Abstract
Little is known about the signals that promote early B lineage differentiation from common lymphoid progenitors (CLPs). Using a stromal-free culture system, we show that interleukin (IL)-7 is sufficient to promote the in vitro differentiation of CLPs into B220+ CD19+ B lineage progenitors. Consistent with current models of early B cell development, surface expression of B220 was initiated before CD19 and was accompanied by the loss of T lineage potential. To address whether IL-7 receptor (R) activity is essential for early B lineage development in vivo, we examined the frequencies of CLPs and downstream pre–pro- and pro-B cells in adult mice lacking either the α chain or the common gamma chain (γc) of the IL-7R. The data indicate that although γc −/− mice have normal frequencies of CLPs, both γc −/− and IL-7Rα−/− mice lack detectable numbers of all downstream early B lineage precursors, including pre–pro-B cells. These findings challenge previous notions regarding the point in B cell development affected by the loss of IL-7R signaling and suggest that IL-7 plays a key and requisite role during the earliest phases of B cell development.
Keywords: B lymphocytes, hematopoiesis, cellular differentiation, cytokine, development
Introduction
IL-7 is a stromal-derived lymphoid-specific cytokine known to regulate lymphocyte development and homeostasis. In developing B cells engagement of the IL-7R, composed of the common γ chain (γc) and IL-7R α chain (IL-7Rα), promotes the proliferation and survival of B lineage precursors (1–3) and is thought to be required for the effective differentiation of B220+ CD43+ pro-B cells into downstream B220+ CD43− pre-B cells (4–8). Although these studies clearly demonstrate that efficient lymphocyte development requires IL-7, the precise identity of the B lineage precursors receptive to IL-7, and how IL-7R–derived signals are integrated within these cells to regulate survival, proliferation, and differentiation, are currently unknown.
Current models indicate that the earliest B lineage–restricted precursors in adult bone marrow (BM) derive from common lymphoid progenitors (CLPs) defined by their capacity to give rise exclusively to each lymphoid lineage (9, 10). Here, we report that IL-7R activity is both necessary and sufficient for CLPs to differentiate into early B lineage precursors. First, we describe in vitro experiments in which freshly sorted CLPs readily differentiated into B lineage–committed precursors in IL-7–supplemented cultures lacking stromal elements. Second, we present evidence that B cell development is arrested earlier than previously appreciated in adult mice lacking components of the IL-7R. Although γc −/− mice exhibit normal frequencies of CLPs, we did not detect appreciable numbers of bona fide early B lineage precursors in either γc −/− or IL-7Rα−/− adults. These findings contrast with previous characterizations of B cell development in IL-7R–deficient mice, and indicate that although the development of CLPs does not require IL-7R activity, IL-7 plays an essential role in the earliest phases of adult B cell development.
Materials and Methods
Mice.
6–10-wk-old C57BL/6 and B6.Ly5.2 (referred to herein as B6.Ly5SJL) mice were purchased from the National Cancer Institute animal facility. IL-7Rα−/− mice (5) were purchased from The Jackson Laboratory and backcrossed with C57BL/6 mice for 12 generations before procurement. γc −/− and μMT−/− mice were provided by David Wiest (Fox Chase Cancer Center, Philadelphia, PA) and John Monroe (University of Pennsylvania Cancer Center, Philadelphia, PA), respectively.
Antibodies, Cell Sorting, and Analytical Flow Cytometry.
For sorting experiments, BM suspensions were prepared and stained with optimal dilutions of directly conjugated fluorescent antibodies as previously described (10), and then sorted on an 11-parameter MoFlo® cell sorter (Cytomation, Inc.). Antibodies used were fluorescein (FL) anti-lineage markers B220 (RA3–6B2), CD11b (M1/70), Gr-1 (8C5), Ter-119, and CD3 (2C11), PE-labeled anti-CD127/IL-7Rα (A7R34; reference 11), PE-Cy5–anti-CD117/c-kit (2B8), allophycocyanin (APC) anti-AA4 (AA4.1; reference 12), and biotin (BI)-anti–Sca-1/Ly6 A/E (E13-161.7) revealed with streptavidin (SA)-coupled APC-Cy7. Additional antibodies used for flow cytometric analyses are described in the appropriate figure legend and were purchased from BD Biosciences except for PE–Cy5-CD117/c-kit and APC–Cy7-B220 (Caltag), A7R34 (eBioscience/Cytomation), anti-IgM (Jackson ImmunoResearch Laboratories), and RA3-6B2 and AA4, which were purified and conjugated by standard methods in our laboratory. All flow cytometric analyses were performed on our MoFlo® or a dual laser FACSCalibur® (Becton Dickinson) and analyzed by uploading files into FlowJo® (Tree Star, Inc.).
