Abstract
Adhesion of bone cells to the extracellular matrix is a crucial requirement for osteoblastic development and function. Adhesion receptors connect the extracellular matrix with the cyto-skeleton and convey matrix deformation into the cell. We tested the hypothesis that sex hormones modulate mechanoperception of human osteoblastic cells (HOB) by affecting expression of adhesion molecules like fibronectin and the fibronectin receptor. Only dihydrotestosterone (DHT), but not 17β-estradiol, stimulated fibronectin (137%) and fibronectin receptor (252%) protein expression. The effects of deformation strain on HOB metabolism were investigated in a FlexerCell® strain unit. Cyclically applied strain (2.5% elongation) increased DNA synthesis (125%) and interleukin-6 (IL-6) production (170%) without significantly affecting alkaline phosphatase (AP) activity, type I collagen (PICP), or osteoprotegerin (OPG) secretion. 10 nM DHT pretreatment abolished the mitogenic response of HOB to strain and increased AP activity (119%), PICP (163%), and OPG production (204%). In conclusion, mechanical strain stimulates bone remodeling by increasing HOB mitosis and IL-6 production. DHT enhances the osteoanabolic impact of deformation strain by increasing bone formation via increased AP activity and PICP production. At the same time, bone resorption is inhibited by decreased IL-6 and increased OPG secretion into the bone microenvironment.
Keywords: mechanoperception, osteoblasts, fibronectin, integrins, androgens
Introduction
Human bone cells are anchored to the organic bone matrix via adhesion receptors of the integrin superfamily. Integrin receptors are cell surface glycoproteins consisting of an α-chain that is noncovalently linked to a β-chain (1). Among the various subunits, α5 and β1 are the most common integrins of bone cells that constitute the fibronectin receptor (FNR) (2). FNRs of human bone cells interact with abundant bone matrix proteins like fibronectin (FN) and type I collagen via a minimal tripeptide sequence (Arg-Gly-Asp) (1, 3). They connect the extracellular matrix to the actin cytoskeleton via cytoskeleton-associated proteins (4). Deformation strain of bone tissue by external mechanical stress can thus be transformed into morphological changes of the cytoskeleton and of cellular shape (5). Therefore, integrins are excellent candidates for mechanoreceptors of bone, physically connecting the extracellular bone matrix via actin-associated proteins within focal adhesions with cytoskeletal microfilaments. This physical link between extracellular matrix and the cytoskeleton enables osteoblasts to respond more rapidly to deformation strain than could be provided by any diffusion-based signaling system. Mechanotransduction via the β1-integrin subunit enhances tyrosine phosphorylation of proteins physically associated with the cytoskeleton and increases the activation of the focal adhesion kinase (FAK) and mitogen-activated protein (MAP) kinase (6). Therefore, mechanotransduction via β1-integrins may affect metabolic functions of human osteoblastic cells (HOB) that are anchored to the organic bone matrix when external mechanical strain is exerted onto bone tissue. Interestingly, the application of cyclic deformation strain to human osteoblastic cells in vitro increased cell number and collagen production (7), which provides a physiological basis for the positive impact of mechanical strain on bone metabolism. Several years ago, Harold Frost (8) advanced the hypothesis that postmenopausal bone loss is the consequence of an impaired ability of bone tissue to perceive the osteoanabolic impact of mechanical strain (8). Frost's hypothesis implies that sex steroids modulate the sensitivity of bone tissue to perceive mechanical strain, which may be due to an involvement of sex steroid receptors in the adaptive responses of osteoblasts to mechanical strain (9, 10). The observation of decreased bone density in long distance running athletes with amenorrhea compared with eumenorrheic athletes is consistent with an important osteoanabolic interaction of mechanical strain and sex steroids (11). In this paper, we tested the possibility that sex steroids regulate the response of human osteoblastic cells to deformation strain by improving osteoblastic anchorage via stimulating gene expression of the most abundant osteoblastic adhesion molecule, the FNR.
Materials and Methods
Cells.
