Abstract
Toll-like receptors (TLRs) mediate recognition of a wide range of microbial products including lipopolysaccharides, lipoproteins, flagellin, and bacterial DNA, and signaling through TLRs leads to the production of inflammatory mediators. In addition to TLRs, many other surface receptors have been proposed to participate in innate immunity and microbial recognition, and signaling through some of these receptors is likely to cooperate with TLR signaling in defining inflammatory responses. In this report we have examined how dectin-1, a lectin family receptor for β-glucans, collaborates with TLRs in recognizing microbes. Dectin-1, which is expressed at low levels on macrophages and high levels on dendritic cells, contains an immunoreceptor tyrosine-based activation motif–like signaling motif that is tyrosine phosphorylated upon activation. The receptor is recruited to phagosomes containing zymosan particles but not to phagosomes containing immunoglobulin G–opsonized particles. Dectin-1 expression enhances TLR-mediated activation of nuclear factor κB by β-glucan–containing particles, and in macrophages and dendritic cells dectin-1 and TLRs are synergistic in mediating production of cytokines such as interleukin 12 and tumor necrosis factor α. Additionally, dectin-1 triggers production of reactive oxygen species, an inflammatory response that is primed by TLR activation. The data demonstrate that collaborative recognition of distinct microbial components by different classes of innate immune receptors is crucial in orchestrating inflammatory responses.
Keywords: lectin, ITAM, zymosan, dendritic cell, macrophage
Introduction
The innate immune system uses a wide variety of microbial recognition receptors including Toll-like receptors (TLRs),*lectins, scavenger receptors, and integrins to identify potential pathogens (1). Microbial recognition by phagocytes triggers phagocytosis, induction of microbial killing, production of inflammatory cytokines and chemokines, and initiates the development of adaptive immunity. All microbes do not induce these responses equally, and the constellation of innate immune recognition receptors activated by a given microbe likely defines the nature of the effector response. Activation of one response such as phagocytosis does not necessarily require activation of other responses. For example, phagocytosis of some particles such as apoptotic cells is noninflammatory (2). Similarly, inflammatory signaling does not require initiation of phagocytosis because many soluble microbial products such as LPS and bacterial lipoproteins are potent inducers of inflammation (3–6). Nevertheless, the fact that complex microbial surfaces present ligands for phagocytic as well as proinflammatory receptors and the fact that these receptors share many intracellular signaling molecules suggests that these different classes of receptors interact with each other in functionally important ways (1). As a model for elucidating the consequences of these interactions, we have examined the role for receptor cooperation in phagocyte responses to zymosan.
Zymosan is a cell wall preparation of Saccharomyces cerevisiae that has been used for over 50 yr as a model phagocytic and inflammatory stimulus both in vivo and in vitro (7, 8). The preparation is composed primarily of β-glucans, mannans, mannoproteins, and chitin, and each of these compounds has been implicated in recognition of yeast by the innate immune system (1, 8–10). In myeloid cells zymosan stimulates phagocytosis, the production of inflammatory cytokines and chemokines, production of reactive oxygen and nitrogen species, and is a potent adjuvant in stimulating adaptive immune responses. We have previously observed that zymosan-induced inflammatory cytokine production by macrophages is mediated by TLRs and does not require particle internalization (11, 12).
TLRs are a family of 10 innate immune recognition receptors that are required for detection of a broad range of microbial products including LPS, flagellin, and bacterial lipoproteins (3–6). Several TLR family members are actively recruited to phagosomes during microbe internalization where they sample the contents of the phagosomes to determine the nature of the microbes being ingested. Thus, TLR1, TLR2, and TLR6 are all recruited to phagosomes containing zymosan particles (11, 13). We have previously demonstrated that both TLR2 and TLR6 are required for activation of nuclear factor (NF)-κB and production of inflammatory cytokines such as TNF-α by zymosan particles, although TLR activation is not required for recruitment to phagosomes because TLRs are also recruited to phagosomes during internalization of IgG-opsonized sheep erythrocytes by Fc receptors, a process that does not stimulate TLRs (11, 13). Thus, TLRs are poised to recognize ligands should they be present in the phagosome. TLR activation is not sufficient to trigger phagocytosis because expression of TLR2 in nonphagocytic cells does not confer on the cells the ability to internalize zymosan (11).
A variety of receptors including those for mannans and β-glucans have been implicated in recognition and phagocytosis of zymosan particles (1). Brown and Gordon (14) and Brown et al. (15) recently identified a C-type lectin called dectin-1 that is expressed on monocytes, macrophages, and dendritic cells as a phagocytic receptor for β-glucan containing particles including zymosan and Candida albicans. The receptor is related to a variety of lectin-type NK receptors, is encoded in the NK gene complex, and may function as a dendritic cell coreceptor for T cell activation by binding an unidentified target on T cells via a site distinct from the β-glucan binding site (16–20). The extracellular COOH terminus of this type 2 transmembrane protein contains a single C-type lectin domain (17). The short intracellular NH2 terminus contains an immunoreceptor tyrosine-based activation motif (ITAM)-like signaling motif, a structure found on a variety of proinflammatory signaling receptors including Fc receptors, T cell receptors, and NK receptors (21, 22), suggesting that dectin-1 might play an important role in inflammatory responses to β-glucan–containing particles in addition to its role in phagocytosis. Therefore, we examined the capacity for dectin-1 to cooperate with TLR2 in defining inflammatory responses to zymosan.
Materials and Methods
Reagents and Plasmids.
Zymosan (Sigma-Aldrich), LPS (List), and the synthetic lipopeptide PAM3CSK4 (EMC Microcollections) were prepared as previously described (11). FITC-conjugated anti-V5 antibody (Invitrogen), unlabeled V5 epitope antibody (P-K; Serotec), anti–sheep red blood cell antibody (Intercell), and sheep red blood cells (ICN Biomedicals/Cappel) were used as previously described (12, 13). Murine IL-12 and TNF-α ELISA kits were purchased from R&D Systems and the sensitivity of detection was 5 pg/ml for both. Laminarin (Sigma-Aldrich) was prepared as a 10-mg/ml stock solution, sterile filtered, and stored frozen until use. Depleted zymosan was prepared by boiling 250 μg zymosan in 1 ml 10 M sodium hydroxide for 30 min and washing three times with sterile PBS.
Murine TLR2 and CD14 expression vectors have been previously described (11). The murine dectin-1 expression vector was constructed by amplifying the full coding region of murine dectin-1 (sequence data available from GenBank/EMBL/DDBJ under accession no. AF262985) from RAW 264.7 cDNA and cloning the resulting fragment into pEF6/V5-HIS-TOPO (Invitrogen), thus adding a V5-HIS epitope tag to the COOH terminus of the expressed protein. Tyrosine 15 was mutated to serine (pEF6-DectinY15S) and the NH2-terminal 38 amino acids were replaced with a new ATG start codon (pEF6-DectinΔ38) by PCR. The dectin-1 coding region was fused to the 3′ end of eGFP (CLONTECH Laboratories, Inc.) by PCR. The identities of all expression vectors were confirmed by sequencing. Mutant versions of dectin-1 were expressed in HEK 293 and RAW 264.7 cells equally as efficiently as the epitope-tagged wild-type protein and were expressed at the cell surface in a manner indistinguishable from the wild-type protein (unpublished data).
