Abstract
Whether or how the activation of Lck and Fyn during T cell receptor (TCR) signaling is coordinated, and their delivery of function integrated, is unknown. Here we show that lipid rafts function to segregate Lck and Fyn in T cells before activation. Coaggregation of TCR and CD4 leads to Lck activation within seconds outside lipid rafts, followed by its translocation into lipid rafts and the activation of colocalized Fyn. Genetic evidence demonstrates that Fyn activation is strictly dependent on receptor-induced translocation of Lck. These results characterize the interdependence of Lck and Fyn function and establish the spatial and temporal distinctions of their roles in the cellular activation process.
Keywords: Src kinases, T cell, lipid rafts, colocalization, sequential activation
Introduction
It is well established that the initial membrane proximal signals induced upon engagement of the TCR are dependent on two Src family tyrosine kinases, Lck and Fyn (1). The regulation of Lck activation and its delivery of function in MHC class II restricted T cells is well characterized, in part due to the high stoichiometry of its interaction with CD4 (2). The juxtaposition of CD4-Lck with TCR/CD3 results in the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) on CD3 chains, providing docking sites for downstream signaling elements (3).
In contrast, the subcellular distribution of Fyn and the events supporting its activation and delivery of function are not well characterized. The vast majority of Fyn is not constitutively associated with any known ligand. Further, biochemical and genetic evidence demonstrates redundancy of Lck and Fyn function (4, 5). Whether or how the activation and delivery of function by these two kinases is interrelated and/or convergent during the process of TCR/CD3-induced proximal signaling remains obscure.
Recent reports highlight the essential role of specialized cholesterol rich membrane micro-domains, or lipid rafts, in coordinating (6), and sustaining (7), TCR signaling. Here we demonstrate that lipid rafts function to physically segregate Fyn and Lck in T cells before activation. Using antibodies specific for the phosphorylated forms of the regulatory tyrosine residues, Y394 and Y417, the phosphorylation of which are required to achieve full kinase activity of Lck and Fyn, respectively (8, 9), and the direct assessment of kinase activity, we demonstrate a temporal uncoupling of Lck and Fyn activation. Within the first 10 s after coaggregation of TCR and CD4, Lck is activated outside lipid rafts and translocates into lipid rafts where it induces Fyn activation. These results provide the molecular basis for both the spatial and temporal coordination of Lck and Fyn involvement in T cell activation and demonstrate that their roles are inter-dependent and nonredundant.
Materials and Methods
Mice.
6–8-wk-old C57BL/6 male mice were purchased from the The Jackson Laboratory. LGF-transgenic mice on an Lck−/− background have been described previously (10, 11).
Antibodies and Reagents.
Polyclonal rabbit anti–mouse Lck (12), Fyn (13), and pY394 Lck (9) specific antibodies have been described elsewhere. For use in immunoprecipitation, antibodies were covalently coupled to Sepharose 4B. Rabbit anti–mouse pY418 Src, that crossreacts with pY417 Fyn, was purchased from Biosource International. Biotinylated anti–mouse CD4 specific mAb, GK1.5, and biotinylated as well as unconjugated anti–mouse TCRCβ-specific mAb, H57, were purchased from BD Biosciences. Phosphotyrosine specific mAb, 4G10, was purchased from Upstate Biotechnology. Cholera toxin B-HRP (CTB-HRP), Streptavidin, and Brij58 were purchased from Sigma-Aldrich.
Isolation and Activation of Primary T Cells.
Primary CD4+ lymph node T cells were isolated using Mouse CD4 Cell enrichment Immunocolumns (Cedarlane). 0.5–10 × 106 CD4+ cells per sample were precoated with 1 μg/ml of biotinylated anti-TCRCβ and 0.3 μg/ml of biotinylated anti-CD4 for 30 min at 4°C. Cells were washed and resuspended in 30 μl of ice cold PBS and prewarmed to 37°C for 1 min. Coaggregation of TCR and CD4 was achieved by addition of Streptavidin to a final concentration of 50 μg/ml at 37°C. Aliquots were taken from a single tube at the indicated time points, and lysed in various buffers depending on the subsequent analysis.
Isolation of Lipids Rafts.
Preparation of lipid rafts by equilibrium density gradient centrifugation (EDGC) was as described previously (14). 5–40 μl of the indicated fractions were transferred to nitrocellulose and probed for the GM1 ganglioside (GM1) distribution as a marker for lipid rafts using CTB-HRP.
