Abstract
Experiments in nonobese diabetic (NOD) mice that lacked expression of glutamic acid decarboxylase (GAD) in β cells have suggested that GAD represents an autoantigen essential for initiating and maintaining the diabetogenic immune response. Several attempts of inducing GAD-specific recessive tolerance to support this hypothesis have failed. Here we report on successful tolerance induction by expressing a modified form of GAD under control of the invariant chain promoter resulting in efficient epitope display. In spite of specific tolerance insulitis and diabetes occurred with normal kinetics indicating that GAD is not an essential autoantigen in the pathogenesis of diabetes.
Keywords: diabetes, NOD mouse, tolerance, GAD65, autoimmunity
Introduction
Failure of self-tolerance can result in tissue-specific autoimmunity. Various types of experimental autoimmune phenomena can be elicited in rodents by deliberate immunization with particular self-antigens, suggesting that a breakdown of tolerance to a single self-antigen can be sufficient to trigger an autoimmune organ destruction. However, it is still a matter of debate in how far such a scenario is representative of the natural etiology of spontaneous autoimmune diseases, i.e., whether one distinct self-antigen is obligatorily involved in the initiation and, perhaps, even the perpetuation of autoimmune tissue destruction. The eventual existence of such essential autoantigens is of great clinical importance, as they represent promising targets for therapeutic intervention with specific, tolerogenic regimens that avoid the side-effects of nonspecific immune suppressive strategies. The definition of an essential autoantigen can be based on two experimentally testable predictions: (a) specific induction of tolerance to the respective self-antigen and/or (b) ablation of expression of the antigen in the target organ would prevent autoimmunity. Here, we have used the NOD mouse model of insulin-dependent diabetes mellitus (IDDM),* a well characterized model of spontaneous autoimmunity, to address the role of glutamic acid decarboxylase (GAD) 65 as an autoantigen in IDDM.
IDDM results from T cell–mediated destruction of insulin producing pancreatic β cells (1). The NOD mouse is a widely used animal model of IDDM sharing major disease characteristics with the human disease (2). Because CD4+T cell responses and auto-antibodies against GAD65 are among the first to be detected in NOD mice (3, 4) and humans (5) it was suggested that GAD65 is a major autoantigen in the initiation of the immune response against β cells. In fact the disease could be prevented in NOD mice by intrathymic (4), intravenous (3), nasal (6), and oral (7) administration of GAD65. The mechanisms of tolerance remained elusive in these experiments, and protection may have resulted from induction of regulatory T cells (dominant tolerance) rather than elimination of GAD-reactive T cells (recessive tolerance; reference 8). Additional strong evidence indicating that GAD may be an essential autoantigen was provided by Yoon and coworkers, who prevented IDDM by silencing GAD expression with a β cell specific antisense approach (9). Strikingly, GADless islets transplanted into diabetic animals were protected from autoimmune destruction suggesting that GAD is not only essential for initiating but also for maintaining an ongoing anti-β cell immune response (9). The various findings have led to an initiative for clinical trials using GAD65-specific tolerance.
Nevertheless the results of Yoon et al. have met with considerable skepticism (9, 10), e.g., it was argued that absence of GAD expression may have rendered the β cells resistant to apoptosis or may have induced an unknown protective metabolic effect. Another finding that argues against an essential role of GAD in IDDM is that diabetes developed normally in GAD65 knockout NOD mice (11), although the interpretation of this observation in complicated by the fact that GAD65 shares several epitopes with GAD67. Several attempts to induce specific tolerance by transgenic overexpression of GAD65 have failed and thus could not address the question whether or not GAD represents an essential autoantigen (12, 13). This failure could be due to possible resistance of NOD mice to tolerance induction (13, 14) or the fact that GAD epitopes were insufficiently presented (12). It was the aim of the present study to express a suitably modified GAD construct with enhanced routing to the class II MHC loading pathway in order to induce GAD-specific tolerance.
Materials and Methods
Mice
NOD/Ltj mice were purchased from The Jackson Laboratory or bred in our facility. Diabetes incidence in females was 90%. NOR, C57/B6, and Balb/c were bred and maintained in the DFCI animal facility. For timed mating experiments animals were mated and carefully examined for plugs. The morning when plugs were detected was counted as day 0.5. Diabetes development was monitored by tail bleeding analyzed with Accu-Chek Advantage device (Roche Diagnostics). Two subsequent measurements >200 mg/dl were considered to indicate IDDM. All animal experiments were performed according to NIH guidelines and experimental protocols were approved by the animal care and user committee of the DFCI.
Antibodies
Antibodies used: anti-CD4-APC, anti-CD4-biotin, anti-CD8-PE, anti-CD8-biotin, anti-CD3-biotin, anti-CD19-PE, anti-B220-Cyc, anti-TCRβ-PE, anti-CD11b-PE, anti-GR1-PE, and anti-CD11c-biotin (all BD Biosciences). Cells were analyzed on a four color FACSCalibur™ (Becton Dickinson).
Generation of GAD65 Transgenic Mice
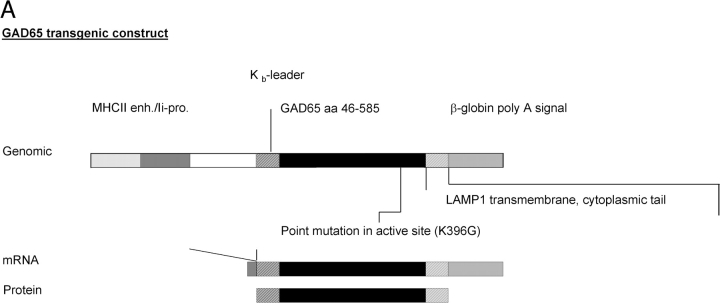
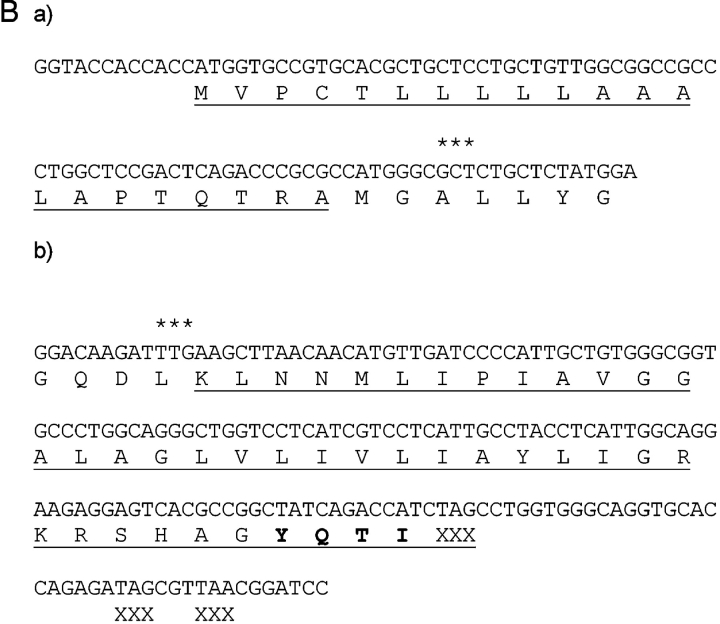
The H-2Kb-leader was fused to GAD65, from which the first 44 amino acids were deleted. The H-2Kb-leader GAD65 boundaries are shown in Fig. 1 B. The H-2Kb-leader was generated as double stranded oligonucleotide and cloned in via KpnI, NcoI restriction sites. The plasmid containing murine GAD65 was kindly provided by A. Lehuen (INSERM, Paris, France). The active site of GAD65 was inactivated by site directed mutagenesis (K396G) and the carboxyterminal end was fused to the LAMP1 cytoplasmic tail containing the Tyr-x-x-hydrophob motif for routing the protein into late endosomes, lysosomes (Fig. 1 B; reference 15). The Lamp1-GAD65 boundaries are shown in Fig. 1 B. The fragment was cloned in via HindIII, BamHI restriction sites. The coding sequence was followed by an β-globin polyadenylation signal. Expression of the construct was driven by a hybrid invariant chain promotor (16). The purified DNA was injected into fertilized NOD oocytes and reimplanted into foster mothers. Offspring was screened by PCR of genomic DNA using primers GAD65 (5′-ATGGTGTTTGATGGGAAGCCTC-3′) and LAMPrv (5′-TGCAAAGCTTATCGATGGATCCGTTAACGCTATCTCTGGTGCA-CCTGCCCAC-3′) for GAD65 transgenic animals.
