Abstract
Toll-like receptor 4 (TLR4)-mediated recognition of lipopolysaccharide (LPS) is required for efficient recognition of Gram-negative bacterial infections. Two commonly occurring mutations in the human TLR4 gene (Asp299Gly and Thr399Ile) have recently been shown to be associated with blunted physiological responses to inhaled LPS, and with increased risk of Gram-negative bacteraemia in sepsis patients and reduced risk of atherosclerosis in an Italian population. Here we show that monocytes from individuals heterozygous for both mutations in the TLR4 gene exhibit no deficit in recognition of LPS of Escherichia coli, Neisseria meningitidis, Bacteroides fragilis, Yersinia pestis, Chlamydia trachomatis, Porphyromonas gingivalis, or Pseudomonas aeruginosa. We propose that the relatively high frequency of these mutations in the Caucasian population may reflect modified responses of carriers to alternative TLR4 agonists.
Keywords: lipopolysaccharides, Toll-like receptor 4, single nucleotide polymorphism, monocytes, innate immunity
Introduction
The innate immune responses of mammalian species have evolved to provide the host with a rapid detection of and reaction toward microbial products. Such responses are essential to allow efficient combatting of infection. A family of pattern recognition proteins termed the Toll-like receptors (TLRs) has recently been discovered and shown to be capable of recognizing a wide range of conserved pathogen–associated molecules. The human genome contains genes coding for at least ten TLRs (1), most of which have been shown to be capable of recognizing microbial products. The majority of bacterial lipopolysaccharides, for example, are recognized by TLR4, though there have been reports that the LPS of Porphyromonas gingivalis and Leptospira interrogans may be recognized by TLR2 (2, 3).
Two common mutations in the human TLR4 gene, Asp299Gly and Thr399Ile, have been observed to occur at a frequency of between 6 and 10% in Caucasian populations (4). Individuals heterozygous for these, typically cosegregating, mutations have been shown to have reduced airway responsiveness to inhaled Escherichia coli LPS (5). Furthermore, primary airway epithelial cells of individuals heterozygous for these mutations were shown to be incapable of producing IL-1α in response to LPS challenge, whereas this response was intact in cells extracted from wild-type individuals (5).
As these mutations have been shown to exist at a relatively high frequency in the Caucasian population (4), we set out to determine whether expression of the mutant allele conferred on carriers any advantage in terms of their ability to respond to the LPS of organisms other than E. coli. The component of LPS recognized by TLR4 has been determined to be the lipid A moiety (6). For this reason, we assembled a panel of seven different LPS types representing diverse lipid A structures (for a review, see reference 7) to determine if presence of the mutation altered responses to the LPS of other bacterial species.
Materials and Methods
Detection of TLR4 Polymorphisms.
80 Scottish residents were screened for the presence of the Asp299Gly and Thr399Ile alleles according to the method of Lorenz et al. (4). Briefly, DNA was prepared from whole blood using the ReadyAmp genomic DNA purification system (Promega) according to manufacturer's instructions. Genomic DNA (5 μl) was added to 25 μl of ReadyTaq PCR mix (Sigma-Aldrich) supplemented with 0.2 μl (20 pmol) of each primer and 19.6 μl of nuclease free water. Primers for the Asp299Gly allele were (forward: 5′-GATTAGCATACTTAGACTACTACCTCCATG-3′, reverse: 5′-GATCAACTTCTGAAAAAGCATTCCCAC-3′). Primers for the Thr399Ile allele were (forward: 5′-GGTTGCTGTTCTCAAAGTGATTTTGGGAGAA-3′, reverse: 5′-ACCTGAAGACTGGAGAGTGAGTTAAATGCT-3′). Thermal cycling was: 95°C for 4 min, then 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Asp299Gly or Thr399Ile PCR products (20 μl) were digested with NcoI (Promega) or HinfI (Promega), respectively, and visualized on a 3% high resolution agar gel (Nusieve 3.1; Flowgen). Genotypes of the six individuals recruited for further testing were confirmed by sequencing. Approval for the study was granted by the Lothian Research Ethical Committee (LREC no. 2000/5/35).
Preparation of LPS.