Cell Lysis, Immunoprecipitations, Immunoblotting, and Immune Complex Kinase Assays.
4G10 and pY394 Lck immunoblotting was performed on aliquots of 5 × 105 and 2 × 106 cells, respectively, directly lysed in loading buffer. pY417 Fyn levels were assessed in anti-Fyn immunoprecipitates from 3 × 106 cell equivalents prepared in Fyn lysis buffer: 20 mM Tris, pH 8, 150 mM NaCl, 200 mM Na3VO4, 0.9% NP-40, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 1 mM PMSF, followed by immunoblotting with anti-pY418 Src.
Rafts (R) and soluble (S) fractions were diluted fivefold in Fyn lysis buffer before immunoprecipitation with anti-Fyn. The supernatants were then adjusted with Tris, pH 8, EDTA, pH 8.0, and NaF to final concentrations of 50, 20, and 50 mM, respectively, before Lck immunoprecipitation. Immunopreciptates were split for pY394 Lck or pY417 Fyn immunoblotting and immune complex kinase assays as described previously (13, 15).
Densitometry.
Densitometric analyses were performed on a GS800 densitometer using volume analysis of Quantity One quantification software (Bio-Rad Laboratories). All densitometric values obtained were calculated from nonsaturated signals.
Results
Coaggregation of TCR and CD4 Results in the Sequential Activation of Lck and Fyn.
A model of T cell activation was established enabling the tracking of the activation states of Lck and Fyn during the first seconds after TCR/CD4 engagement. Primary CD4+ T cells were precoated with biotin-conjugated antibodies specific for TCRCβ and CD4, followed by the addition of streptavidin. Coaggregation of TCR and CD4 induces robust global tyrosine phosphorylation of cellular substrates throughout the time course analyzed, peaking at 10 s and subsiding over the ensuing 80 s (Fig. 1 a). Of note is that aggregation of TCRCβ alone did result in an increase in phosphotyrosine content of select substrates and will be discussed further (Fig. 1 a, lane 2).
Figure 1.
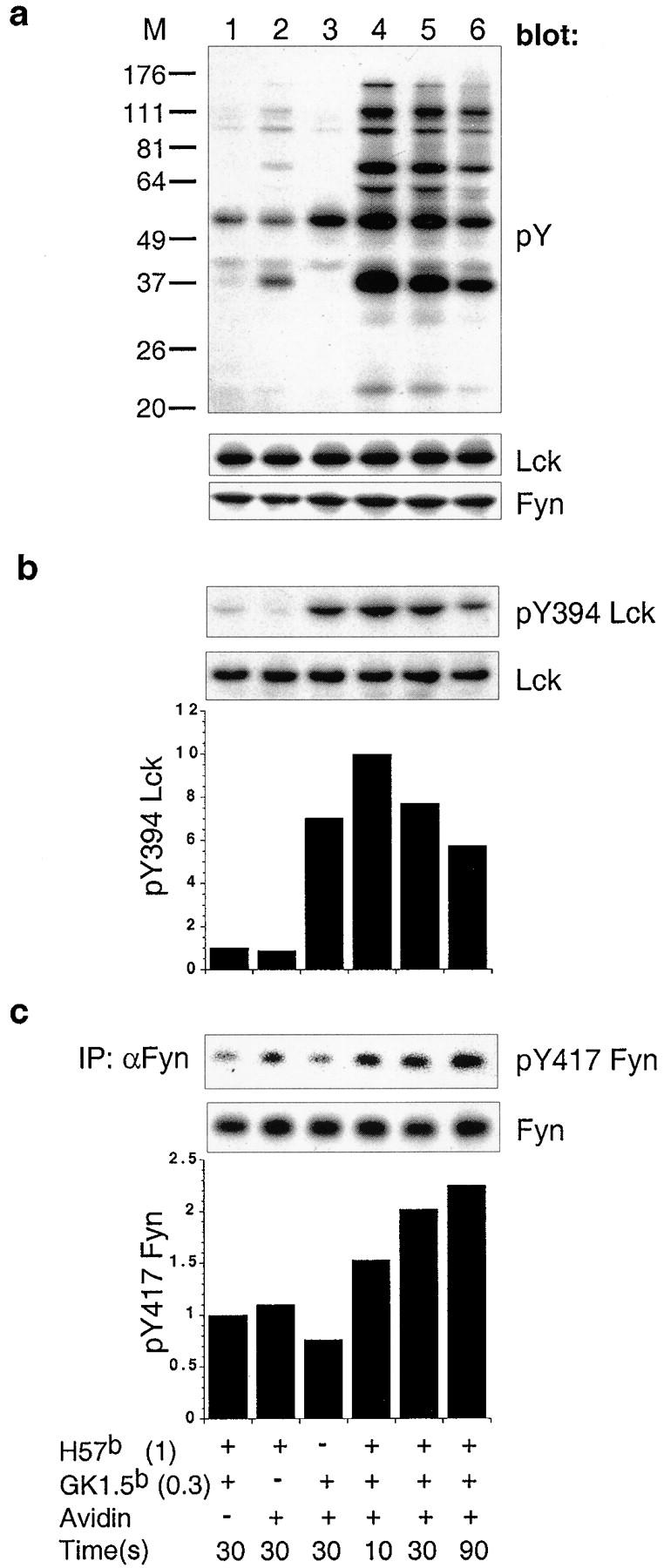