Mice.
MyD88−/− mice (129/SvJ′ C57Bl/6 background; reference 23) were backcrossed for six generations with C57Bl/6 mice. Mice from the F3 or F6 generation were used and were compared with littermate controls (MyD88+/+). TLR2−/− mice (129/SvJ′ C57Bl/6 background; reference 24) were backcrossed for two generations with C57Bl/6 mice. Mac-1 (CD11b)−/− and C57Bl/6 mice were purchased from The Jackson Laboratory.
Cell Lines.
HEK 293 cells (American Type Culture Collection [ATCC] no. CRL-1573) were maintained in DMEM (Invitrogen) and the mouse macrophage cell line RAW 264.7 cells (ATCC no. TIB-71) was maintained in RPMI (Invitrogen). Both media were made complete with 10% heat-inactivated fetal bovine serum (Hyclone), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (GIBCO BRL). RAW cells transfected with an NF-κB luciferase reporter have been described (25). The coding regions for mouse wild-type and truncated dectin-1 (V5-HIS tagged) were cloned into pFB-Neo (Stratagene), allowing for expression of dectin-1 and neomycin resistance from single polycystronic mRNAs. These plasmids were transfected into RAW 264.7 cells by electroporation (13) or Effectene (QIAGEN), and populations of G418- (0.4 mg/ml; Invitrogen) resistant cells were generated. Several populations expressing wild-type and mutant dectin-1 were recovered and data from one population of each are presented. The remaining populations responded to zymosan in manners consistent with the data presented and proportional to their levels of dectin-1 expression. Phagocytosis was quantified as previously described (11).
Primary Cell Culture.
Bone marrow–derived macrophages were prepared by 7 d of culture in 20% L929 cell–conditioned media. Phagocytosis was quantified as previously described (11). Bone marrow–derived dendritic cells were cultured using a previously described method (26) with minor alterations. Bone marrow was collected from femurs of adult mice and resuspended in complete RPMI containing 10 ng/ml GM-CSF (R&D Systems) and 20 ng/ml IL-4 (R&D Systems). After 2 d in culture, media and nonadherent cells were aspirated and replaced with fresh media, media was replaced again after 4 d, and assays were performed on day 5. Purity of dendritic cell preparations was assayed by flow cytometry using the following panel of antibodies (all from BD Biosciences, except where noted), and found to be at least 75% pure): anti–CD11c-FITC, anti–CD11b-FITC, anti–CD40-biotin, anti–GR-1–PE, anti–CD14-FITC, anti–F4/80-biotin (Caltag Laboratories). Dendritic cell cultures were found to be of the immature phenotype by low level staining with anti–I-Ab–biotin, anti–B7-1–biotin, and Streptavidin-PE (Caltag Laboratories) compared with activated controls. Unelicited peritoneal macrophages were isolated by peritoneal lavage and plated directly into 96-well luminometer plates (Costar) in complete DMEM and incubated overnight. Nonadherent cells were removed and the cells were placed in 100 μl fresh media containing 50 U/ml recombinant interferon γ (PeproTech) for 4 h before use in the chemiluminescence oxidase assay described below.
Real-Time PCR Analysis.
RAW 264.7 cells were plated at 0.5 × 106 per 1 ml complete RPMI for 24 h before start of assay. Total RNA was prepared with RNeasy columns (QIAGEN) using the manufacturer's protocols. Synthesis of cDNA was completed with MMLV Reverse Transcriptase (Promega) according to the manufacturer's recommendations and primed with oligo d(T) (Promega). Quantitative real-time PCR was performed on an ABI 7700 (Applied Biosystems) using TaqMan Universal PCR Master Mix (Applied Biosystems). Probes (IDT) labeled with 5′ FAM and 3′ TAMRA modifications were used at a final concentration of 0.9 mM, and primers were used at 0.2 mM (GIBCO BRL). The PCR program was as follows: 50°C for 2 min and 95°C for 10 min (95°C for 15 s and 60°C for 1 min) for 40 cycles. All data were normalized to EF1α expression in the same cDNA set. Data is presented in relative mRNA units and represents the average of at least three values ± standard deviation. Each experiment was performed independently at least three times and the results of one representative experiment are shown. Sequences are as follows: IL-12 p40 probe: GGAAACACATGCCCACTTGCTGCA, forward primer: GCTCAGGATCGCTATTACAATTCC, reverse primer: TCTTCCTTAATGTCTTCCACTTTTCTT; TNF-α probe: TCAGCCTCT-TCTCATTCCTGCTTG, forward primer: TCCAGGCGGTGCCTATGT, reverse primer: CACCCCGAAGTTCAGTAG-ACAGA; and EF1α probe: CACCTGAGCAGTGAAGCC-AGCTGCT, forward primer: GCAAAAACGACCCACCA-ATG, reverse primer: GGCCTGGATGGTTCAGGATA.
Luciferase Assays.
HEK 293 cells were transfected by calcium phosphate precipitation in 96-well plates. Each well received 250 ng ELAM luciferase reporter and 125 ng TK-RL (Promega) transfection control reporter together with 250 ng TLR2, dectin-1, CD14 expression vectors, or pEF6 vector control as indicated for a total of 875 ng DNA. Cells were transfected overnight and stimulated with the indicated reagents for 4 h. The cells were lysed and firefly and Renilla luciferase activities were determined using the Dual Luciferase Assay kit (Promega). Luciferase activity is expressed as the ratio of the ELAM luciferase (firefly) activity to the TK-RL (Renilla) activity. Assays were performed in triplicate with standard deviations indicated and are representative of at least three independent experiments.
Assay of Luminol-enhanced Chemiluminescence.
Production of reactive oxygen species (ROS) was assayed by luminol-enhanced chemiluminescence (27). RAW 264.7 cells were suspended in culture medium at 400,000 cells/ml, warmed to 37°C in water bath for 30 min, and then 100-μl aliquots were placed in a 96-well luminometer plate (Costar). 100 μl medium containing 100 μM luminol (Fluka) with or without zymosan and laminarin at the indicated concentrations was added. Chemiluminescence was measured at 2.5-min intervals. Luminescence is expressed as relative luciferase units/minute/1,000 cells and was not reduced in the presence of inhibitors of inducible nitric oxide synthase (unpublished data).
Immunoprecipitations and Phosphotyrosine Analysis.
RAW cells expressing epitope-tagged wild-type dectin-1 were stimulated in 6-well dishes as indicated for 15 min in the presence of 1 mM sodium orthovanadate. The cells were lysed in 250 μl lysis buffer I (1% SDS, 10 mM Tris, 0.2 mM sodium orthovanadate, pH 7.4, with protease inhibitors) and insoluble material was pelleted. 750 μl lysis buffer II (1% Triton X-100, 50 mM NaCl, 10 mM Tris, 0.2 mM sodium orthovanadate, pH 7.4, with protease inhibitors) was added together with 15 μl PY20 agarose beads (Zymed Laboratories). The mixture was incubated for 2 h at 4°C, the beads were washed twice with lysis buffer II, and tyrosine phosphorylated proteins were separated by SDS-PAGE. Recovery of epitope-tagged dectin-1 was determined by blotting with anti-V5 antibody (P-K; Serotec).
