Abstract
We studied the function of antitumor T and natural killer T (NKT) cells from the blood and tumor bed in 23 patients with premalignant gammopathy, nonprogressive myeloma, or progressive multiple myeloma. We show that antitumor killer T cells can be detected in patients with both progressive or nonprogressive myeloma. Vα24+Vβ11+ invariant NKT cells are detectable in the blood and tumor bed of all cohorts. However, freshly isolated NKT cells from both the blood and tumor bed of patients with progressive disease, but not nonprogressive myeloma or premalignant gammopathy, have a marked deficiency of ligand-dependent interferon-γ production. This functional defect can be overcome in vitro using dendritic cells pulsed with the NKT ligand, α-galactosylceramide (α-GalCer). Fresh myeloma cells express CD1d, and can be efficiently killed by autologous NKT cells. We hypothesize that presentation of tumor derived glycolipids by myeloma cells leads to NKT dysfunction in vivo. These data demonstrate that clinical progression in patients with monoclonal gammopathies is associated with an acquired but potentially reversible defect in NKT cell function and support the possibility that these innate lymphocytes play a role in controlling the malignant growth of this incurable B cell tumor in patients.
Keywords: multiple myeloma, NKT cells, dendritic cells, interferon-γ, immunotherapy
Introduction
Clonal expansion of a transformed cell is a prerequisite for the development of cancer, but such expansions can remain stable for prolonged periods without the development of overt clinical malignancy in patients (1–3). Monoclonal gammopathies are characterized by the proliferation of a transformed clone of plasma cells (3, 4). However the clinical outcome of this clonal expansion can vary greatly. Patients with monoclonal gammopathies of undetermined significance (MGUS)* often remain stable for several years, and are thought to have a clinically “premalignant” state. Even some patients with greater clonal burden meeting diagnostic criteria for myeloma can remain stable for prolonged periods (“nonprogressive myeloma”; NPM), while those with progressive myeloma (PM) have a limited survival, even after high dose chemotherapy (4). Clinical management of these patients is therefore a challenge, because current therapies are not curative and tumor progression in patients with MGUS or myeloma is difficult to predict. This is particularly puzzling because in recent studies, nearly all of the cytogenetic changes initially described in myeloma tumor cells have now also been observed in plasma cells from MGUS (5, 6). Furthermore, recent studies of gene expression profiles have also failed to demonstrate clear differences between purified plasma cells in MGUS or myeloma (7). One possibility that we address here, is that the host response plays a key role in controlling the “malignant transformation” of this cancer.
In mice, components of both the adaptive (cytolytic killer and helper T lymphocytes) and the innate immune system (natural killer [NK], and NKT cells), as well as IFN-γ are important for the surveillance of spontaneous as well as carcinogen induced tumors (8–10). In human cancer, multiple myeloma provides a valuable opportunity to study these innate and adaptive defense mechanisms, because of the ease of access to the tumor and immune cells in the tumor bed, the bone marrow, without the need for enzymatic manipulations and in vitro culture. Recently we observed that freshly isolated T cells from the tumor bed of patients with progressive myeloma do not react detectably to their autologous tumor (11). However, both cytolytic and IFN-γ producing response to autologous myeloma could be reliably generated following stimulation with tumor-loaded dendritic cells (DCs), even though these patients had clinically progressive tumors (11).
Human natural killer T (NKT) cells are distinct lymphocytes, which often use a restricted T cell receptor (Vα24-Vβ11 in humans) that recognizes glycolipid ligands in the context of a nonpolymorphic antigen presenting molecule, CD1d (for reviews, see references 12–14). NKT cells can be activated by a synthetic ligand, α-galactosyl ceramide (α-GalCer; reference 15), leading to rapid production of several cytokines such as IL-4 and IFN-γ. In mice, α-GalCer stimulation of NKT cells regulates autoimmunity, as well as resistance to infections, and tumors (16–21), and preliminary evidence for such effects are evident with human cells (22, 23) (for reviews, see references 12–14). Deficiencies in IFN-γ production and the proliferative potential of NKT cells have recently been described in some patients with advanced cancer (24, 25). However, it is difficult to interpret the significance of an alteration in immune function, unless one understands its relationship to the development or clinical outcome of a disease, and the benefit of its functional restoration, particularly in the context of autologous fresh tumor. Here we measure antitumor killer T and NKT cell function in clinically distinct cohorts of patients with monoclonal gammopathies.
Materials and Methods
Study Subjects.
Peripheral blood and bone marrow samples were obtained from patients with monoclonal gammopathies following informed consent approved by the institutional review board. Patients were classified as MGUS or myeloma, according to standard diagnostic criteria (4). Myeloma patients were further classified either as progressive if they experienced disease progression by standard criteria (4), or as nonprogressive, if they did not experience disease progression or require therapy for at least 3 mo after the blood draw. All patients with progressive disease were studied before the initiation of any chemotherapy.
Isolation of Tumor and Immune Cells.
Mononuclear cells from blood or bone marrow were obtained by density gradient centrifugation using Ficoll Hypaque. DCs were generated from monocytes by culture of adherent blood mononuclear cells in the presence of GM-CSF (Immunex) and IL-4 (R&D Systems) as described (26). Nonadherent cells were used as a source of blood T cells. Bone marrow mononuclear cells were separated into tumor (CD138+ve) and nontumor (CD138-ve) fractions using CD138 magnetic beads (Miltenyi Biotec). Tumor cells were cryopreserved in aliquots for use as sources of antigen, as well as targets. Non tumor cell fractions were also cryopreserved for use as control targets, as well as a source of T cells from the marrow.
Generation of Antigen-loaded DCs.
Autologous tumor cells were opsonized using anti-syndecan-1, γ-irradiated, and then fed to immature DCs as described (26). DCs were then induced to mature by overnight culture in the presence of a cytokine cocktail consisting of TNF-α (10 ng/ml), IL-1β (10 ng/ml), IL-6 (1,000 U/ml; all from R&D Systems), and PGE2 (1 μg/ml; Amersham Biosciences). For stimulation of NKT cells, α-GalCer (100 ng/ml; kindly provided by Kirin Breweries, Gunma, Japan) was loaded on DCs overnight on day 6–7 of culture, together with the cytokine cocktail for maturation.
Stimulation of T/NKT Cell Responses in Culture.
To stimulate tumor specific T cells, tumor-loaded mature DCs (or unpulsed as the negative control) were cocultured with autologous T cells (2 × 105 cells/well) from either the marrow or blood at a DC:T cell ratio of 1:10–30 in 200 μl of 5% pooled human serum (11, 26). Cultures were supplemented with IL-2 (Chiron Corp.) on days 4 and 7 (10 U/ml). In some experiments, IL-7 (R&D Systems; 5 ng/ml) was added to cultures on day 7. After 12–16 d in most experiments, T cells from several micro-cultures were pooled and tumor-specific killer T cells were assayed by cytotoxicity and ELISPOT assays, as described (11).
To expand NKT cells in culture, Mφ-depleted cells or PBMCs (105/well) were cultured with autologous α-GalCer loaded DCs (or unpulsed DCs as a control) in the presence of recombinant IL-2 (50 U/ml) for 1–3 wk, as described (27). IL-2 (50 U/ml) was supplemented weekly. α-GalCer–dependent expansion of NKT cells was monitored by quantifying Vα24+Vβ11+ cells by FACS®.
Single Cell ELISPOT Assay for Quantifying NKT and T Cells.