Cell Cultures.
1,000 CLPs were cultured in round-bottom 96-well plates in 100 μl complete medium (Opti-MEM or RPMI 1640 with 5% FCS (Iscove) containing 10 mM glutamine, 10 mM Hepes, 0.5 mg/ml gentamycin, and 5 × 10−5 2-ME). This medium was supplemented with IL-7, stem cell factor (SCF), and/or fetal liver tyrosine kinase-3 (Flt-3)L (R&D Systems), each at a final concentration of 10 ng/ml.
Multilineage (Myeloid/Lymphoid) Progenitor (MLP) Assay.
The MLP assay was previously described (10, 13, 14). For the limiting dilution experiments described here, 3–100 freshly sorted CLPs were placed in hanging drop cultures in Terasaki plates with irradiated (2,700 rads) day 15 fetal thymi for 24 h before placement on polycarbonate filters (Millipore). Filters were then floated in 2 ml complete medium containing 3 ng/ml IL-3, 10 ng/ml IL-7, and 10 ng/ml SCF (R&D Systems), and then cultured and analyzed as previously described (10, 13, 14).
Intrathymic Transfers.
Intrathymic transfers were performed as previously described (15). In brief, 3,000 freshly isolated or cultured CLPs from female C57BL/6 (Ly5B6) adults were injected into the thymi of anesthetized female B6.Ly5SJL mice given 500 rads 18 h earlier.
Results
IL-7 Is Sufficient to Induce the Step-wise Differentiation of CLPs into B220+ CD19+ Cells In Vitro.
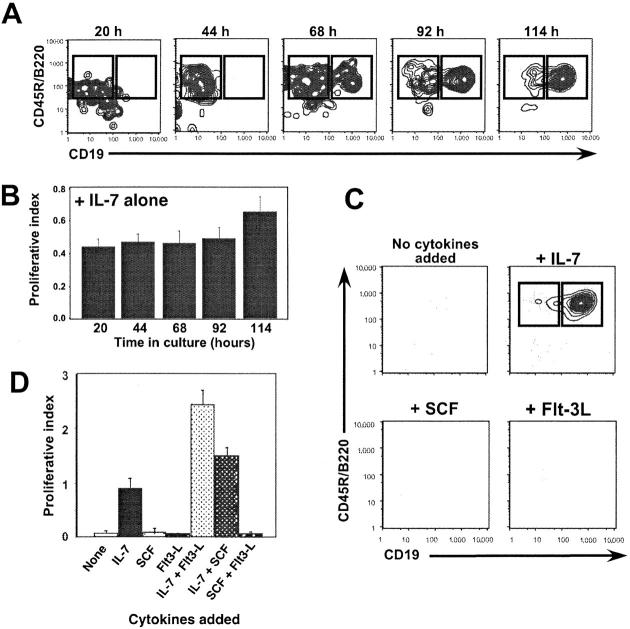
Previous studies illustrate that CLPs readily yield B lineage precursor cells when placed in stromal cultures supplemented with IL-7 (10, 16), or in methylcellulose supplemented with IL-7, SCF, and Flt-3L (9). To more precisely define the signals responsible for promoting B lineage differentiation, 1,000 lineage marker (B220, CD3, CD11b/Mac-1, Gr-1, Ter-119, Lin)− IL-7Rα+ AA4+ Sca-1low CLPs (10) were sorted and cultured in stromal-free conditions in media supplemented with IL-7. As shown in Fig. 1 A, IL-7 alone promoted the survival and step-wise differentiation of CLPs into B220+ CD19− and B220+ CD19+ cells for over 5 d in culture. Numbers of B220+ CD19− and B220+ CD19+ cells in these cultures mirrored input cell numbers (Fig. 1, B and D), suggesting minimal proliferation of CLPs as a consequence of IL-7R signaling. Moreover, CLPs cultured without IL-7 did not survive (Fig. 1 C), all B220+ CD19+ cells were also AA4+ (not depicted), and IL-7 was sufficient to induce this effect regardless of whether Opti-MEM (Fig. 1) or RPMI (unpublished data) culture medium was used. Finally, when cytokines to which CLPs are known to be responsive were used in isolation, namely IL-7, SCF, and Flt-3L (9, 10), only IL-7 drove early B cell differentiation. Combining SCF or Flt-3L with IL-7 led to increased recoveries of B220+ CD19+ cells, but CLP survival and B lineage differentiation did not occur without the inclusion of IL-7 (Fig. 1, C and D). Together, these data demonstrate that IL-7 is sufficient to promote early B cell differentiation from CLPs and are consistent with current models of early B cell development that predict that B220 expression precedes CD19 expression during early B cell development (17, 18).