LPS of Escherichia coli R1 (NCTC 13114), Neisseria meningitidis (MPRL-2843), Bacteroides fragilis (NCTC 9343), Pseudomonas aeruginosa (PAC 611), and P. gingivalis (MPRL-1675) were reconstituted from frozen laboratory stocks previously prepared using either the phenol/chloroform/petroleum (E. coli, B. fragilis, P. aeruginosa), triton/MgCl2 (N. meningitidis), or phenol/water method (P. gingivalis) as described previously (8). LPS of Chlamydia trachomatis (LGV-1) and Y. pestis were gifts of Dr. A. Eley (University of Sheffield, Sheffield, UK) and Dr. P. Oyston (DSTL, Porton Down, UK), respectively. All LPS samples (except that of C. trachomatis, of which only 8 μg was available) were repurified to remove protein and lipoprotein contamination according to the method of Hirschfeld et al. (9). Lipoteichoic acid of S. aureus was purchased from Sigma-Aldrich.
Separation of Monocytes.
Whole venous blood was collected from three individuals homozygous for the wild type TLR4 alleles, and three individuals heterozygous for the Asp299Gly and Thr399Ile polymorphisms. All volunteers were healthy at time of testing and were not undergoing any drug therapy other than oral contraceptives. Blood diluted 1:3 in PBS (40 ml) was layered onto 10 ml of Histopaque-1077 (Sigma-Aldrich) and centrifuged at 300 g for 30 min. Peripheral blood mononuclear cells were removed from the interface and washed twice (7 min, 300 g) in PBS before being counted and resuspended in RPMI supplemented with antibiotics and 10% human AB serum (Sigma-Aldrich) at 106 monocytes per ml. Cells were then plated at 100 μl per well of a 96 well plate (Iwaki) and incubated for 1 h at 37°C/5%CO2. Non-adherent cells were removed with four rinses with PBS.
Challenge of Monocytes.
Serial 10-fold dilutions of each LPS were made in RPMI supplemented with 10% human AB serum (Sigma-Aldrich) and 100 μl of each dilution was added to cells. Control cultures were incubated in medium alone. After 4 h incubation, supernatants were assayed for IL-1β production using the Duoset ELISA system (R&D Systems) according to the manufacturer's instructions.
Results and Discussion
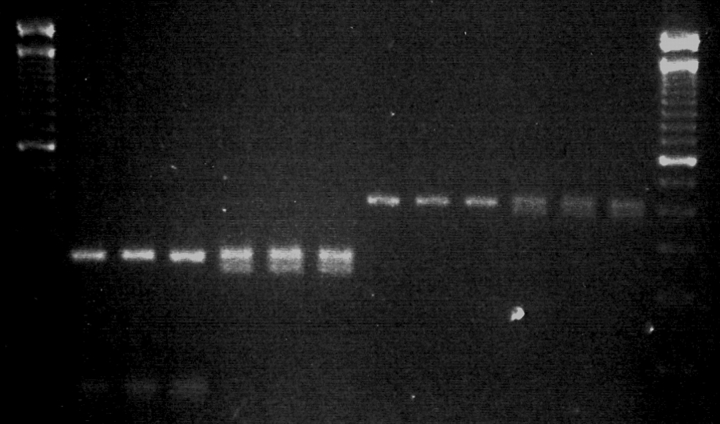
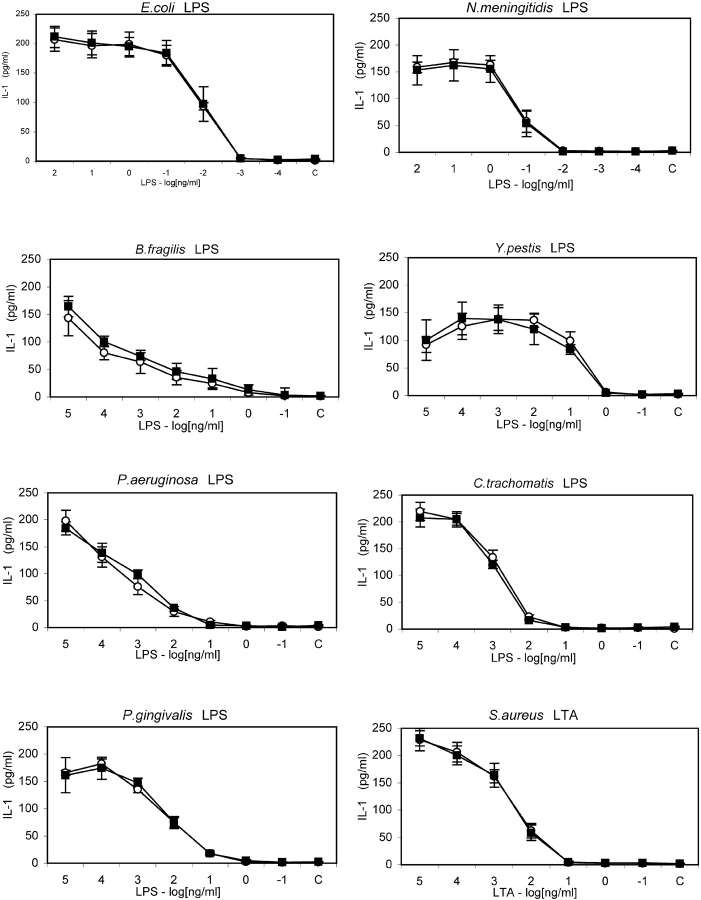
A screen of 80 Scottish residents revealed 8 individuals heterozygous for the Asp299Gly and Thr399Ile polymorphisms in the TLR4 gene. Each individual carried both mutations, confirming the earlier observation that these mutations are rarely seen to segregate individually (10). Fig. 1 shows the results of genotyping of three individuals heterozygous for the TLR4 mutations and three individuals expressing wild-type TLR4. Monocytes from these six, roughly sex and age matched, individuals were challenged with LPS of E. coli, N. meningitidis, B. fragilis, Y. pestis, C. trachomatis, P. gingivalis, P. aeruginosa, and