Primary HOB were obtained from femoral bone biopsies of healthy adults who underwent trauma surgery (12). Cells originating from male and female donors, unless otherwise stated, were cultured in DMEM supplemented with 10% (vol/vol) newborn calf serum, and 1% (vol/vol) penicillin/streptomycin. This work was approved by the local ethics commission of the University of Heidelberg.
Stock Solutions and In Vitro Incubation.
10-mM stock solutions of 5α-dihydrotestosterone (DHT) and 17β-estradiol (E2; Sigma-Aldrich) were prepared in ethanol. PP1 (Alexis Biochemicals) was dissolved in DMSO as a 10-mM solution. All experiments were performed in phenol red–free and serum-free DMEM. The maximal final solvent concentration was 0.005%.
Measurement of FN and FNR Expressed by HOB In Vitro.
HOB of both genders were grown in the presence of either DHT or E2 (1–100 nM) for 72 h. Triton extracts (0.1% Triton X-100 in 12.5 mM Tris, 12.5 mM NaHCO3, and 0.01% sodium azide, pH 7.0) were analyzed for FN using a fibronectin EIA kit (TaKaRa Shuzo Co., Ltd) and normalized to cellular protein content (nanogram FN per milligram protein). Protein was quantified using the BCA protein assay kit (Pierce Chemical Co.). The β1 subunit of the FNR was quantified with a fibronectin receptor EIA kit (TaKaRa Shuzo Co., Ltd) and also normalized to cellular protein (nanogram FNR per milligram protein).
Indirect Immunofluorescence.
For indirect immunofluorescence, HOB were seeded on glass slides and grown to 75% confluence. Cells were treated with 10 nM DHT or the solvent for 24 h and fixed in ice-cold 80% methanol for 5 min, followed by incubation in acetone (5 min). The primary antibody, specific for β1-integrin (CHEMICON International, Inc.), was applied overnight at 4°C. Cells were washed in PBS and incubated with a fluorochrome-conjugated antibody (MoBiTec) for 1 h at room temperature. Slides were photographed using a Leica DMRE microscope.
RNA Isolation and Northern Blot.
Total RNA was isolated from cultured HOB of male and female donors using the TRIzol® method (GIBCO BRL). 15 μg RNA/lane was separated on a 1.2% formaldehyde-MOPS agarose gel and transferred to Hybond™-N (Amersham Biosciences). The cDNA probes were prepared by reverse transcription of total RNA (Thermoscript; GIBCO BRL) followed by a gene-specific PCR. Primer sequences for FNR amplification (β1 subunit) were as follows: for FNR-F, 5′-ATCCCAGAGGCTCCAAAGATA-3′; and for FNR-R, 5′-TGGTGCAGTTCTGTTCACTTG-3′ (GIBCO BRL). Annealing was performed at 47°C. Expected fragment size was 399 bp. Primers for GAPDH amplification were as follows: for GAPDH-F, 5′-ACCACAGTCCATGCCATCAC-3′ and for GAPDH-R, 5′-TCCACCACCCGTTTGCTGTAG-3′ (Sigma-Aldrich). Annealing temperature was 58°C. Expected fragment size was 450 bp. DNA probes were denatured and labeled with α-[32P]-dCTP (Amersham Biosciences) using the Prime-a-Gene labeling kit (Promega). Hybridization and evaluation of autoradiographs were performed as described previously (12).
Western Blot Analysis.
HOB from male and female donors were grown to 90% confluence and lysed with 1 mM sodium orthovanadate, 1% SDS, and 10 mM Tris, pH 7.4. Extracts were denatured and mixed 1:2 with loading buffer (160 mM Tris-HCl, pH 6.9, 20% glycerol, 4% SDS, 200 mM dithiothreitol, and 0.01% bromophenol blue). 30 μg protein/lane was separated on an SDS-PAGE gel and transferred to Hybond™-P (Amersham Biosciences). After blocking with 5% nonfat dry milk in TBS-T (Tris-buffered saline, pH 7.6, 0.1% Tween 20), the membrane was incubated with the antiintegrin β1-antibody (Transduction Laboratories), followed by thorough rinsing in TBS-T and treatment with the peroxidase-linked secondary antibody (Amersham Biosciences). Chemiluminescent detection was performed with the ECL™ + Plus reagent (Amersham Biosciences).
