Abstract
Differentiation therapy for acute myeloid leukemia uses transcriptional modulators to reprogram cancer cells. The most relevant clinical example is acute promyelocytic leukemia (APL), which responds dramatically to either retinoic acid (RA) or arsenic trioxide (As2O3). In many myeloid leukemia cell lines, cyclic adenosine monophosphate (cAMP) triggers growth arrest, cell death, or differentiation, often in synergy with RA. Nevertheless, the toxicity of cAMP derivatives and lack of suitable models has hampered trials designed to assess the in vivo relevance of theses observations. We show that, in an APL cell line, cAMP analogs blocked cell growth and unraveled As2O3-triggered differentiation. Similarly, in RA-sensitive or RA-resistant mouse models of APL, continuous infusions of 8-chloro-cyclic adenosine monophosphate (8-Cl-cAMP) triggered major growth arrest, greatly enhanced both spontaneous and RA- or As2O3-induced differentiation and accelerated the restoration of normal hematopoiesis. Theophylline, a well-tolerated phosphodiesterase inhibitor which stabilizes endogenous cAMP, also impaired APL growth and enhanced spontaneous or As2O3-triggered cell differentiation in vivo. Accordingly, in an APL patient resistant to combined RA–As2O3 therapy, theophylline induced blast clearance and restored normal hematopoiesis. Taken together, these results demonstrate that in vivo activation of cAMP signaling contributes to APL clearance, independently of its RA-sensitivity, thus raising hopes that other myeloid leukemias may benefit from this therapeutic approach.
Keywords: theophylline, arsenic, retinoic acid, transgenic mice, clinical trial
Introduction
The different forms of acute myeloid leukemias (AMLs)* are characterized by the arrest of cell differentiation, the induction of cell proliferation, and the repression of normal hematopoiesis. These malignancies are often associated with recurrent chromosomal translocations, most of which encode fusion proteins derived from transcription factors (1). Functional analyses of several of these fusion proteins have shown that they behave like potent transcriptional repressors (2). Repression is often achieved through the modification of chromatin structure by histone desacetylases which blocks the expression of unidentified genes that control myeloid differentiation. Transcription therapy attempts to induce the reexpression of these genes and thus restore differentiation. The inhibition of desacetylases by a variety of compounds has been fairly effective in cell culture (3) and in animal models of leukemia (4), but as yet, there only is slight evidence of its beneficial effects in clinical settings (5). To date, the only real clinical success of transcription/differentiation therapy has been in acute promyelocytic leukemia (APL), for which two drugs, retinoic acid (RA) and arsenic trioxide (As2O3) induce remissions (6, 7). The action of these drugs is remarkable because they both target the oncogenic promyelocytic leukemia (PML)–retinoic acid receptor α (RARA) fusion protein and reverse PML/RARA–mediated repression (8). Cyclic adenosine monophosphate (cAMP) also differentiates many AML cell line and strongly synergizes with other differentiating agents (references 9 and 10; for a review, see reference 11). Moreover, in APL cells, cAMP can decrease the concentrations of RA required for differentiation to almost physiological ones (12). However, a number of acute toxic reactions have precluded or severely limited trials using cAMP derivatives (13). Here, we used an animal model of APL derived from PML/RARA transgenic mice (14) to study the effects of cAMP in vivo. We showed that cAMP induces major cell growth arrest together with differentiation and synergizes with both As2O3 and RA to clear RA-sensitive or RA-resistant APL. In an APL patient who had become resistant to RA and As2O3 therapy, addition of theophylline, an inhibitor of cAMP intracellular degradation, induced a clinical remission. That activation of cAMP signaling is beneficial in APL, independently of its sensitivity to RA, raises hopes for the successful treatment of therapy-resistant forms of AML.
Materials and Methods
Morphology and differentiation of the APL cell line NB4 were evaluated on May-Grünwald Giemsa–stained cytospins. Differentiation was quantified by reduction of nitroblue-tetrazolium (NBT). 8-chloro-adenosine 3-5′ cyclic monophosphate (8-Cl-cAMP) and 8-(4-chlorophenylthio)adenosine 3-5′ cyclic monophosphate (8-CPT-cAMP; reference 15) were respectively obtained from the Biology Life Research Institute and Sigma-Aldrich. 8-CPT-cAMP was used at a concentration of 2.10−4 M.