Coaggregation of TCR and CD4 results in sequential activation of Lck then Fyn. (a) Total phosphotyrosyl content of each sample precoated with 1 μg/ml of biotinylated anti-TCRCβ (H57b (1)) and/or 0.3 μg/ml of biotinylated anti-CD4 (GK1.5b (0.3)) and coaggregated with 50 μg/ml of streptavidin for 10, 30, and 90 s, was assessed by immuno-blotting with phosphotyrosine specific mAb. The filter was stripped and successively probed with anti-Lck followed by anti-Fyn. (b) Cell lysates probed with pY394 Lck. The filter was stripped and probed with anti-Lck. (c) Fyn immunoprecipitates were probed with pY418 Src. The filter was stripped and probed with anti-Fyn. Histograms in b and c show the quantification of pY394 Lck and pY417 Fyn normalized to total kinase signals. The nonaggregated control sample was given a reference value “1.” All sample lanes in a, b, and c are aligned over a common legend.
The activities of Lck and Fyn were tracked through the use of antibodies specific for regulatory tyrosine residues Y394 and Y417, respectively (8, 9). Within the first 10 s, TCR/CD4 coaggregation induced a maximum pY394 Lck signal >10-fold higher than that observed in noncoaggregated control cells (Fig. 1 b, lane 4). This signal decreased roughly twofold over the ensuing 80 s (Fig. 1 b, lane 6). Aggregation of CD4 alone induced pY394 Lck (reference 16; Fig. 1 b, lane 3), but not global tyrosine phosphorylation (Fig. 1 a, lane 3). A 1.5-fold increase in pY417 Fyn coincided with the peak induction of pY394 Lck, and continued to increase over the subsequent 80 s (Fig. 1 c).
This analysis reveals the temporal uncoupling of Lck and Fyn activation. We reasoned that a plausible mechanism underpinning this result could be based on the distinct subcellular localization of these two kinases in unstimulated cells.
Lipid Rafts Partition Lck and Fyn in a Cellular Activation-dependent Fashion.
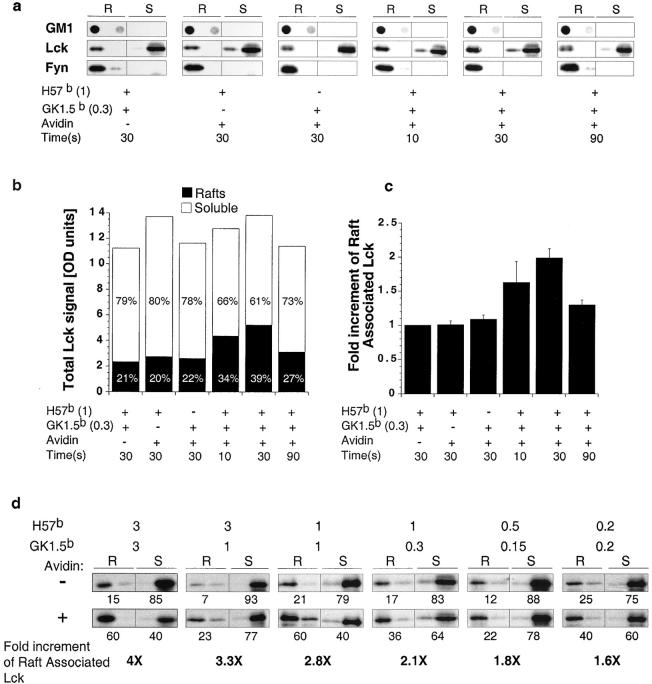
Isolation of lipid rafts from unstimulated CD4+ T cells and analysis of Lck and Fyn distribution reveals that while 75–95% of Lck is found in soluble fractions, >98% of Fyn localizes to lipid rafts (Fig. 2 and Fig. 3 , a and d). Strikingly, within the first 10 s after TCR/CD4 coaggregation the amount of Lck associated with lipid rafts increases, reaching a peak of twofold at 30 s (Fig. 3, a-c). Lck redistribution to lipid rafts is dependent on antibody concentration and can result in up to a fourfold increase at the highest concentrations anti-CD4 and anti-TCRCβ used for coaggregation (Fig. 3 d). Fyn distribution remained unaltered in all conditions analyzed (Fig. 3 a).
