fAβ deposition in vivo.
Figure 8.
Intracerebral fAβ-induced recruitment of microglia is CD36 dependent. CD36+/+ (A and B) or CD36−/− (C and D) mice stereotaxically microinjected with 2 μg fAβ (A and C) or revAβ (B and D) into opposite hemispheres. 48 h later, mice were killed and 20-μm frozen sections were stained with F4/80 (green) and DAPI (blue; original magnification, ×4). The fluorescence intensity of F4/80 staining representing the number of microglia per injection site was quantified by digital imaging analysis (E). A higher magnification image showing details of cytoplasmic F4/80 and nuclear DAPI staining is shown in F. Each data point is the average ± SEM of five different sections per mouse and is a representative of five to seven mice per genotype with similar results. *, P < 0.005.
Discussion
Recruitment of activated leukocytes to sites of tissue injury is an important initial step in the inflammatory process (26). In the case of AD, the initial injurious inflammatory stimulus is thought to be the deposition of insoluble extracellular aggregates of fAβ. These fAβ deposits lead to an innate host response characterized by the accumulation of activated microglia (2, 27). The molecular events that occur between the deposition of fAβ and recruitment and activation of microglia have not been identified. The data presented here show that the class B2 scavenger receptor CD36 is necessary for fAβ-induced activation of microglia and macrophages. Binding of fAβ to CD36 mediates activation of microglia to produce ROS, the proinflammatory cytokines IL-1β and TNFα, and a number of chemokines active on microglial cells. ROS and cytokines are thought to initiate and/or propagate the neurotoxic effects of fAβ (1, 3, 6, 28), and we have proposed that CD36-dependent chemokine secretion promotes the recruitment of additional activated microglia, which would amplify the local neurotoxic inflammatory response. In fact, using two different in vivo assays we demonstrated that fAβ induces the recruitment of microglia and macrophages in vivo in a CD36-dependent fashion. CD36 is therefore a key molecular link between recognition of extracellular fAβ and microglial activation.
Our use of stereotaxic intracerebral microinjection of fAβ into genetically deficient mice represents a novel extension of this technique previously used in wild-type primates and rats (5, 6). Using this approach, we were able to demonstrate a critical role for a single gene, CD36, in initiating the inflammatory response to fAβ in the brain in vivo. Thus, stereotaxic intracerebral microinjection of fAβ in genetically deficient mice might be used as a rapid in vivo method for dissecting the molecular and cellular components of the brain's response to fAβ. This assay also can be used for the rapid screening of novel therapeutics for their ability to interfere with this pathologic process.
Although the data in this work clearly indicate that CD36 is necessary for fAβ-induced signal transduction in macrophages and microglia, other receptors on these cells participate in the interaction with fAβ, as we found that CD36−/− macrophages and microglia adhered normally to fAβ-coated surfaces. Indeed, another scavenger receptor, SR-A, has been shown to play a nonredundant role in adhesion of macrophages to fAβ (9) and data using a polyclonal antibody to the receptor for advanced glycation end products also suggested that it may play a role in fAβ-induced microglial activation (30). It is likely that SR-A and CD36 and perhaps receptor for advanced glycation end products play complementary roles in the macrophage response to fAβ. Interestingly, it has been proposed that SR-A and CD36 are similarly involved in the macrophage response to oxidized low density lipoprotein (29). Thus, CD36 is a necessary signaling component of a pattern recognition receptor complex on macrophages and microglia that recognize modified host proteins. This interaction leads to cellular activation and initiation of a local inflammatory reaction that is a defining feature of chronic inflammatory diseases, such as AD and atherosclerosis.
Recent attention has been focused on the Toll-like receptor family of “pattern recognition receptors” as critical activators of the innate immune system. Toll-like receptors are activated by pathogen-associated molecular structures (31) as well as endogenous molecular structures such as heat shock proteins (32) and IgM-chromatin immune complexes (33). Our study now extends this concept to a less well understood family of pattern recognition receptors, the scavenger receptors. Although the scavenger receptors may have evolved to clear the body of nonnative proteins, we show here that when host proteins are modified to form fibrils they can bind scavenger receptors and trigger a local chronic inflammatory response. Our study has clearly demonstrated that CD36 is a critical component of this innate receptor complex that mediates a wide range of proinflammatory events induced by fAβ. CD36 as such represents a defined molecular target for therapeutic intervention in AD and, perhaps, other chronic inflammatory diseases.