Figure 1.
Lin− IL-7Rα+AA4+ Sca-1low BM cells (CLPs) yield B lineage precursors when cultured in IL-7 without stromal cells. (A) 1,000 sorted CLPs were cultured in stromal-free conditions with 10 ng/ml IL-7 for the indicated times before staining with antibodies to CD45R/B220, CD19, and AA4. (B) Cell recoveries for triplicate cultures for the experiment shown in A. (C) 1,000 sorted CLPs were cultured in stromal-free conditions with or without the indicated cytokine for 5 d, and then analyzed as described in A. (D) 1,000 sorted CLPs were cultured in triplicate in the presence or absence of the indicated cytokines(s), each at 10 ng/ml, and then analyzed 5 d later as described in B. All cells remained AA4+ (not depicted). Data are representative of eight separate experiments. Proliferative indices for B and D were calculated by dividing the number of viable cells recovered by the input cell number.
Exposure of CLPs to IL-7 Results in Loss of T Lineage Potential.
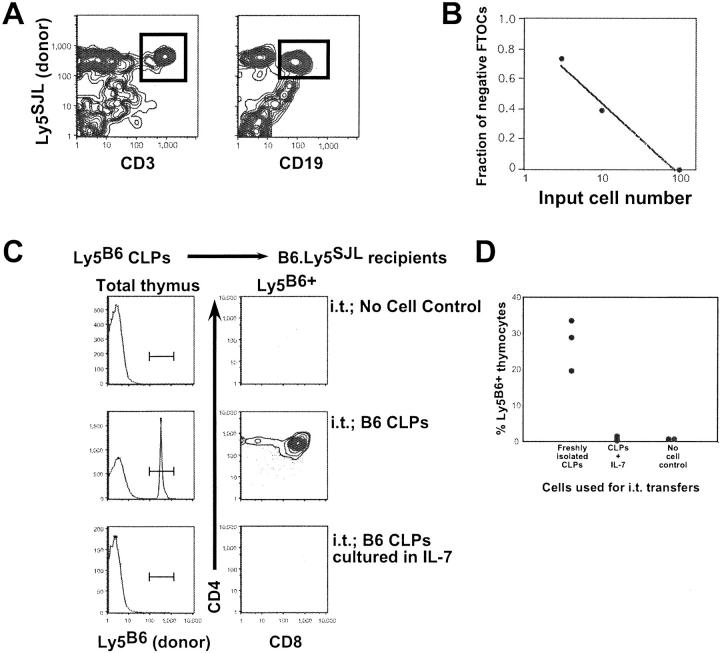
We also examined whether the IL-7–mediated up-regulation of B220 and CD19 expression on CLPs in vitro coincided with the loss of T lineage potential. Two previous studies have concluded that CLP-derived B and T lineage cells derive from clonogenic CLPs (9, 10). However, because each of these studies used a rather small sample size, it remained possible that this population also contained B- and T-committed precursors. Therefore, to test for heterogeneity within this population, graded doses of freshly sorted Lin− IL-7Rα+ AA4+ Sca-1low BM cells were cocultured with irradiated Ly5 disparate fetal thymi in hanging drop cultures before placement in the MLP assay. In this system, cytokine supplementation of fetal thymic organ cultures (FTOCs) provides a microenvironment supportive of B and T (and myeloid) development from early progenitors (10, 13, 14). Two relevant points can be derived from the data. First, MLP FTOCs seeded with limiting numbers of Lin− IL-7Rα+ AA4+ Sca-1low BM, including those in which donor-derived (Ly5SJL+) cells were detected after coculture of irradiated thymi with only three sorted cells, routinely contained donor-derived CD19+ B lineage and CD3+ T lineage cells (Fig. 2, A and B) . Second, the limiting dilution graph shown in Fig. 2 B demonstrates single hit kinetics for B/T progenitors in this system, suggesting that Lin− IL-7Rα+ AA4+ Sca-1low BM cells are a relatively homogeneous population of B/T progenitors.