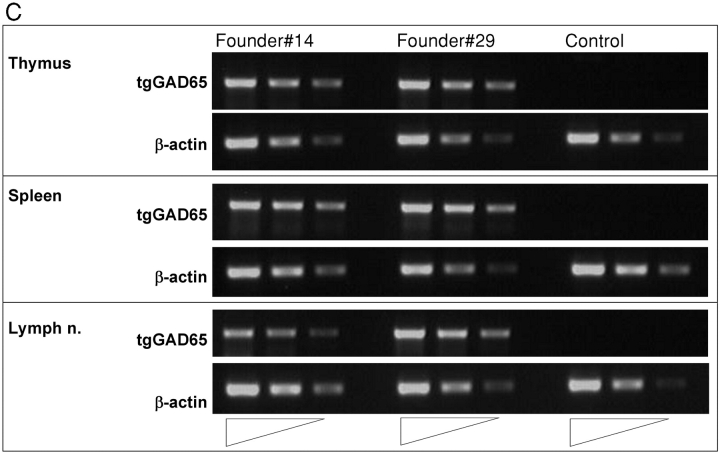
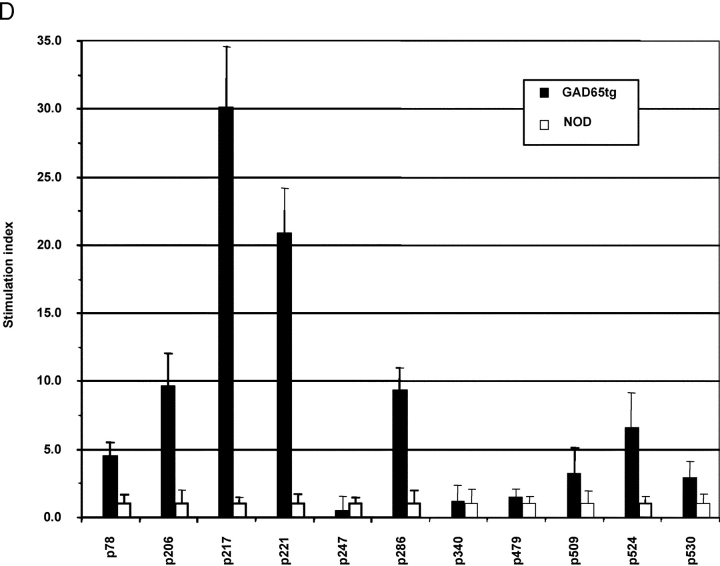
Figure 1.
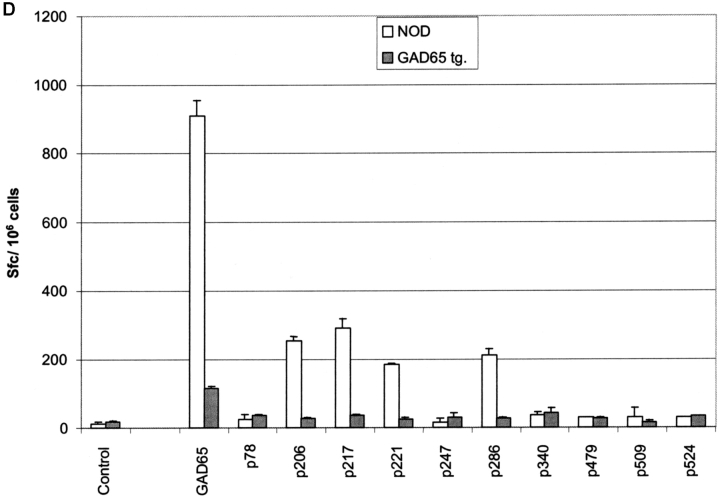
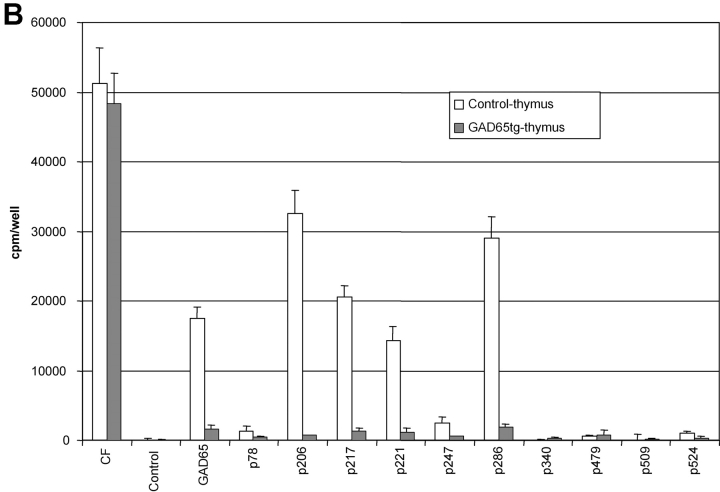
Characterization of GAD65 transgenic mice. (A) Diagram of the transgenic GAD65 construct. (B) Details of the transgenic construct being used. (a) Kb-leader (underlined) fusioned with amino acid 46 (***) of the GAD65 protein. (b) Amino acid 585 (***) of GAD65 fusioned with the transmembrane, cytoplasmic tail of the LAMP1 protein (underlined). The lysosomal targeting signal is in boldface. XXX indicate stop codons. (C) Expression of GAD65 mRNA in lymphoid compartments of 6-wk-old female NOD or GAD65-tg. mice by reverse transcriptase PCR using fivefold serial dilutions. β-actin cDNA was amplified as an internal control. (D) Epitope display of thymic dendritic cells. 11 different GAD65 reactive T cell hybridomas recognizing I-Ag7 restricted GAD65 epitopes were tested against thymic DCs from GAD65tg. (black bars) and NOD control mice (open bars). Stimulation indices were calculated as cpm sample/cpm control. Background values were between 300 cpm to 3,300 cpm/well depending on the hybridoma used. Stimulation indices for peptide pulsed DCs were between 10–70 for the hybridomas shown.
Expression Analysis
Total RNA was prepared from frozen tissue samples using TRIZOL (Life Technologies). 2 μg total RNA was reverse transcribed using oligo-dT12–15 priming. Fivefold serial dilutions of cDNA were amplified using forward primer (5′-AGACACACAGCAGCAGCAGCAGC-3′) and reverse primer (5′-CCCAATTATACTCTTGAAGAAGC-3′) for GAD65 transgenic animals. Primers were spanning a 1.3 kb intron. β-actin cDNA was amplified as an internal control using intron spanning primers (5′-TGGAATCCTGTGGCATCCATGAAAC-3′) and (5′-TAAAACGCAGCTCAGTAACAGTCCG-3′).