Spleen-derived leukemic blasts (107) were serially passaged in syngeneic FVB/n mice 6 wk old, weighing 20 g, as described previously (14). Both RA-sensitive leukemias (strain 935) or RA-resistant leukemias (strain 4048; reference 16) were used. Mice were treated according to institutional guidelines. All experiments involving mice were repeated between two and eight times, usually with two mice in each treatment arm. 0.5 μl/h alzet pumps were loaded with 20 mg/ml 8-Cl-cAMP and implanted subcutaneously on the back of treated mice. Aminophylline, a stabilized precursor of theophylline, was injected intraperitoneally (100 μl/day of a 25 mg/ml solution). RA and As2O3 treatments, autopsies, and cell or tissue analyses were performed as described previously (14). For Western blot analysis, a p21 mAb (BD PharMingen) was used at a 1:500 dilution. Plasma 8-Cl-cAMP was measured by HPLC using a C18 column (Chromosep Inertil 5 ODS3) with a 15% methanol/50 mM phosphate buffer, pH 5.85, as a mobile phase and UV detection at 254 nm. The patient studied gave informed consent to the use of theophylline to enhance RA/As2O3 differentiation. The patient's daily treatment consisted of 45 mg/m2 RA per os, 10 mg As2O3 intravenously, and 250 mg theophylline per os.
Results
cAMP Synergizes with As2O3 to Promote APL Cell Differentiation.
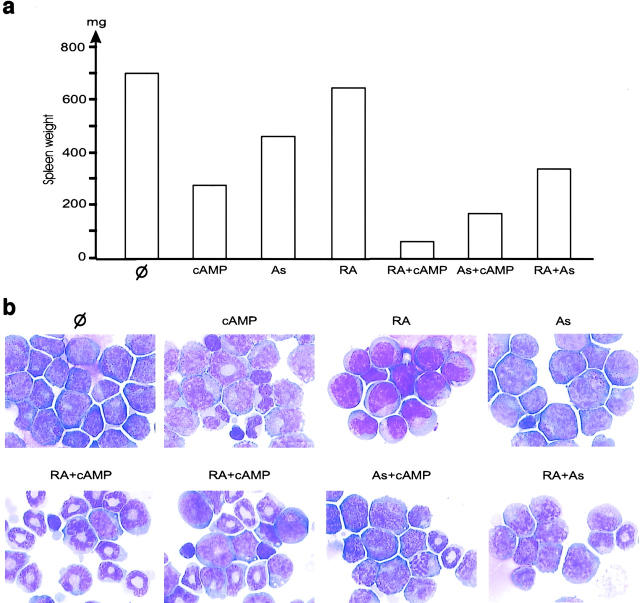
It is well known that cAMP greatly enhances the RA-induced differentiation of many cell lines derived from embryonal carcinoma or myeloid leukemias, including APL (10). As low concentrations of As2O3 were found to induce only incomplete differentiation in an APL cell line (7), we tested the hypothesis that cAMP would also enhance As2O3-induced differentiation. Even very low doses of As2O3 combined with 8-CPT-cAMP (a low toxicity cAMP analog) induced NBT reduction in 40% of the NB4 cells, whereas cAMP or As2O3 alone did not have significant effects (unpublished data, see Fig. 2 a). However, high As2O3 concentrations inhibited NBT reduction. Similarly, only combined As2O3 and 8-CPT-cAMP induced morphologic differentiation of NB4 cells into myelocyte-like cells (unpublished data), as reported recently (17).
Figure 2.
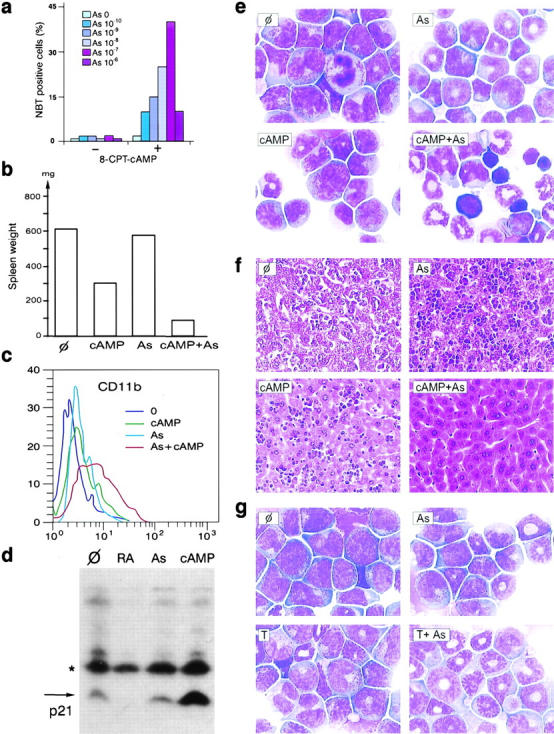
Arsenic and cAMP synergize to induce tumor regression and differentiation in RA-sensitive APL. (a) 8-CPT-cAMP potentiates As2O3-induced NBT reduction at 4 d in the APL cell line NB4. (b) In a mouse model of APL, continuous infusion of 8-Cl-cAMP for 3 d greatly reduces spleen weight and synergizes with As2O3 (As) to induce tumor reduction (one representative experiment). (c) CD11b expression on bone marrow cells after 24 h of in vivo treatments, as indicated. (d) 8-Cl-cAMP induces p21 expression in leukemic bone marrow after 24 h of in vivo treatment. *Cross-reactive protein. (e and f) 8-Cl-cAMP synergizes with As2O3 to eradicate leukemia. 3 d of combined treatment restores normal hematopoiesis in the bone marrow (e) and induces tumor clearance from the liver (f). (g) Theophylline (T) therapy (3 d) induces blast differentiation. Note the synergistic action of As.
