Abstract
In the central nervous system, release of Ca2+ from intracellular stores contributes to numerous functions, including neurotransmitter release and long-term potentiation and depression. We have investigated the developmental profile and the regulation of inositol 1,4,5-trisphosphate receptor (IP3R) and ryanodine receptor (RyR) in primary cultures of cerebellar granule cells. The expression of both receptor types increases during development. Whereas the expression of type 1 IP3R appears to be regulated by Ca2+ influx through L type channels or N-methyl-d-aspartate (NMDA) receptors, RyR levels increase independently of Ca2+. The main target of Ca2+-influx-regulating IP3R expression is the Ca2+ calmodulin-dependent protein phosphatase calcineurin, because pharmacological blockade of this protein abolishes IP3R expression. Although calcineurin has been shown to regulate the phosphorylation state of the IP3R, the effect described here is at the transcriptional level because IP3R mRNA changes in parallel with protein levels. Thus, calcineurin plays a dual role in IP3R-mediated Ca2+ signaling: it regulates IP3R function by dephosphorylation in the short-term time scale and IP3R expression over more extended periods.
Mammalian cells contain two main channels responsible for Ca2+ efflux from intracellular stores, inositol 1,4,5-trisphosphate receptors (IP3Rs; gated by inositol 1,4,5-trisphosphate) (1, 2) and ryanodine receptors (RyRs; gated by Ca2+ and by the pyridine nucleotide cyclic ADP ribose) (2, 3). Both IP3Rs and RyRs are the products of multigene families. At least three members have been described for each family (IP3R1–3 and RyR1–3) with different regional distribution patterns (1, 4, 5). The present work investigates the developmental pattern of expression of the two receptor types in neurons and their dependence on Ca2+. As shown for other proteins involved in Ca2+ signaling (6, 7), IP3R expression depends on Ca2+ influx, whereas RyR expression does not appear to be regulated in the same manner.
Calcineurin is a Ca2+/calmodulin-dependent phosphatase that has been shown to play a central role in the regulation of gene expression (8). In T lymphocytes, when an antigen binds to its receptor, Ca2+ influx activates calcineurin (9), which dephosphorylates the transcription factor NF-AT (nuclear factor of activated T cells). The latter then enters the nucleus and triggers IL-2 transcription, leading to T lymphocyte activation (9). The central role played by calcineurin in immune responses has been demonstrated by two of the most important immunosuppressants, FK506 and cyclosporin A, which have turned out to be powerful calcineurin inhibitors (9). Calcineurin’s regulation of transcription has also been shown to be central in the development of normal heart morphogenesis (10, 11) as well as in the etiology of cardiac hypertrophy (12, 13).
Because calcineurin is abundant in the brain (14), we decided to explore its involvement in the transcriptional regulation of IP3R in cultured cerebellar granule cells, one of the most robust and best established primary neuronal cultures (15). FK506 and cyclosporin A completely abolished IP3R expression: the regulation was at the transcriptional level because IP3R mRNA decreased in parallel with the protein. The immunosuppressant rapamycin, a structural analogue of FK506 devoid of an inhibitory action on calcineurin, did not interfere with IP3R transcription or expression. Thus, the data show that calcineurin regulates gene transcription in the central nervous system. This phosphatase had been previously shown to bind to and modulate the phosphorylation state of the IP3R complex (16). The results described here show that calcineurin has an additional role in controlling Ca2+-release from intracellular stores, because it also regulates the expression of the IP3R.
MATERIALS AND METHODS
Cell Culture.
Granule cells were dissociated from the cerebella of 7-day-old Wistar rats as described (15, 17) and plated in DMEM Hepes modification (Sigma) containing 1.8 mM CaCl2 supplemented with heat-inactivated 10% fetal calf serum (Life Technologies, Grand Island, NY or Sigma), 100 μg/ml gentamicin, 7 μM p-aminobenzoic acid, 100 μg/ml pyruvate, 100 microunits/ml insulin, on poly-l-lysine-treated plates at a density of 2–3 × 105 cells per cm2. After 24 h, 10 μM cytosine arabinofuranoside was added to the culture to inhibit mitotic active cell’s growth. Neuronal survival was estimated by measuring the amount of colored formazan by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (18).