Results
Dectin-1 and TLR2 Collaboration.
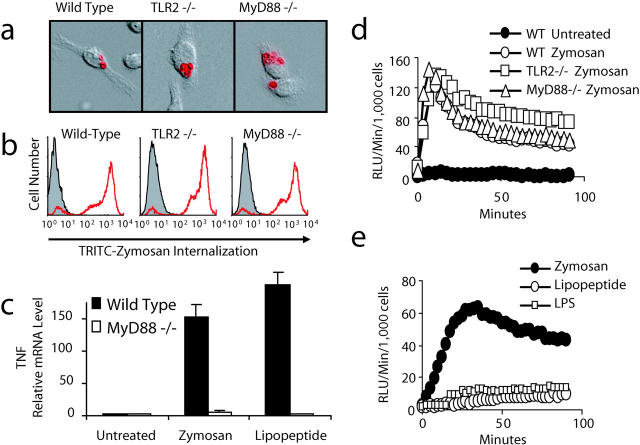
We have previously observed that expression of inhibitory forms of TLR2, TLR6, and MyD88 (an adaptor molecule required for TLR signaling; references 4, 5, and 28) do not inhibit recognition and phagocytosis of zymosan by macrophages (11, 13). We have extended these observations using macrophages from TLR2– and MyD88-deficient mice and examining the production of ROS. Phagocytosis of unopsonized zymosan is unaffected in bone marrow–derived macrophages from mice deficient in TLR2 or MyD88, indicating that additional receptor(s) recognize the particle and trigger intracellular signaling for phagocytosis (Fig. 1 , a and b). Macrophages from mice lacking MyD88 fail to produce inflammatory cytokines such as TNF-α in response to the pure TLR2 stimulus, PAM3CSK4 lipopeptide (a synthetic tri-palmitoylated bacterial lipopeptide; reference 13), or in response to the more complex TLR2 stimulus zymosan (Fig. 1 c). Zymosan particles trigger the production of ROS in murine macrophages and although TLR activation is required for cytokine production, TLR2−/− and MyD88−/− macrophages still produce ROS in response to zymosan (Fig. 1 d). TLR stimulation alone is not sufficient to induce ROS because PAM3CSK4 lipopeptide and LPS fail to trigger ROS production in a mouse macrophage cell line (Fig. 1 e) or in primary peritoneal macrophages (unpublished data). Thus, recognition through receptors independent of TLRs mediates ROS production and phagocytosis.
Figure 1.
Zymosan engages receptors on macrophages other than TLRs. (a and b) Bone marrow–derived macrophages from wild-type, TLR2−/−, or MyD88−/− mice were incubated with tetramethylrhodamine isothiocyanate–labeled zymosan for 15 min. Phagocytosis was visualized by immunofluorescence microscopy (a) and quantified by flow cytometry (b). (c) Bone marrow macrophages from MyD88−/− or wild-type mice were stimulated with 100 μg/ml zymosan or 100 ng/ml PAM3CSK4 lipopeptide for 4 h and TNF-α mRNA production was measured by quantitative real-time PCR. (d and e) Production of ROS was assayed by luminol-enhanced chemiluminescence in peritoneal macrophages from wild-type, TLR2−/−, or MyD88−/− mice (d) stimulated with 200 μg/ml zymosan or in RAW 264.7 macrophages (e) stimulated with 100 μg/ml zymosan, 100 ng/ml LPS, or 100 ng/ml PAM3CSK4 lipopeptide.
It is not clear whether in the presence of TLR-mediated inflammatory signaling additional receptors contribute to modulation of gene expression. Therefore, we explored whether recognition by a phagocytic receptor for zymosan (dectin-1) could contribute to TLR-mediated signaling. As we and others have previously observed, HEK 293 cells (that do not normally express TLR2 or respond to TLR2 stimuli) transiently transfected with TLR2 and an NF-κB luciferase reporter plasmid respond strongly to the soluble TLR2 stimulus PAM3CSK4 lipopeptide. However, the cells respond poorly to zymosan, suggesting that additional recognition molecules are required for efficient TLR2–mediated detection of zymosan (Fig. 2 a). Coexpression of dectin-1 (that is not endogenously expressed by HEK 293 cells; reference 16) enhanced TLR2–mediated recognition of zymosan to levels comparable to stimulation by the soluble lipopeptide (Fig. 2 a). Although expression of dectin-1 alone in HEK 293 cells conferred the ability to phagocytose zymosan (unpublished data), it did not confer NF-κB activation by zymosan in this system (Fig. 2 a), suggesting that dectin-1 signaling alone is not sufficient to activate NF-κB and associated downstream inflammatory responses. Dectin-1–enhanced signaling was clearly dependent on TLR2 activation because expression of a signaling-deficient mutant of TLR2, TLR2-P681H (11), blocked NF-κB activation (Fig. 2 a). Dectin-1 signaling is required for enhanced TLR2 responses because mutation of dectin-1 either by truncation of the intracellular amino terminal 38 acids (Dectin-Δ38) or by substitution of tyrosine 15 (in the ITAM-like motif) to serine (DectinY15S) abrogated the enhancement (Fig. 2 b). Dectin-enhanced signaling was dependent on recognition of β-glucan on the particle because laminarin (a soluble β-glucan from the brown seaweed Laminaria digitata) completely blocked the enhancement (Fig. 2 c). Although we have observed dectin-1 and TLR2 enriched together on phagosomes containing zymosan, we have not detected direct physical interactions between the receptors, suggesting that cooperation occurs by parallel activation (unpublished data). We have previously observed that CD14 (a soluble or GPI-linked protein) can facilitate TLR2–mediated inflammatory responses to zymosan (11), so we compared the requirement for β-glucan recognition by CD14 and dectin-1. Laminarin had no effect on intracellular signaling by TLR2 coexpressed with CD14 (Fig. 2 c), demonstrating that neither CD14 nor TLR2 directly recognizes β-glucan. Taken together, these observations demonstrate that dectin-1 enhances TLR signaling in response to β-glucan–containing particulate stimuli.
Figure 2.