Adhesion of HOB to FN-coated Plates.
HOB of both genders were treated with 1–100 nM DHT or the solvent for 72 h. Cells were scraped off the dish and plated onto FN-coated 6-well plates. 4 h later, nonadherent cells were removed by rinsing the wells twice with PBS. Adherent cells were trypsinized and counted in a hemocytometer.
Binding of FN-coated Latex Beads to HOB.
Latex beads (11.9–25.7 μm in diameter; Sigma-Aldrich) were coated with 2 mg FN/ml PBS for 24 h and added to the DHT- or solvent-treated cultures at a density of 1,000 cells/well (in 24-well plates). After 4 h, supernatants together with unbound latex beads were removed and cells were washed with PBS. 1 M NaOH was added and the remaining latex beads were counted in a hemocytometer.
Application of Deformation Strain.
Application of deformation strain was performed with a Flexercell® strain unit (model FX-4000™; Flexcell International). This system uses a vacuum to apply defined deformation to cells attached on flexible-bottomed 6-well plates coated with type I collagen (BioFlex®). Male HOB cultures were plated at a density of 100,000 cells/well. After 24 h, the medium was changed and the plates, containing a uniformly distributed cell layer within each well, were inserted into the baseplate of the strain device and strained according to the programmed regimen. Control plates were left unstrained for the same period of time. Strain (2.5% elongation = 25,000 μstrain = 17 kPa) was intermittently applied (1-h strain, 3-h rest) for 48 h in a sinus wave with 5-s increase and 5-s decrease. The results are expressed in percentages of the unstrained controls ± SD. Pretreatment with 10 nM DHT was performed for 24 h. 25 ng/ml PP1 was directly added into the medium before strain application. The compounds were present during the following 48-h experimental period.
DNA Synthesis Assay.
6 h before the end of the strain regimen, 2 μCi/ml methyl-[3H]thymidine (NEN Life Science Products) was added to each well and the regimen was continued. Cells were washed with PBS and lysed with 0.25 M NaOH. Incorporated tritium was determined in a scintillation counter and is expressed as counts per minute/well.
Alkaline Phosphatase Activity.
Cells were lysed with 500 μl Triton buffer/well. The alkaline phosphatase (AP) activity was determined by mixing 50 μl Triton extract, 50 μl H2O, and 200 μl of a solution containing 30 mM paranitrophenylphosphate, 150 mM bicarbonate buffer, and 1 mM MgCl2, pH 10.3. We measured the change in absorbency at 405 nm against a reference wavelength of 492 nm after 30-min incubation at 37°C. The specific AP activity is expressed in milliunits per milligram of cellular protein.
Type I Procollagen Peptide Secretion.
Type I procollagen peptide (PICP) released into the culture medium was determined as a parameter for type I collagen synthesis. It was quantified in the supernatants of HOB cultures using a PICP [125I]-labeled radioimmunoassay kit (Orion Diagnostica). The remaining cell layer was rinsed with PBS and dissolved by the addition of Triton buffer. Secreted PICP is expressed as nanogram per milligram of cellular protein.
Osteoprotegerin and IL-6.
Quantitative determination of secreted osteoprotegerin (OPG) was conducted using an OPG-ELISA assay kit (Immundiagnostik). IL-6 was quantified in the medium using an IL-6 ELISA assay kit (R&D Systems). Both glycoproteins are expressed as picogram per milligram cellular protein.
Results
Effects of DHT and E2 on FN and FNR Expression.
First, we examined the effects of DHT and E2 on FN and FNR expression to test the possibility that sex steroids affect osteoblastic anchorage. DHT (Table I) but not E2 (not depicted) dose-dependently stimulated FN and FNR synthesis in male and female cells. The potent stimulation of FNR protein expression by DHT is visualized in Fig. 1 . In solvent-treated HOB cultures, functional active integrin β1 subunit of FNR was faintly detectable and the fluorescence appeared in an array-like pattern within the cytoplasm or at the cell margins, respectively (Fig. 1 A). In marked contrast, DHT-stimulated cultures exhibited a more pronounced fluorescent signal. Herein, integrin β1 immunolocalization clearly decorated the cytoplasm (Fig. 1 B), suggesting an enhancement of ligand engagement of the integrin at the cell undersurface.