Antileukemic Effects of cAMP in Two Mouse Models of APL.
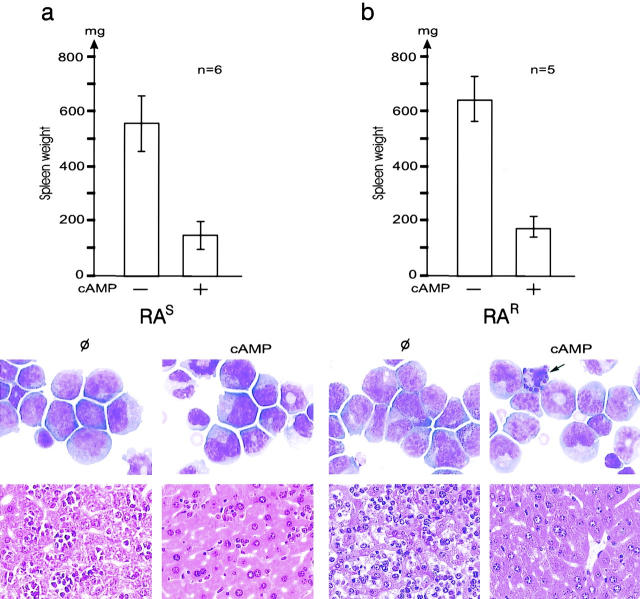
To assess the possible in vivo efficiency of cAMP, we used transplantation APL model (14) derived from RA-sensitive PML/RARA transgenic mice (18). We developed a similar transplantation model for RA-resistant APL, using leukemic cells from PML/RARA transgenic mice in which a point mutation in the transgene impairs the binding of RA to PML/RARA (16). In vivo growth of this leukemia is much slower than in the RA-sensitive model, liver, or spleen invasion is not as pronounced and either RA or As2O3 have mild antiproliferative effects in the absence of significant differentiation (see Fig. 4). Mice bearing established leukemias were treated with 8-Cl-cAMP, As2O3, RA, or combinations of these drugs and killed 1–7 d after treatment. Continuous 8-Cl-cAMP infusions allowed significant plasma concentrations to be reached (1 μM on an average at day 3). Despite its toxicity, 8-Cl-cAMP induced major antileukemic effects in both RA-sensitive and RA-resistant APLs, as assessed by spleen weight (Fig. 1) and liver or bone marrow infiltration (Fig. 1). Myeloid cells undergoing differentiation were always observed in the marrow of RA-resistant APL after 7 d of treatment, and were often found upon treatment of RA-sensitive APL (Fig. 1). In leukemic cells infiltrating the liver, a sharp reduction in the number of mitoses was observed after 8-Cl-cAMP treatment (3% post- versus 28% pretreatment) and a few condensed nuclei were seen (unpublished data). Apoptosis was also noted in the bone marrow (arrow in Fig. 1 b). Taken together, these results show that in these RA-sensitive or RA-resistant mouse models of APL, cAMP triggers a combination of cell growth arrest, differentiation, and apoptosis, leading to dramatic regressions of the leukemia. Nevertheless, in most cases, cAMP was unable to eradicate APL.
Figure 4.
cAMP synergizes with RA in RA-resistant APL. (a) Spleen weight after 7 d of treatment. (b) Corresponding bone marrow morphology. Two examples of marrows from mice exposed to RA and cAMP are shown.
Figure 1.