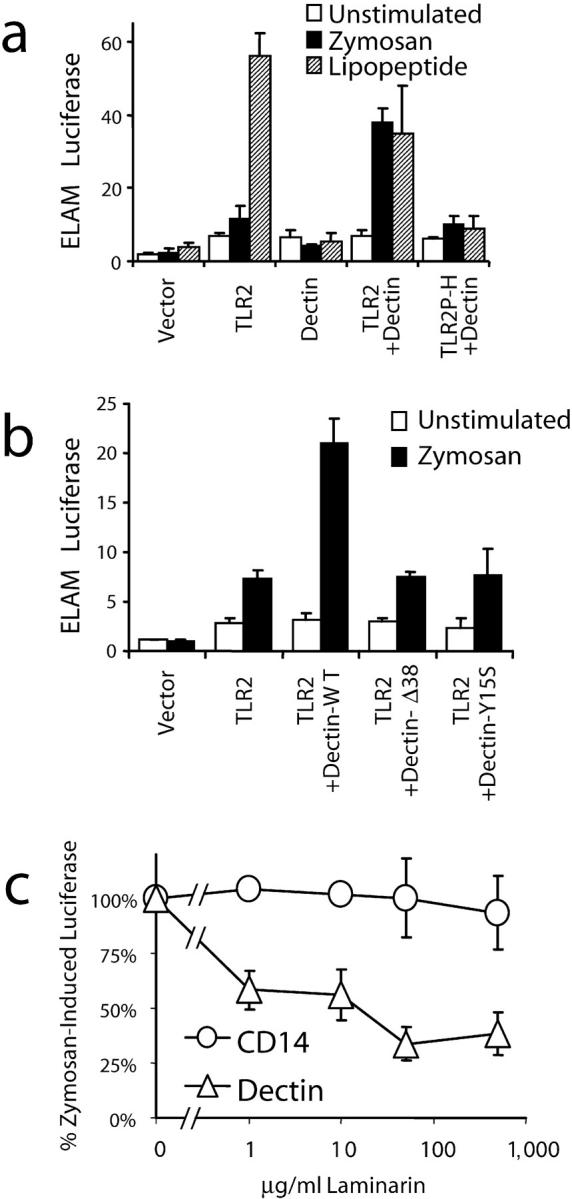
Dectin-1 and TLR2 collaborate in zymosan recognition by HEK 293 cells. (a and b) Activation of NF-κB (ELAM luciferase) was measured in HEK 293 cells transiently transfected with the indicated expression vectors and stimulated with 100 μg/ml zymosan or 100 ng/ml PAM3CSK4 lipopeptide for 4 h. (c) Activation of NF-κB was measured in HEK 293 cells transfected with expression vectors for TLR2 (together with either CD14 or dectin-1) and stimulated with 100 μg/ml zymosan in the indicated concentrations of laminarin.
Dectin-1 Enhances Cytokine Production in Macrophages.
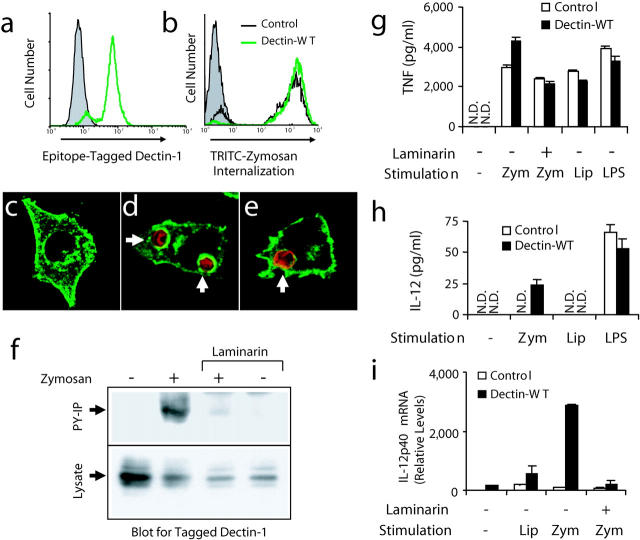
To examine the role of β-glucan recognition in zymosan-induced inflammatory signaling in macrophages, we established a model system using the RAW 264.7 (RAW) mouse macrophage cell line. RAW cells express surface CD14, low levels of mRNA for dectin-1, and bind and internalize zymosan. We generated a stable population of cells overexpressing an epitope-tagged dectin-1. By real-time PCR, these cells express 34-fold (±3) more dectin-1 mRNA than the parental cells. As demonstrated by flow cytometry, the population uniformly expresses epitope-tagged dectin-1 (Fig. 3 a) and immunofluorescence microscopy revealed that the receptor is expressed evenly at the cell surface and is also found in some intracellular compartments (Fig. 3 c).
Figure 3.
Zymosan triggers activation of dectin-1 and enhanced inflammatory responses in mouse macrophages. (a) V5 epitope–tagged dectin-1 expression was measured by flow cytometry in RAW cells stably overexpressing wild-type dectin-1 (green line) and in control RAW cells (shaded). (b) Control cells (black line) and cells overexpressing dectin-1 (green line) were incubated with tetramethylrhodamine isothiocyanate zymosan for 1 h and phagocytosis was measured by flow cytometry and compared with unfed control cells (shaded). (c–e) The cellular distribution of epitope-tagged dectin-1 (green) was examined by immunofluorescence microscopy in resting cells (c), cells fed zymosan (red) for 5 min (d), or in cells fed IgG-opsonized sheep red blood cells (red) for 5 min (e). (f) Cells expressing V5-tagged dectin-1 were fed 100 μg/ml zymosan for 15 min in the presence or absence of 50 μg/ml laminarin, total cell lysates were prepared, and the protein was detected in all lysates by immunoblot using an antibody for the tag (bottom). Tyrosine-phosphorylated proteins were immunoprecipitated from the lysates and recovery of dectin-1 was analyzed by immunoblot (top). (g and h) Induction of inflammatory responses was measured by ELISA in control RAW cells and cells overexpressing dectin-1 stimulated with 100 μg/ml zymosan (zym), 100 ng/ml PAM3CSK4 lipopeptide (Lip), or 100 ng/ml LPS for 4 (g, TNF-α) or 24 h (h, IL-12 p40) in the presence or absence of 500 μg/ml laminarin as indicated. N.D., none detected. (i) Induction of IL-12 p40 mRNA was measured by quantitative real-time PCR in cells stimulated for 4 h as in g.
Although dectin-1 expression in nonphagocytic cells such as NIH3T3 cells (14) or HEK 293 cells (unpublished data) is sufficient to confer the ability to internalize zymosan particles, phagocytosis of fluorescently labeled zymosan was only mildly enhanced in RAW cells overexpressing dectin-1 (Fig. 3 b). Parental RAW cells bind and internalize zymosan efficiently and this internalization is not substantially inhibited by soluble β-glucans (unpublished data), indicating that in these cells receptors in addition to β-glucan receptors mediate zymosan phagocytosis. We have previously reported that TLR2 is recruited to phagosomes containing zymosan particles and that this recruitment does not require ligand binding because TLR2 is also recruited to phagosomes containing IgG-opsonized particles (13). Therefore, we examined whether dectin-1 is similarly recruited to phagosomes. Dectin-1 is strongly enriched on zymosan phagosomes (Fig. 3 d), but unlike TLR2, dectin-1 is not significantly recruited to phagosomes containing IgG-opsonized particles (Fig. 3 e). Further, dectin-1 is not significantly enhanced on IgG-opsonized particles after stimulation of TLR2 with PAM3CSK4 lipopeptide, nor is dectin-1 enriched on phagosomes containing Staphylococcus aureus (unpublished data), supporting a model in which β-glucan binding is required for dectin-1 recruitment to phagosomes.
The cytoplasmic tail of dectin-1 contains two tyrosines and these amino acids are arranged in a sequence similar to the consensus sequence for an ITAM motif. Therefore, we examined whether dectin-1 is tyrosine phosphorylated upon stimulation with zymosan. RAW cells expressing V5 epitope–tagged dectin-1 were stimulated with zymosan, lysates were prepared, and tyrosine phosphorylated proteins were immunoprecipitated. Dectin-1 was detected by immunoblotting in all lysates but was recovered by antiphosphotyrosine immunoprecipitation only after binding zymosan (Fig. 3 f). Tyrosine phosphorylation of dectin-1 was blocked by the addition of soluble laminarin, demonstrating that binding to particulate β-glucans triggers tyrosine phosphorylation of the ITAM-like motif in dectin-1 (Fig. 3 f). Thus, these cells provide a model for studying the contribution of dectin-1 to zymosan-induced inflammatory responses in macrophages.