Table I.
Effects of DHT Treatment (72 h) on FN and FNR Production by HOB
| DHT | FN | FNR |
|---|---|---|
| nM | % control ± SD | % control ± SD |
| 1 | 110 ± 5 | 158 ± 4a |
| 10 | 130 ± 12a | 226 ± 15a |
| 100 | 137 ± 7a | 252 ± 21a |
The results are representative for male and female HOB. Control: for FN, 30 ng/mg protein; for FNR, 7.5 ± 1.2 ng/mg protein. Mean ± SD, n = 6.
P < 0.01.
Figure 1.
Detection of functional active β1-integrin in HOB from a 39-yr-old man by indirect immunofluorescence. While in solvent-treated cells, the β1 subunit was faintly detectable at the perinuclear region and the cytoplasm and cell margins, respectively (A, arrowheads), fluorescence appeared reinforced after 24-h DHT (10 nM) treatment (B, arrowheads). Bar, 25 μm.
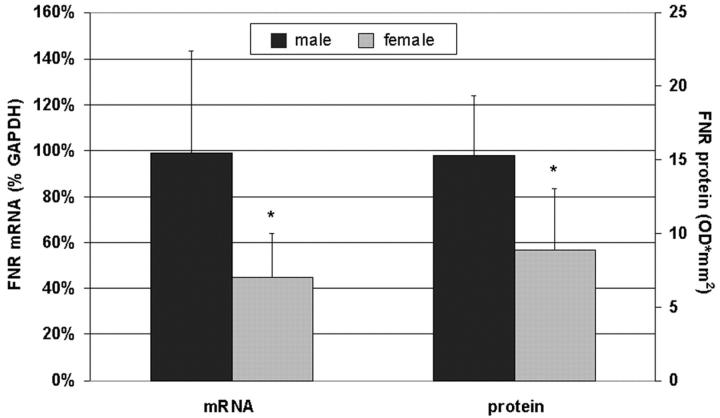
Because DHT increased FNR gene expression in HOB cultures in vitro, we reasoned that untreated primary HOB isolated from male donors (with higher androgen levels compared with females) ex vivo also exhibit a higher FNR gene expression than HOB from female donors. Indeed, male HOB expressed significantly higher FNR mRNA and FNR protein levels compared with female HOB (Fig. 2) .
Figure 2.
Comparison of FNR mRNA (Northern blot) and protein (Western blot) expression in primary HOB from five male and seven female donors, respectively. Mean ± SD, unpaired two-tailed t test. *, P (of male vs. female level) < 0.05.
Effect of DHT on Adhesion of HOB to FN-coated Surfaces.
To test the functional significance of an increased FNR gene expression, we determined the number of HOB adhering to FN-coated culture vessels after pretreatment with DHT for 72 h. DHT dose-dependently increased the number of cells adhering to the FN-coated surface (Table II). In addition, DHT pretreatment significantly increased the number of FN-coated latex beads adhering to HOB (Table II), indicating enhanced anchorage of HOB to FN-coated surfaces.
Table II.
Effects of DHT Treatment (72 h) on HOB Adhesion to FN-coated Surfaces and on Binding of FN-coated Latex Beads to HOB Cultures
| DHT | HOB adhesion to FN-coated plates |
Binding of FN-coated latex beads to HOB |
|---|---|---|
| nM | % control ± SD | % control ± SD |
| 1 | 182 ± 10a | 126 ± 9a |
| 10 | 184 ± 22a | 140 ± 8a |
| 100 | 254 ± 21a | 161 ± 13a |
The results are representative for male and female HOB. Control: for HOB adhesion, 96,700 ± 8,200 cells; for latex beads, 119 ± 9 beads. Mean ± SD, n = 6.
P < 0.01.
Effects of Elongation Strain on HOB Metabolism with/without DHT Pretreatment.