Figure 2.
Lin− IL-7Rα+ AA4+ Sca-1low BM cells (CLPs) are a relatively homogeneous population of B/T progenitors that lose T lineage potential when cultured in IL-7. (A) Representative flow cytometric data from a lobe cocultured with three CLPs and containing detectable donor-derived cells. Freshly sorted CLPs from B6.Ly5SJL adults were cocultured with irradiated fetal thymi in hanging drop culture at a dose of 100, 10, or 3 cells/lobe for 24 h before placement in the MLP FTOC assay as described in Materials and Methods. Individual lobes were assessed 16 d later for the presence of donor-derived (B6.Ly5SJL+) B and T lineage cells as determined by staining with antibodies to CD19 (1D3) and CD3 (2C11), respectively. (B) Each data point is derived from a total of 20 thymi. (B) (C) Intrathymic transfers were performed using 3,000 C57BL/6 (Ly5B6) CLPs either freshly isolated or cultured in IL-7 4 d before transfer into B6.Ly5SJL recipients. After 18 d, thymocytes from each recipient were stained for the expression of CD4, CD8, and the B6 allele of CD45/Ly5. (D) Percentage of donor-derived (Ly5B6+) cells present in each B6.Ly5SJL host thymi. Each dot represents one recipient. Data are representative of two separate experiments.
These data suggest that the capacity of IL-7 to promote Lin− IL-7Rα+ AA4+ Sca-1low BM cells to differentiate into B220+ CD19+ cells might reflect both early B cell differentiation and B lineage commitment. To test this possibility, we compared the capacity of freshly isolated versus IL-7–cultured CLPs to yield T lineage precursors upon intrathymic transfer. As shown, when CLPs were cultured in IL-7 for 4 d before transfer, the resulting cells failed to give rise to T lineage cells after transfer (Fig. 2, C and D). This contrasted with freshly isolated CLPs, which readily and consistently yielded T lineage cells (Fig. 2, A and B), suggesting that the IL-7–induced up-regulation of B220 and CD19 in CLPs is also associated with the loss of T cell potential.
IL-7Rα– and γc–deficient Adults Lack Pro-B Cells.
The findings described above raise the question of whether IL-7–derived signals are required for the earliest phases of B cell development in vivo. Previous analyses of B cell development in IL-7– and IL-7Rα–deficient mice led others to conclude that deficiencies in IL-7 production or IL-7R expression result in an incomplete arrest at the B220+ CD43+ pro-B cell to B220+ CD43− pre-B cell transition that allows for the accumulation of significant numbers of peripheral B cells (5, 6, 8). However, a recent study by Carvalho et al. (19) suggests that most peripheral B cells in IL-7−/− mice derive from IL-7–independent fetal or perinatal precursors. These findings led us to more closely examine the B220+ CD43+ compartment in C57BL/6, μMT−/−, IL-7Rα−/−, and γc −/− adults. For these experiments, we chose to discriminate bona fide B lineage precursors from non-B lineage B220+ cells and potential recirculating B cells through surface expression of AA4 and the pan B lineage–restricted protein CD19. μMT−/− adults were used as a control because these mice are characterized by a clear and profound arrest in B cell development at the pro- to pre-B cell transition (20).