Generation of Recombinant GAD65
Full length murine GAD65 cDNA (with mutated active site) was fused carboxyterminally to a 6*His tag and cloned into the pET3a-expression vector (Novagen). The vector was transformed into BL21DE3 Lys S cells. GAD65 expression was induced by 4 mM IPTG. Baculovirus derived full-length murineGAD65–6*His was produced according to a published protocol with a baculovirus obtained from N. Sarvetnick (Scripps Research Institute, La Jolla, CA; reference 17). Insect cells and bacteria were lysed in buffer containing Guanidin-HCl buffer and purified over Ni-NTA agarose (QIAGEN) according to standard protocols. Protein was further purified on a 10% preparative SDS-page gel and protein band was made visible with negative Zn-Immidazol staining (Bio-Rad Laboratories). Recombinant protein was electroeluted. Protein solution was successively refolded by dialysis against refolding buffer (5 mM reduced glutathione, 25 mM Tris-HCl pH 7.4, 192 mM glycine, 0.02% SDS). Concentration of SDS was subsequently lowered to 0.01 and 0.001%. The protein was then extensively dialyzed against PBS. Protein solution was then incubated with End-X B15 beads (Associates of Cape Cod, MA) to remove endotoxin. Finally solution was filtered and tested for endotoxin content using LAL-lysate (Associates of Cape Cod). Endotoxin concentration was below 0.3 U/ml. Quality and quantity of protein was tested with SDS page gels, Coomassie stains, and Western blots using anti-GAD6 monoclonal antibody.
Epitope Display
Thymi or spleens of 4-wk-old animals were minced with scissors and digested with collagenase/dispase (0.2 mg/ml collagenase D, 0.2 mg/ml dispase I; Roche Diagnostics). Dendritic cells (DCs) were enriched by density centrifugation on 16% (wt/wt) accudens (Accurate Chemical & Scientific Corp.), stained with biotin-antiCD11c and positively enriched using SA-Macs beads (Miltenyi Biotech). 18 different GAD65 reactive T cell hybridomas recognizing 11 I-Ag7 restricted GAD65 epitopes (p78, p206, p217, p221, p246, p286, p340, p479, p509, p524, p530) were tested against the various DC preparations. We used two hybridomas against some determinants and show the one with the best signal to noise ratio in Fig. 1 D. GAD65 peptides used: p78–97 (KPSNSPKGDVNYAFLHATDL), p206–220 (TYEIAPVFVLLEYVT), p217–236 (EYVTLKKMREIIGWPGGSGD), p221–240 (LKKMREIIGWPGGSGDGIFS), p246–266 (SNMYAMLIARYKMFPEVKEKG), p286–300 (KKGAAAIGIGTDSVI), p340–359 (VYGAFDPLLAVADISKKYK), p479–493 (EYLYTIIKNREGYEM), p509–528 (VPPSLRTLEDNEERMSRLSK), p524–543 (SRLSKVAPVIKARMMEYGTT).
104 DCs were incubated for 48 h with 2 × 105 T cell hybridomas in RPMI, 10% FCS, 100 U/ml penicillin/100 μg/ml streptomycin, 55 μM 2-mercaptoethanol, and 0.3 mg/ml glutamine (P/S/M/G). The IL-2 amount in the supernatant was tested with a CTLL assay. Measurements were performed in triplicates and peptide-loaded (10 μg/ml) DCs were used as positive controls to test viability of the DC preparation and the responsiveness of the hybridoma. Stimulation indices were calculated as cpm sample/ cpm control.
Testing for Tolerance
4 × 105 cells of draining popliteal and inguinal lymph nodes of 10-wk-old NOD mice 8 d after immunization with GAD65 (E. coli derived) in CFA or IFA injected into the foot pad were incubated for 96 h with 10 μg/ml GAD65 (baculovirus derived) or GAD65 peptides (10 μg/ml) in HL1 medium (BioWhittaker) containing (P/S/M/G). Culture filtrate protein (CFP) was used as positive control in recall assays (provided by College of Veterinary Medicine and Biomedical Sciences, University of Colorado, produced through funds from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, contract NO1-AI-75320 entitled “Tuberculosis Research and Vaccine Testing”). Culture was pulsed for final 18 h with [3H]-thymidine and incorporated radioactivity was measured using scintillation fluid in a β-counter. Measurements were performed in triplicates. Stimulation indices were calculated as cpm sample/cpm control.
Tetramer Staining
The SC-2 drosophila cell line that produces Ag7-p286 molecules was kindly provided by Luc Teyton (Scripps Research Institute, La Jolla, CA). Monomers of Ag7-p286 were purified as described (18) and biotinylation of purified molecules was performed with Bir A enzyme (Avidity) according to the manufacturer's instructions. Biotinylated molecules were tetramerized at 4°C overnight using PE-labeled streptavidin (Biosource International).
Single cell suspensions from lymph nodes were incubated with 0.5 mg/ml of streptavidin and Fc block in FACS buffer for 1 h at room temperature (RT). Cells were then stained with PE-labeled Ag7-p286 tetramer (50 μg/ml) for 1 h at RT. For costaining of surface markers APC-anti-CD4, FITC-anti-CD44, from BD Biosciences were used, as well as PE-Cy7-anti-CD8 and PE-Texas Red-anti-CD45R (B220) from Caltag. Exclusion of dead cells was done by adding 0.1 μg/ml of Hoechst 33342 (Molecular Probes, Inc.). Flow cytometry was performed using a MoFlow instrument (DakoCytomation) and the data analyzed using Summit software (DakoCytomation). Analysis was gated (in dependent order) on a lymphocyte gate in FSC versus SSC-plot, a B220 negative and HOECHST negative gate, a CD4 positive and CD8 negative gate, and analyzed in a CD4/tetramer PE FACS-plot.
ELISPOT
5 × 105 draining lymph node cells were cultured in 200 μl HL-1 medium (P/S/M/G) for 18 h at 37°C in Immunospot M200 plates (Cellular Technologies Limited) coated with anti–IFN-γ mAB (R4–6A2; BD Biosciences). Plates were washed and incubated with biotin anti–IFN-γ (XMG1.2; BD Biosciences). Spots were developed using streptavidin-alkaline phosphatase (Mabtech), and NBT and BCIP were used as substrate (PromegaI). Spots were analyzed on a ELISPOT reader (Cellular Technologies Limited).
Measurement of insulin-autoantibodies was performed by radiobinding assay as recently described (19).
Transfer Experiments
Bone Marrow Transplants.
Bone marrow from 4–5-wk-old, female GAD65 transgenic and NOD control mice was depleted of erythrocytes by osmotic shock. T cells were removed using biotinylated anti-CD3, anti-CD4, and anti-CD8 followed by negative selection using streptavidin-Macs beads (AmCell). 3 × 106 T cell–depleted bone marrow cells were used to reconstitute 4-wk-old lethally irradiated (2 × 600 rads) female NOD mice. Proliferative response of draining lymph nodes cells 8 d after immunization with GAD65 in CFA were tested 8 wk after reconstitution.
Thymic Chimeras.