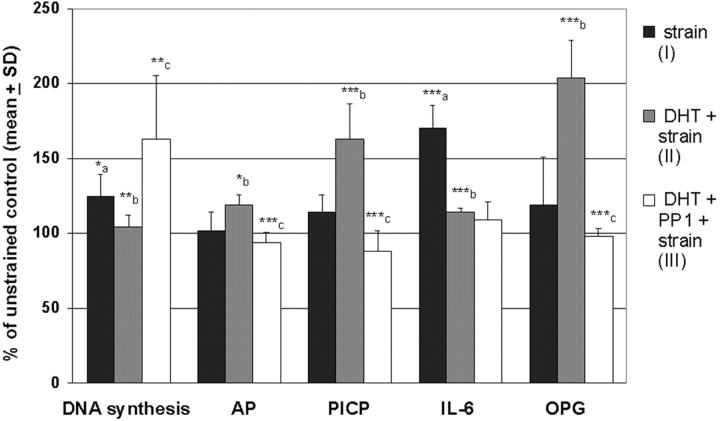
HOB from male individuals were used for the following experiments because male HOB possess higher FNR gene expression than female cells (Fig. 2), and a tight attachment appears crucial for the subsequent strain application experiments. The cultures were exposed to 2.5% elongation strain in order to study the effects of androgens on the osteoblastic response induced by cellular deformation. This strain pattern stimulated DNA synthesis and had a potent stimulatory effect on the IL-6 production, both evidence for a high turnover state of bone metabolism, whereas there were no significant effects on AP activity, type I collagen, and OPG production (Fig. 3, I) . However, pretreatment with 10 nM DHT markedly reduced the stimulatory effects of elongation strain on DNA synthesis and IL-6 production but increased AP activity, type I collagen, and OPG secretion (Fig. 3, II). The presence of the specific FAK inhibitor PP1 (Fig. 3, III) enhanced DNA synthesis but abolished the stimulatory effects of DHT pretreatment plus strain on AP, PICP, and OPG and did not affect IL-6 secretion. Interestingly, when repeating these experiments using female HOB, we did not observe reproducibly significant effects (unpublished data).
Figure 3.
Effect of intermittently applied elongation strain (2.5%; 1-h strain, 3-h rest for 48 h) on HOB metabolism in vitro. Cells were submitted to strain either without any pretreatment (I), pretreatment with 10 nM DHT for 24 h (II), or pretreatment with DHT and 25 ng/ml PP1 (III), respectively. Mean ± SD (n = 6), unpaired two-tailed t test, *, P < 0.05; **, P < 0.01; ***, P < 0.001. (a) Strained cultures (I) versus unstrained controls. (b) DHT pretreatment (II) versus non–DHT-treated cells (I). (c) DHT + PP1 pretreatment (III) versus DHT pretreatment (II).
Discussion
This paper demonstrates a novel mechanism whereby androgens modify the impact of mechanical strain by directing bone metabolism from a high turnover and, therefore, bone-losing state into an osteoanabolic process. On the molecular level, DHT induces gene expression of the fibronectin receptor and its ligand, fibronectin, thereby enhancing anchorage of osteoblastic cells to the organic bone matrix (13). This effect is androgen dependent because we did not find a consistent stimulatory effect of E2 on FNR or FN production in male and female osteoblastic cell cultures, although HOB of both genders possess similar numbers of estrogen and androgen receptors (12, 14). However, if androgens stimulate FNR gene expression in HOB in vitro, male-derived primary HOB ex vivo should express higher FNR levels than female-derived HOB due to 10-fold higher androgen serum levels in men (female DHT serum levels range ∼1 nM). We found, indeed, significantly higher FNR mRNA and protein levels in primary male HOB compared with female-derived, age-matched HOB. Therefore, androgens stimulate FNR gene expression in HOB of both genders but due to the higher androgen serum levels in men, male HOB possess a greater number of FNR.