lipoteichoic acid of S. aureus. Fig. 2 shows that no appreciable difference exists in the responses of wild-types and heterozygotes to any of the LPS tested. Statistical analysis reveals that no significant difference exists in the response of wild type or heterozygote monocytes to any of the panel of LPS investigated at any of the concentrations tested (Table I). It is also clear when viewing the data in this way that the capacity of the different LPSs to stimulate cells varies substantially. For example, responses to the LPS of E. coli and N. meningitidis are strongest, while responses to B. fragilis, P. gingivalis, and Y. pestis LPS are somewhat reduced and LPS of C. trachomatis and P. aeruginosa show the lowest activity.
Figure 1.
Detection of wild-type, Asp299Gly, and Thr399Ile alleles in the human TLR4 gene. Genomic DNA of individuals recruited into the current study was amplified according to the method of Lorenz et al. (reference 4). PCR products were digested with the enzymes NcoI (299 allele) or HinfI (399 allele). Lanes 1 and 14, 100 base pair marker. Lanes 2–7, RFLP of the 299 section of TLR4 gene from three wild-type donors and three heterozygotes. Lanes 8–13, RFLP of the 399 section of TLR4 gene from three wild-type donors and three heterozygotes. Top band represents presence of wild-type allele, bottom band indicates presence of mutant allele. Genotypes were confirmed by sequencing.
Figure 2.
IL-1β secretion of wild type and Asp299Gly/Thr399Ile heterozygote TLR4 monocytes in response to LPS and LTA challenge. Human monocytes were challenged with ten-fold serial dilutions of E. coli, N. meningitidis, Y. pestis, C. trachomatis, B. fragilis, P. aeruginosa, P. gingivalis LPS or S. aureus LTA, or medium alone (C). Supernatants were assayed for IL-1β content after 4 h incubation. Open circles represent mean IL-1β secretion ± SEM from three wild-type donors. Filled squares represent mean IL-1β secretion ± SEM from three donors heterozygous for both TLR4 mutations.
Table I.
Comparison of Homozygous Wild-Type and Wild-Type/Asp299Gly,Thr399Ile Heterozygote TLR4-bearing Monocyte Recognition of Various LPS
| Stimulus | Minimum concentration activating wild-type monocytes (ng/ml)a |
Minimum concentration activating TLR4+/− monocytes (ng/ml)a |
|---|---|---|
| E. coli LPS | 0.01 | 0.01 |
| N. meningitidis LPS | 0.1 | 0.1 |
| B. fragilis LPS | 1 | 1 |
| P. gingivalis LPS | 10 | 10 |
| Y. pestis LPS | 10 | 10 |
| C. trachomatis LPS | 100 | 100 |
| P. aeruginosa LPS | 100 | 100 |
| S. aureus LTA | 100 | 100 |
Homozygous wild-type or Asp299Gly/Thr399Ile heterozygous (TLR4+/−) human monocytes were challenged with 10-fold serial dilutions of E. coli, N. meningitidis, Y. pestis, C. trachomatis, B. fragilis, P. aeruginosa, P. gingivalis LPS, or S. aureus LTA or medium alone.