Acknowledgments
We thank Dr. E. Antonio Chiocca from the Neurosurgical Service at Massachusetts General Hospital for use of the stereotaxic instruments and Marie McKee from the Renal Unit at Massachusetts General Hospital for assistance with the processing of frozen sections.
This work was supported by National Institutes of Health grants NS41330 (to J. El Khoury), R01AG20255-01 (to K.J. Moore), P01 DK50305 (to M.W. Freeman and A.D. Luster), R01 HL45098 (to M.W. Freeman), and grants from the Alzheimer's Disease Research Program of the American Health Assistance Foundation and the Harvard Center for Neurodegeneration and Repair (to J. El Khoury).
Footnotes
Abbreviations used in this paper: AD, Alzheimer's disease; CIV, collagen IV; DAPI, 4′,6-diamidine-2′-phenylindole dihydrochloride; fAβ, β-amyloid fibril deposits; MCP-1, monocyte chemoattractant protein 1; MIP, macrophage inflammatory protein; NBT, nitro blue tetrazolium; Q-PCR, quantitative real-time PCR; ROS, reactive oxygen species; SR-A, class A scavenger receptor.
References
- 1.Itagaki, S., P.L. McGeer, H. Akiyama, S. Zhu, and D. Selkoe. 1989. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 24:173–182. [DOI] [PubMed] [Google Scholar]
- 2.McGeer, P.L., S. Itagaki, H. Tago, and E.G. McGeer. 1987. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 79:195–200. [DOI] [PubMed] [Google Scholar]
- 3.Giulian, D., L.J. Haverkamp, J.H. Yu, W. Karshin, D. Tom, J. Li, J. Kirkpatrick, L.M. Kuo, and A.E. Roher. 1996. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J. Neurosci. 16:6021-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frautschy, S.A., F. Yang, M. Irrizarry, B. Hyman, T.C. Saido, K. Hsiao, and G.M. Cole. 1998. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 152:307–317. [PMC free article] [PubMed] [Google Scholar]
- 5.Geula, C., C.K. Wu, D. Saroff, A. Lorenzo, M. Yuan, and B.A. Yankner. 1998. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat. Med. 4:827–831. [DOI] [PubMed] [Google Scholar]
- 6.Weldon, D.T., S.D. Rogers, J.R. Ghilardi, M.P. Finke, J.P. Cleary, E. O'Hare, W.P. Esler, J.E. Maggio, and P.W. Mantyh. 1998. Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 18:2161–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornemann, K.D., K.H. Wiederhold, C. Pauli, F. Ermini, M. Stalder, L. Schnell, B. Sommer, M. Jucker, and M. Staufenbiel. 2001. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am. J. Pathol. 158:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selkoe, D.J. 1991. The molecular pathology of Alzheimer's disease. Neuron. 6:487–498. [DOI] [PubMed] [Google Scholar]
- 9.El Khoury, J., S.E. Hickman, C.A. Thomas, L. Cao, S.C. Silverstein, and J.D. Loike. 1996. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 382:716–719. [DOI] [PubMed] [Google Scholar]
- 10.Paresce, D.M., R.N. Ghosh, and F.R. Maxfield. 1996. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 17:553–565. [DOI] [PubMed] [Google Scholar]
- 11.Christie, R.H., M. Freeman, and B.T. Hyman. 1996. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer's disease. Am. J. Pathol. 148:399–403. [PMC free article] [PubMed] [Google Scholar]
- 12.Honda, M., H. Akiyama, Y. Yamada, H. Kondo, Y. Kawabe, M. Takeya, K. Takahashi, H. Suzuki, T. Doi, A. Sakamoto, et al. 1998. Immunohistochemical evidence for a macrophage scavenger receptor in Mato cells and reactive microglia of ischemia and Alzheimer's disease. Biochem. Biophys. Res. Commun. 245:734–740. [DOI] [PubMed] [Google Scholar]
- 13.Coraci, I.S., J. Husemann, J.W. Berman, C. Hulette, J.H. Dufour, G.K. Campanella, A.D. Luster, S.C. Silverstein, and J.B. El-Khoury. 2002. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 160:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antic, A., K.A. Dzenko, and J.S. Pachter. 2000. Engagement of the scavenger receptor is not responsible for beta-amyloid stimulation of monocytes to a neurocytopathic state. Exp. Neurol. 161:96–101. [DOI] [PubMed] [Google Scholar]