Regulation of FNR-mediated cellular anchorage to the extracellular matrix by sex steroids has also been observed in other mesenchymal tissues (15). Disturbance of the extracellular matrix composition impairs the susceptibility of bone tissue to perceive mechanical loading (16). Anchorage of bone cells to the extracellular matrix is a prerequisite for integrin-mediated signal transduction that affects osteoblastic differentiation and supports osteoblastic survival (17, 18). The β1-integrin subfamily is crucial for the development of stromal precursor cells into functional osteoblast-like cells (19). There is also evidence for an interaction between the postreceptor signaling pathway of bone morphogenetic proteins and collagen–integrin-dependent signaling (20). Therefore, the observed induction of the FNR gene by androgens may enhance paracrine signaling by growth factors of the TGFβ family in the bone microenvironment. Importantly, androgens elicit part of their positive effects on bone metabolism by stimulating the expression of the TGFβ2 gene in human bone cells (21) and growth factors of the TGFβ family have been shown to elevate gene expression of fibronectin and adhesion protein receptors (22).
The observation that the application of both testosterone and TGFβ2 are effective in the prevention of unloading-induced osteopenia (23, 24) lead us to investigate whether mechanoperception of bone cells is affected by androgens. This work and other authors (25) demonstrate that deformation strain is mitogenic for osteoblastic cells and stimulates IL-6 production, which suggests a stimulation of bone remodeling because paracrine IL-6 secretion induces osteoclastogenesis (7, 26) and, due to the stimulation of osteoblastic cell growth, resorbed bone can be replaced effectively. If androgens increase the number of FNR on the osteoblastic cell surface and at least some of the effects of deformation strain on bone cell metabolism are mediated via integrin-associated signaling (27), then one might expect a quantitatively increased response of HOB to deformation after androgen treatment. Surprisingly, androgen treatment of male HOB does not induce a quantitatively increased response, but qualitatively shifts the osteoblastic response pattern. Androgen pretreatment inhibits the mitogenic and stimulates the AP response to deformation strain and allows HOB to secrete significantly increased amounts of collagen type I and osteoprotegerin. OPG as the soluble decoy receptor for the osteoblastic cell surface protein RANKL inhibits osteoclastic bone resorption by intercepting with the RANK–RANKL system (28). The androgen-induced increase of OPG production and simultaneous decrease of IL-6 secretion by strained HOB implicate a potent inhibition of bone resorption. Simultaneously, androgens enable HOB to respond to deformation strain with an increased AP activity and collagen I production, which indicates increased bone formation. Considering the observed enhanced anchorage of male HOB to the bone matrix due to higher androgen serum levels in men, it is possible that the development of a higher mean peak bone mass in men (29) is, at least partly, due to this enhanced anchorage of HOB in male bone.
Bone cell growth is regulated by several kinases (30), whereby a specific integrin function in regulating responses of human bone cells to mechanical strain via tyrosine kinases and MAP kinases has been demonstrated (31). Stretch-triggered induction of MAP kinase cascades stimulates DNA binding activity of the bone-specific transcriptional regulator Cbfa1 (32), which has a pivotal role in osteoblast function (31). These findings indicate that osteoblastic deformation activates multiple integrin-associated signaling pathways. To further elucidate the specific integrin function in osteoblastic mechanoperception and its modulation by androgens, we added an inhibitor that blocks signaling by FAKs (33) while HOB cultures were exposed to deformation strain. Inhibition of integrin-mediated signaling via FAK enhanced the mitogenic effect after DHT treatment but decreased the positive effect of osteoblastic deformation on AP, PICP, and OPG production and did not affect IL-6 secretion. Thus, FAK activity is required for the enhanced AP, PICP, and OPG production of strained HOB but not for the mitogenic response or for the decreased IL-6 secretion induced by deformation strain. Possibly, the promoter regions of the AP, OPG, and collagen gene possess force-responsive elements that bind transcription factors activated by FAK activity (34).
These in vitro observations indicate that androgens in vivo may have a modulatory role in the regulation of bone cell metabolism, whereby androgens gear the impact of mechanical strain on bone metabolism from remodeling into an osteoanabolic mode. As a corollary, these findings may also help to understand the high turnover state of bone metabolism induced by castration (26, 35). However, a clinical study is required to address the long-term effects of combined load and androgen treatment on bone tissue in vivo.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Ka 682/2-3 and Ka 682/7-1).