Figure 2.
Lck and Fyn localize to different subcellular compartments. Primary CD4+ lymph node T cells were lysed in TKM + 0.5% Brij58 buffer and subjected to sucrose EDGC. The presence of GM1 (top panel), Lck (middle panel), and Fyn (bottom panel) were assessed by probing with CTB-HRP, anti-Lck, and anti-Fyn, respectively.
Figure 3.
Coaggregation of TCR and CD4 induces the translocation of Lck into lipid rafts. (a) 107 primary CD4+ lymph node T cells were precoated with biotinylated anti-TCRCβ (H57b) and/or biotinylated anti-CD4 (GK1.5b) followed by the addition of Streptavidin for 10, 30, or 90 s, as indicated. Distribution of GM1, Lck, and Fyn was assessed in fractions 1–2 and 9–10, representing lipid raft (R) and soluble fractions (S), respectively. (b) Quantification of Lck subcellular distribution. Numbers represent the proportion of total Lck detected in R and S fractions. (c) Quantification of Lck enrichment in lipid rafts. The values represent the mean of three independent experiments performed as described for panel a with one SD indicated. (d) 107 primary CD4+ lymph node T cells were precoated with the indicated concentrations of H57b and GK1.5b and coaggregated or not by the addition of Streptavidin for 30 s. Distribution of Lck was assessed as described above. Quantification of the signals revealed was performed by densitometric analysis.
Since the total Lck signal detected throughout the time course (Fig. 3 b) is not significantly altered, the enrichment of lipid raft-associated Lck reflects translocation of Lck from the soluble fraction. Moreover, this translocation is predicated by TCR/CD4 coaggregation, as coaggregation of neither CD28 nor MHC class I with TCR perturbed the subcellular distribution of Lck (not shown). Further, aggregation of TCRCβ alone has no impact on either Lck activity (Fig. 1 b, lane 2), or distribution (Fig. 3, a–c), and while aggregation of CD4 alone does result in an increase in pY394 Lck (Fig. 1 b, lane 3), it does not alter the subcellular distribution of Lck (Fig. 3, a–c). These results characterize Lck as a mobile signaling element during the first seconds after TCR/CD4 coaggregation.
Activation and Subcellular Redistribution of Lck Regulates Sequential Fyn Activation.
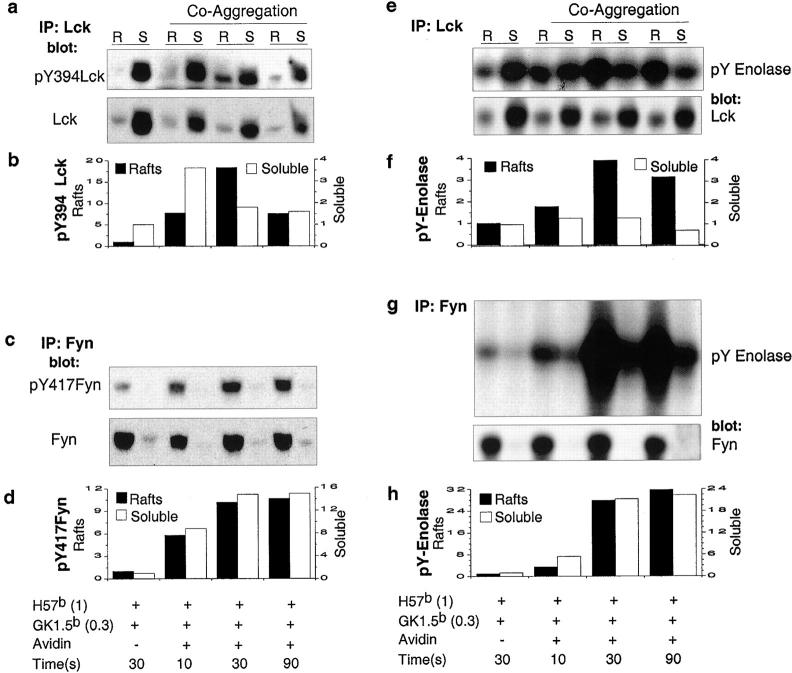
The temporal and spatial relationships governing the activation of Lck and Fyn were characterized by analyzing the kinetics of induction and subcellular distribution of pY394 Lck and pY417 Fyn in soluble fractions and lipid rafts isolated from primary T cells over the coaggregation time course. Virtually all pY394 Lck resides in the soluble fraction in cells before activation (Fig. 4 a). Of note is that this pY394 Lck signal is consistent with the basal kinase activity of Lck in unstimulated primary T cells, as previously reported (15). At 10 s after TCR/CD4 coaggregation there is a >3-fold increase in the level of pY394 Lck, that decreases to basal levels by 90 s (Fig. 4, a and b). pY394 Lck in lipid rafts increases to 7- and 18-fold over unstimulated levels at 10 and 30 s after coaggregation, respectively (Fig. 4, a and b).