Freshly isolated PBMCs (5 × 105/well) were incubated for 16 h either with or without α-GalCer (100 ng/ml) in 5% heat inactivated human serum containing RPMI (complete medium or CM) in ELISPOT plates precoated with anti–IFN-γ or IL-4 antibodies, as described (27). In prior studies with healthy donors, we have shown that α-GalCer reactive IFN-γ producers in this assay are depleted after the removal of Vα24+ or Vβ11+ cells and correlate only with the number of NKT, but not number of NK or T cells, strongly suggesting that the reactivity in this ELISPOT assay is a quantitative measure of NKT function (27). This is also supported by the finding of Gumperz et al., who also did not find cytokine production by nontetramer binders in response to overnight α-GalCer stimulation of fresh human PBMCs in vitro (28).
For detecting influenza virus–specific T cells, PBMCs were infected with influenza virus (MOI = 2) before plating in the ELISPOT assay, as described (29). PHA (10 μg/ml) and staphylococcal enterotoxin A (SEA; 50 ng/ml) were used as a positive control. The presence of tumor-reactive IFN-γ–producing T cells in both blood and marrow T cells was also quantified by ELISPOT both directly ex vivo, and after in vitro stimulation (as described above), with autologous tumor loaded DCs, as described (11, 26). For some experiments, T cells expanded using α-GalCer pulsed DCs were separated into Vα24 positive or negative fractions and tested for recognition of unpulsed or α-GalCer pulsed DCs using an ELISPOT assay for IFN-γ or IL-4 production.
Flow Cytometric Analyses.
FITC-labeled murine IgG, PE-labeled murine IgG2a, anti–human CD3, anti-CD8, anti-CD4, anti-CD14, and anti-CD56 mAbs were purchased from Becton Dickinson, and FITC-labeled anti-Vα24 and PE-labeled anti-Vβ11 from Beckman Coulter. In samples with low frequencies of NKT cells, analyses were repeated with acquisition of at least 1–3 × 105 lymphocyte gated events, to confirm the frequency of NKT cells. To test the functional properties of freshly isolated uncultured NKT cells, PBMCs were cultured in 96-well plates with α-GalCer (100 ng/ml) and purified anti-CD28 (3 μg/ml; BD Biosciences), or anti-CD28 alone as a control, in the presence of 0.7 μg/ml of Golgistop (from Cytofix/CytoPerm Plus Kit from BD Biosciences). Preliminary studies had shown the need for anti-CD28 for enhancing the sensitivity of reliable detection of α-GalCer reactive cytokine producers in this system, as shown previously (30). After overnight (12–16 h) culture at 37°C, cells were washed and stained for surface markers, and then washed two more times in staining buffer. Cells were then fixed and permeabilized in 100 μl cytofix/cytoperm solution using manufacturer's directions, and stained for intracellular cytokines (IFN-γ and IL-4). Reagents used for staining were anti-Vα24-FITC, (Immunotech), anti-CD3-Cychrome, anti-CD4 APC, and anti–IFN-γ-PE or anti–IL-4-PE (BD Biosciences). Samples were analyzed on FACSort™ or FACSCalibur™ (Becton Dickinson) instrument using CELLQuest™ software. Lymphocyte events collected were typically 1–3 × 105 events per sample. Functional aspects of NKT cells expanded using α-GalCer pulsed DCs were also evaluated by monitoring intracellular cytokine (IFN-γ, IL-4) expression by flow cytometry after 6 h stimulation with PMA (50 ng/ml) and ionomycin (1 μM).
Cytolysis Assays.
The presence of tumor specific killer T cells in bulk T cell cultures after in vitro stimulation with tumor cell–loaded DCs was evaluated using standard 5 h 51Cr release assays, with autologous tumor cells (CD138+) as targets, with autologous nontumor fractions from the tumor bed of the same patient as controls, as described (11, 26). To test cytolysis by NKT cells, bulk cultures expanded using α-GalCer pulsed DCs were separated into Vα24 positive and negative fractions using magnetic beads and tested in cytolysis assays. Purity of fractions was monitored by FACS® and was >80%. For some experiments, target cells were tested with or without pulsing with 100 ng/ml α-GalCer, or pretreated with anti-human CD1d antibody (5 μg/ml, clone 42; BD Biosciences) or isotype control.
Statistical Analysis.
Data for immune reactivity between two groups was compared using Student's t test, and Mann Whitney test. Kruskall Wallis test was used when more than two groups were compared. Significance was set at P = 0.05.
Results
Comparable Antitumor Killer T Cell Function in Progressive and Nonprogressive Myeloma.
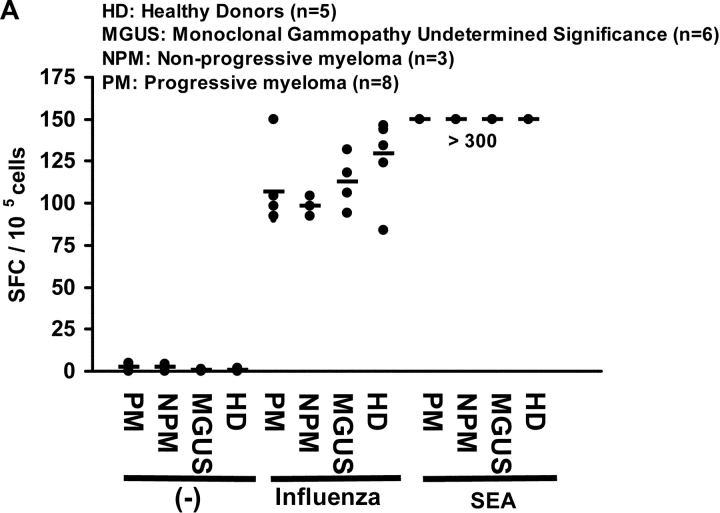
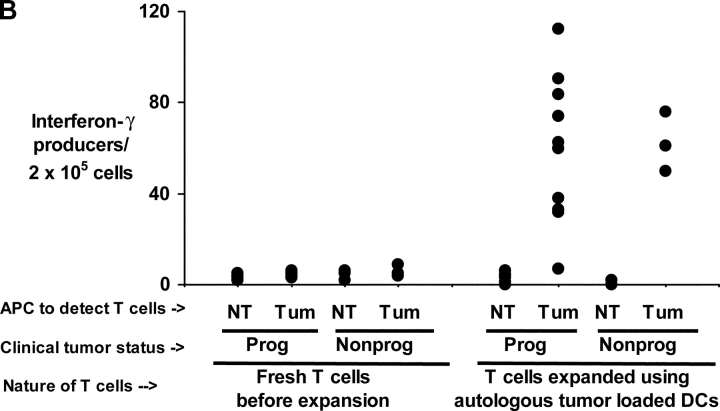
To assess general immune competence, we first quantified influenza virus–specific T cells, as well as response to superantigen SEA in fresh lymphocytes from 4 groups of individuals: patients with MGUS, nonprogressive myeloma (defined as asymptomatic and stable disease for >3 mo), progressive myeloma, and healthy controls. Immune reactivity to influenza (P = 0.3) and SEA was comparable in all 4 cohorts indicating general immune competence (Fig. 1 A). We next hypothesized that the state of clinical stability in patients with nonprogressive myeloma may be due to enhanced activation and expansion of antitumor T cells with rapid effector function. To test this, we measured the immune reactivity of T cells from 3 consecutive patients with nonprogressive myeloma, and compared it to the data from 8 patients with progressive tumors. Freshly isolated circulating T cells from patients with nonprogressive tumors did not react detectably with autologous tumor for IFN-γ production (Fig. 1 B). Similar findings were observed when T cells from the tumor bed of these patients were tested (unpublished data). However, after 14–16 d of stimulation using autologous tumor loaded DCs, reactivity against autologous tumor (but not against nontumor cells in the tumor bed) was generated from all three patients. Contrary to our expectations, this reactivity was quantitatively similar to the reactivity of T cells from patients with clinically progressive disease, which develop tumor reactive killer cells as reported (11) when cultured under identical conditions (P = 0.76, Fig. 1 B). Thus, antitumor effector T cell function, as measured in our assays with both fresh and cultured T cells for reactivity against autologous tumor, was similar between patients with clinically progressive or nonprogressive myeloma.