References
- 1.Horton, M.A., and J. Davis. 1989. Adhesion receptors in bone. J. Bone Miner. Res. 4:803–807. [DOI] [PubMed] [Google Scholar]
- 2.Hughes, D.E., D.M. Salter, S. Dedhar, and R. Simpson. 1993. Integrin expression in human bone. J. Bone Miner. Res. 8:527–533. [DOI] [PubMed] [Google Scholar]
- 3.Xiong, J., T. Stehle, R. Zhang, A. Joachimiak, M. Frech, S.L. Goodman, and M.A. Arnaout. 2002. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science. 296:151–155. [DOI] [PubMed] [Google Scholar]
- 4.Ruoslahti, E., and M.D. Pierschbacher. 1987. New perspectives in cell adhesion: RGD and integrins. Science. 238:491–497. [DOI] [PubMed] [Google Scholar]
- 5.Wang, N., J.P. Butler, and D.E. Ingber. 1993. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 260:1124–1127. [DOI] [PubMed] [Google Scholar]
- 6.Pommerenke, H., C. Schmidt, F. Dürr, B. Nebe, F. Lüthen, P. Müller, and J. Rychly. 2002. The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J. Bone Miner. Res. 17:603–611. [DOI] [PubMed] [Google Scholar]
- 7.Kaspar, D., W. Seidl, C. Neidlinger-Wilke, and L. Claes. 2000. In vitro effects of dynamic strain on the proliferative and metabolic activity of human osteoblasts. J. Musculoskel. Neuron. Interact. 1:161–164. [PubMed] [Google Scholar]
- 8.Frost, H.M. 1992. The role of changes in mechanical usage set points in the pathogenesis of osteoporosis. J. Bone Miner. Res. 7:253–261. [DOI] [PubMed] [Google Scholar]
- 9.Damien, E., J.S. Price, and L.E. Lanyon. 2000. Mechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as females. J. Bone Miner. Res. 15:2169–2177. [DOI] [PubMed] [Google Scholar]
- 10.Jessop, H.L., M. Sjöberg, M.Z. Cheng, G. Zaman, C.P.D. Wheeler-Jones, and L.E. Lanyon. 2001. Mechanical strain and estrogen activate estrogen receptor α in bone cells. J. Bone Miner. Res. 16:1045–1055. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson, U., B.M. Stalnacke, G.M. Ahlenius, K. Henriksson-Larsen, and R. Lorentzon. 1999. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif. Tissue Int. 64:117–125. [DOI] [PubMed] [Google Scholar]
- 12.Kasperk, C., A. Helmboldt, I. Börcsök, S. Heuthe, O. Cloos, F. Niethard, and R. Ziegler. 1997. Skeletal site-dependent expression of the androgen receptor in human osteoblastic cell populations. Calcif. Tissue Int. 61:464–473. [DOI] [PubMed] [Google Scholar]
- 13.Gronthos, S., K. Stewart, S.E. Graves, S. Hay, and P.J. Simmons. 1997. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 12:1189–1197. [DOI] [PubMed] [Google Scholar]
- 14.Manolagas, S.C., and S. Kousteni. 2001. Perspective: nonreproductive sites of action of reproductive hormones. Endocrinology. 142:2200–2204. [DOI] [PubMed] [Google Scholar]
- 15.Woodward, T.L., A.S. Mienaltowski, R.R. Modi, J.M. Bennett, and S.Z. Haslam. 2001. Fibronectin and the α5β1 integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology. 142:3214–3222. [DOI] [PubMed] [Google Scholar]
- 16.Ishijima, M., K. Tsuji, S.R. Rittling, T. Yamashita, H. Kurosawa, D.T. Denhardt, A. Nifuji, and M. Noda. 2002. Resistance to unloading-induced three dimensional bone loss in osteopontin-deficient mice. J. Bone Miner. Res. 17:661–667. [DOI] [PubMed] [Google Scholar]
- 17.Ilic, D., E. Almeida, D. Schlaepfer, P. Dazin, S. Aizawa, and C. Damsky. 1998. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 143:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacouture, M.E., J.L. Schaffer, and L.B. Klickstein. 2002. A comparison of type I collagen, fibronectin, and vitronectin in supporting adhesion of mechanically strained osteoblasts. J. Bone Miner. Res. 17:481–492. [DOI] [PubMed] [Google Scholar]