Figure 4.
Lck-dependent Fyn activation is spatially and temporally regulated. Lck and Fyn immunoprecipitates from lipid rafts (R) and soluble fractions (S) derived from noncoaggregated control samples and those aggregated for the indicated time were probed with pY394 Lck (a, top panel) and pY417 Fyn (c, top panel), respectively. Both filters were stripped and probed with anti-Lck (a, bottom panel) or anti-Fyn (c, bottom panel), respectively. Quantification of pY394 Lck (b) and pY417 Fyn (d) were determined subsequent to normalizing for the presence of total Lck and Fyn. In parallel, immune complex kinase assays were performed using immunoprecipitates from each of the above samples. The phospho-enolase signals (pY Enolase) for Lck (e, top panel) and Fyn (g, top panel) were normalized to total Lck (e, bottom panel) and Fyn (g, bottom panel) content, respectively. Specific kinase activity was expressed as a ratio between pY Enolase and total Lck (f) and Fyn (h), respectively. Levels of pY394 Lck and pY417 Fyn as well as their specific kinase activities in nonaggregated control samples, were given a reference value “1.” All sample lanes in a–d and e–h are aligned over common figure legends. Bars representing R and S fractions from the same sample are grouped.
Lipid raft-associated pY417 Fyn increases with the first detectable translocated pY394 Lck (Fig. 4, c and d). At 10 s after TCR/CD4 coaggregation pY417 Fyn levels are fivefold over those observed in unstimulated cells, and increase to 10-fold by 90 s (Fig. 4, c and d). Longer exposure of pY417 Fyn immunoblots reveal that the small pool of nonraft associated Fyn also increases pY417 content with kinetics similar to raft associated Fyn (Fig. 4, c and d).
Immune complex kinase assays were performed in parallel with the above experiments and yielded convergent results. A 1.2–1.3-fold increase in kinase active Lck in the soluble fraction at 10 s after TCR/CD4 coaggregation is observed (Fig. 4, e and f). Consistent with the kinetics of TCR/CD4 induced redistribution of pY394 Lck into rafts, the appearance of raft-associated Lck kinase activity initiates within 10 s, and peaks at 30 s (Fig. 4, e and f). The effect of the redistribution of kinase active Lck on the activity of raft-associated Fyn was profound. Fyn kinase activity increased roughly 2.5-fold over basal levels at 10 s, and 19- and 23-fold at 30 and 90 s, respectively (Fig. 4, g and h). Fyn kinase activity in the soluble fraction increased in tandem (Fig. 4, g and h).
Genetic Evidence for Lck-dependent Fyn Activation.
Formal demonstration that the activation of raft-associated Fyn is dependent on the translocation of Lck is derived from the use of conditional Lck-deficient animals. These animals express an Lck transgene under the control of the Lck proximal promoter, and provide a model system in which the fundamental roles of Lck in intrathymic T cell development (10), and mature T cell TCR signaling (11), are uncoupled (17). Thus, while immature thymocytes are Lck sufficient, upon their maturation expression of the Lck transgene is down-regulated and the resultant mature peripheral T lymphocytes are severely deficient in Lck expression (11).