Figure 1.
(A) Influenza and SEA reactive T cell response in monoclonal gammopathies. ELISPOT assay quantifying the presence of influenza and SEA reactive T cells in patients with m MGUS (n = 6), NPM (n = 3), PM (n = 8), or healthy donors (HD, n = 5). Each dot represents an individual patient. (B) Antitumor T cell function in progressive or nonprogressive myeloma. ELISPOT assay testing IFN-γ production in response to autologous tumor (CD138+) or nontumor (CD138-ve) cells from bone marrow, by blood T cells tested either fresh immediately after isolation, or after in vitro expansion using autologous tumor loaded DCs. Each dot represents an individual patient.
Detection of NKT Cells in Patients with Monoclonal Gammopathies.
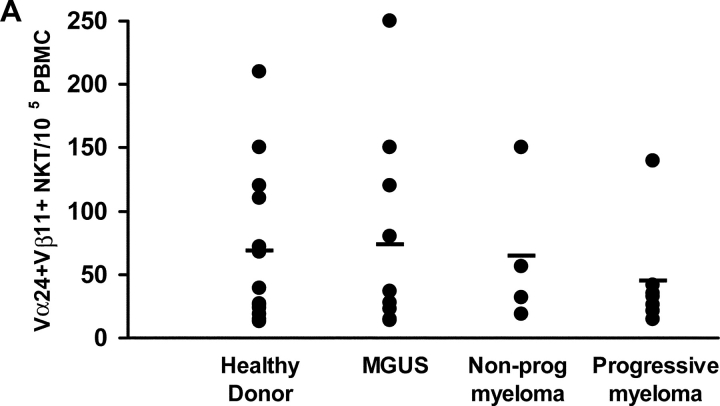
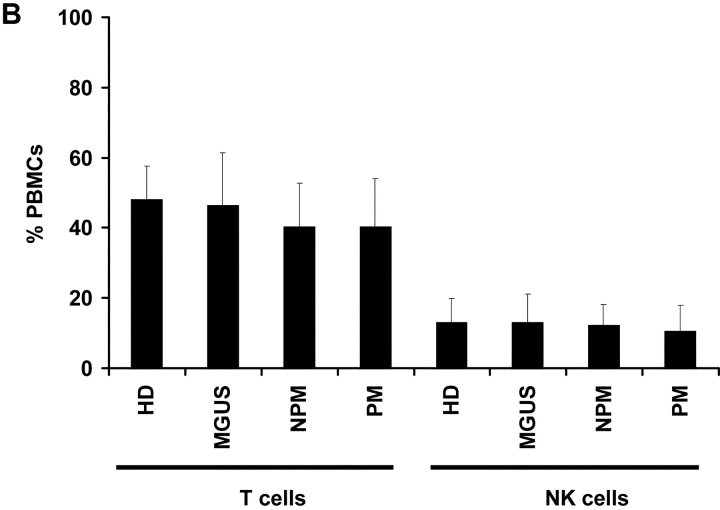
Given the difficulty in identifying clear distinctions in antitumor CD8+ T cells in patients with progressive or nonprogressive myeloma, we focused on the natural killer T (NKT) cells. These bear a restricted T cell receptor (Vα24-Vβ11) and can be activated by a synthetic ligand, α-galactosyl ceramide (α-GalCer; references 12–14). Vα24+Vβ11+ NKT cells could be detected by flow cytometry in PBMCs from patients with progressive (n = 7) or nonprogressive myeloma (n = 4), MGUS (n = 10), or healthy controls (n = 13) (Fig. 2 A). The frequency of NKT cells in progressive myeloma (mean ± SD; 0.045 ± 0.04%) overlapped with that in patients with nonprogressive disease (0.064 ± 0.05%), MGUS (0.073 ± 0.07%), or healthy donors (0.068 ± 0.06%; P = 0.8; Kruskall Wallis). Similarly, the frequency of T (CD3+ve) and NK cells (CD3-ve, CD56+ve) were also comparable between these cohorts (P = 0.8 for comparison of NK cells, and P = 0.7 for comparison of T cells; Fig. 2 B).
Figure 2.
(A and B) Quantitation of NKT (Vα24+ Vβ11+) (A), NK (CD3−CD56+), and T cells (B) by flow cytometry in PBMCs of healthy donors (HD; n = 13), or patients with MGUS (n = 10), NPM (n = 4), or PM (n = 7).
Decreased NKT Effector Function in Progressive Myeloma.
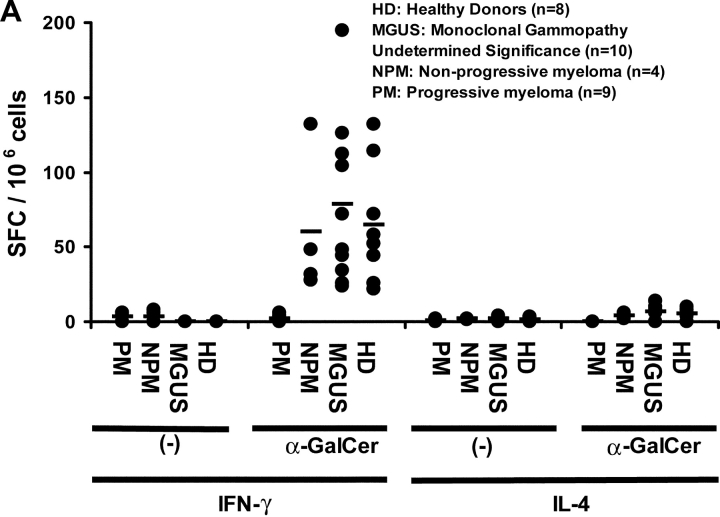
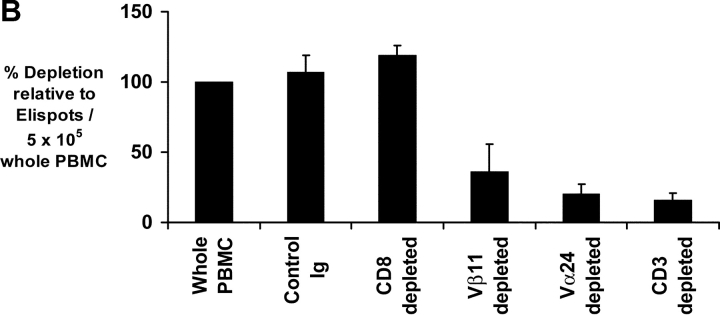
We have recently shown that the presence of functional human NKT cells in fresh blood can be readily quantified using an ELISPOT assay measuring cytokine production in response to NKT ligand, α-GalCer (27). α-GalCer reactive IFN-γ producting NKT cells could be readily detected in fresh PBMCs from healthy donors, patients with MGUS or nonprogressive myeloma (Fig. 3 A). In contrast, there was a marked decline in α-GalCer reactive IFN-γ production in patients with progressive myeloma (Fig. 3 A). Thus the mean (±SD) α-GalCer reactive IFN-γ producers were significantly lower in fresh blood of patients with progressive myeloma (1.8 ± 2.4 per 106 PBMCs) than in patients with nonprogressive disease (60.5 ± 48.8), MGUS (78.5 ± 37.7), or healthy controls (65 ± 39.6; P < 0.001). This loss of IFN-γ production by NKT cells in progressive myeloma was not associated with detectable α-GalCer–reactive IL-4 production (Fig. 3 A). PHA induced T cell activation served as positive control for IL-4 production in these assays (unpublished data). IFN-γ producers detected in this assay were NKT dependent because they could be removed by the depletion of Vα24+ or Vβ11+ fraction, before use in the assay (Fig. 3 B). When patients with progressive myeloma (who lacked detectable functional NKT cells) were excluded, detection of α-GalCer–reactive IFN-γ producers in fresh blood in the ELISPOT assay correlated with the number of NKT (r2 = 0.8), but not NK cells or T cells by flow cytometry (unpublished data), similar to prior studies in healthy donors (27).