- 15.Moore, K.J., J. El Khoury, L.A. Medeiros, K. Terada, C. Geula, A.D. Luster, and M.W. Freeman. 2002. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J. Biol. Chem. 277:47373–47379. [DOI] [PubMed] [Google Scholar]
- 16.Thomas, C.A., Y. Li, T. Kodama, H. Suzuki, S.C. Silverstein, and J. El Khoury. 2000. Protection from lethal gram-positive infection by macrophage scavenger receptor–dependent phagocytosis. J. Exp. Med. 191:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu, A., J.K. Krady, M. O'Malley, S.D. Styren, S.T. DeKosky, and S.W. Levison. 2002. The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J. Neurosci. 22:6071–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Khoury, J., C.A. Thomas, J.D. Loike, S.E. Hickman, L. Cao, and S.C. Silverstein. 1994. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J. Biol. Chem. 269:10197–10200. [PubMed] [Google Scholar]
- 19.Ishizuka, K., T. Kimura, R. Igata-yi, S. Katsuragi, J. Takamatsu, and T. Miyakawa. 1997. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin. Neurosci. 51:135–138. [DOI] [PubMed] [Google Scholar]
- 20.Meda, L., P. Baron, E. Prat, E. Scarpini, G. Scarlato, M.A. Cassatella, and F. Rossi. 1999. Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid. J. Neuroimmunol. 93:45–52. [DOI] [PubMed] [Google Scholar]
- 21.Sheng, J.G., W.S. Griffin, M.C. Royston, and R.E. Mrak. 1998. Distribution of interleukin-1-immunoreactive microglia in cerebral cortical layers: implications for neuritic plaque formation in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 24:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grammas, P., and R. Ovase. 2001. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol. Aging. 22:837–842. [DOI] [PubMed] [Google Scholar]
- 23.Sly, L.M., R.F. Krzesicki, J.R. Brashler, A.E. Buhl, D.D. McKinley, D.B. Carter, and J.E. Chin. 2001. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res. Bull. 56:581–588. [DOI] [PubMed] [Google Scholar]
- 24.Meda, L., M.A. Cassatella, G.I. Szendrei, L. Otvos, Jr., P. Baron, M. Villalba, D. Ferrari, and F. Rossi. 1995. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 374:647–650. [DOI] [PubMed] [Google Scholar]
- 25.Combs, C.K., J.C. Karlo, S.C. Kao, and G.E. Landreth. 2001. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 21:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luster, A.D. 1998. Chemokines–chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436–445. [DOI] [PubMed] [Google Scholar]
- 27.Dickson, D.W., J. Farlo, P. Davies, H. Crystal, P. Fuld, and S.H. Yen. 1988. Alzheimer's disease. A double-labeling immunohistochemical study of senile plaques. Am. J. Pathol. 132:86–101. [PMC free article] [PubMed] [Google Scholar]
- 28.El Khoury, J., S.E. Hickman, C.A. Thomas, J.D. Loike, and S.C. Silverstein. 1998. Microglia, scavenger receptors, and the pathogenesis of Alzheimer's disease. Neurobiol. Aging. 19:S81–S84. [DOI] [PubMed] [Google Scholar]
- 29.Maxeiner, H., J. Husemann, C.A. Thomas, J.D. Loike, J. El Khoury, and S.C. Silverstein. 1998. Complementary roles for scavenger receptor A and CD36 of human monocyte-derived macrophages in adhesion to surfaces coated with oxidized low-density lipoproteins and in secretion of H2O2. J. Exp. Med. 188:2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lue, L.F., D.G. Walker, L. Brachova, T.G. Beach, J. Rogers, A.M. Schmidt, D.M. Stern, and S.D. Yan. 2001. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp. Neurol. 171:29–45. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452–456. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558–561. [DOI] [PubMed] [Google Scholar]
- 33.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]