4-wk-old NOD mice were thymectomized. 2 wk later GAD65 transgenic or control thymi from 14-d-old embryos were transplanted under the kidney capsule. After another 2 wk mice were reconstituted with 3 × 106 T cell–depleted bone marrow cells from NOD-Thy1.1 mice. 8 wk later proliferative responses after immunization with GAD65 were analyzed.
Histology
Thin section from formaldehyde fixed organs embedded in paraffin were examined for the presence of insulitis after hematoxylin-eosin staining. Multiple sections were taken from different levels and graded. Grade 0 (normal islets), Grade 1 (mononuclear infiltrate largely in the periphery in less than 25% of the islet), Grade 2 (25% to 50% of the islet), Grade 3 (over 50% of the islet), and Grade 4 (small retracted islet with minor infiltrate). 32–64 islets were scored per animal.
Statistical Analysis
All results of epitope display, proliferation-assays, ELISPOT analysis, and tetramer-staining were analyzed by Student's t test. All differences reported in the results were significant (P < 0.05). The diabetes onset between transgenic and nontransgenic animals was compared by Kaplan-Meier Analysis.
Results
GAD Transgenic Mice.
GAD65 displays a number of particular features that may interfere with efficient presentation of GAD-derived epitopes and thus may impede efforts to experimentally induce specific tolerance. First, the presence of a strongly hydrophobic NH2-terminal sequence (20) may interfere with efficient degradation. As no epitopes were reported to derive from the NH2 terminus of GAD65 the amino-terminal region containing 45 mostly hydrophobic amino acids (3, 21–24) was deleted. Second, in vitro translation assays in the presence of pancreatic microsomes showed that GAD65 cannot translocate efficiently into the endoplasmic reticulum, most likely due to the lack of a leader sequence (unpublished data). We tested several leader constructs and found that amino-terminal fusion of the H-2Kb leader resulted in improved translocation (unpublished data). Next the enzymatically active site was destroyed by mutagenesis (K396G) to prevent metabolic effects of the transgene. Finally the construct was carboxy-terminally fused to the LAMP-1 signaling sequence to route the protein efficiently into the class II MHC peptide-loading pathway (15; Fig. 1 A). Expression of the modified sequence was controlled by a hybrid invariant chain promoter to achieve efficient expression in antigen presenting cells (16; Fig. 1 A).
Two independent GAD65 transgenic lines on the NOD background were established showing comparable mRNA expression in thymus, spleen, and lymph nodes (Fig. 1 C). Besides expression in lymphoid tissue, signals were detected in liver, lung, kidney, and brain (unpublished data). Epitope presentation by DCs from GAD65 transgenic mice was tested using a panel of 18 GAD-specific T cell hybridomas covering all 11 I-Ag7–restricted epitopes reported to date (3, 22–24). As shown in Fig. 1 D the epitopes p206, 217, 221, and 286 were efficiently presented by thymic DCs from GAD65 transgenic mice, and weaker stimulation was found for the epitopes p78 and p524. It is noteworthy that, in accord with data by others (22, 23), some of the epitopes that were not presented can only be defined by stimulating T cells with peptides but are not displayed when APCs such as NOD splenocytes or DCs are incubated with GAD65 protein. In the latter case only epitopes 206, 217, 221, and 286 are presented (unpublished data) and it remains to be shown that in vivo any of the other epitopes is even generated from endogenous GAD65 (24). Taken together, all epitopes that can reasonably be believed to be derived from GAD65 in vivo were presented by DCs in the GAD65 transgenic mice. In the GAD transgenic mice, total thymocytes and thymocyte subsets defined by CD4 and CD8 were as in nontransgenic littermate controls as were T and B lymphocytes, DCs, and macrophages in spleen and lymph node (unpublished data).
GAD65-specific Tolerance.
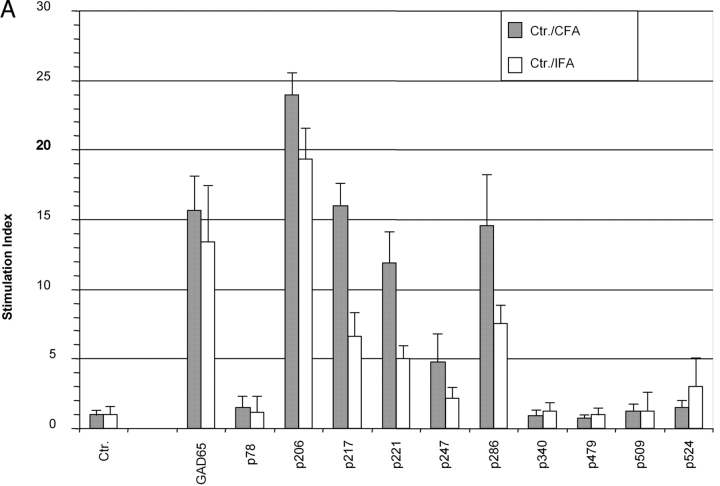
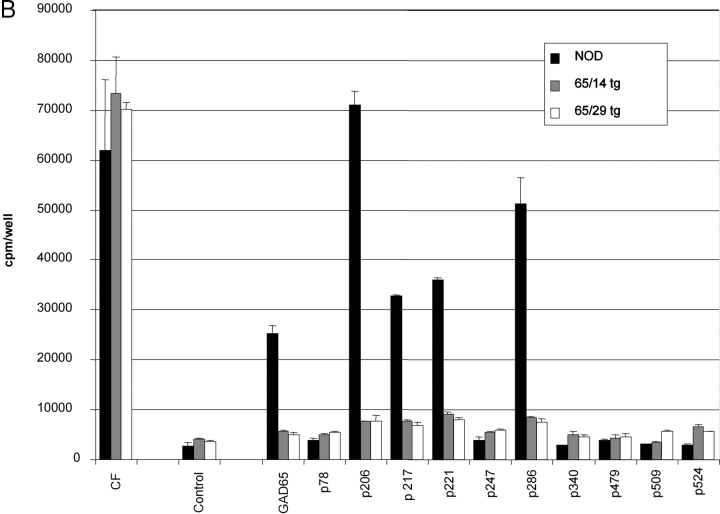
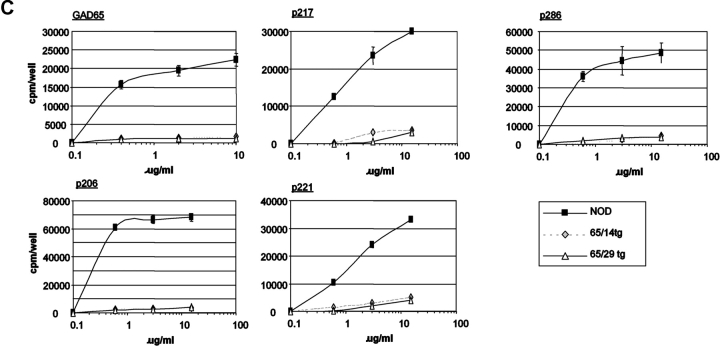
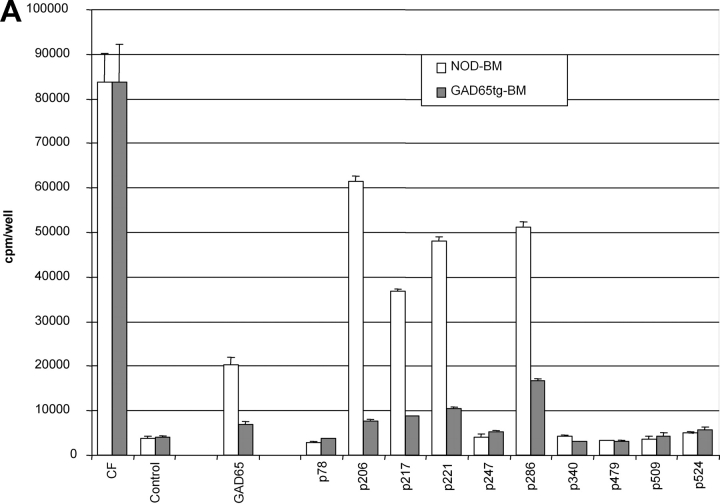