Figure 3.
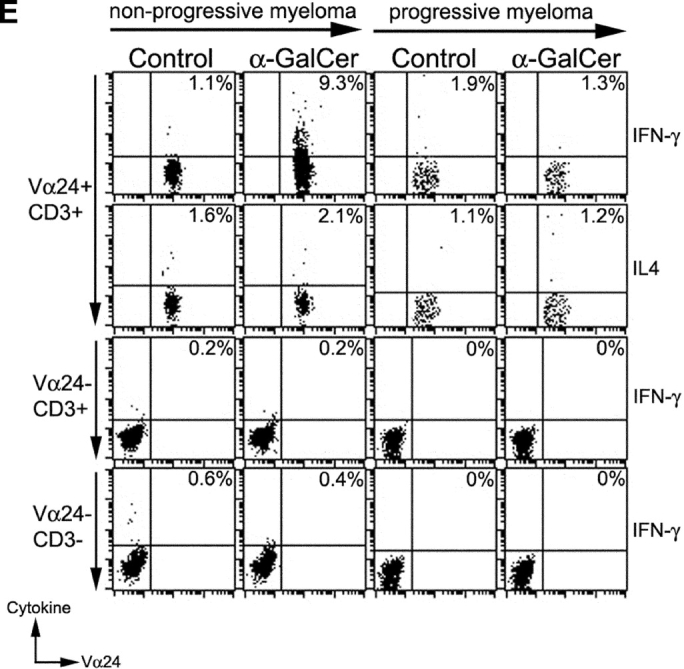
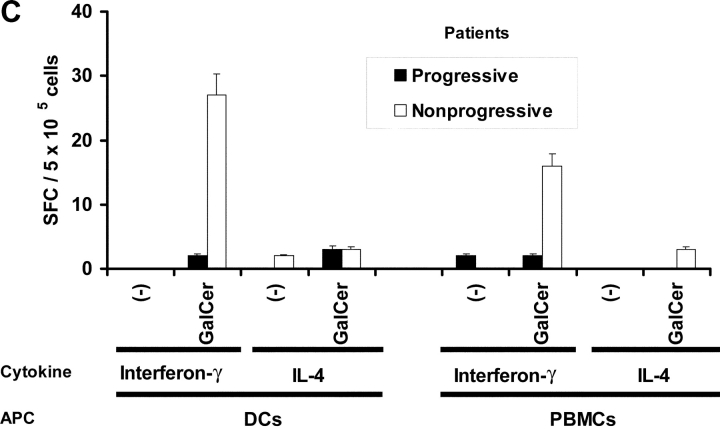
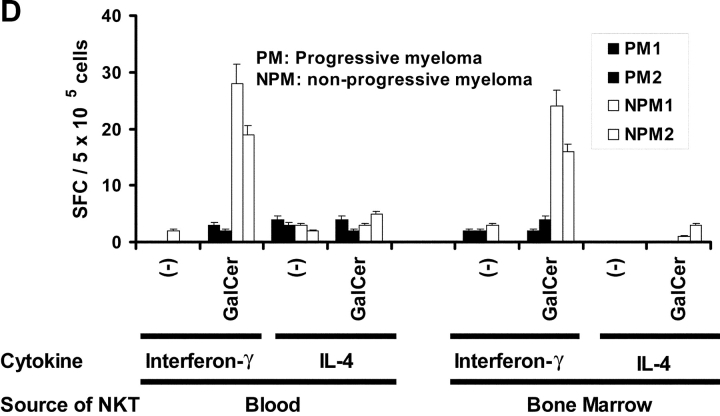
Function of fresh uncultured NKT cells in monoclonal gammopathies. (A) Loss of α-GalCer–reactive IFN-γ production in fresh NKT cells in progressive myeloma. ELISPOT assay quantifying the presence of α-GalCer reactive IFN-γ/IL-4 producers in fresh PBMCs from healthy donors (HD; n = 13), or patients with MGUS (n = 10), NPM (n = 4), or PM (n = 9). (B) Effect of depletion of various cell populations on α-GalCer–reactive IFN-γ production in fresh PBMCs. PBMCs were depleted before the α-GalCer stimulated ELISPOT assay using anti-Vα24+, Vβ11+, CD3+, CD8+, or isotype control antibody, followed by magnetic bead depletion. Data shown are percentage of reactivity in whole PBMCs. (C) Fresh NKT cells from progressive myeloma do not react to ligand bearing DCs. ELISPOT assay quantifying the presence of α-GalCer–reactive IFN-γ/IL-4 producers in fresh PBMCs from a representative patient with progressive or nonprogressive myeloma, using PBMCs alone or autologous mature DCs (added to PBMCs at PBMC:DC ratio of 20:1) as APCs during the 16 h ELISPOT assay. Data represent two patients from each cohort. (D) Loss of effector NKT function in the tumor bed of patients with PM, but not those with NPM myeloma. ELISPOT assays quantifying the presence of α-GalCer–reactive IFN-γ/IL-4 producers in fresh mononuclear from blood or bone marrow (tumor bed) of two patients with progressive or nonprogressive myeloma. (E) Detection of ligand reactive cytokine production by uncultured NKT cells of patients with progressive or nonprogressive myeloma using flow cytometry. PBMCs were stimulated with α-GalCer + anti-CD28, or anti-CD28 alone (as a control), in the presence of monensin. FACS® plots show cytokine production by NKT cells from patients with progressive or nonprogressive myeloma. Data shown are representative of similar experiments on five additional patients.
The loss of α-GalCer–reactive IFN-γ producers in myeloma patients was not due to the loss of resident APC function in myeloma PBMCs, as influenza infection of these PBMCs led to efficient activation of influenza specific T cells (Fig. 1 A). We have recently shown that the nature of the APC expressing NKT ligand has a significant impact on activation of resting human NKT cells (27). Thus mature DCs were superior to monocytes for activation of NKT cells and improve the detection of NKT function in fresh blood (27). The use of α-GalCer–pulsed mature DCs did lead to improved detection of functioning NKT cells in the PBMCs from blood of patients with nonprogressive myeloma, but again, no IFN-γ producers could be detected in the blood of patients with clinically progressive disease, when tested immediately after venapuncture (Fig. 3 C, compare open with closed bars).