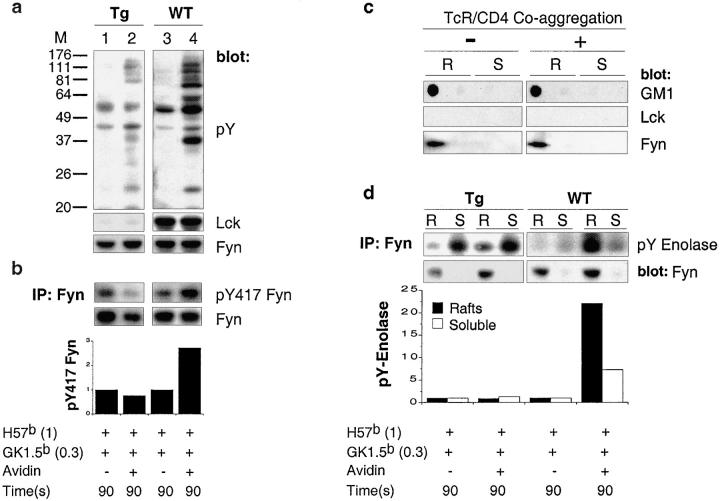
Less than 1% of the Lck signal observed in wild-type cells was detected in either lysates (Fig. 5 a) or in lipid rafts (Fig. 5 c) prepared from CD4+ transgenic peripheral T cells. This impacts profoundly on the induction of global tyrosine phosphorylation observed at 90 s after TCR/CD4 coaggregation (Fig. 5 a). There is induction of phosphorylation of select substrates in these transgenic T cells, specifically those induced in Lck sufficient cells upon the aggregation of TCRCβ alone (compare Figs. 1 a, track 2, and 5 a, track 2). This result is consistent with the role of CD3 associated Fyn. Importantly, the lack of Lck precluded the induction of pY417 Fyn observed in Lck sufficient T cells upon TCR/CD4 coaggregation (Fig. 5 b).
Figure 5.
Activation of lipid raft-associated Fyn is Lck dependent. TCRCβ and CD4 were coaggregated on CD4+ lymph node cells derived from wild-type (WT) or LGF+Lck−/− (Tg). (a) Total phosphotyrosyl content of each sample was assessed by immunoblotting with phophotyrosine-specific mAb (top panel). The filter was stripped and successively probed with anti-Lck (middle panel) followed by anti-Fyn (bottom panel). (b) Fyn immunoprecipitates were probed with pY418 Src and developed with Protein A-HRP (top panel). The filter was stripped and probed with anti-Fyn (middle panel). Quantification of pY417 Fyn normalized to total Fyn (bottom panel). (c) Distribution of GM1, Lck and Fyn in sucrose EDGC fractions derived from Tg cells subjected or not to streptavidin-mediated aggregation for 90 s. (d) Fyn immunoprecipitates derived from R and S fractions of WT and Tg cells were subjected to immune complex kinase assays. The phospho-enolase signals (pY Enolase) for Fyn (top panel) were normalized to total Fyn content (middle panel) and specific kinase activity was expressed as a ratio between pY Enolase and total Fyn (bottom panel). Bars representing R and S fractions from the same sample are grouped. Levels of pY417 Fyn as well as Fyn specific kinase activity in nonaggregated control samples were given a reference value “1.” Sample lanes in panels a and b are aligned over a common figure legend.
Fyn distribution in these transgenic T cells was identical to that observed in wild-type cells (Fig. 5 c). However, the kinase activity of raft-associated Fyn did not increase over control at 90 s after TCR/CD4 coaggregation, at which point there is a 20-fold increase in wild-type cells (Fig. 5 d). The pool of Fyn observed in the soluble fractions derived from transgenic T cells was constitutively active, and in contrast to observations in wild-type cells, this activity did not increase upon TCR/CD4 coaggregation (Fig. 5 d). The mechanism(s) underpinning this phenotype remain obscure. Taken together, these results provide formal proof that the activation of the majority of cellular Fyn, that which is raft-associated, is strictly dependent on receptor induced translocation of kinase active Lck into lipid rafts.
Discussion
The results presented are the first to characterize the distinct yet interdependent roles of Lck and Fyn in the propagation of signals emanating from the T cell antigen receptor. Lipid rafts are shown to spatially segregate 75–95% of Lck from >98% of Fyn in unstimulated primary CD4+ T cells. This might suggest parallel yet independent functions regulated by these two kinases. However, the demonstration of TCR/CD4-induced translocation of activated Lck into lipid rafts, and the ensuing Lck-dependent activation of colocalized Fyn, supports the conclusion that their functions are interdependent, yet tightly regulated.