Preparation of Membranes.
Cerebella were dissected from rat brains and homogenized in 5 mM Tris/HCl (pH 7.5)/320 mM sucrose/5 μg/ml pepstatin, antipain, and leupeptin with a loose Potter-homogenizer. A crude synaptosomal membrane fraction was obtained after centrifuging the postnuclear supernatant at 12,000 × g for 10 min at 4°C. The supernatant was again centrifuged at 100,000 × g for 1 h at 4°C. The 100,000 × g precipitated material was defined as the microsomal fraction.
Granule cells were resuspended at 5–10 × 106 cells per ml in 10 mM Tris⋅HCl (pH 8.0)/1 mM EDTA/5 μg/ml leupeptin/5 μg/ml aprotinin/5 μg/ml pepstatin/75 μg/ml PMSF/1 mM DTT and subjected to three cycles of freeze and thaw. The particulate fraction was sedimented at 15,000 × g for 15 min. The resulting protein pellet was resuspended in 5 mM Tris⋅HCl (pH 8.0)/10% sucrose and frozen at −70°C.
Western Blotting.
Proteins were separated by using SDS/PAGE (19) and transferred to nitrocellulose or poly(vinyldenedifluoride) (PVDF)membranes (20). The membranes were incubated with isoform specific polyclonal antibodies against IP3R1, IP3R3, or a polyclonal antibody that recognizes all three IP3R isoforms (21). RyRs were detected with isoform-specific antibodies kindly donated by A. Conti and V. Sorrentino (University of Milan, Italy) (22). The monoclonal antibody against PMCA4 was a kind gift of J. Penniston (Mayo Clinic, Rochester, MN). The polyclonal antibody against SERCA2b was a gift of F. Wuytack (Catholic University, Leuven, Belgium). The blots were developed with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) or with cytidine 5′-diphosphate–Star (Tropix, Bedford, MA).
Isolation of RNA, Reverse Transcription–PCR (RT-PCR), and Southern and Northern Blotting.
Total RNA was prepared from granule cells by using the Trizol reagent according to manufacturer’s instructions (GIBCO) and was either used for Northern blotting as described elsewhere (23) or was reverse-transcribed by using a random octamer primer (first-strand cDNA synthesis kit, Amersham Pharmacia) according to the manufacturer’s protocol.
The following nucleotides were used for PCR: IP3R1 (forward: GTGGAGGTTTCATCTGCAAGC; reverse: GCTTTCGTGGAATA CTCGGTC), IP3R2 (forward: GCTCTTGTCCCTGACATTG; reverse: CCC ATGTCTCCATTCTCATAGC); IP3R3 (forward: CTGCCCAAGAGGAGG AGGAAG; reverse: GAACAGCGCGGCAATGGAGAAG); RyR1 (forward: GCTT AGCTGAGGTCTGCAGCTGG; reverse: AGGGGGTGTAGCACAGG ATTTAT); RyR2 (forward: TTCATTGCATGTTTATTATGC; reverse: AAAA GATGGCCTGTCAAGGCGTC); RyR3 (forward: CCTGAGTTCACG ACAAGCTAC; reverse: TAGCTGCTTAAAGCTTTTC); G3PDH (forward: CCAAAAGGGTCATCATCTCC; reverse: GTAGGCCATGAGGTCC ACCAC); c-fos (forward: AAGTCTGCGTTGCAGAAGGAACC; reverse: GTCTGCTGCATAGAAGGAACC).
The conditions for the PCR reactions were as suggested from Perkin–Elmer for the Taq Gold polymerase. PCR products were separated on 1.8% agarose gels or on 8% PAGE. All reactions resulted in a single band except for the IP3R1 experiments, where two bands, corresponding to two splicing variants were amplified according to the treatments. The identity of the PCR-generated fragments was verified by DNA sequencing (23). For Southern and Northern blotting (23), the 113-bp fragment from position 5412 and 5525 of the published IP3R1 sequence (24) was used as a probe.