Next, we examined the numbers and function of NKT cells in the tumor bed of myeloma patients. The numbers of Vα24+Vβ11+ NKT cells detected by flow cytometry in the bone marrow mononuclear cells were comparable to those in the fresh blood, in patients with either progressive or nonprogressive myeloma (unpublished data). Again however, patients with progressive disease had fewer α-GalCer–reactive IFN-γ producers in their tumor bed (Fig. 3 D). Although human bone marrow has been shown to contain Th2-like, noninvariant TCR, CD1d reactive cells (31), no α-GalCer–reactive IL-4 producers were detected among marrow T cells (Fig. 3 D). To directly detect cytokine production in response to α-GalCer by uncultured NKT cells, we also examined intracellular cytokine production by flow cytometry (30). A fraction of Vα24+ cells secreting IFN-γ in response to α-GalCer stimulation could be readily detected in patients with MGUS or nonprogressive myeloma, similar to prior studies (Fig. 3 E; references 28 and 30). In contrast, no ligand reactive IFN-γ production was detected in NKT cells from patients with nonprogressive myeloma, confirming our studies with the ELISPOT assay (Fig. 3 E). The proportion of invariant TCR (Vα24+Vβ11+) positive T cells among Vα24+ cells in progressive myeloma was comparable to all other study cohorts (unpublished data). α-GalCer reactive cytokine production in these assays was restricted to the CD3+Vα24+ T cells, and not the Vα24− T cells, or CD3 negative population (Fig. 3 E), which is similar to prior studies showing that cytokine production after α-GalCer stimulation was detected only in a fraction of CD1d tetramer binding NKT cells (28). These data support our conclusion that the α-GalCer–reactive IFN-γ producers in the ELISPOT assay are predominantly NKT cells. Together, these data suggest that freshly isolated NKT cells from patients with progressive myeloma have a defect in IFN-γ production in response to ligand stimulation.
DC-mediated Expansion of Functional NKT Cells in Patients with Progressive Myeloma.
We and others have recently shown that mature DCs loaded with α-GalCer are efficient APCs for the expansion of human NKT cells in culture (27, 32). The use of autologous DCs led to expansion of NKT cells in all four cohorts (Fig. 4, A and B) . Importantly, NKT cells expanded in cultures with autologous DCs in all cohorts were functional and secreted both IFN-γ and IL-4 in response to stimulation with PMA and ionomycin (unpublished data), as previously shown with healthy donors (27). These expanded Vα24+ NKT cells also secreted both IFN-γ and IL-4, in response to α-GalCer loaded DCs (Fig. 4 C). Thus, although fresh NKT cells from patients with progressive myeloma did not produce IFN-γ in response to ligand bearing APC, NKT cells expanded from these patients using α-GalCer–loaded DCs, secreted both IFN-γ and IL-4 in response to the ligand.
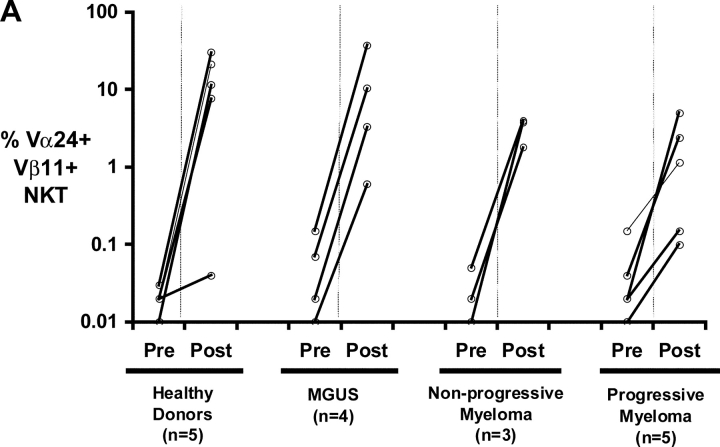
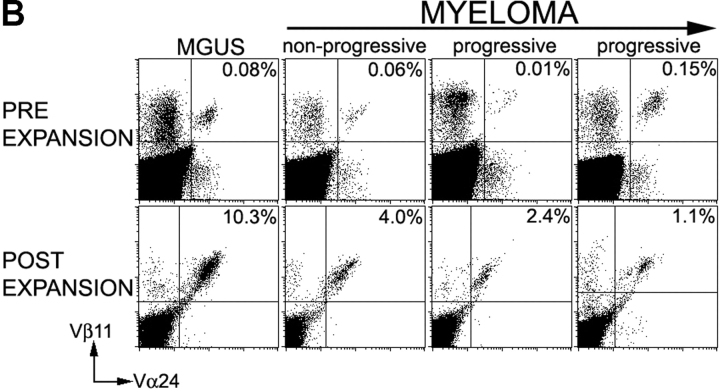
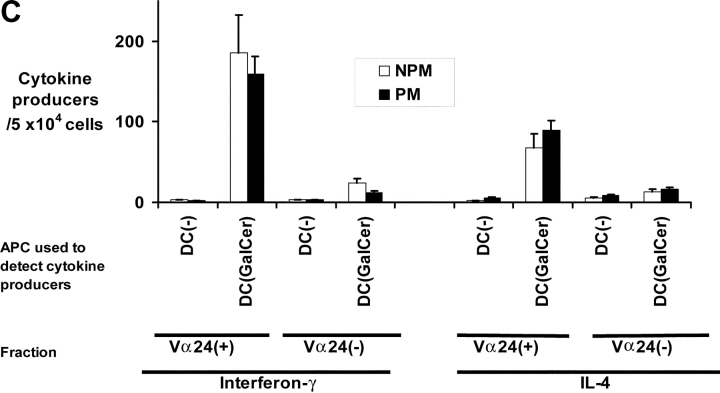
Figure 4.
Functional NKT cells can be expanded from patients with monoclonal gammopathies using α-GalCer pulsed mature DCs. (A) Expansion of Vα24+Vβ11+ NKT cells in culture using autologous α-GalCer pulsed mature DCs from healthy donors (HD; n = 5), or patients with MGUS (n = 4), NPM (n = 3), or PM (n = 5). Data shown are % NKT cells in PBMCs determined by flow cytometry before and after 2–3 wk of expansion. (B) Representative FACS plots showing detection of NKT cells (Vα24+ Vβ11+) in fresh PBMCs (pre) of patients with MGUS, nonprogressive or progressive myeloma, or after PBMC expansion (post) using α-GalCer pulsed mature DCs. Percentages refer to percent NKT cells in total recovered PBMCs. (C) Cytokine production by expanded NKT cells in response to ligand-bearing APCs. T cells expanded using autologous α-GalCer pulsed autologous mature DCs as in panel A, were separated into NKT (Vα24+) and non-NKT (Vα24-ve) fractions using magnetic beads. Individual fractions were then tested for reactivity to autologous α-GalCer pulsed mature DCs (DC: responder ratio of 1:10), using a 16 h Elispot assay for IFN-γ or IL-4 producing cells. Data shown are representative of two patients each with PM or NPM.
Expression of CD1d by Primary Myeloma Cells and Cell Lines.
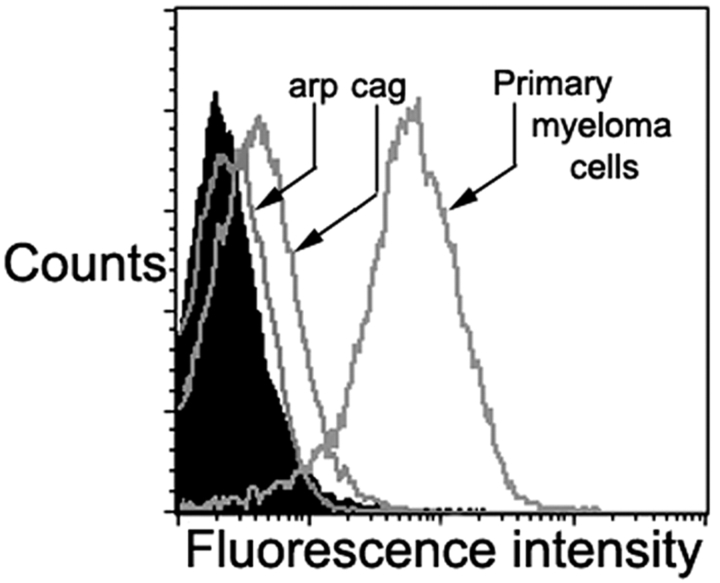
Expression of CD1d by myeloma tumor cells was tested by flow cytometry. Several myeloma cell lines tested (arp, ark, RPMI 8226, U266) lacked detectable CD1d expression. Low but detectable CD1d expression was noted in one of the cell lines (cag cells; Fig. 5) . However in distinct contrast to cell lines, all fresh tumor cells from myeloma patients (n = 5) expressed much higher levels of CD1d (Fig. 5).