8-Cl-cAMP induces growth arrest in RA-sensitive (RAS, a) or RA-resistant (RAR, b) APL in mice. (Top) Spleen weight after 7 d of 8-Cl-cAMP treatment or no therapy (Φ). (Middle) Bone marrow sample after May-Grünwald Giemsa staining. Note an apoptotic cell (arrow) in the treated sample. (Bottom) Liver from the same mice.
Since cAMP greatly increased As2O3-triggered differentiation ex vivo in the NB4 cell line (Fig. 2 a), we combined 8-Cl-cAMP and As2O3 treatments in vivo. With this combination, the spleen, liver, and bone marrow of mice with RA-sensitive APL became free of leukemia between days 1 and 3, whereas mice treated with As2O3 or cAMP alone retained a significant tumor burden consisting of differentiating leukemic cells (Fig. 2, b, e, and f). Note that erythroblasts and megakaryocytes were extremely numerous in a cell-rich marrow (Fig. 2 e), in agreement with the idea that cAMP promotes the regrowth of these cells. In keeping with our ex vivo results, cAMP synergized with As2O3 to trigger differentiation, as shown by CD11b expression on bone marrow blasts at day 1 (Fig. 2 c), strongly suggesting that enhanced differentiation of the leukemic blasts contributes to these accelerated remissions. The level of the cdk inhibitor p21, a known cAMP target implicated in growth arrest, differentiation, and apoptosis, rose strikingly in bone marrow APL blasts upon treatment in vivo (Fig. 2 d). Synergy was also noted between 8-Cl-cAMP and RA with respect to both spleen weight and marrow cell differentiation, although this synergy was dimmed by the stronger differentiating effect of RA (Fig. 3) .
Figure 3.
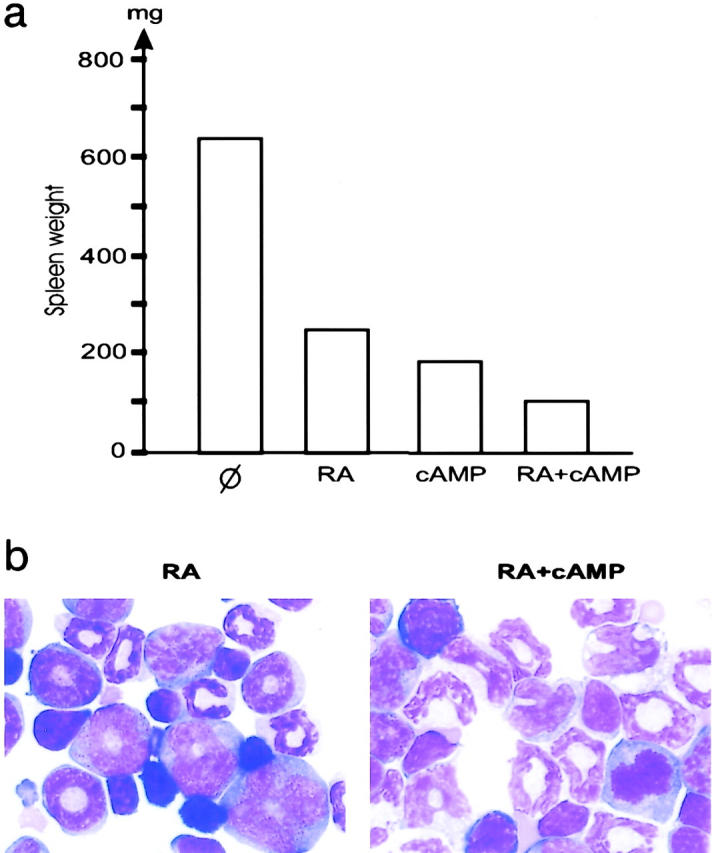
RA and cAMP synergize to induce tumor regression and differentiation. (a) Spleen weight after 7 d of treatment or untreated (Φ). (b) Corresponding bone marrow morphology.
8-Cl-cAMP induced even more dramatic regressions in RA-resistant APL, with some morphologically complete clearances (Figs. 1 and 4) . Unexpectedly, major enhancements of leukemia clearance and differentiation were always observed when RA was combined with 8-Cl-cAMP (Fig. 4). Although As2O3 alone triggered a slight antiproliferative effect in the absence of significant differentiation, the RA/As2O3 combination triggered minor, but reproducible, differentiation (Fig. 4 b). Additive effects between cAMP and As2O3 were also noted (Fig. 4), but the significance of this interaction remains unclear.