GAD65-specific tolerance was tested in nonprimed animals (proliferation-assay/ELISPOT/tetramer-staining) and after immunization with recombinant murine GAD65 produced in an E. coli system. Unprimed cells from spleen and pancreatic lymph nodes displayed no consistent response upon culture in the presence of GAD65 or its peptide epitopes as measured by proliferation or ELISPOT analysis. Similar difficulties in obtaining reproducible “spontaneous” responses of nonprimed T cells have been reported by other groups (25, 26). To achieve a more reliable assessment of GAD-specific T cell responses animals were immunized with recombinant GAD65 protein produced in an E. coli system. Proliferative recall responses were then tested using either GAD65 peptides or whole protein. GAD65 protein used for restimulation was produced in a baculoviral expression system to prevent recall responses to contaminants from the E. coli preparation used as an immunogen. In nontransgenic mice, the qualitative spectrum (responses against GAD65 protein and p206, p217, p221, p286) of the immune response was similar after immunization with GAD65 in either complete or incomplete Freund's adjuvants ruling out an alteration of the GAD65 epitope hierarchy by the use of agents stimulating APCs (Fig. 2 A). GAD65tg mice exhibited tolerance to the entire GAD65 protein as well as to all tested peptide epitopes (Fig. 2, B and C). Although GAD65-specific tolerance in NOD mice could only be induced by transgenic expression of a modified form of GAD65, tolerance was seen in recall assays using the entire, unmodified GAD65 protein, thereby excluding that the modifications altered epitopes that can be derived from the wild-type protein. No responses to epitopes p78, p246, p340, p479, p509, and p530 were detected in either immunized GAD transgenic or NOD mice indicating that these epitopes are only recognized after specific immunization/stimulation with the respective peptides but are not part of the normal T cell response to the GAD protein.
Figure 2.
T cell responses of GAD65 transgenic animals against GAD65 and its peptide epitopes. (A) Proliferation assay of draining lymph node cells of 10-wk-old NOD mice 8 d after immunization with GAD65 in complete (black bars) and incomplete Freund's adjuvants (open bars) into foot pads. Representative result from five independent experiments. (B and C) Proliferative response of draining lymph node cells of NOD control mice (black bars) and two GAD65 transgenic lines (gray bars: line 14; open bars: line 29) to GAD65 and its peptide epitopes (all 10 μg/ml) 8 d after immunization with GAD65 in CFA. CF, culture filtrate protein of M. tuberculosis as positive control. (C) Titration of the positive proliferative recall responses as seen in B. (▪ NOD controls; ⋄ GAD65tg. line 14; Δ GAD65tg. line 29).
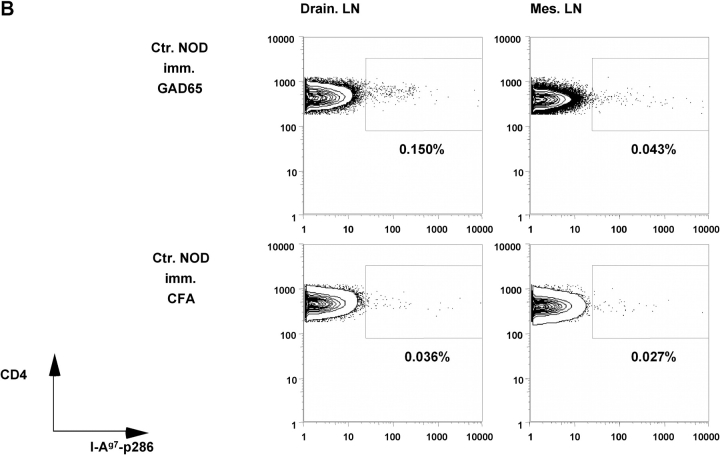
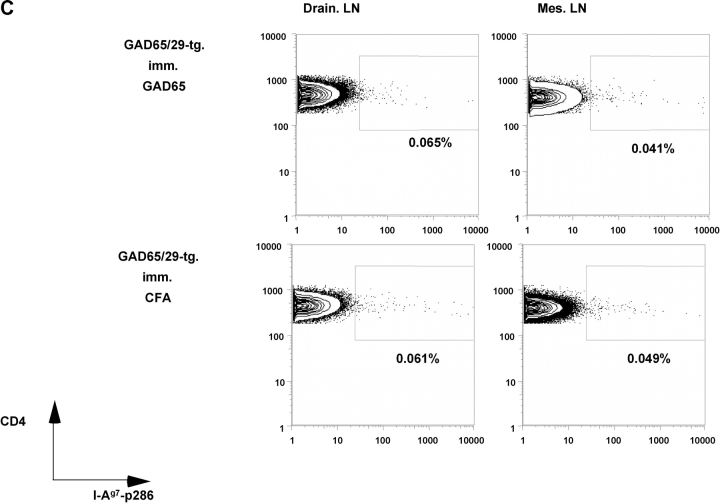
Staining with an I-Ag7-GAD65-p286 tetramer (18) of draining lymph nodes 8 d after immunization with GAD65 protein demonstrated the absence of p286-reactive cells in GAD transgenic mice but not in NOD control mice (Fig. 3, A–C) . Likewise no IFN-γ–producing cells were found in draining lymph nodes of immunized GAD65 transgenic mice by ELISPOT assays (Fig. 3 D). These results were reproducible in all 10 GAD65 transgenic mice that were tested.
Figure 3.
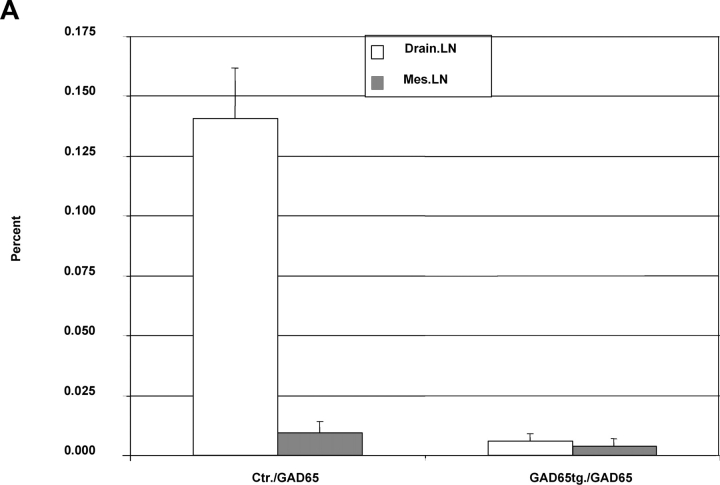
T cell responses of GAD65 transgenic animals against GAD65 and its peptide epitopes. (A) Percent of CD4+ cells from lymph nodes (draining lymph nodes: open bars; mesenteric lymph nodes: gray bars) of NOD control and GAD65tg. mice 8 d after immunization with GAD65 in CFA as measured by FACS® analysis. Percent values of background tetramer staining (animals just immunized with CFA [0.034–0.062%]) were subtracted from each sample (n = 4). (B) Representative FACS-plot of I-Ag7-p286 tetramer staining of lymph node cells from NOD control mice (B) and GAD65tg. mice (C) immunized with GAD65 in CFA (top two panels) or CFA alone (bottom two panels). (D) Number of IFN-γ–secreting cells from draining lymph nodes of NOD-control (open bars) and GAD65tg. mice (gray bars) after immunization with GAD65 as determined by ELISPOT analysis.