Figure 5.
Expression of CD1d on myeloma cells. CD1d expression on myeloma cell lines (arp and cag) as well as purified myeloma cells, as assessed by flow cytometry. Filled histogram reflects isotype control. Data on primary myeloma cells are representative of five different patients.
Cytotoxicity of Expanded NKT Cells Against Primary Human Myeloma and Cell Lines.
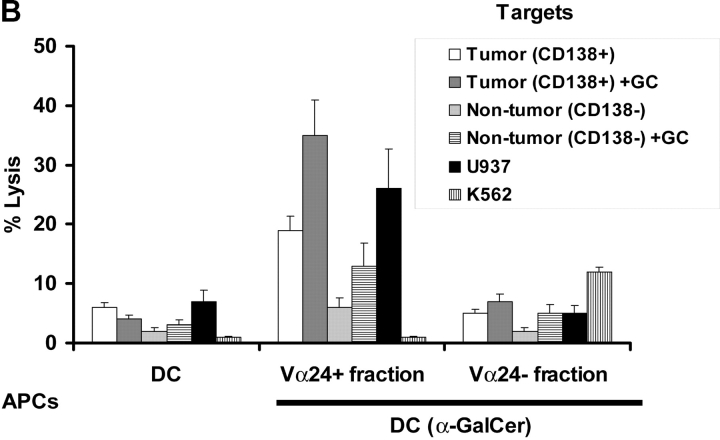
To test the cytolytic capacities of NKT cells expanded in culture from patients with monoclonal gammopathies, the expanded cultures were separated into Vα24+ and negative fractions using magnetic beads, and then tested for killing of myeloma cell lines and primary myeloma cells. NKT cells expanded from patients with MGUS or nonprogressive myeloma could kill CD1d expressing but not CD1d negative myeloma cell lines in a ligand (α-GalCer)–dependent manner (Fig. 6 A). Then we tested if NKT cells generated from patients with nonprogressive myeloma could kill purified autologous tumor cells. Indeed, Vα24+ NKT cells (not the Vα24− fraction) killed purified autologous myeloma cells (CD138+ve) but not nonmyeloma cells (CD138-ve) from the same tumor bed (Fig. 6 B). Importantly, killing of primary myeloma cells by activated purified NKT cells did not require the addition of exogenous α-GalCer to the targets in the cytolytic assay as was the case with cell lines, although an increase in killing was noted with the addition of exogenous α-GalCer (Fig. 6 B). Killing of tumor cells was inhibited by preincubation of targets with anti-human CD1d antibody (unpublished data).
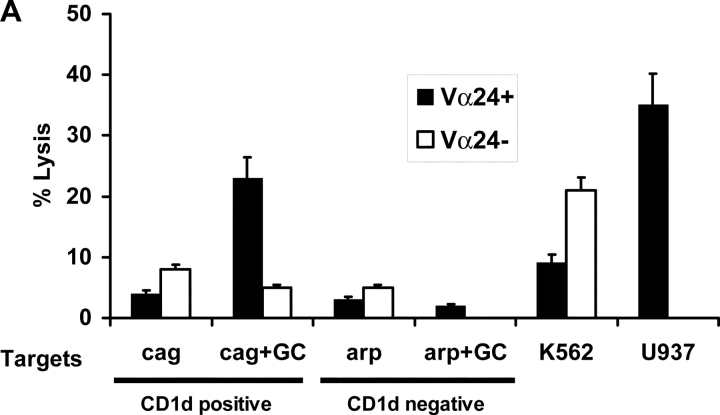
Figure 6.
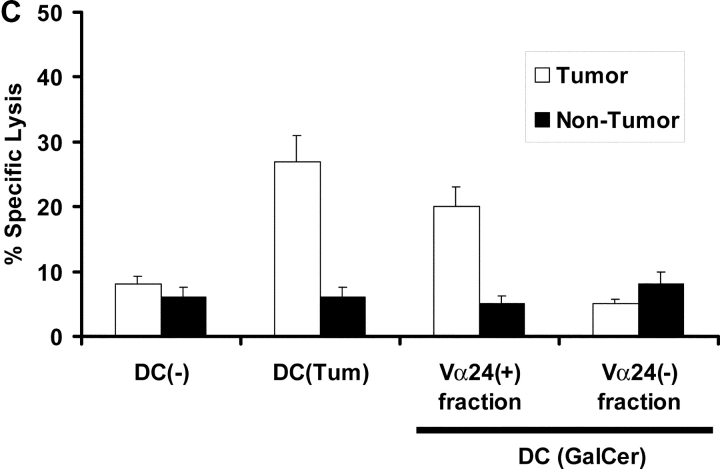
Cytolytic function of NKT cells from patients with gammopathies. (A) Lysis of CD1d positive (cag) or negative (arp) myeloma cell lines (the lines were tested with or without in vitro pulsing with α-GalCer [+GC]), by Vα24 positive or negative fractions of cultures after in vitro expansion with α-GalCer pulsed DCs. Additional positive control targets were U937 histiocytic lymphoma (NKT sensitive) and K562 erythroleukemia cells (NK sensitive). (B) Killing of autologous primary tumor cells (CD138 positive) versus nontumor cells (CD138 negative) in the tumor bed, by Vα24 positive or negative fraction of cells expanded using α-GalCer (GC) pulsed DCs (versus unpulsed DCs) from a patient with nonprogressive myeloma. The tumor cells were tested without or with supplementation with α-GalCer (GC) during the lytic assay. Additional control targets were U937 histiocytic lymphoma and K562 erythroleukemia cells. Data are representative of similar experiments on two patients. (C) Killing of autologous primary tumor cells (CD138+ve) versus nontumor cells (CD138-ve) from the tumor bed of a patient with progressive myeloma. Killing was measured with bulk T cells expanded using DC only (DC(−)) and DCs loaded with autologous tumor (DC-tum), and for the latter, Vα24 positive or negative fractions from cultures expanded using α-GalCer pulsed DCs (DC(Gal-Cer)).
In one of the patients with progressive myeloma, adequate numbers of expanded Vα24+ NKT and autologous tumor cells were available to test killing of fresh autologous tumor from a clinically progressive patient. In this patient, Vα24+, but not the negative fraction could mediate killing of autologous tumor cells, to a similar extent to the killing by bulk T cells generated using tumor cell–loaded DCs (Fig. 6 C). Killing of primary tumor cells by the Vα24+ fraction again did not require exogenous α-GalCer, and nontumor cells from the same tumor bed were not killed (Fig. 6 C). Killer T cells generated using tumor loaded DCs are distinct from CD1d restricted NKT cells and mediate lytic activity in a MHC class I–restricted fashion, as shown previously (11). These initial studies suggest that the NKT cells in patients with progressive myeloma are dysfunctional, lacking rapid effector function to ligand specific stimulation when studied directly from patients, but capable of rescue by antigen presenting DCs to expand and to lyse autologous tumor cells.