The stable cAMP derivative used here induces massive diuresis, precluding any long-term use, and may be further metabolized into potentially cytotoxic nucleotide analogs (15). To ensure that the antileukemic effect indeed resulted from the activation of cAMP signaling, we used theophylline, a phosphodiesterase inhibitor which stabilized pools of endogenous intracellular cAMP. Like 8-Cl-cAMP, the-ophylline blocked growth in RA-sensitive APL and induced some apoptosis, accompanied by non-terminal differentiation (Fig. 2 g and unpublished data). As expected, the enhancement of differentiation was more pronounced with As2O3 than with RA (Fig. 2 g and unpublished data). However, there was no obvious boost in normal hematopoiesis, possibly reflecting low production of endogenous cAMP in normal cells.
Theophylline Induces Remission in an RA- and As-resistant APL Patient.
On the basis of these promising results in mouse APL, an RA/As2O3-resistant APL patient was offered an experimental course of combined RA/As2O3/theophylline therapy. Previously, a month of the RA/As2O3 combination had led to a slow decrease in bone marrow blasts, a peak of differentiating myeloid cells in the blood (Fig. 5) , but normal hematopoiesis was not restored and the leukemia had reappeared 5 wk later (Fig. 5, a and b). However, with combined RA/As2O3/theophylline treatment, leukemic cells underwent differentiation and normal erythroblasts appeared rapidly (Fig. 5, a and c). The patient no longer required red cell or platelet transfusions and white blood cells and hemoglobin reached levels close to the normal ones. The patient remained leukemia free for 4 mo, but then the leukemic clone reappeared. Paradoxically, with ongoing RA/As2O3/theophylline therapy and despite the leukemia relapse, normal hematopoiesis has been maintained to date (5 mo since relapse), an extremely unusual situation in APL, in which cytopenia is the first sign of relapse.
Figure 5.
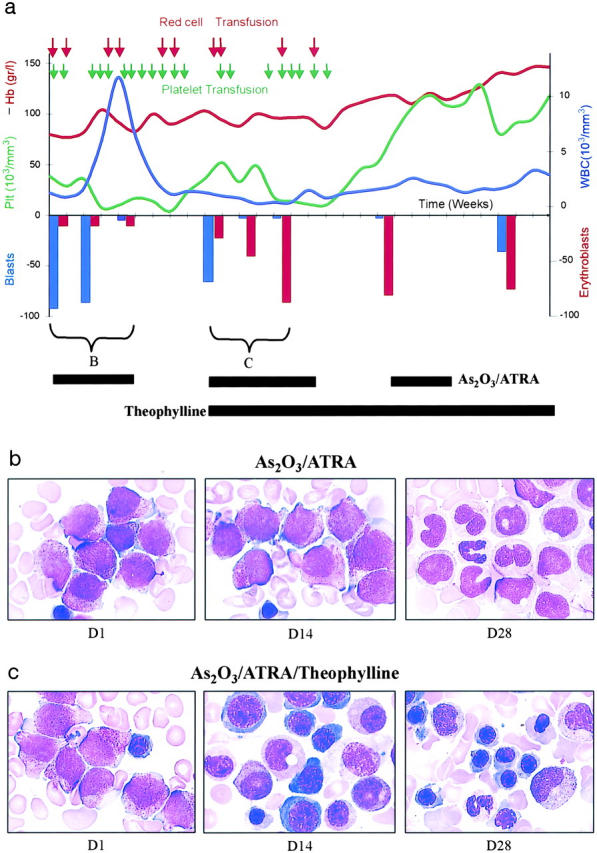
Theophylline synergizes with RA/As2O3 therapy in a multirelapsed patient with RA/As2O3-resistant APL. (a) Schematic representation of clinical events. (b and c) Representative images of bone marrow samples taken at days 0, 14, and 28 of the initial RA/As2O3 course (b) or the following RA/As2O3/theophylline course (c). Note the rapid differentiation in (c) with the reappearance of normal erythroblasts at day 14, as well as the maintenance of normal hematopoiesis despite leukemia relapse. Hb, hemoglobin; Plt, platelet; WBC, white blood cell.