We stimulated control RAW cells and cells overexpressing dectin-1 with zymosan, PAM3CSK4 lipopeptide, or LPS and measured cytokine production by ELISA. Dectin-1 expression enhanced TNF-α expression in response to zymosan but not in response to the other TLR stimuli, and this enhancement was blocked by soluble laminarin (Fig. 3 g). In contrast to TNF-α, RAW cells do not produce detectable amounts of IL-12 in response to zymosan or PAM3CSK4 lipopeptide (Fig. 3, h and i). Dectin-1 expression facilitated zymosan-induced IL-12 p40 production as measured by ELISA (Fig. 3 h) and quantitative real-time PCR (Fig. 3 i) whereas lipopeptide and LPS responses were not affected. Thus, dectin-1 expression specifically enhances zymosan-induced cytokine production in macrophages.
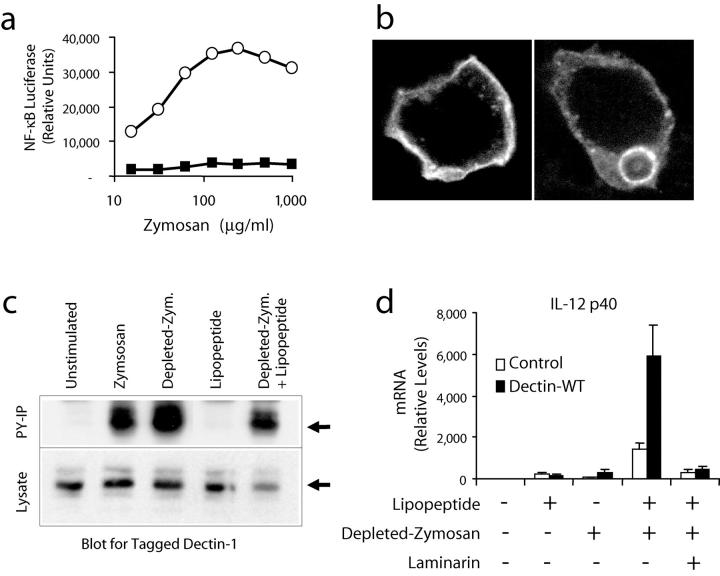
To separate the dectin-1– and TLR2–activating components of zymosan, we treated zymosan with hot alkali to remove all of its TLR-stimulating properties. In a very sensitive assay for NF-κB activation in RAW cells (25), this depleted zymosan had no activity compared with untreated zymosan (Fig. 4 a), although these macrophages still bound and internalized the particles (unpublished data). The depleted zymosan particles were still specifically recognized by dectin-1. GFP-tagged dectin-1 expressed in HEK 293 cells was found at the cell surface (Fig. 4 b, left) and bound to and internalized depleted zymosan particles (Fig. 4 b, right). Untransfected HEK 293 cells do not bind or internalize depleted zymosan (unpublished data). In addition, these particles trigger phosphorylation of dectin-1 in RAW cells overexpressing dectin-1 (Fig. 4 c). Thus, depleted zymosan activates dectin-1 without stimulating TLRs. PAM3CSK4 lipopeptide alone fails to induce phosphorylation on dectin-1 and also fails to enhance dectin-1 phosphorylation in response to depleted zymosan particles (Fig. 4 c), suggesting that TLR2 activation does not play a role in the tyrosine phosphorylation of dectin-1. Therefore, we treated RAW cells or RAW cells overexpressing dectin-1 with depleted zymosan alone or together with PAM3CSK4 lipopeptide and measured induction of IL-12 p40 mRNA (Fig. 4 d). Although neither PAM3CSK4 lipopeptide nor depleted zymosan induced significant amounts of IL-12, together the stimuli were strongly synergistic. The synergy was amplified in cells overexpressing dectin-1 and was blocked by laminarin. Similar results were obtained for the induction of TNF-α mRNA (unpublished data). Taken together, these data demonstrate that signaling by dectin-1 in macrophages collaborates with TLR signaling to shape inflammatory responses to β-glucan–containing particles.
Figure 4.
Zymosan depleted of its TLR stimulatory activity synergizes with a TLR2 agonist through activation of dectin-1. (a) RAW cells stably transfected with an NF-κB luciferase reporter (ELAM luciferase) were stimulated for 4 h with the indicated concentrations of zymosan (○) or depleted zymosan (▪), and luciferase activity was measured. (b) HEK 293 cells were transiently transfected with dectin-1 fused to green fluorescent protein at the receptor's intracellular amino terminus and dectin-1 localization was visualized before (left) and after (right) exposure to depleted zymosan. (c) Cells expressing V5-tagged dectin-1 were stimulated with 100 μg/ml zymosan, 100 μg/ml depleted zymosan, or PAM3CSK4 lipopeptide as indicated for 15 min in the presence or absence of 50 μg/ml laminarin. Total cell lysates were prepared and the protein was detected by immunoblot using an antibody for the tag (bottom). Tyrosine-phosphorylated proteins were immunoprecipitated from the lysates and recovery of dectin-1 was analyzed by immunoblot (top). (d) IL-12 p40 mRNA production was measured by quantitative real-time PCR in control RAW cells and cells overexpressing dectin-1 stimulated for 4 h with 100 ng/ml PAM3CSK4 lipopeptide or 100 μg/ml depleted zymosan in the presence or absence of 500 μg/ml laminarin.
Dectin-1 Enhances Cytokine Production in Dendritic Cells.