Discussion
Pioneering studies with spontaneous as well as carcinogen induced tumors have suggested a role for T lymphocytes, IFN-γ, NK, and NKT cells in immune-surveillance and “immune-editing” of cancers in mice (8–10, 33, 34). However, the clinical picture of stable versus progressive clonal expansions of transformed cells (as with MGUS/myeloma) has been difficult to model in mice, although stable clonal expansions of transformed cells are now increasingly apparent in healthy humans (2). In this paper we have shown that clinical progression in patients with monoclonal gammopathies is associated with the loss of ligand-dependent IFN-γ production by invariant NKT cells. In contrast, there was no detectable difference in the antitumor killer T cell function between patients with progressive or nonprogressive tumors. The observed NKT cell dysfunction could however be restored, with the induction of strong lytic activity against autologous tumor and ligand-dependent IFN-γ secretion in vitro. These data suggest that NKT effector function represents an important host factor maintaining control of tumor growth in vivo in patients with clinically stable gammopathies. IFN-γ production by these innate lymphocytes may also contribute to growth control of myeloma by nonimmune mechanisms, for example by mediating angiostasis (35), controlling tumor growth via decreased IL-6 or STAT-3–mediated transcription (36, 37), and inhibiting osteoclastogenesis (38), all features of progressive myeloma.
The loss of ligand and NKT dependent IFN-γ production in progressive myeloma is not simply due to the loss of invariant NKT cells, as they were detectable by flow cytometric assay with lower sensitivity than the ELISPOT assay. The presence of these cells in patients with progressive myeloma is also supported by the observation that they can be readily expanded using α-GalCer pulsed mature DCs. Another possibility, that we favor, is that the observed loss of rapid effector function in fresh NKT cells in these patients reflects the induction of NKT cell dysfunction in association with tumor progression. Recent studies in mice suggest that the presentation of α-GalCer by non-DCs in vivo may lead to the induction of NKT cell dysfunction (39). Therefore the presentation of tumor derived glycolipids by tumors themselves, or by other APCs in the tumor bed (as opposed to DCs), could lead to NKT cell dysfunction with tumor progression in vivo. Glycolipids shed from these tumor cells in vivo may also inhibit NKT cell function (40). In other words, a critical step in the transition to progressive tumors may be an acquired capacity of the tumor cells to disable the NKT arm of host resistance. Recent studies have suggested functionally distinct subsets among NKT cells (28, 41). However this is unlikely to account for our findings, as both subsets identified in these studies predominantly make IFN-γ. Further studies are needed to characterize both the molecular nature of the glycolipid ligands expressed by these tumor cells in vivo, and the functional aspects of tumor specific NKT cells, particularly for potential tumor promoting properties in response to tumor derived glycolipids (21).
Although IFN-γ production by freshly isolated NKT cells was deficient in progressive myeloma, these cells could be expanded after in vitro stimulation with α-GalCer pulsed DCs. Also the expanded NKT cells were functional and able to secrete IFN-γ. However, this expansion was reduced compared with NKT cells from MGUS or healthy donors. Diminished expansion of IFN-γ producing NKT cells has also been noted in some patients with advanced cancer, although DCs were not used in these studies and cytokine production was only tested after prior in vitro culture (24, 25).
To our knowledge, this is the first report to correlate NKT cell function against fresh autologous tumor with clinical outcome in humans. NKT cells may mediate immune control of tumors both via direct cytotoxicity, as well as indirectly via release of cytokines and enhancement of NK or T cell function (18, 22, 42, 43). Although the existing data for the lysis of tumor cells by human NKT cells is largely limited to tumor cell lines, our data extend it to fresh uncultured autologous tumor cells (44), including to autologous myeloma. Importantly, autologous nontumor cells in the tumor bed were not killed, which may help allay some of the concerns about potential toxicity if one would attempt to enhance NKT function therapeutically with DCs in myeloma. The marked difference in CD1d expression between cell lines and primary tumors in our disease model also urges the need to test CD1d expression in other primary human tumors and explore primary uncultured tumor instead of tumor cell lines as targets in immune assays testing antitumor NKT function.
Taken together, our data support the hypothesis that NKT effector function may play a role in the control of malignant growth of transformed plasma cells in gammopathies. This would extend the putative role of NKT cells from immune surveillance (34) to immune control of malignant behavior of cancer cells in vivo. This is also distinct from the “immune editing” of tumors described in mice (8), as progressive tumors remain quite sensitive to NKT cells activated ex vivo. These data provide impetus for clinical studies to boost or restore NKT cell function in myeloma. Measurement of NKT cell function may be a useful predictor of clinical outcome in these patients.
Acknowledgments
We thank Ralph M. Steinman for his mentorship and critical reading of this manuscript, and all our patients for their support and encouragement of this research.
Supported in part by funds from the National Institutes of Health (CA81138, CA84512, MO-1 RR00102), New York Community Trust, Cancer Research Institute, Irma T. Hirschl Foundation, Fund to Cure Myeloma, and DRCRF/Eli Lilly clinical investigator award from the Damon Runyon Cancer Research Fund. M.V. Dhodapkar is an Irene Diamond Assistant Professor in Immunology and this work is also supported in part by funds from the Irene Diamond Foundation.
Footnotes
Abbreviations used in this paper: α-GalCer, α galactosylceramide; DC, dendritic cell; MGUS, monoclonal gammopathy of undetermined significance; NKT, natural killer T; NPM, nonprogressive myeloma; PM, progressive myeloma; SEA, staphylococcal enterotoxin A.
References
- 1.Bernards, R., and R.A. Weinberg. 2002. A progression puzzle. Nature. 418:823. [DOI] [PubMed] [Google Scholar]
- 2.Rawstron, A.C., M.J. Green, A. Kuzmicki, B. Kennedy, J.A. Fenton, P.A. Evans, S.J. O'Connor, S.J. Richards, G.J. Morgan, A.S. Jack, and P. Hillmen. 2002. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood. 100:635–639. [DOI] [PubMed] [Google Scholar]
- 3.Kyle, R.A., and S.V. Rajkumar. 1999. Monoclonal gammopathies of undetermined significance. Hematol. Oncol. Clin. North Am. 13:1181–1202. [DOI] [PubMed] [Google Scholar]
- 4.Tricot, G. 2000. Multiple myeloma and related plasma cell disorders. Hematology: Principles and Practice. R. Hoffman, editor. Churchill Livingstone, New York. 1398–1415.