Discussion
Our data demonstrate that in vivo activation of cAMP signaling was beneficial in two distinct mouse models of APL, as well as in an RA/As2O3-resistant APL patient. The relative balance between growth arrest, apoptosis, and differentiation presumably depends on the dose of 8-Cl-cAMP, the microenvironment of the APL blasts (marrow versus liver metastasis, for example) and their nature (RA-sensitive or RA-resistant cells). cAMP-triggered growth arrest may result from induction of the cdk inhibitor p21, which was previously found to be involved in RA-induced APL cell differentiation (19). Enhancement of RA-, As2O3-, or rexinoid-triggered differentiation by cAMP may also result from induction of G-CSF expression (20). In F9 embryonal carcinoma cells, cAMP was shown to modulate RA-triggered differentiation directly through RARA phosphorylation (21). Since RARA plays a critical role in myeloid differentiation, including in its regulation by IL-3 and GM-CSF (22), the cAMP triggered myeloid differentiation may similarly be caused by modulation of RARA signaling. At present, we cannot explain the greater sensitivity to 8-Cl-cAMP of RA-resistant APL cells, but it might be due to their slower growth rate. In European trials, therapy-resistant patients only exceptionally exhibit mutations in PML/RARA, which does not favor a direct parallel between the case of this patient and RA-resistant APL mice. The RA/8-Cl-cAMP synergy for differentiation in RA-resistant APL was unexpected. Indeed, a cell line (NB4-LR2) that harbors a mutation in PML/RARA very similar to the one present in the RA-resistant APL cells used here, failed to differentiate upon combined RA/8-Cl-cAMP treatment, but only matured upon combined cAMP/rexinoid exposure (20). Therefore, our observations might reflect an in vivo metabolism of RA to rexinoids and activation of this parallel pathway. Absence of major synergy between cAMP and As2O3 in RA-resistant APL, compared to RA-sensitive disease, may reflect the low potency of As2O3 and the striking efficacy of 8-Cl-cAMP in this setting. As2O3 was initially believed to trigger apoptosis, but recent observations have suggested that differentiation was the dominant mechanism (for a review, see reference 23). The dramatic enhancement of As2O3-triggered differentiation by 8-Cl-cAMP both ex vivo and in vivo (Fig. 2 and reference 17), greatly strengthens this idea. In the patient, as in the APL mice, cAMP induced rapid regrowth of normal erythroblasts and megakaryocytes. This might have resulted from a direct positive effect on the normal progenitors, as reported ex vivo (24). Alternatively, APL cells might secrete inhibitors of normal hematopoiesis (25, 26) whose synthesis or downstream signaling, may be blocked by cAMP. In the clinical setting, such an in vivo stimulatory effect on normal hematopoiesis could be as important as leukemia inhibition.
The low toxicity of theophylline, its ability to accelerate RA- or As2O3–triggered remissions and the observation that the rapid induction of ex vivo differentiation is predictive of outcome in APL (27), could favor the use of theophylline for de novo APL patients. cAMP is active both in RA-sensitive and RA-resistant APL. In addition, unlike RA and As2O3, cAMP does not obviously target PML/RARA and might therefore be beneficial in malignancies other that APL. Indeed, patients with chronic lymphocytic leukemia experienced faster clinical remissions when theophylline was combined with low-dose chemotherapy (28). Similarly, a patient on Viagra®, another phosphodiesterase inhibitor, exhibited regression of B cell chronic lymphocytic leukemia (29). Many AML-derived cell lines are very sensitive to cAMP-triggered differentiation, particularly in the presence of other differentiation inducers. Like histone desacetylase inhibitors, which unravel RA-induced differentiation (3, 30), the-ophylline may greatly increase the potency of RA and other differentiation inducers in AML patients.
Acknowledgments
We warmly thank Marika Pla and all those who provided animal house facilities for their invaluable assistance, Catherine Lavau for comments on the manuscript, M. Dreyfus for English language revisions, Pascal Houzé for providing aminophylline, Jean Francois Bourges for FACS® analyses, and the Laboratoire Photo Hemato (LBH) for the artwork. We also thank H.F. Genieser (Biology Life Research Institute) for providing 8-Cl-cAMP.
We would also like to thank the Fondation de France, Centre Evian pour l'Eau, and ARECA for financial support. D. Vitoux was supported by an AP/CNRS grant. S. Kogan is the 32nd Edward Mallinckrodt Junior Scholar and is the recipient of a Burroughs Wellcome Fund Career Award.
M.-C. Guillemin and E. Raffoux contributed equally to this article.
Footnotes
Abbreviations used in this paper: 8-Cl-cAMP, 8-chloro-cyclic adenosine monophosphate; AML, acute myeloid leukemia; APL, acute PML; cAMP, cyclic adenosine monophosphate; NBT, nitroblue-tetrazolium; PML, promyelocytic leukemia; RA, retinoic acid; RARA, retinoic acid receptor α.