As shown in Fig. 3 , B220+ CD43+ BM cells in adult C57BL/6 mice can be subdivided into both AA4+ CD19+ pro-B cells and a AA4− CD19+ population that was not detected in μMT−/− BM. Significantly, although B220+ CD43+ AA4+ CD19+ CD24/HSA+ pro-B cells were clearly apparent in the BM of C57BL/6 and μMT−/− adults, we could not detect these cells in either IL-7Rα−/− or γc −/− adults (Fig. 3), indicating an arrest in B cell development before the pro-B cell stage in IL-7R–deficient mice. Consistent with this interpretation, among these populations only cells derived from the AA4+ CD19+ population proliferated in IL-7–supplemented stromal cultures, and additional analyses demonstrated that the B220+ CD43+ CD19+ AA4− population (Fig. 3, upper right) expressed high levels of sIgM (unpublished data), and may therefore constitute recirculating CD43+B1 B cells (21). Furthermore, although we readily detected B cells in the spleens of IL-7Rα−/− and γc −/− adults, these cells expressed a sIgMhigh sIgDlow surface phenotype indicative of fetal-derived B1 B cells in accordance with the recent findings of Carvalho et al. (19; unpublished data). Finally, it should be noted that an AA4− CD19low population of unknown identity was detected in a subset of IL-7Rα−/− and γc −/− adults. Together, these data indicate that the loss of IL-7R activity leads to an arrest in B cell development before the development of pro-B cells.
Figure 3.
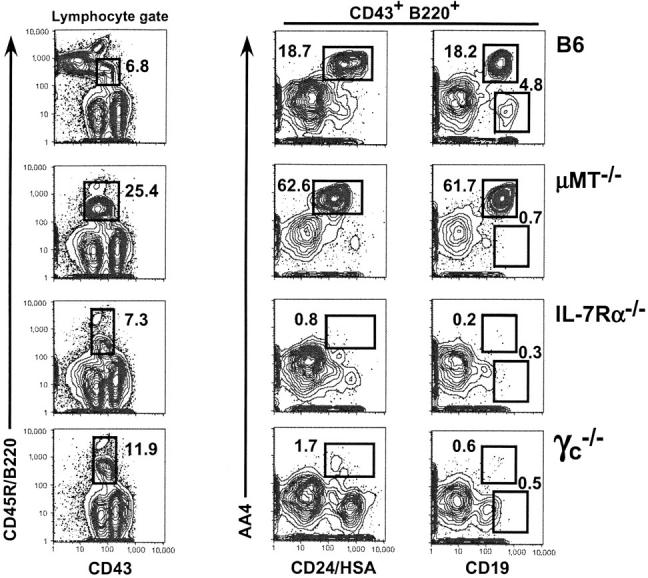
Lack of AA4+ B220+ CD43+ pro-B cells in adults lacking components of the IL-7R. BM cells from 8-wk-old C57BL/6, μMT−/−, IL-7Rα+, and γc −/− mice were stained with FL-CD43 (S7), PE-CD19 (1D3), APC-Cy7-B220, APC-AA4, and BI–anti-CD24/HSA (30F1) revealed with SA-PE-TR. 200,000 events per tube were subsequently analyzed on a MoFlo® flow cytometer as described in Materials and Methods. Data are representative of four separate experiments. Numbers indicate the fraction of events among the parent population falling within the indicated gate and were consistent with three or more mice per group.
Disruption of the IL-7R Leads to Loss of the Earliest Stage of B Lineage Development without Affecting the Development of CLPs.
Because IL-7Rα−/− and γc −/− mice lack bona fide pro-B cells, we next sought to define the precise point in early B lineage development affected by the inability to assemble a functional IL-7R. Accordingly, we compared the frequency of CLPs and the earliest identified B lineage precursors termed pre–pro-B cells in C57BL/6, μMT−/−, IL-7Rα−/−, and γc −/− adults. To characterize AA4+ B220+ CD19− B lineage pre–pro-B cells, we coupled the findings of Li et al. (17), which demonstrate that these cells are HSA− and AA4+, with the recent findings of Tudor et al. (18), which show that the HSA− AA4+ B220+ pool in normal mouse BM is heterogeneous with functional B lineage precursors being restricted to those cells lacking surface expression of Ly6C.
As shown, although the determination of the frequency of IL-7Rα+ cells is precluded in IL-7Rα−/− mice, C57BL/6, μMT−/−, and γc −/− adults had comparable frequencies of CLPs (Fig. 4 A). In sharp contrast, both C57BL/6 and μMT−/− but not IL-7Rα−/− and γc −/− adults had detectable numbers of Ly6C− HSA− AA4+ B220+ pre–pro-B cells (Fig. 4 B). Thus, these data indicate that although the loss of the IL-7R does not perturb CLP development, the differentiation of all B lineage precursor populations downstream of CLPs requires the expression of both components of the IL-7R.