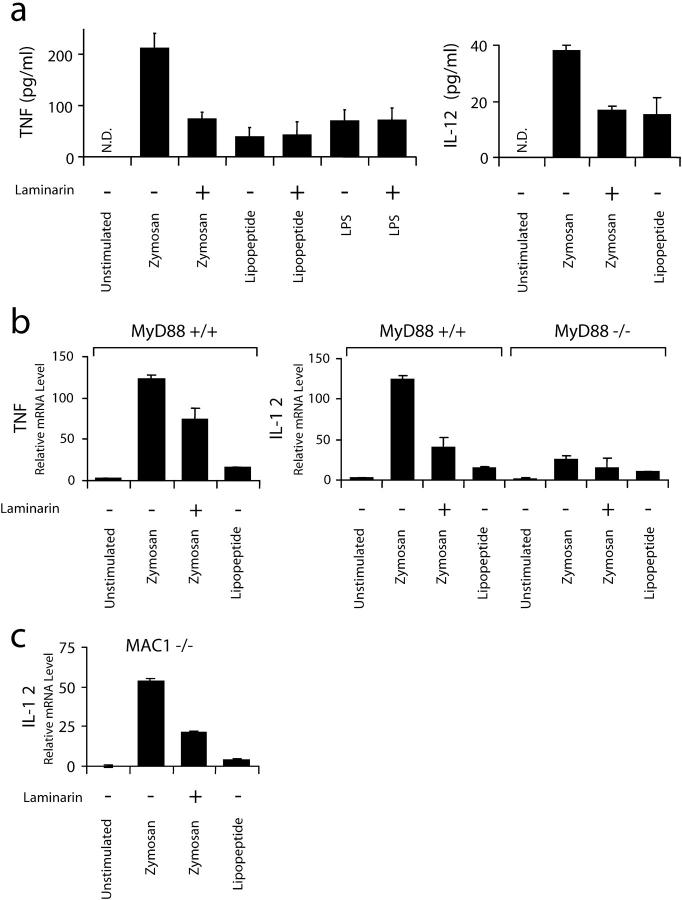
The observation that amplified dectin-1 expression increases TLR-mediated responses in a macrophage cell line suggested that in vivo dectin-1 might be especially important for inflammatory responses in dendritic cells that naturally expresses high levels of dectin-1 (17, 18, 20). Mouse bone marrow–derived dendritic cells (BMDCs) express 127-fold (±34) more dectin-1 mRNA than RAW cells as measured by quantitative real-time PCR, and we observed that dendritic cell responses to zymosan including induction of IL-12 and TNF-α were reduced in the presence of soluble β-glucan (Fig. 5 , a and b). Although β-glucan recognition was very important for BMDC responses to zymosan, these responses were still dependent on TLR signaling because they were abrogated in BMDCs from MyD88-deficient mice (Fig. 5 b). Although the doses of zymosan (100 μg/ml) and PAM3CSK4 lipopeptide (100 ng/ml) used are maximal for induction of inflammatory responses in macrophages, in BMDCs zymosan is a much stronger activator of both TNF-α and IL-12 than PAM3CSK4 lipopeptide, suggesting especially strong cooperation between TLR2 and β-glucan receptor signaling in the BMDCs (Fig. 5, a and b). Indeed, similar to our observations in macrophages overexpressing dectin-1 (Fig. 4), zymosan depleted of its TLR-stimulating activity, although a poor inducer of IL-12 in BMDCs alone, is strongly synergistic with PAM3CSK4 lipopeptide for induction of IL-12 (unpublished data).
Figure 5.
TLRs and dectin-1 collaborate in primary bone marrow dendritic cells. (a) Induction of cytokines in mouse BMDCs was measured by ELISA. The cells were stimulated for 4 h with 100 μg/ml zymosan or 100 ng/ml PAM3CSK4 lipopeptide in the presence or absence of 500 μg/ml laminarin. N.D., none detected. (b) Induction of cytokines was measured by quantitative real-time PCR in primary dendritic cells from MyD88+/+ and MyD88−/− mice stimulated for 4 h as in a. (c) Induction of IL-12 p40 was measured by quantitative real-time PCR in dendritic cells from Mac-1(CD11b)−/− mice stimulated as in b.
In addition to dectin-1, Mac-1 (CD11b) has been suggested to participate in recognition of β-glucans (29). Therefore, we examined the effect of soluble β-glucan on zymosan-induced responses in BMDCs from Mac-1–deficient mice. Zymosan-induced IL-12 and TNF-α production and inhibition by soluble β-glucan were identical in Mac-1−/− and wild-type BMDCs (Fig. 5 c and unpublished data), suggesting that dectin-1, not Mac-1, is the β-glucan receptor responsible for the observed modulation of BMDC responses to zymosan.
Dectin-1 Triggers NADPH Oxidase Activation.
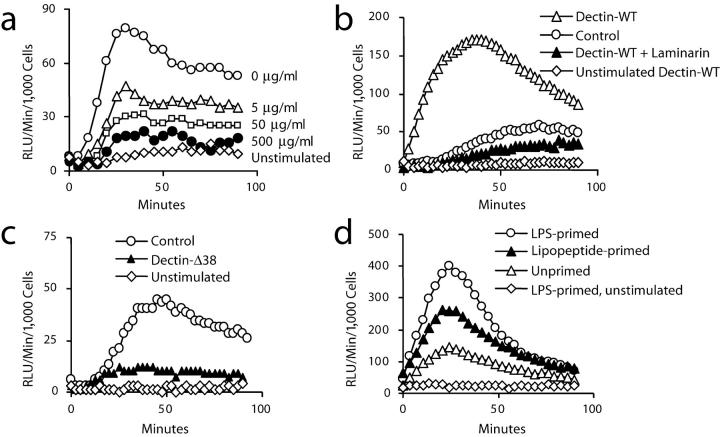
Because zymosan activates the production of ROS in leukocytes and β-glucan recognition is implicated in the response (30–32), we examined whether dectin-1 mediates zymosan-induced activation of ROS production independent of its ability to enhance TLR-mediated signaling. Zymosan-induced ROS production in macrophages was inhibited in a dose-dependent manner by laminarin (Fig. 6 a), indicating that these cells require β-glucan recognition to trigger ROS production. Similarly, we observed that depleted zymosan strongly activates ROS production in a laminarin-inhibitable fashion (unpublished data), demonstrating that TLR activation is not required for ROS production. Therefore, we examined the production of ROS in cells overexpressing dectin-1 and we observed that cells overexpressing dectin-1 exhibit faster kinetics and greater magnitude of ROS production in response to zymosan (Fig. 6 b). The enhanced response was also strongly inhibited by soluble β-glucans (Fig. 6 b). In RAW cell populations expressing the signaling-deficient Dectin-Δ38 mutant, zymosan-induced ROS production was reduced nearly to baseline levels (Fig. 6 c) although particle binding and internalization were not inhibited (unpublished data). Taken together, these data demonstrate that β-glucan recognition by dectin-1 triggers ROS production. In addition, these data demonstrate that low level expression of dectin-1 in RAW cells is responsible for zymosan-induced ROS production and that the signaling-deficient form of the receptor acts as a dominant-negative inhibitor of the endogenous receptor.
Figure 6.
Dectin-1 stimulates production of ROS in macrophages. (a) Production of ROS was assayed by luminol-enhanced chemiluminescence in RAW cells in the presence of 100 μg/ml zymosan together with the indicated concentrations of laminarin. (b) Zymosan-induced production of ROS in RAW cells was compared with RAW cells overexpressing wild-type dectin-1 in the presence or absence of 500 μg/ml laminarin. (c) Zymosan-induced production of ROS in RAW cells was compared with RAW cells overexpressing a signaling-deficient (Δ38) form of dectin-1. (d) Zymosan-induced production of ROS was measured in RAW cells overexpressing wild-type dectin-1 with or without 4 h prestimulation with 100 ng/ml LPS or 100 ng/ml PAM3CSK4 lipopeptide.
It has long been appreciated that TLR stimuli such as LPS can potentiate inflammatory responses such as ROS production and arachidonic acid metabolism that are triggered by other receptors (33, 34). Therefore, we examined whether dectin-1 can trigger inflammatory responses that are primed but not induced by TLRs. Despite the heightened production of ROS in RAW cells overexpressing dectin-1, ROS production induced by zymosan is still further potentiated by prestimulating the cells with the TLR stimuli LPS or PAM3CSK4 lipopeptide, demonstrating that TLR activation can prime a response that is triggered by dectin-1 (Fig. 6 d). Thus, in addition to direct cooperation between TLR2 and dectin-1 in zymosan recognition and inflammatory cytokine production, dectin-1 can also cooperate with additional TLRs in a sequential manner to induce potent antimicrobial responses.