- 19.Gronthos, S., P.J. Simmons, S.E. Graves, and P.G. Robey. 2001. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 28:174–181. [DOI] [PubMed] [Google Scholar]
- 20.Suzawa, M., Y. Tamura, S. Fukumoto, K. Miyazono, T. Fujita, S. Kato, and Y. Takeuchi. 2002. Stimulation of Smad1 transcriptional activity by Ras-extracellular signal-regulated kinase pathway: a possible mechanism for collagen-dependent osteoblastic differentiation. J. Bone Miner. Res. 17:240–248. [DOI] [PubMed] [Google Scholar]
- 21.Kasperk, C., R. Fitzsimmons, D. Strong, S. Mohan, J. Jennings, J. Wergedal, and D. Baylink. 1990. Studies on the mechanism by which androgens enhance mitogenesis and differentiation in bone cells. J. Clin. Endocrinol. Metab. 71:1322–1329. [DOI] [PubMed] [Google Scholar]
- 22.Ignotz, R.A., and J. Massague. 1987. Cell adhesion protein receptors as targets for transforming growth factor β action. Cell. 51:189–197. [DOI] [PubMed] [Google Scholar]
- 23.Wimalawansa, S.M., and S.J. Wimalawansa. 1999. Simulated weightlessness-induced attenuation of testosterone production may be responsible for bone loss. Endocrine. 10:253–260. [DOI] [PubMed] [Google Scholar]
- 24.Machwate, M., E. Zerath, X. Holy, M. Hott, D. Godet, A. Lomri, and P.J. Marie. 1995. Systemic administration of transforming growth factor β2 prevents the impaired bone formation and osteopenia induced by unloading in rats. J. Clin. Invest. 96:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaspar, D., W. Seidl, C. Neidlinger-Wilke, A. Ignatius, and L. Claes. 2000. Dynamic cell stretching increases human osteoblast proliferation and CICP synthesis but decreases osteocalcin synthesis and alkaline phosphatase activity. J. Biomech. 33:45–51. [DOI] [PubMed] [Google Scholar]
- 26.Manolagas, S.C., and R.L. Jilka. 1995. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathogenesis of osteoporosis. N. Engl. J. Med. 332:305–311. [DOI] [PubMed] [Google Scholar]
- 27.Chen, C.S., M. Mrksich, S. Huang, G.M. Whitesides, and D.E. Ingber. 1997. Geometric control of cell life and death. Science. 276:1425–1428. [DOI] [PubMed] [Google Scholar]
- 28.Khosla, S. 2001. The OPG/RANKL/RANK system. Endocrinology. 142:5050–5055. [DOI] [PubMed] [Google Scholar]
- 29.Looker, A.C., T.J. Beck, and E.S. Orwoll. 2001. Does body size account for gender differences in femur bone density and geometry? J. Bone Miner. Res. 16:1291–1299. [DOI] [PubMed] [Google Scholar]
- 30.Pouyssegur, J. 2000. An arresting start for MAPK. Science. 290:1515–1518. [DOI] [PubMed] [Google Scholar]
- 31.Lin, T.H., A.E. Aplin, Y. Shen, M. Schaller, L. Romer, I. Aukhil, and R.L. Juliano. 1997. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J. Cell Biol. 136:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziros, P.G., A.-P. Rojas Gil, T. Georgakopoulos, I. Habeos, D. Kletsas, E.K. Basdra, and A.G. Papavassiliou. 2002. The bone specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J. Biol. Chem. 277:23934–23941. [DOI] [PubMed] [Google Scholar]
- 33.Schlaepfer, D.D., K.C. Jones, and T. Hunter. 1998. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol. Cell. Biol. 18:2571–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, J., M. Su, J. Fan, A. Seth, and C.A. McCulloch. 2002. Transcriptional regulation of a contractile gene by mechanical forces applied through integrins in osteoblasts. J. Biol. Chem. 277:22889–22895. [DOI] [PubMed] [Google Scholar]
- 35.Stepan, J.J., M. Lachman, J. Zverina, V. Pacovsky, and D.J. Baylink. 1989. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J. Clin. Endocrinol. Metab. 69:523–527. [DOI] [PubMed] [Google Scholar]