As all MHC class II–positive APC can express the modified form of GAD65 it was of interest to dissect the respective roles of hematopoietic APC versus thymic epithelial cells for tolerance induction. Transplantation of GAD65tg. bone marrow into irradiated NOD mice resulted in GAD65-specific tolerance indicating that expression by hematopoietic cells was sufficient for tolerance induction (Fig. 4 A). Likewise, transplantation of irradiated GAD65tg E14 fetal thymi into NOD mice and subsequent reconstitution with nontransgenic bone marrow resulted in GAD-specific tolerance (Fig. 4 B). This indicates that GAD expression by thymic epithelial cells suffices to induce tolerance in developing T cells.
Figure 4.
Cells mediating GAD65-specific tolerance. (A) NOD mice were lethally irradiated and reconstituted with bone marrow from NOD (open bars) or GAD65tg. mice (gray bars). Proliferative recall responses after immunization with GAD65. CF, culture filtrate protein of M. tuberculosis as positive control. Representative result out of three independent experiments. (B) Thymectomized NOD mice were engrafted with E14 fetal thymi from NOD (open bars) or GAD65tg. mice (gray bars) and, after ablative irradiation, reconstituted with NOD bone marrow. Proliferative recall responses after immunization with GAD65.
Insulitis/Diabetes.
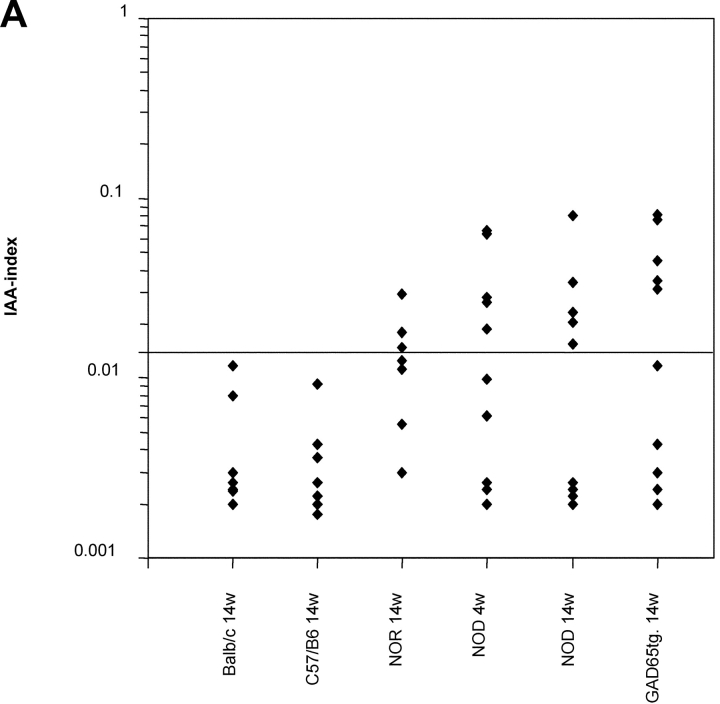
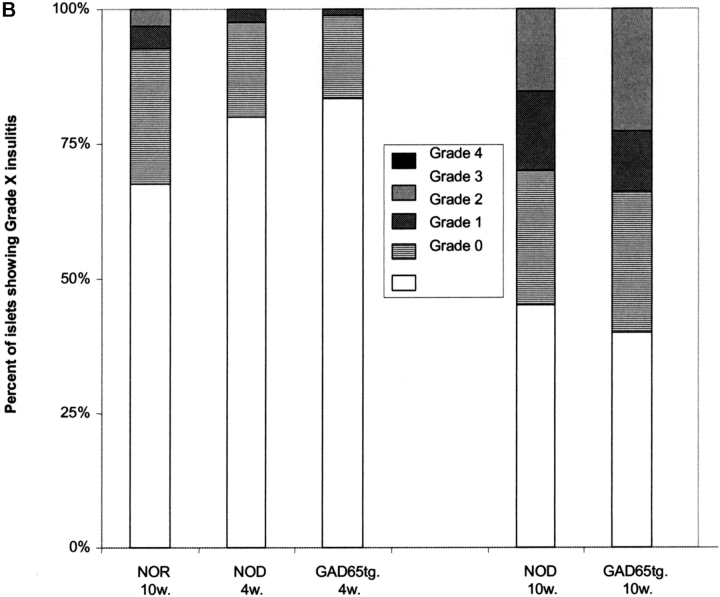
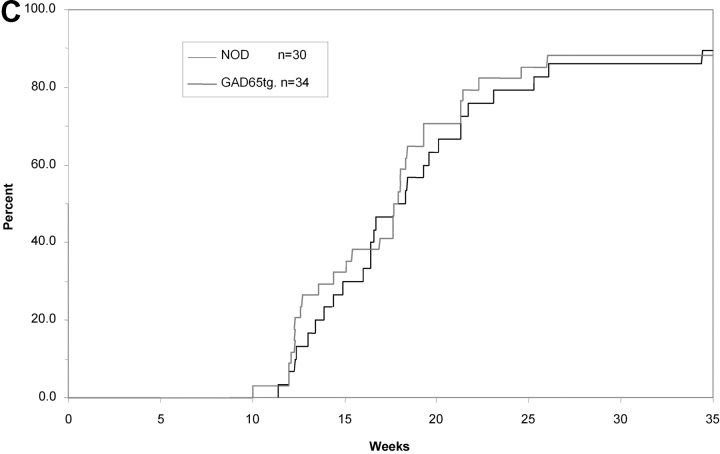
In GAD65 tolerant mice, we investigated anti-β cell immunity in order to find out whether recognition GAD represents an essential event for the development of diabetes. In spite of GAD-specific tolerance, titers of insulin-autoantibodies, an accepted marker of a spontaneous anti–β-cell immune response in the NOD mouse (19), were similar in 14-wk-old GAD65tg. and NOD control mice (Fig. 5 A). More importantly, GAD65tg mice exhibited a similar incidence and severity of insulitis as an early sign of the autoimmune disease at ten weeks of age (Fig. 5 B). Furthermore, GAD65 transgenic females developed IDDM with the same incidence and kinetics as female NOD mice (Fig. 5 C). The results were similar for two independent founder lines of GAD65 transgenic mice.
Figure 5.
Spontaneous anti-insulin immune response, insulitis, and IDDM in GAD65 transgenic mice. (A) Insulin-autoantibodies in 14-wk-old, female GAD transgenic, NOD mice as well as in controls (NOR, non obese diabetes resistant mice; C57/B6, Balb/c mice) measured by radiobinding assay. There was no statistical difference between 14w old NOD mice and GAD65-tg. animals. (B) Histological grading of insulitis in pancreas sections of 4 and 10 week old mice (n = 4 per group). (C) Cumulative incidence of development of IDDM in NOD mice (n = 30; black curve) and GAD65 transgenic mice (n = 34; gray curve). Data of two independent GAD65 founder lines were combined. There was no statistical difference in diabetes onset between transgenic and nontransgenic mice according to Kaplan-Meier Analysis.