Discussion
We have identified dectin-1 as an important partner for TLR2 on macrophages and dendritic cells for the production of inflammatory cytokines in response to particulate stimuli containing β-glucans. Collaboration between dectin-1 and TLR2 in orchestrating immune responses provides a valuable model for elucidating the mechanisms of interaction between multiple innate immune recognition receptors during microbial recognition. During macrophage or dendritic cell recognition of zymosan, both dectin-1 and TLR2 are recruited to phagosomes where dectin-1 binds β-glucans and TLR2/CD14 binds distinct component(s) of the yeast cell wall yet to be characterized (Fig. 7) . We have demonstrated that dectin-1 ligation results in tyrosine phosphorylation of the receptor's ITAM-like signaling motif, generating intracellular signals that mediate phagocytosis and activation of the NADPH oxidase, contributing to microbial killing. Many groups have demonstrated that TLR stimulation leads to activation of NF-κB and production of proinflammatory cytokines such as TNF-α and IL-12. Using a reconstitution system, macrophage cell lines overexpressing dectin-1 and primary dendritic cells, we have established that concurrent engagement of dectin-1 enhances these TLR2–mediated responses. Although it is clear that many receptors participate in microbial recognition by the innate immune system, studies on TLRs as well as on mice deficient in TLR signaling have surprisingly demonstrated an almost complete requirement for this family of molecules in a broad range of inflammatory responses. Our data suggest that one important role of other innate immune recognition receptors (such as dectin-1) in inflammatory gene regulation is to enhance specific TLR-mediated signaling. Additionally, as demonstrated by the observation that exposure to LPS or bacterial lipoproteins primes phagocytes for reactive oxygen production triggered by dectin-1, TLR signaling enhances responses triggered by other receptors. Thus, microbial components are directly recognized by both dectin-1 and TLRs and these receptors trigger independent and cooperative inflammatory responses.
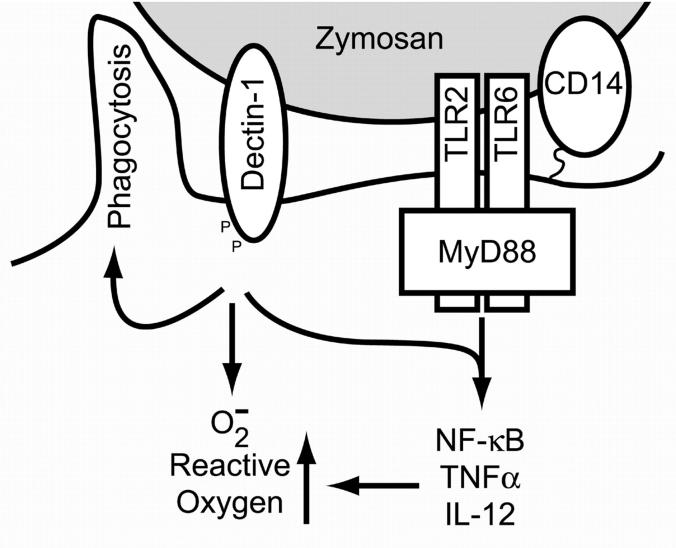
Figure 7.
Model of dectin-1/TLR collaboration. Zymosan particles are recognized simultaneously by dectin-1, TLRs, and CD14. Together these receptors facilitate inflammatory responses to the particle. Dectin-1 directly triggers phagocytosis and stimulates the production of ROS, thus contributing to microbial killing. TLRs induce signaling through NF-κB that leads to the production of inflammatory cytokines and these responses are enhanced by dectin-1. Similarly, TLR signaling enhances responses such as the production of ROS that are triggered by dectin-1.
We have demonstrated that signaling through dectin-1 requires tyrosine phosphorylation of an ITAM-like motif. Although dectin-1 is the first direct innate immune recognition receptor demonstrated to signal through an ITAM-like motif, these motifs are found in many receptors including B and T cell antigen receptors, Fc receptors, and NK activating receptors. One recent report has suggested that activation of autoreactive B cells by chromatin–IgG complexes occurs synergistically through the B cell receptor and a TLR (35). Also, Fc receptor ligation in macrophages has been reported to inhibit subsequent induction of IL-12 by LPS through a mechanism requiring intracellular calcium fluxes (36, 37). We have observed that dectin-1 signaling potentiates IL-12 production by TLR2 stimuli, suggesting that although dectin-1 triggers phagocytosis like Fc receptors, important differences in signaling exist. Fc receptors have been reported to associate with a variety of other surface molecules including Mac-1, CD9, and lipid rafts (29, 38, 39), and it is possible that coactivation of signaling through additional receptors participates in the reported inhibition of LPS-induced IL-12. Alternately, ITAM-based signaling in Fc receptors and dectin-1 might be different. The ITAM-like motif in dectin-1 is imperfect. Although the consensus ITAM motif consists of two YXXL/I repeats (40, 41), the membrane distal repeat in dectin-1 lacks the L/I, which may engage different signaling mechanisms. Nonetheless, as for Fc receptors, tyrosine phosphorylation of the ITAM-like motif in dectin-1 is required for receptor function.
Other investigators have recently observed that highly purified TLR2 stimuli are poor inducers of IL-12 in immature dendritic cells (42, 43). Pulendran et al. (42) demonstrated that Escherichia coli LPS (a TLR4 stimulus) activated IL-12 production by highly purified CD8α+ mouse dendritic cells whereas Porphyromonas gingivalis LPS (a TLR2 stimulus) did not. These authors further demonstrated that when used as adjuvants, this different profile of responses caused E. coli LPS to induce a Th1 response whereas P. gingivalis induced a more Th2-like response. However, Th1-type immune responses are critical for defense against many pathogens that activate innate immunity primarily through TLR2 such as the fungal pathogen Aspergillus fumigatus (44–46). Therefore, additional components of the microbes must contribute to induction of Th1 cytokines such as IL-12. Our data indicate that dectin-1–mediated recognition of β-glucans found in many fungi (8, 47–49) may focus Th1-type responses by enhancing production of IL-12.
Microbial recognition by innate immune cells occurs through multiple receptors including TLRs and lectins, and the inflammatory consequence of this recognition is dependent on both the repertoire of receptors that are expressed and functional cooperation between the signals generated downstream of receptor activation. These interactions shape the cytokine response and antimicrobial functions of the cell, and tailor the immune response to be effective against specific pathogens. In this context, we have observed that dectin-1 collaborates with TLRs in promoting responses associated with Th1 immunity including production of IL-12 and activation of antimicrobial killing (ROS).
Acknowledgments
We would like to thank Dr. Alan Aderem for helpful conversations and critical reading of the manuscript.
This work was supported by National Institutes of Health grant RO1 GM62995 to D.M. Underhill.
Footnotes
Abbreviations used in this paper: BMDC, bone marrow–derived dendritic cell; ITAM, immunoreceptor tyrosine-based activation motif; NF, nuclear factor; ROS, reactive oxygen species; TLR, Toll-like receptor.