Minimum concentration represents the lowest concentration at which individual stimuli induce production of significantly more IL-1β (P < 0.05) than cells incubated with medium alone.
It was expected that the responses of wild-type and heterozygote monocytes to S. aureus LTA should be broadly similar as the signaling of this molecule is thought to be TLR4 independent (11). This differential signaling could also explain the identical responses made toward P. gingivalis LPS as this molecule has been shown to be capable of signaling via TLR2 (2), though it should be pointed out that the LPS of at least one strain of P. gingivalis has been shown to signal via TLR4 (12). However, the responses of heterozygotes to every other LPS tested are at odds with recent reports of the reduced functional capacity of cells isolated from such donors. Arbour and coworkers have reported that individuals heterozygous for these mutations have blunted physiological responses to inhaled endotoxin and while airway epithelia from wild-type individuals are capable of secreting IL-1α in response to LPS in vitro, cells from heterozygotes were shown to be incapable of this response (5).
There are several possible explanations for the discrepancy between the findings of these workers and the current study. First, airway responses to LPS are known to be highly variable between healthy donors (13). Arbour and colleagues categorized study subjects challenged with inhaled endotoxin as ‘LPS responsive’ if their forced expiratory volume in one second (FEV1) was reduced by at least 20% at any stage following endotoxin challenge. Using this cut off, they state that the mutation was present in 3 of 52 normal responders and 7 of 24 hyporesponders. Using Fisher's one-tailed exact test of these ratios, the group cite a P value of 0.029, though the more appropriate chi-squared analysis of these ratios would have indicated no significant difference in allele frequency between these two groups (P = 0.0539). Most significantly, however, the authors point out that not everyone in their study with the TLR4 mutation was hyporesponsive to LPS and that some of the individuals hyporesponsive to inhaled LPS in their study expressed wild-type TLR4. With this in mind, we feel it is more likely that the differences in airway responsiveness to LPS observed by Arbour and colleagues are determined not by the presence of these mutations, but instead by such factors as the circulating levels of LBP and soluble CD14, the number of LPS responsive cells present in the airway at time of testing and the current expression level of TLR4 – itself understood to rise and fall in response to various stimuli.
To remove bias from modulating factors such as these, we investigated the LPS responsiveness of monocytes derived from different donors in vitro. Using this approach allowed much of the interindividual variability to be removed, as factors such as cell number and differences in serum constituents could be controlled for. Arbour and colleagues investigated the in vitro responses of airway epithelial cells and showed that while epithelia from wild-type individuals were capable of secreting IL-1α in response to LPS, cells from heterozygotes appeared to be incapable of this response (5). This finding is clearly in contrast with the observations of the current study, a difference which may perhaps be explained in two ways. First, it is possible that the mechanisms for regulation of TLR4 are different in monocytes and airway tissues, such that presence of the mutation in the latter may lead to down-regulation of TLR4 expression. Second, it is worth noting that the LPS used in Arbours study was a commercially bought sample of E. coli serotype O111:B4 LPS. Some commercial preparations of LPS have been shown to contain substantial amounts of protein and lipoprotein contamination (2), a property of most LPS preparations prepared using traditionally employed methods (14). It has been shown that removal of these contaminants can be achieved using a phenol reextraction technique (9). For this reason, each of the LPS used in the present study was repurified according to the protocol described by Hirschfeld et al. (9) (except that of C. trachomatis of which not enough material was available to facillitate efficient purification). Thus, it remains possible that the blunted responses documented by Arbour et al. occurred as a result of impaired recognition of either heat shock protein or another TLR4 agonist present in their preparations. Certainly, the concentration of LPS used in their study (100 ng/ml) was ∼10,000 times more than that required to detect pure LPS signaling, allowing the possibility that alternative TLR4 agonists may exist at a high enough concentration in their experiments to evoke additional TLR4 signaling. Further experiments using purified LPS will be required to resolve this issue.
Researchers from the same group have also contributed to several studies suggesting that the presence of these mutations correlates with outcome in a number of clinical conditions. As each of these studies has relied heavily on the citation of Arbours observation that the allele confers LPS hyporesponsiveness, it is worth considering the findings of these studies in the light of the current study.