We conclude therefore that GAD is not an essential autoantigen for either initiating or maintaining diabetes in NOD mice.
Discussion
The protocol described here, is the first to induce GAD-specific tolerance in NOD mice. These findings argue against the notion that NOD mice may be generally refractory to tolerance induction against autoantigens and that this is the reason why diabetes develops in these mice (13, 14). While it is difficult to rule out subtle differences in susceptibility to tolerance induction in NOD mice, expression of modified transgenic GAD65 resulted in a robust state of CD4+ T cell tolerance in this mouse strain, thereby enabling us to conclusively investigate the role of GAD as an autoantigen in the natural development of IDDM in NOD mice. Tolerance was demonstrated to the entire, unmodified GAD65 protein indicating tolerance to all epitopes that can be naturally derived from this protein. In spite of specific tolerance insulitis and diabetes occurred with normal kinetics indicating that GAD is not an essential autoantigen in the pathogenesis of diabetes. Although tolerance was demonstrated in proliferation assays, ELISPOT, and MHC II-tetramer staining, we cannot completely rule out that there were some GAD65-reactive, low-affinity T cells, which cannot be detected by our assays. According to the titration in Fig. 2 C, these cells would be of extremely low affinity and thus of questionable relevance. On the other hand, the NOD mouse contains a substantial number of highly reactive anti-GAD T cells as shown by ELISPOT and proliferation assays that were deleted in transgenic mice (12, 13). If T cells against GAD had a major role in the pathogenesis of IDDM in the NOD mouse one would expect these high affinity T cells to be of greater relevance than putative low affinity T cells. Yet deletion of these high affinity cells did not result in any delay of diabetes onset.
We also cannot rule out that the GAD transgenic mice still contain some class I MHC-restricted, GAD65-specific T cells as reported by Quinn (27). However, it is not clear at present whether such cells can be activated in vivo and transfer experiments failed to show that these cells cause diabetes. In any case, it is quite likely that such cells would be tolerized by the numerous cells in the GAD transgenic mice that express the protein intracellularly.
One needs to explain why on the one hand various regimens of administration of GAD (intrathymic, intranasal, oral, intravenous) resulted in delayed onset of diabetes (3, 4, 7, 28) while on the other hand tolerogenic expression of transgenic GAD had no effect on the onset of disease. We consider it likely that dominant tolerance is induced by the former approaches resulting in the generation of regulatory cells that may locally suppress immune responses against a variety of β cell–derived antigens in a bystander fashion. Importantly, such a modulation of diabetes on the basis of dominant tolerance toward GAD cannot be taken to indicate an essential role of GAD65 as target antigen in the natural disease process. In contrast, transgenic expression of GAD most likely resulted in deletional (recessive) tolerance, i.e., elimination of GAD-specific T cells rather than generation of regulatory. This conclusion is supported by the absence of detectable frequencies of tetramer-positive cells in draining lymph nodes of immunized GAD-transgenic animals. With regard to the possible induction of regulatory T cells in our transgenic mice, we did not observe suppression or delay of diabetes upon cotransfer of 2 × 107 splenocytes from GAD65 transgenic mice with 2 × 107 diabetogenic T cells into irradiated NOD mice compared with the cotransfer of 2 × 107 splenocytes from age matched control mice (containing comparable numbers of CD4+CD25+ and CD4+CD25− T cells) with diabetogenic splenocytes. In contrast development of diabetes was prevented by cotransfer of 2 × 107 thymocytes (positive control) from 4-wk-old NOD mice as previously reported (29; unpublished data).
Whatever the reason for tolerance in the GAD transgenic mice is, the fact that T cells do not respond to GAD and disease progresses normally clearly indicate that GAD is not an essential autoantigen. In this context it is interesting to note that CD4+ T cells from GAD65-specific T cell receptor transgenic NOD mice are not diabetogenic (30).
Our data suggest that the protection observed in the GAD-antisense experiments has no immunological basis (9), i.e., the absence of a GAD-specific T cell response is most likely not the reason for the observed protection. In this context it would be of interest to find out in which way pancreatic islet cells might be protected from immunological attack by GAD antisense transgenic mice (9).
While the above experiments ruled out GAD as an autoantigen essential for the development of diabetes, they have documented that it is feasible to induce antigen-specific tolerance in NOD mice. The same approach might be used to determine whether tolerance to other individual β-cell–specific autoantigens such as preproinsulin can prevent diabetes or whether tolerance to a combination of such autoantigens can be used to interfere with the disease. Transgenic NOD mice may be useful to identify such antigens which through presentation by thymic stroma as well as by bone marrow–derived hemopoietic cells can induce specific tolerance. Future research in this field will be important to develop antigen-specific intervention protocols for the treatment of patients at risk of developing IDDM or for patients receiving islet cell transplantation.
Acknowledgments
The authors would like to thank M. Anderson (Joslin Diabetes Center, Boston, MA) for help in determining the insulin-autoantibody titers, M. Lipes (Joslin Diabetes Center, Boston, MA) for the injection of the GAD65 construct, R.N. Smith (Dept. of Pathology, Massachusetts General Hospital, Boston, MA) for evaluation of histological slides, H. McDevitt (Stanford University, CA) for providing T cell hybridomas against GAD65 p205, p221, and p286, K. Jensen and E. Sercarz (La Jolla Inst. For Allergy & Immunology, CA) for providing T cell hybridomas against GAD65 p78, p206, p217, p221, p246, p340, p479, p509, p524, p530, N. Sarvetnick (Scripps Research Inst., La Jolla, CA) for providing the GAD65 baculovirus, H. van Santen for providing the modified invariant chain promotor, and L. Teyton (Scripps Research Inst., La Jolla, CA) for the permission to use the I-Ag7p286 tetramers. The GAD65 cDNA was kindly provided by A. Lehuen (INSERM, Paris, France).
Supported in part by grant 9-1998-1005 from Juvenile Diabetes Research Foundation (to H. von Boehmer), by grant 281541 of the JDRF Center for Islet Cell Transplantation at Harvard (to H. von Boehmer, E. Jaeckel) and fellowship grant JA977/1-1 by the German Research Foundation (to E. Jaeckel). H. von Boehmer was supported by the Körber Foundation. N. Martin-Orozco was supported by a mentor-based postdoctoral fellowship to Diane Mathis from the American Diabetes Association.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; GAD, glutamic acid decarboxylase; IDDM, insulin-dependent diabetes mellitus.