While the biochemical analyses undertaken in this study precluded the use of antigen mediated TCR/CD4 coaggregation, recent studies demonstrate that antigen-dependent and specific T cell–APC conjugation does result in the translocation of TCR, CD4, and Lck, into lipid rafts (7, 18). Further, the recent demonstration that antigen mediated induction of pY394 Lck and its recruitment to the T cell-APC interface (9, 19), supports the conclusion that the antibody mediated TCR/CD4 coaggregation model used in this study accurately recapitulates antigen induced changes in Lck physiology. Moreover, as 75–95% of cellular Lck is CD4 associated, and CD4 is excluded in the vast majority from lipid rafts in primary unstimulated T cells, CD4 could be viewed as the “gate keeper” maintaining Lck outside of lipid rafts. Whether or how alterations in palmitoylation of CD4 and Lck induced upon activation supports their translocation into lipid rafts remains to be determined.
As previously reported, sustaining pY394 Lck during the first minutes of antigen-mediated T cell activation is dependent on CD28 signaling (9). The results presented here are consistent. Levels of raft-associated pY394 Lck and associated kinase activity peak at 30 s after TCR/CD4 coaggregation, and fall rapidly over the next 60 s. In contrast, pY417 Fyn and associated kinase activity increased in parallel throughout the time course of coaggregation. This demonstrates that in contrast to Lck, sustaining Fyn activity appears CD28 independent over the time course tested, and may reflect the recruitment of SHP-1 to lipid rafts, functioning as an Lck-specific phosphatase (20). The differential persistence of Fyn kinase activity suggests that the interdependence of Lck and Fyn is unidirectional. Specifically, while Fyn activation is preceded by the translocation of activated Lck, active Fyn appears not to impact on the activity of colocalized Lck.
Two key molecules are involved in regulating pY levels of critical tyrosine residues on Fyn and Lck. The COOH-terminal Src kinase (Csk) phosphorylates negative regulatory COOH-terminal tyrosines, and the CD45 phosphatase opposes this action (1). Some CD45 is associated with lipid rafts in unstimulated T cells (21), and in light of the present and a previous report (22), Fyn is likely the predominant target. Counterbalancing this activity could be maintained through negative feedback involving the opposing action of Csk. Thus, Fyn-mediated phosphorylation of raft-associated Cbp/PAG would provide a Csk docking site (22). The balance of CD45/Csk activities in maintaining basal Fyn function in unstimulated cells would be disrupted by TCR/CD4-induced translocation of active Lck into rafts, culminating in the amplification of Fyn activity.
The results presented here demonstrate that Lck-dependent Fyn activation is initiated before the formation of a stabilizing immunological synapse. This is consistent with the increase of both pY394 Lck and pY319 ZAP70 within minutes of TCR engagement at the periphery of the T cell-APC interface (19). Thus, lipid rafts play a central role in supporting the full sequelae of signals preceding the formation of the immunological synapse. During the first seconds, in parallel with Lck-mediated hyperphosphorylation of CD3-ζ and the de novo recruitment and activation of ZAP70 (3), the translocation of activated Lck into lipid rafts would initiate a scaffolding process through the sequential activation of Fyn, its ensuing phosphorylation of Pyk2 and Fyb (23, 24), and initiation of the cascade culminating in de novo IL-2 transcription (24). This would be accompanied by aggregation of lipid rafts, shown to be dependent on raft-associated Lck (25). The formation of the immunological synapse through the coalesence of these raft aggregates would sustain signaling that ultimately leads to T cell proliferation (7).
Acknowledgments
This work was supported by a grant from the Canadian Institute of Health Research.
S.D. Levin's present address is Zymogenetics, Seattle, WA 98102.