The Asp299Gly mutation, for example, has been seen to occur at a higher frequency in septic shock patients than in healthy controls (15) and a study of premature birth in Finnish infants revealed a statistically significant correlation between presence of the mutation in the infant and premature birth among singletons (P = 0.024; reference 16). An association study investigating risk of atherogenesis in an Italian cohort consisting of 810 individuals revealed 55 individuals harboring the Asp299Gly mutation (10). These individuals demonstrated lower plasma levels of certain proinflammatory cytokines, acute-phase reactants, and fibrinogen. Also, these individuals were found to be more susceptible to severe bacterial infections, but demonstrated a significantly lower risk of carotid atherosclerosis and a smaller intima-media thickness in the common carotid artery (10). In contrast to the findings of these studies, however, a study of 237 patients receiving hematopoietic stem cell transplantation revealed that the frequency of onset of Gram-negative bacteraemia in patients with TLR4 mutations was not significantly different from wild-type individuals (17) and an unrelated group has reported no association between presence of the Asp299Gly mutation and either susceptibility to or severity of meningococcal disease in a study of over 1,000 patients (18).
It is worth noting that where significant correlations were shown in these studies, no correction for multiple analysis was offered. Furthermore, the study of septic patients found 5 of 91 patients and 0 of 73 controls to harbor the Asp299Gly allele in the absence of the normally cosegregating Thr399Ile allele (15). The authors cite a P value of 0.05 for this comparison using Fisher's one-sided exact test, though the more appropriate chi-squared analysis of these ratios would have indicated a P value of 0.115.
It remains possible, however, that expression of variant TLR4 is capable of altering the host response to TLR4 ligands other than LPS. Indeed, several other microbial and host-derived products have also been shown to signal via this molecule. For example, F-protein of respiratory syncitial virus (19), extra domain A of fibronectin (20), and both human (21) and bacterial heat shock protein (22) have all been shown capable of activating cells via TLR4. Therefore, while the responses to LPS may be identical between wild-types and heterozygotes, this does not preclude the possibility that these mutations may alter the ability of individuals to detect other microbial products. Larger association studies may yet therefore discover a correlation between presence of the mutation and disease progression. It is possible, for example, that efficient recognition of Gram-negative heat shock proteins may be significant in combatting infection and it has already been shown that recognition of human heat shock proteins has a role to play in atherogenesis (23).
In summary, our initial hypothesis that the described mutations in the TLR4 gene may alter host responses to the LPS of bacteria other than E. coli has proven to be unfounded. Instead, we have shown that presence of these mutations (at least in heterozygote carriers) has no effect on the capacity of human monocytes to detect LPS of seven different strains of Gram-negative bacteria. The marked differences in in vivo LPS responsiveness between individuals is therefore determined far more significantly by other genetic and acquired traits of individuals, than by the presence of the Asp299Gly and Thr399Ile TLR4 mutations. Further experiments will be required to determine whether expression of these variant receptors results in modified capacity to respond to alternative TLR4 agonists.
Acknowledgments
We thank Dr. Adrian Eley for the kind gift of C. trachomatis LPS and Dr. Petra Oyston for the kind gift of Y. pestis LPS. We also thank Dr. Paddy Gibb for assistance in venipuncture.
This work was funded by the Wellcome Trust through a 4-year PhD studentship to C. Erridge.
References
- 1.Aderem, A., and R.J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–786. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfeld, M., J.J. Weis, V. Toschchakov, C.A. Salkowski, M.J. Cody, D.C. Ward, N. Quereshi, S.M. Michalek, and S.N. Vogel. 2001. Signalling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werts, C., R.I. Tapping, J.C. Mathison, T.H. Chuang, V. Kravchenko, I. Saint Girons, D.A. Haake, P.J. Godowski, F. Hayashi, A. Ozinsky, et al. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346–352. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz, E., K.L. Frees, and D.A. Schwartz. 2001. Determination of the TLR4 genotype using allele-specific PCR. Biotechniques. 31:22–24. [DOI] [PubMed] [Google Scholar]
- 5.Arbour, N.C., E. Lorenz, B.C. Schutte, J. Zabner, J.N. Kline, M. Jones, K. Frees, J.L. Watt, and D.A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187–191. [DOI] [PubMed] [Google Scholar]
- 6.Tanamoto, K., U. Zahringer, G.R. Mckenzie, C. Galanos, E.T. Rietschel, O. Luderitz, S. Kusumoto, and T. Shiba. 1984. Biological activities of synthetic lipid A analogues: Pyrogenicity, lethal toxicity, anticomplement activity and induction of gelation of limulus amoebocyte lysate. Infect. Immun. 44:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erridge, C., E. Bennett-Guerrero, and I.R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837–851. [DOI] [PubMed] [Google Scholar]
- 8.Hancock, I.C., and I.R. Poxton. 1998. Bacterial Cell Surface Techniques. Wiley, Chichester, UK. 488 pp.