Figure 4.
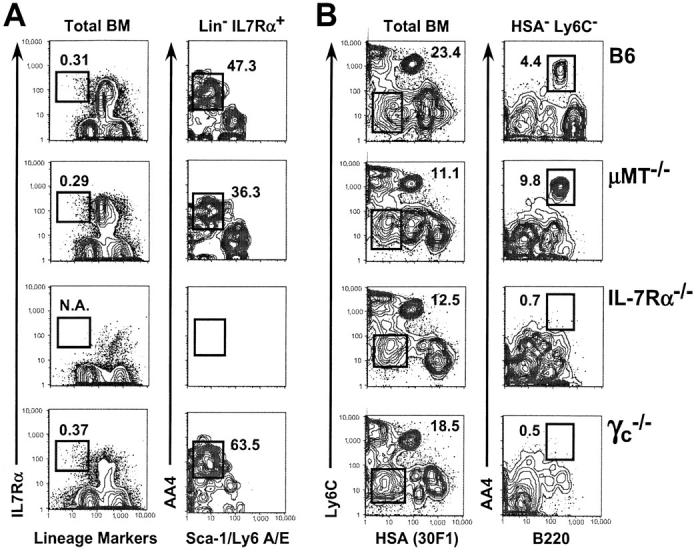
Frequencies of very early B lineage precursors, but not CLPs, are diminished in adult mice lacking components of the IL-7R. (A) BM cells from 8-wk-old C57BL/6, μMT−/−, IL-7Rα+, and γc −/− mice were stained with FL lineage markers (see Materials and Methods), PE-anti–IL-7Rα, APC-AA4, and BI-anti–Sca-1. N.A., not applicable. (B) BM cells from the aforementioned adults were stained with FL-Ly6C (AL-21), PE-B220, APC-AA4, and BI-CD24/HSA (30F1). Biotinylated antibodies were revealed with SA-PerCP-Cy5.5 and 200,000 events per tube were collected on a FACSCalibur® flow cytometer as described in Materials and Methods. Numbers indicate the fraction of events among the parent population falling within the indicated gate and were consistent for three or more mice per group. Data are representative of four separate experiments.
Discussion
Two lines of evidence support the conclusion that IL-7R signaling is critical for the development of CLPs into very early B cell precursors. First, CLPs cultured in IL-7 without stromal cells adopted a B220+ CD19+ surface phenotype associated with pro-B cells (Fig. 1), showing that although stromal elements may provide additional signals required for the expansion of precursor cells within this pathway, direct interactions between CLPs and stromal cells and additional stromal-derived cytokines are not requisite for early B lineage differentiation. Moreover, the up-regulation of B220 and CD19 occurred in a step-wise manner consistent with current models of early B cell development (Fig. 1 A; references 15 and 17), thus providing direct evidence that B220+ CD19− AA4+ pre–pro-B cells are a developmental intermediate between CLPs and CD19+ pro-B cells. Finally, although CLPs are also responsive to SCF and Flt-3L (9), neither of these factors promoted B lineage differentiation in vitro unless IL-7 was included (Fig. 1), and CLPs cultured in IL-7 for 4 d lost the capacity to generate T cells (Fig. 2). Second, contrary to conclusions from previous studies in which Ly6C+ B220+ BM cells were not excluded from analysis (22), IL-7Rα−/− and γc −/− adults exhibit a profound and very early block in initial B cell development characterized by normal frequencies of CLPs and low to undetectable frequencies of pre–pro-B cells and all downstream progenitor populations (Figs. 3 and 4).
Although elucidating the mechanism by which IL-7 promotes early B lineage differentiation will require additional study, it is noteworthy that CLPs failed to survive in vitro without added IL-7 (Fig. 1 C), and IL-7 stimulation of CLPs resulted in the loss of T lineage potential (Fig. 2). These data suggest that IL-7 functions to promote survival as well as induce B lineage differentiation of CLPs. However, because ectopic expression of Bcl-2 does not rescue early B cell development in IL-7Rα−/− and γc −/− mice (23–25), it is likely that IL-7 provides CLPs with both trophic and mechanistic signals required for early B lineage differentiation. In this regard, it is noteworthy that Corcoran et al. (8) demonstrated diminished mRNA levels for Pax5 in B lineage cells from IL-7Rα−/− mice. Given that Pax5 provides a mechanistic signal essential for maintaining B lineage commitment (26, 27), one might speculate that IL-7R signaling stimulates early B cell differentiation from CLPs by directly up-regulating Pax5 transcription. However, because IL-7Rα−/− adults lack bona fide pro-B cells (Figs. 3 and 4), perhaps this issue might be more clearly resolved through a comparison of Pax5 levels in normal and IL-7R–deficient CLPs.