- 5.Fonseca, R., R.J. Bailey, G.J. Ahmann, S.V. Rajkumar, J.D. Hoyer, J.A. Lust, R.A. Kyle, M.A. Gertz, P.R. Greipp, and G.W. Dewald. 2002. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 100:1417–1424. [PubMed] [Google Scholar]
- 6.Kuehl, W.M., and P.L. Bergsagel. 2002. Multiple myeloma: evolving genetic events and host interactions. Nat. Rev. Cancer. 2:175–187. [DOI] [PubMed] [Google Scholar]
- 7.Zhan, F., J. Hardin, B. Kordsmeier, K. Bumm, M. Zheng, E. Tian, R. Sanderson, Y. Yang, C. Wilson, M. Zangari, et al. 2002. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 99:1745–1757. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran, V., H. Ikeda, A.T. Bruce, J.M. White, P.E. Swanson, L.J. Old, and R.D. Schreiber. 2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 410:1107–1111. [DOI] [PubMed] [Google Scholar]
- 9.Smyth, M.J., N.Y. Crowe, and D.I. Godfrey. 2001. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int. Immunol. 13:459–463. [DOI] [PubMed] [Google Scholar]
- 10.Street, S.E., J.A. Trapani, D. MacGregor, and M.J. Smyth. 2002. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J. Exp. Med. 196:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhodapkar, M.V., J. Krasovsky, and K. Olson. 2002. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong tumor specific cytolytic responses to autologous tumor loaded dendritic cells. Proc. Natl. Acad. Sci. USA. 99:13009–13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey, D.I., K.J. Hammond, L.D. Poulton, M.J. Smyth, and A.G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today. 21:573–583. [DOI] [PubMed] [Google Scholar]
- 13.Benlagha, K., and A. Bendelac. 2000. CD1d-restricted mouse V alpha 14 and human V alpha 24 T cells: lymphocytes of innate immunity. Semin. Immunol. 12:537–542. [DOI] [PubMed] [Google Scholar]
- 14.Park, S.H., and A. Bendelac. 2000. CD1-restricted T-cell responses and microbial infection. Nature. 406:788–792. [DOI] [PubMed] [Google Scholar]
- 15.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 16.Toura, I., T. Kawano, Y. Akutsu, T. Nakayama, T. Ochiai, and M. Taniguchi. 1999. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J. Immunol. 163:2387–2391. [PubMed] [Google Scholar]
- 17.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, H. Sato, E. Kondo, M. Harada, H. Koseki, T. Nakayama, et al. 1998. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA. 95:5690–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metelitsa, L.S., O.V. Naidenko, A. Kant, H.W. Wu, M.J. Loza, B. Perussia, M. Kronenberg, and R.C. Seeger. 2001. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J. Immunol. 167:3114–3122. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa, R., K. Motoki, H. Ueno, R. Iijima, H. Nakamura, E. Kobayashi, A. Shimosaka, and Y. Koezuka. 1998. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res. 58:1202–1207. [PubMed] [Google Scholar]
- 20.Moodycliffe, A.M., D. Nghiem, G. Clydesdale, and S.E. Ullrich. 2000. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1:521–525. [DOI] [PubMed] [Google Scholar]
- 21.Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D.D. Donaldson, D.P. Carbone, W.E. Paul, and J.A. Berzofsky. 2000. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515–520. [DOI] [PubMed] [Google Scholar]
- 22.Kawano, T., T. Nakayama, N. Kamada, Y. Kaneko, M. Harada, N. Ogura, Y. Akutsu, S. Motohashi, T. Iizasa, H. Endo, et al. 1999. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 59:5102–5105. [PubMed] [Google Scholar]
- 23.Nieda, M., A. Nicol, Y. Koezuka, A. Kikuchi, T. Takahashi, H. Nakamura, H. Furukawa, T. Yabe, Y. Ishikawa, K. Tadokoro, and T. Juji. 1999. Activation of human Valpha24NKT cells by alpha-glycosylceramide in a CD1d-restricted and Valpha24TCR-mediated manner. Hum. Immunol. 60:10–19. [DOI] [PubMed] [Google Scholar]
- 24.Yanagisawa, K., K. Seino, Y. Ishikawa, M. Nozue, T. Todoroki, and K. Fukao. 2002. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J. Immunol. 168:6494–6499. [DOI] [PubMed] [Google Scholar]
- 25.Tahir, S.M., O. Cheng, A. Shaulov, Y. Koezuka, G.J. Bubley, S.B. Wilson, S.P. Balk, and M.A. Exley. 2001. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J. Immunol. 167:4046–4050. [DOI] [PubMed] [Google Scholar]
- 26.Dhodapkar, K., J. Krasovsky, B. Williamson, and M. Dhodapkar. 2002. Anti-tumor monoclonal antibodies enhance cross presentation of cellular antigens and the generation of tumor specific killer T cells by dendritic cells. J. Exp. Med. 195:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii, S.I., K. Shimizu, R.M. Steinman, and M.V. Dhodapkar. 2003. Detection and activation of human V alpha 24+ natural killer T cells using alpha galactosyl ceramide pulsed dendritic cells. J. Immunol. Methods. 272:147–159. [DOI] [PubMed] [Google Scholar]
- 28.Gumperz, J.E., S. Miyake, T. Yamamura, and M.B. Brenner. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhodapkar, M.V., R.M. Steinman, M. Sapp, H. Desai, C. Fossella, J. Krasovsky, S.M. Donahoe, P.R. Dunbar, V. Cerundolo, D.F. Nixon, and N. Bhardwaj. 1999. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Invest. 104:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandberg, J.K., N.M. Fast, E.H. Palacios, G. Fennelly, J. Dobroszycki, P. Palumbo, A. Wiznia, R.M. Grant, N. Bhardwaj, M.G. Rosenberg, and D.F. Nixon. 2002. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 76:7528–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Exley, M.A., S.M. Tahir, O. Cheng, A. Shaulov, R. Joyce, D. Avigan, R. Sackstein, and S.P. Balk. 2001. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J. Immunol. 167:5531–5534. [DOI] [PubMed] [Google Scholar]
- 32.van der Vliet, H.J., N. Nishi, Y. Koezuka, B.M. von Blomberg, A.J. van den Eertwegh, S.A. Porcelli, H.M. Pinedo, R.J. Scheper, and G. Giaccone. 2001. Potent expansion of human natural killer T cells using alpha-galactosylceramide (KRN7000)-loaded monocyte-derived dendritic cells, cultured in the presence of IL-7 and IL-15. J. Immunol. Methods. 247:61–72. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan, D.H., V. Shankaran, A.S. Dighe, E. Stockert, M. Aguet, L.J. Old, and R.D. Schreiber. 1998. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 95:7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowe, N.Y., M.J. Smyth, and D.I. Godfrey. 2002. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 196:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coughlin, C.M., K.E. Salhany, M.S. Gee, D.C. LaTemple, S. Kotenko, X. Ma, G. Gri, M. Wysocka, J.E. Kim, L. Liu, et al. 1998. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 9:25–34. [DOI] [PubMed] [Google Scholar]
- 36.Catlett-Falcone, R., T.H. Landowski, M.M. Oshiro, J. Turkson, A. Levitzki, R. Savino, G. Ciliberto, L. Moscinski, J.L. Fernandez-Luna, G. Nunez, et al. 1999. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 10:105–115. [DOI] [PubMed] [Google Scholar]
- 37.Portier, M., X.G. Zhang, E. Caron, Z.Y. Lu, R. Bataille, and B. Klein. 1993. Gamma-interferon in multiple myeloma: inhibition of interleukin-6 dependent myeloma cell growth and down-regulation of IL-6 receptor expression in vitro. Blood. 81:3076–3082. [PubMed] [Google Scholar]
- 38.Takayanagi, H., K. Ogasawara, S. Hida, T. Chiba, S. Murata, K. Sato, A. Takaoka, T. Yokochi, H. Oda, K. Tanaka, et al. 2000. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 408:600–605. [DOI] [PubMed] [Google Scholar]
- 39.Fujii, S., K. Shimuzu, M. Kronenberg, and R.M. Steinman. 2002. Prolonged interferon-g producing NKT responses induced with a-galactosyl ceramide loaded dendritic cells. Nat. Immunol. 3:867–874. [DOI] [PubMed] [Google Scholar]
- 40.Sriram, V., S. Cho, P. Li, P.W. O'Donnell, C. Dunn, K. Hayakawa, J.S. Blum, and R.R. Brutkiewicz. 2002. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc. Natl. Acad. Sci. USA. 99:8197–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee, P.T., K. Benlagha, L. Teyton, and A. Bendelac. 2002. Distinct functional lineages of human V(alpha)24 natural killer T cells. J. Exp. Med. 195:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicol, A., M. Nieda, Y. Koezuka, S. Porcelli, K. Suzuki, K. Tadokoro, S. Durrant, and T. Juji. 2000. Human invariant valpha24+ natural killer T cells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology. 99:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth, M.J., E. Cretney, K. Takeda, R.H. Wiltrout, L.M. Sedger, N. Kayagaki, H. Yagita, and K. Okumura. 2001. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nieda, M., A. Nicol, Y. Koezuka, A. Kikuchi, N. Lapteva, Y. Tanaka, K. Tokunaga, K. Suzuki, N. Kayagaki, H. Yagita, et al. 2001. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 97:2067–2074. [DOI] [PubMed] [Google Scholar]