References
- 1.Hermiston, M.L., Z. Xu, R. Majeti, and A. Weiss. 2002. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J. Clin. Invest. 109:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham, N., M.C. Miceli, J.R. Parnes, and A. Veillette. 1991. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 350:62–66. [DOI] [PubMed] [Google Scholar]
- 3.Nel, A.E. 2002. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J. Allergy Clin. Immunol. 109:758–770. [DOI] [PubMed] [Google Scholar]
- 4.Chan, A.C., M. Iwashima, C.W. Turck, and A. Weiss. 1992. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 71:649–662. [DOI] [PubMed] [Google Scholar]
- 5.Groves, T., P. Smiley, M.P. Cooke, K. Forbush, R.M. Perlmutter, and C.J. Guidos. 1996. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 5:417–428. [DOI] [PubMed] [Google Scholar]
- 6.Janes, P.W., S.C. Ley, A.I. Magee, and P.S. Kabouridis. 2000. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23–34. [DOI] [PubMed] [Google Scholar]
- 7.Bromley, S.K., W.R. Burack, K.G. Johnson, K. Somersalo, T.N. Sims, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. [DOI] [PubMed] [Google Scholar]
- 8.Veillette, A., and M. Fournel. 1990. The CD4 associated tyrosine protein kinase p56lck is positively regulated through its site of autophosphorylation. Oncogene. 5:1455–1462. [PubMed] [Google Scholar]
- 9.Holdorf, A.D., K.H. Lee, W.R. Burack, P.M. Allen, and A.S. Shaw. 2002. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat. Immunol. 3:259–264. [DOI] [PubMed] [Google Scholar]
- 10.Trobridge, P.A., K.A. Forbush, and S.D. Levin. 2001. Positive and negative selection of thymocytes depends on Lck interaction with the CD4 and CD8 coreceptors. J. Immunol. 166:809–818. [DOI] [PubMed] [Google Scholar]
- 11.Trobridge, P.A., and S.D. Levin. 2001. Lck plays a critical role in Ca(2+) mobilization and CD28 costimulation in mature primary T cells. Eur. J. Immunol. 31:3567–3579. [DOI] [PubMed] [Google Scholar]
- 12.Veillette, A., I.D. Horak, and J.B. Bolen. 1988. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 2:385–401. [PubMed] [Google Scholar]
- 13.Davidson, D., L.M. Chow, M. Fournel, and A. Veillette. 1992. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J. Exp. Med. 175:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmor, M.D., and M. Julius. 2001. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood. 98:1489–1497. [DOI] [PubMed] [Google Scholar]
- 15.Haughn, L., B. Leung, L. Boise, A. Veillette, C. Thompson, and M. Julius. 1998. Interleukin 2-mediated uncoupling of T cell receptor alpha/beta from CD3 signaling. J. Exp. Med. 188:1575–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo, K.X., and B.M. Sefton. 1990. Cross-linking of T-cell surface molecules CD4 and CD8 stimulates phosphorylation of the lck tyrosine protein kinase at the autophosphorylation site. Mol. Cell. Biol. 10:5305–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen, J.M., K.A. Forbush, and R.M. Perlmutter. 1992. Functional dissection of the lck proximal promoter. Mol. Cell. Biol. 12:2758–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balamuth, F., D. Leitenberg, J. Unternaehrer, I. Mellman, and K. Bottomly. 2001. Distinct patterns of membrane microdomain partitioning in Th1 and th2 cells. Immunity. 15:729–738. [DOI] [PubMed] [Google Scholar]
- 19.Lee, K.H., A.D. Holdorf, M.L. Dustin, A.C. Chan, P.M. Allen, and A.S. Shaw. 2002. T cell receptor signaling precedes immunological synapse formation. Science. 295:1539–1542. [DOI] [PubMed] [Google Scholar]
- 20.Chiang, G.G., and B.M. Sefton. 2001. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 276:23173–23178. [DOI] [PubMed] [Google Scholar]
- 21.Edmonds, S.D., and H.L. Ostergaard. 2002. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J. Immunol. 169:5036–5042. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda, K., M. Nagafuku, T. Shima, M. Okada, T. Yagi, T. Yamada, Y. Minaki, A. Kato, S. Tani-Ichi, T. Hamaoka, and A. Kosugi. 2002. Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk- binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 169:2813–2817. [DOI] [PubMed] [Google Scholar]
- 23.Katagiri, T., T. Takahashi, T. Sasaki, S. Nakamura, and S. Hattori. 2000. Protein-tyrosine kinase Pyk2 is involved in interleukin-2 production by Jurkat T cells via its tyrosine 402. J. Biol. Chem. 275:19645–19652. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths, E.K., and J.M. Penninger. 2002. Communication between the TCR and integrins: role of the molecular adapter ADAP/Fyb/Slap. Curr. Opin. Immunol. 14:317–322. [DOI] [PubMed] [Google Scholar]
- 25.Valensin, S., S.R. Paccani, C. Ulivieri, D. Mercati, S. Pacini, L. Patrussi, T. Hirst, P. Lupetti, and C.T. Baldari. 2002. F-actin dynamics control segregation of the TCR signaling cascade to clustered lipid rafts. Eur. J. Immunol. 32:435–446. [DOI] [PubMed] [Google Scholar]