Although IL-7 regulates both trophic and differentiative aspects of early B and T cell development (28), there are also several key differences in the impact of IL-7R activity on progenitors for each lineage. For T lineage precursors, αβ but not γδ T cell development is partially restored via the expression of a Bcl-2 transgene in γc −/− and IL-7Rα−/− mice (23–25, 29), and IL-7 provides a mechanistic signal required for the rearrangement of the TCR-γ locus (23–25, 29) and is apparently requisite in both fetal and adult life. In contrast, for B lineage precursors, Bcl-2 transgenes do not complement IL-7R deficiencies (23–25), a clear connection between IL-7R activity and IgH rearrangement remains to be established, and IL-7 is required during adult but not fetal/perinatal life (19 and data herein). Thus, it is likely that IL-7R signaling and/or access to IL-7 target genes is regulated by lineage- and stage-specific mechanisms in early lymphoid precursors and their downstream B and T lineage progeny that result in qualitative differences in the outcome of IL-7R activity.
Given our demonstration that IL-7 is sufficient to promote the differentiation of CLPs into B lineage precursors and the critical role IL-7 plays in early thymocyte development (28), the question arises as to how early lymphoid progenitors in the thymus avoid B lineage differentiation in the presence of IL-7. Because Notch signaling is required for T lineage commitment (30), one possibility is that Notch signals override or otherwise modify IL-7 signals to drive T lineage commitment. Thus, IL-7 and Notch signaling might be coordinately regulated in early lymphoid progenitors to result in B lineage commitment in the BM and T lineage commitment in the thymus. However, it should be emphasized that it is presently unclear whether the adult thymus is seeded by CLPs and/or earlier BM-derived lymphoid precursors. Therefore, it is also possible that signals leading to B and T cell commitment target qualitatively distinct progenitors. Additional studies will be required to address how environmental cues such as those derived from IL-7 and Notch regulate the earliest phases of lymphoid development in the BM and thymus.
Acknowledgments
We thank Drs. Jennifer Punt, Michael Cancro, and Warren Pear for helpful discussions and critically reviewing this manuscript, and Kristina Rudd for technical support.
D. Allman is supported by National Institutes of Health (NIH) grants AI52861 and AG20818 and an intramural grant (IRG-78-002-23) from the University of Pennsylvania Cancer Center. R. Gerstein is supported by NIH grant AI43534.
D. Izon's present address is TVW Telethon Institute for Child Health Research, 100 Roberts Road, Subiaco WA 6008, Australia.