- 9.Hirschfeld, M., Y. Ma, J.H. Weis, S.N. Vogel, and J.J. Weis. 2000. Cutting edge:repurification of lipopolysaccharide eliminates signalling through both human and murine toll-like receptor 2. J. Immunol. 165:618–622. [DOI] [PubMed] [Google Scholar]
- 10.Kiechl, S., E. Lorenz, M. Reindl, C.J. Wiedermann, F. Oberhollenzer, E. Bonora, J. Willeit, and D.A. Schwartz. 2002. Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 347:185–192. [DOI] [PubMed] [Google Scholar]
- 11.Lien, E., T.J. Sellati, A. Yoshimura, T.H. Flo, G. Rawadi, R.W. Finberg, J.D. Carroll, T. Espevik, R.R. Ingalls, J.D Radolf, and D.T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419–33425. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa, T., Y. Asai, M. Hashimoto, O. Takeuchi, T. Kurita, Y. Yoshikai, K. Miyake, and S. Akira. 2002. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4 and myeloid differentiation factor 88–dependent signaling pathway. Int. Immunol. 14:1325–1332. [DOI] [PubMed] [Google Scholar]
- 13.Kline, J.N., J.D. Cowden, G.W. Hunninghake, B.C. Schutte, J.L. Watt, C.L. Wohlford-Lenane, L.S. Powers, M.P. Jones, and D.A. Schwartz. 1999. Variable airway responsiveness to inhaled lipopolysaccharide. Am. J. Respir. Crit. Care Med. 160:297–303. [DOI] [PubMed] [Google Scholar]
- 14.Manthey, C.L., and S.N. Vogel. 1994. Elimination of trace endotoxin protein from rough chemotype LPS. J. Endotoxin Res. 1:84–92. [Google Scholar]
- 15.Lorenz, E., J.P. Mira, K.L. Frees, and D.A. Schwartz. 2002. Relevance of mutations in the TLR4 receptor in patients with Gram-negative septic shock. Arch. Intern. Med. 162:1028–1032. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz, E., M. Hallman, R. Martilla, R. Haataja, and D.A. Schwartz. 2002. Association between the Asp299Gly polymorphisms in the Toll-like receptor 4 and premature births in the Finnish population. Pediatr. Res. 52:373–376. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz, E., D.A. Schwartz, P.J. Martin, T. Gooley, M.T. Lin, J.W. Chien, J.A. Hansen, and J.G. Clark. 2001. Association of TLR4 mutations and the risk for acute GVHD after HLA matched sibling hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 7:384–387. [DOI] [PubMed] [Google Scholar]
- 18.Read, R.C., J. Pullin, S. Gregory, R. Borrow, E.B. Kaczmarski, F.S. Giovine, S.K. Dower, C. Cannings, and A.G. Wilson. 2001. A functional polymorphism of Toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J. Infect. Dis. 184:640–642. [DOI] [PubMed] [Google Scholar]
- 19.Kurt-Jones, E.A., L. Popova, L. Kwinn, L.M. Haynes, L.P. Jones, R.A. Tripp, E.E. Walsh, M.W. Freeman, D.T. Golenbock, L.J. Anderson, and R.W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398–401. [DOI] [PubMed] [Google Scholar]
- 20.Okamura, Y., M. Watari, E.S. Jerud, D.W. Young, S.T. Ishizaka, J. Rose, J.C. Chow, and J.F. Strauss iii. 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276:10229–10233. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor 4 complex. J. Immunol. 164:558–561. [DOI] [PubMed] [Google Scholar]
- 22.Sasu, S., D. La Verda, N. Qureshi, D.T. Golenbock, and D. Beasly. 2001. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via Toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ. Res. 89:244–250. [DOI] [PubMed] [Google Scholar]
- 23.Xu, Q. 2002. Role of heat shock proteins in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 22:1547–1559. [DOI] [PubMed] [Google Scholar]