References
- 1.Tisch, R., and H. McDevitt. 1996. Insulin-dependent diabetes mellitus. Cell. 85:291–297. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, M.A., and E.H. Leiter. 1999. The NOD mouse model of type 1 diabetes: as good as it gets? Nat. Med. 5:601–604. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman, D.L., M. Clare-Salzler, J. Tian, T. Forsthuber, G.S. Ting, P. Robinson, M.A. Atkinson, E.E. Sercarz, A.J. Tobin, and P.V. Lehmann. 1993. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 366:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisch, R., X.D. Yang, S.M. Singer, R.S. Liblau, L. Fugger, and H.O. McDevitt. 1993. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 366:72–75. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson, M.A., D.L. Kaufman, L. Campbell, K.A. Gibbs, S.C. Shah, D.F. Bu, M.G. Erlander, A.J. Tobin, and N.K. Maclaren. 1992. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 339:458–459. [DOI] [PubMed] [Google Scholar]
- 6.Tian, J., M.A. Atkinson, M. Clare-Salzler, A. Herschenfeld, T. Forsthuber, P.V. Lehmann, and D.L. Kaufman. 1996. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J. Exp. Med. 183:1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma, S.W., D.L. Zhao, Z.Q. Yin, R. Mukherjee, B. Singh, H.Y. Qin, C.R. Stiller, and A.M. Jevnikar. 1997. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat. Med. 3:793–796. [DOI] [PubMed] [Google Scholar]
- 8.Tisch, R., and H.O. McDevitt. 1994. Antigen-specific immunotherapy: is it a real possibility to combat T-cell-mediated autoimmunity? Proc. Natl. Acad. Sci. USA. 91:437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon, J.W., C.S. Yoon, H.W. Lim, Q.Q. Huang, Y. Kang, K.H. Pyun, K. Hirasawa, R.S. Sherwin, and H.S. Jun. 1999. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science. 284:1183–1187. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson, M., T. Ellis, D. Serreze, J. Tian, and D.L. Kaufman. 2000. Control of autoimmune diabetes. Science. 287:191a. [Google Scholar]
- 11.Kash, S.F., B.G. Condie, and S. Baekkeskov. 1999. Glutamate decarboxylase and GABA in pancreatic islets: lessons from knock-out mice. Horm. Metab. Res. 31:340–344. [DOI] [PubMed] [Google Scholar]
- 12.Jeske, D. 1999. On the mechanisms of autoimmune beta-cell destruction in type I diabetes. Philosophisch-Naturwissenschaftliche Fakultaet. University of Basel, Basel, Switzerland. 1–197.
- 13.Geng, L., M. Solimena, R.A. Flavell, R.S. Sherwin, and A.C. Hayday. 1998. Widespread expression of an autoantigen-GAD65 transgene does not tolerize non-obese diabetic mice and can exacerbate disease. Proc. Natl. Acad. Sci. USA. 95:10055–10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto, H., and J. Sprent. 2001. A defect in central tolerance in NOD mice. Nat. Immunol. 2:1025–1031. [DOI] [PubMed] [Google Scholar]
- 15.Wu, T.C., F.G. Guarnieri, K.F. Staveley-O'Carroll, R.P. Viscidi, H.I. Levitsky, L. Hedrick, K.R. Cho, J.T. August, and D.M. Pardoll. 1995. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc. Natl. Acad. Sci. USA. 92:11671–11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Santen, H., C. Benoist, and D. Mathis. 2000. A cassette vector for high-level reporter expression driven by a hybrid invariant chain promoter in transgenic mice. J. Immunol. Methods. 245:133–137. [DOI] [PubMed] [Google Scholar]
- 17.Tisch, R., R.S. Liblau, X.D. Yang, P. Liblau, and H.O. McDevitt. 1998. Induction of GAD65-specific regulatory T-cells inhibits ongoing autoimmune diabetes in nonobese diabetic mice. Diabetes. 47:894–899. [DOI] [PubMed] [Google Scholar]
- 18.Stratmann, T., V. Apostolopoulos, V. Mallet-Designe, A.L. Corper, C.A. Scott, I.A. Wilson, A.S. Kang, and L. Teyton. 2000. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J. Immunol. 165:3214–3225. [DOI] [PubMed] [Google Scholar]
- 19.Bonifacio, E., M. Atkinson, G. Eisenbarth, D. Serreze, T.W. Kay, E. Lee-Chan, and B. Singh. 2001. International workshop on lessons from animal models for human type 1 diabetes: identification of insulin but not glutamic acid decarboxylase or IA-2 as specific autoantigens of humoral autoimmunity in nonobese diabetic mice. Diabetes. 50:2451–2458. [DOI] [PubMed] [Google Scholar]
- 20.Shi, Y., B. Veit, and S. Baekkeskov. 1994. Amino acid residues 24-31 but not palmitoylation of cysteines 30 and 45 are required for membrane anchoring of glutamic acid decarboxylase, GAD65. J. Cell Biol. 124:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solimena, M., R. Dirkx, Jr., M. Radzynski, O. Mundigl, and P. De Camilli. 1994. A signal located within amino acids 1-27 of GAD65 is required for its targeting to the Golgi complex region. J. Cell Biol. 126:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao, C.C., and H.O. McDevitt. 1997. Identification of immunogenic epitopes of GAD 65 presented by Ag7 in non-obese diabetic mice. Immunogenetics. 46:29–34. [DOI] [PubMed] [Google Scholar]
- 23.Chao, C.C., H.K. Sytwu, E.L. Chen, J. Toma, and H.O. McDevitt. 1999. The role of MHC class II molecules in susceptibility to type I diabetes: identification of peptide epitopes and characterization of the T cell repertoire. Proc. Natl. Acad. Sci. USA. 96:9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zechel, M.A., J.F. Elliott, M.A. Atkinson, and B. Singh. 1998. Characterization of novel T-cell epitopes on 65 kDa and 67 kDa glutamic acid decarboxylase relevant in autoimmune responses in NOD mice. J. Autoimmun. 11:83–95. [DOI] [PubMed] [Google Scholar]
- 25.Hanson, M.S., M. Cetkovic-Cvrlje, V.K. Ramiya, M.A. Atkinson, N.K. Maclaren, B. Singh, J.F. Elliott, D.V. Serreze, and E.H. Leiter. 1996. Quantitative thresholds of MHC class II I-E expressed on hemopoietically derived antigen-presenting cells in transgenic NOD/Lt mice determine level of diabetes resistance and indicate mechanism of protection. J. Immunol. 157:1279–1287. [PubMed] [Google Scholar]
- 26.Kaufman, D.L., R. Tisch, N. Sarvetnick, L. Chatenoud, L.C. Harrison, K. Haskins, A. Quinn, E. Sercarz, B. Singh, M. von Herrath, et al. 2001. Report from the 1st International NOD Mouse T-Cell Workshop and the follow-up mini-workshop. Diabetes. 50:2459–2463. [DOI] [PubMed] [Google Scholar]
- 27.Quinn, A., M.F. McInerney, and E.E. Sercarz. 2001. MHC class I-restricted determinants on the glutamic acid decarboxylase 65 molecule induce spontaneous CTL activity. J. Immunol. 167:1748–1757. [DOI] [PubMed] [Google Scholar]
- 28.Tian, J., M. Clare-Salzler, A. Herschenfeld, B. Middleton, D. Newman, R. Mueller, S. Arita, C. Evans, M.A. Atkinson, Y. Mullen, et al. 1996. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat. Med. 2:1348–1353. [DOI] [PubMed] [Google Scholar]
- 29.Boitard, C., R. Yasunami, M. Dardenne, and J.F. Bach. 1989. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J. Exp. Med. 169:1669–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarbell, K.V., M. Lee, E. Ranheim, C.C. Chao, M. Sanna, S.K. Kim, P. Dickie, L. Teyton, M. Davis, and H. McDevitt. 2002. CD4(+) T cells from glutamic acid decarboxylase (GAD)65-specific T cell receptor transgenic mice are not diabetogenic and can delay diabetes transfer. J. Exp. Med. 196:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]