References
- 1.Namen, A.E., S. Lupton, K. Hjerrild, J. Wignall, D.Y. Mochizuki, A. Schmierer, B. Mosley, C.J. March, D. Urdal, and S. Gillis. 1988. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 333:571–573. [DOI] [PubMed] [Google Scholar]
- 2.Sudo, T., M. Ito, Y. Ogawa, M. Iizuka, H. Kodama, T. Kunisada, S. Hayashi, M. Ogawa, K. Sakai, and S. Nishikawa. 1989. Interleukin 7 production and function in stromal cell–dependent B cell development. J. Exp. Med. 170:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy, R.R., C.E. Carmack, S.A. Shinton, J.D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabstein, K.H., T.J. Waldschmidt, F.D. Finkelman, B.W. Hess, A.R. Alpert, N.E. Boiani, A.E. Namen, and P.J. Morrissey. 1993. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J. Exp. Med. 178:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran, A.E., F.M. Smart, R.J. Cowling, T. Crompton, M.J. Owen, and A.R. Venkitaraman. 1996. The interleukin-7 receptor alpha chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J. 15:1924–1932. [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran, A.E., A. Riddell, D. Krooshoop, and A.R. Venkitaraman. 1998. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 391:904–907. [DOI] [PubMed] [Google Scholar]
- 9.Kondo, M., I.L. Weissman, and K. Akashi. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 91:661–672. [DOI] [PubMed] [Google Scholar]
- 10.Izon, D., K. Rudd, W. DeMuth, W.S. Pear, C. Clendenin, R.C. Lindsley, and D. Allman. 2001. A common pathway for dendritic cell and early B cell development. J. Immunol. 167:1387–1392. [DOI] [PubMed] [Google Scholar]
- 11.Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi, and H. Yoshida. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKearn, J.P., J. McCubrey, and B. Fagg. 1985. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc. Natl. Acad. Sci. USA. 82:7414–7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamoto, H., T. Ikawa, K. Ohmura, S. Fujimoto, and Y. Katsura. 2000. T cell progenitors emerge earlier than B cell progenitors in the murine fetal liver. Immunity. 12:441–450. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto, H., K. Ohmura, and Y. Katsura. 1997. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. Int. Immunol. 9:1011–1019. [DOI] [PubMed] [Google Scholar]
- 15.Allman, D., J. Li, and R.R. Hardy. 1999. Commitment to the B lymphoid lineage occurs before DH-JH recombination. J. Exp. Med. 189:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo, M., D.C. Scherer, T. Miyamoto, A.G. King, K. Akashi, K. Sugamura, and I.L. Weissman. 2000. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 407:383–386. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y.S., R. Wasserman, K. Hayakawa, and R.R. Hardy. 1996. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 5:527–535. [DOI] [PubMed] [Google Scholar]
- 18.Tudor, K.S., K.J. Payne, Y. Yamashita, and P.W. Kincade. 2000. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity. 12:335–345. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho, T.L., T. Mota-Santos, A. Cumano, J. Demengeot, and P. Vieira. 2001. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7−/− mice. J. Exp. Med. 194:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papavasiliou, F., Z. Misulovin, H. Suh, and M.C. Nussenzweig. 1995. The role of Ig beta in precursor B cell transition and allelic exclusion. Science. 268:408–411. [DOI] [PubMed] [Google Scholar]
- 21.Wells, S.M., A.B. Kantor, and A.M. Stall. 1994. CD43 (S7) expression identifies peripheral B cell subsets. J. Immunol. 153:5503–5515. [PubMed] [Google Scholar]
- 22.Egawa, T., K. Kawabata, H. Kawamoto, K. Amada, R. Okamoto, N. Fujii, T. Kishimoto, Y. Katsura, and T. Nagasawa. 2001. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 15:323–334. [DOI] [PubMed] [Google Scholar]
- 23.Maraskovsky, E., L.A. O'Reilly, M. Teepe, L.M. Corcoran, J.J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 89:1011–1019. [DOI] [PubMed] [Google Scholar]
- 24.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. [DOI] [PubMed] [Google Scholar]
- 25.Kondo, M., K. Akashi, J. Domen, K. Sugamura, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 7:155–162. [DOI] [PubMed] [Google Scholar]
- 26.Nutt, S.L., B. Heavey, A.G. Rolink, and M. Busslinger. 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 401:556–562. [DOI] [PubMed] [Google Scholar]
- 27.Rolink, A.G., S.L. Nutt, F. Melchers, and M. Busslinger. 1999. Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature. 401:603–606. [DOI] [PubMed] [Google Scholar]
- 28.Candeias, S., K. Muegge, and S.K. Durum. 1997. IL-7 receptor and VDJ recombination: trophic versus mechanistic actions. Immunity. 6:501–508. [DOI] [PubMed] [Google Scholar]
- 29.Ye, S.K., K. Maki, T. Kitamura, S. Sunaga, K. Akashi, J. Domen, I.L. Weissman, T. Honjo, and K. Ikuta. 1999. Induction of germline transcription in the TCRγ locus by Stat5: implications for accessibility control by the IL-7 receptor. Immunity. 11:213–223. [DOI] [PubMed] [Google Scholar]
- 30.Allman, D., J.A. Punt, D.J. Izon, J.C. Aster, and W.S. Pear. 2002. An invitation to T and more. Notch signaling in lymphopoiesis. Cell. 109:S1–S11. [DOI] [PubMed] [Google Scholar]