References
- 1.Look, A.T. 1997. Oncogenic transcription factors in the human acute leukemias. Science. 278:1059–1064. [DOI] [PubMed] [Google Scholar]
- 2.Minucci, S., C. Nervi, F. Lo Coco, and P.G. Pelicci. 2001. Histone deacetylases: a common molecular target for differentiation treatment of acute myeloid leukemias? Oncogene. 20:3110–3115. [DOI] [PubMed] [Google Scholar]
- 3.Gottlicher, M., S. Minucci, P. Zhu, O.H. Kramer, A. Schimpf, S. Giavara, J.P. Sleeman, F. Lo Coco, C. Nervi, P.G. Pelicci, and T. Heinzel. 2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20:6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He, L.Z., T. Tolentino, P. Grayson, S. Zhong, R.P. Warrell, Jr., R.A. Rifkind, P.A. Marks, V.M. Richon, and P.P. Pandolfi. 2001. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J. Clin. Invest. 108:1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrell, R.P., L.-Z. He, V. Richon, E. Calleja, and P.P. Pandolfi. 1998. Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J. Natl. Cancer Inst. 90:1621–1625. [DOI] [PubMed] [Google Scholar]
- 6.Warrell, R., H. de Thé, Z. Wang, and L. Degos. 1993. Acute promyelocytic leukemia. N. Engl. J. Med. 329:177–189. [DOI] [PubMed] [Google Scholar]
- 7.Chen, G.-Q., X.-G. Shi, W. Tang, S.-M. Xiong, J. Zhu, X. Cai, Z.-G. Han, J.-H. Ni, G.-Y. Shi, P.-M. Jia, et al. 1997. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukaemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 89:3345–3353. [PubMed] [Google Scholar]
- 8.Zhu, J., V. Lallemand-Breitenbach, and H. de The. 2001. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARα catabolism, role of oncogene degradation in disease remission. Oncogene. 20:7257–7265. [DOI] [PubMed] [Google Scholar]
- 9.Olsson, I.L., and T.R. Breitman. 1982. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by retinoic acid and cyclic adenosine 3′:5′-monophosphate-inducing agents. Cancer Res. 42:3924–3927. [PubMed] [Google Scholar]
- 10.Ruchaud, S., E. Duprez, M.C. Gendron, G. Houge, H.G. Genieser, B. Jastorff, S.O. Doskeland, and M. Lanotte. 1994. Two distinctly regulated events, priming and triggering, during retinoid-induced maturation and resistance of NB4 promyelocytic leukemia cell line. Proc. Natl. Acad. Sci. USA. 91:8428–8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoit, G., M. Roussel, F. Pendino, E. Segal-Bendirdjian, and M. Lanotte. 2001. Orchestration of multiple arrays of signal cross-talk and combinatorial interactions for maturation and cell death: another vision of t(15;17) preleukemic blast and APL-cell maturation. Oncogene. 20:7161–7177. [DOI] [PubMed] [Google Scholar]
- 12.Quenech'Du, N., S. Ruchaud, N. Khelef, N. Guiso, and M. Lanotte. 1998. A sustained increase in the endogenous level of cAMP reduces the retinoid concentration required for APL cell maturation to near physiological levels. Leukemia. 12:1829–1833. [DOI] [PubMed] [Google Scholar]
- 13.Langdon, S.P., A.A. Ritchie, M. Muir, M. Dodds, A.F. Howie, R.C. Leonard, P.K. Stockman, and W.R. Miller. 1998. Antitumour activity and schedule dependency of 8-chloroadenosine-3′,5′-monophosphate (8-ClcAMP) against human tumour xenografts. Eur. J. Cancer. 34:384–388. [DOI] [PubMed] [Google Scholar]
- 14.Lallemand-Breitenbach, V., M.-C. Guillemin, A. Janin, M.-T. Daniel, L. Degos, S.C. Kogan, J.M. Bishop, and H. de The. 1999. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 189:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman, C., S. Raffel, S. Ruchaud, M.C. Gendron, J. Kruppa, M. Zorn, S.O. Doskeland, M. Lanotte, and B. Jastorff. 1996. Chloro-substituted cAMP analogues and their adenosine metabolites induce apoptosis of the human cell-line NB4: molecular basis for cell-type specificity. Cell. Pharmacol. 3:417–427. [Google Scholar]