References
- 1.Underhill, D.M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825–852. [DOI] [PubMed] [Google Scholar]
- 2.Henson, P.M., D.L. Bratton, and V.A. Fadok. 2001. Apoptotic cell removal. Curr. Biol. 11:R795–R805. [DOI] [PubMed] [Google Scholar]
- 3.Underhill, D.M., and A. Ozinsky. 2002. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14:103–110. [DOI] [PubMed] [Google Scholar]
- 4.Aderem, A., and R.J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–787. [DOI] [PubMed] [Google Scholar]
- 5.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 6.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 7.Pillemer, L., and E.E. Ecker. 1941. Anticomplementary factor in fresh yeast. J. Biol. Chem. 137:139–142. [Google Scholar]
- 8.Di Carlo, F.J., and J.V. Fiore. 1958. On the composition of zymosan. Science. 127:756–757. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura, K., C. Ishihara, S. Ukei, S. Tokura, and I. Azuma. 1986. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine. 4:151–156. [DOI] [PubMed] [Google Scholar]
- 10.Vecchiarelli, A., M. Puliti, A. Torosantucci, A. Cassone, and F. Bistoni. 1991. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell. Immunol. 134:65–76. [DOI] [PubMed] [Google Scholar]
- 11.Underhill, D.M., A. Ozinsky, A.M. Hajjar, A. Stevens, C.B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 401:811–815. [DOI] [PubMed] [Google Scholar]
- 12.Gold, E.S., N.S. Morrissette, D.M. Underhill, J. Guo, M. Bassetti, and A. Aderem. 2000. Amphiphysin IIm, a novel amphiphysin II isoform, is required for macrophage phagocytosis. Immunity. 12:285–292. [DOI] [PubMed] [Google Scholar]
- 13.Ozinsky, A., D.M. Underhill, J.D. Fontenot, A.M. Hajjar, K.D. Smith, C.B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA. 97:13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, G.D., and S. Gordon. 2001. Immune recognition. A new receptor for beta-glucans. Nature. 413:36–37. [DOI] [PubMed] [Google Scholar]
- 15.Brown, G.D., P.R. Taylor, D.M. Reid, J.A. Willment, D.L. Williams, L. Martinez-Pomares, S.Y. Wong, and S. Gordon. 2002. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willment, J.A., S. Gordon, and G.D. Brown. 2001. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276:43818–43823. [DOI] [PubMed] [Google Scholar]
- 17.Ariizumi, K., G.L. Shen, S. Shikano, S. Xu, R. Ritter III, T. Kumamoto, D. Edelbaum, A. Morita, P.R. Bergstresser, and A. Takashima. 2000. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275:20157–20167. [DOI] [PubMed] [Google Scholar]
- 18.Sobanov, Y., A. Bernreiter, S. Derdak, D. Mechtcheriakova, B. Schweighofer, M. Duchler, F. Kalthoff, and E. Hofer. 2001. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur. J. Immunol. 31:3493–3503. [DOI] [PubMed] [Google Scholar]
- 19.Yokota, K., A. Takashima, P.R. Bergstresser, and K. Ariizumi. 2001. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene. 272:51–60. [DOI] [PubMed] [Google Scholar]
- 20.Hermanz-Falcon, P., I. Arce, P. Roda-Navarro, and E. Fernandez-Ruiz. 2001. Cloning of human DECTIN-1, a novel C-type lectin-like receptor gene expressed on dendritic cells. Immunogenetics. 53:288–295. [DOI] [PubMed] [Google Scholar]
- 21.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 22.Lanier, L.L. 2001. On guard–activating NK cell receptors. Nat. Immunol. 2:23–27. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 25.Hume, D.A., D.M. Underhill, M.J. Sweet, A.O. Ozinsky, F.Y. Liew, and A. Aderem. 2001. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liles, W.C., J.A. Ledbetter, A.W. Waltersdorph, and S.J. Klebanoff. 1995. Cross-linking of CD45 enhances activation of the respiratory burst in response to specific stimuli in human phagocytes. J. Immunol. 155:2175–2184. [PubMed] [Google Scholar]
- 28.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. [DOI] [PubMed] [Google Scholar]
- 29.Ross, G.D. 2000. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit. Rev. Immunol. 20:197–222. [PubMed] [Google Scholar]
- 30.Williams, J.D., N. Topley, H.M. Alobaidi, and M.J. Harber. 1986. Activation of human polymorphonuclear leucocytes by particulate zymosan is related to both its major carbohydrate components: glucan and mannan. Immunology. 58:117–124. [PMC free article] [PubMed] [Google Scholar]
- 31.Astarie-Dequeker, C., E.N. N'Diaye, V. Le Cabec, M.G. Rittig, J. Prandi, and I. Maridonneau-Parini. 1999. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 67:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czop, J.K. 1986. The role of beta-glucan receptors on blood and tissue leukocytes in phagocytosis and metabolic activation. Pathol. Immunopathol. Res. 5:286–296. [DOI] [PubMed] [Google Scholar]
- 33.Pabst, M.J., and R.B. Johnston, Jr. 1980. Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J. Exp. Med. 151:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aderem, A.A., D.S. Cohen, S.D. Wright, and Z.A. Cohn. 1986. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J. Exp. Med. 164:165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]
- 36.Sutterwala, F.S., G.J. Noel, R. Clynes, and D.M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber, J.S., and D.M. Mosser. 2001. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J. Immunol. 166:6861–6868. [DOI] [PubMed] [Google Scholar]
- 38.Kaji, K., S. Takeshita, K. Miyake, T. Takai, and A. Kudo. 2001. Functional association of CD9 with the Fc gamma receptors in macrophages. J. Immunol. 166:3256–3265. [DOI] [PubMed] [Google Scholar]
- 39.Katsumata, O., M. Hara-Yokoyama, C. Sautes-Fridman, Y. Nagatsuka, T. Katada, Y. Hirabayashi, K. Shimizu, J. Fujita-Yoshigaki, H. Sugiya, and S. Furuyama. 2001. Association of FcgammaRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 167:5814–5823. [DOI] [PubMed] [Google Scholar]
- 40.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234. [DOI] [PubMed] [Google Scholar]
- 41.Love, P.E., and E.W. Shores. 2000. ITAM multiplicity and thymocyte selection: how low can you go? Immunity. 12:591–597. [DOI] [PubMed] [Google Scholar]
- 42.Pulendran, B., P. Kumar, C.W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, J.E., A. Warris, E.A. Ellingsen, P.F. Jorgensen, T.H. Flo, T. Espevik, R. Solberg, P.E. Verweij, and A.O. Aasen. 2001. Involvement of CD14 and toll-like receptors in activation of human monocytes by Aspergillus fumigatus hyphae. Infect. Immun. 69:2402–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362–1371. [DOI] [PubMed] [Google Scholar]
- 46.Del Sero, G., A. Mencacci, E. Cenci, C.F. d'Ostiani, C. Montagnoli, A. Bacci, P. Mosci, M. Kopf, and L. Romani. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes. Infect. 1:1169–1180. [DOI] [PubMed] [Google Scholar]
- 47.Cassone, A., P. Marconi, and F. Bistoni. 1987. Cell wall of Candida albicans and host response. Crit. Rev. Microbiol. 15:87–95. [DOI] [PubMed] [Google Scholar]
- 48.Cross, C.E., and G.J. Bancroft. 1995. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and beta-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect. Immun. 63:2604–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kan, V.L., and J.E. Bennett. 1991. Beta 1,4-oligoglucosides inhibit the binding of Aspergillus fumigatus conidia to human monocytes. J. Infect. Dis. 163:1154–1156. [DOI] [PubMed] [Google Scholar]