- 16.Kogan, S.C., S.H. Hong, D.B. Shultz, M.L. Privalsky, and J.M. Bishop. 2000. Leukemia initiated by PMLRARα: the PML domain plays a critical role while retinoic acid-mediated transactivation is dispensable. Blood. 95:1541–1550. [PubMed] [Google Scholar]
- 17.Zhu, Q., J.W. Zhang, H.Q. Zhu, Y.L. Shen, M. Flexor, P.M. Jia, Y. Yu, X. Cai, S. Waxman, M. Lanotte, S.J. Chen, Z. Chen, and J.H. Tong. 2002. Synergic effects of arsenic trioxide and cAMP during acute promyelocytic leukemia cell maturation subtend a novel signaling cross-talk. Blood. 99:1014–1022. [PubMed] [Google Scholar]
- 18.Brown, D., S. Kogan, E. Lagasse, I. Weissman, M. Alcalay, P.G. Pelicci, S. Atwater, and J.M. Bishop. 1997. A PML RAR α transgene initiates murine acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 94:2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casini, T., and P.-G. Pelicci. 1999. A function of p21 during promyelocytic leukemia cell differentiation independent of CDK inhibition and cell cycle arrest. Oncogene. 18:3235–3243. [DOI] [PubMed] [Google Scholar]
- 20.Benoit, G., L. Altucci, M. Flexor, S. Ruchaud, J. Lillehaug, W. Raffelsberger, H. Gronemeyer, and M. Lanotte. 1999. RAR-independent RXR signaling induces t(15;17) leukemia cell maturation. EMBO J. 18:7011–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taneja, R., C. Rochette-Egly, J.-L. Plassat, L. Penna, M.-P. Gaub, and P. Chambon. 1997. Phosphorylation of activation functions AF-1 and AF-2 of RAR α and RAR γ is indispensable for differentiation of F9 cells upon retinoic acid and cAMP treatment. EMBO J. 16:6452–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, B.S., L. Mueller, J. Si, and S.J. Collins. 2002. The cytokines IL-3 and GM-CSF regulate the transcriptional activity of retinoic acid receptors in different in vitro models of myeloid differentiation. Blood. 99:746–753. [DOI] [PubMed] [Google Scholar]
- 23.Zhu, J., Z. Chen, V. Lallemand-Breitenbach, and H. de The. 2002. How acute promyelocytic leukemia revived arsenic. Nat. Rev. Cancer. 2:705–713. [DOI] [PubMed] [Google Scholar]
- 24.Gallicchio, V.S., and M.G. Chen. and M.J. Murphy. 1982. Modulation of murine in vitro erythroid and granulopoietic colony formation by ouabain, digoxin and theophylline. Exp. Hematol. 10:682–688. [PubMed] [Google Scholar]
- 25.Kogan, S.C., D.E. Brown, D.B. Shultz, B.T. Truong, V. Lallemand-Breitenbach, M.C. Guillemin, E. Lagasse, I.L. Weissman, and J.M. Bishop. 2001. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor α chimeric protein (PMLRARα) to block neutrophil differentiation and initiate acute leukemia. J. Exp. Med. 193:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Z.Y., G.L. Sun, S.J. Chen, M.E. Huang, and Z. Chen. 1994. Cytodifferentiation therapy: from concept to realization. In Accomplishments in Cancer Research. J. Fortner and J. Rhoads, editors. J.D. Lippincott, Philadelphia. 33–38.
- 27.Cassinat, B., S. Chevret, F. Zassadowski, N. Balitrand, I. Guillemot, M.L. Menot, L. Degos, P. Fenaux, and C. Chomienne. 2001. In vitro all-trans retinoic acid sensitivity of acute promyelocytic leukemia blasts: a novel indicator of poor patient outcome. Blood. 98:2862–2864. [DOI] [PubMed] [Google Scholar]
- 28.Mentz, F., M.D. Mossalayi, F. Ouaaz, S. Baudet, F. Issaly, S. Ktorza, M. Semichon, J.L. Binet, and H. Merle-Beral. 1996. Theophylline synergizes with chlorambucil in inducing apoptosis of B-chronic lymphocytic leukemia cells. Blood. 88:2172–2182. [PubMed] [Google Scholar]
- 29.Sarfati, M., V. Mateo, S. Baudet, M. Rubio, C. Fernandez, F. Davi, J.L. Binet, J. Delic, and H. Merle-Beral. 2002. Sildenafil (Viagra) and vardenafil, type 5/6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-CLL cells. Blood. In press. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara, F.F., F. Fazi, A. Bianchini, F. Padula, V. Gelmetti, S. Minucci, M. Mancini, P.G. Pelicci, F. Lo Coco, and C. Nervi. 2001. Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 61:2–7. [PubMed] [Google Scholar]