RESULTS AND DISCUSSION
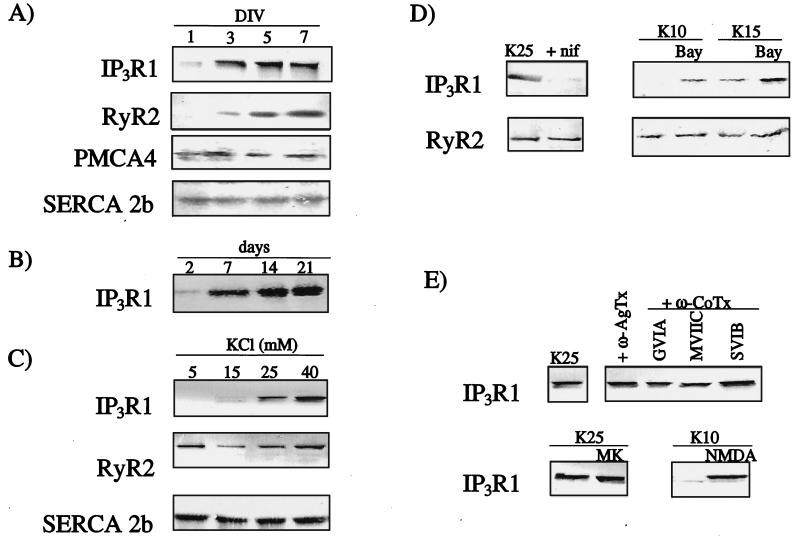
Preliminary Western blotting analysis of the receptor subtypes present in granule cells revealed that RyR1, RyR3 and IP3R3 were absent (data not shown). This was confirmed by RT-PCR, performed by using primers specific for the receptor subtypes (data not shown). Although Western blots on IP3R2 were not performed, RT-PCR experiments showed that this receptor subtype was present in the cultures. However, no further investigation on it was performed because this receptor has been shown to be present only in the astrocytic contamination of these cultures (25), which accounts for <2% of the total cell population (15). Thus, the main intracellular Ca2+-channel subtypes present in cerebellar granule cells were IP3R1 and RyR2 (Fig. 1A). The expression levels of these two receptor subtypes, which were extremely low at the start of the culture, increased with time, reaching a peak 5–9 days after plating, when granule neurons have become mature (15) (Fig. 1A). This was also true of intact cerebellum, where IP3R1 expression increased during development from day 2–21 after birth (Fig. 1B). The isoform 4 of the plasma membrane Ca2+-ATPase (PMCA4) and the isoform 2b of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2b) were used as controls (Fig. 1A), because their levels do not change during development under depolarizing conditions (6).
Figure 1.
Characterization of intracellular Ca2+ channels in developing granule cells by Western immunoblotting. In all cases, 25 μg of crude membrane proteins were separated and immunoblotted with isoform-specific antibodies. (A) Developmental profile in cerebellar granule cells of IP3R1, RyR2, PMCA4, and SERCA2b. DIV, days in vitro. (B) Developmental profile of IP3R1 in developing cerebellum. Days shown are days after birth. (C) Effect of different concentrations of KCl on the expression of IP3R1, RyR2, and SERCA2b. KCl concentrations were adjusted 1 day after plating, and cells were harvested on the fifth day. (D) Effect of agents acting on the L type Ca2+ channel on the expression of IP3R1. nif, nifedipine, 10 μM; BayK, BayerK8644, 1 μM. (E) Effect of agents acting on non-L type Ca2+ channels on IP3R1 expression. Agatoxins and ω-conotoxins all at 1 μM; MK, MK806, 10 μM; NMDA, 130 μM.
Granule cells require chronic partial depolarization of the plasma membrane to survive and to mature (15). This is routinely achieved by increasing K+ in the medium from about 5 to 25 mM, a condition that is thought to mimic synaptic activity. The effects of depolarization on the expression of IP3R1 and RyR2 were thus investigated. RyR2 expression appeared to be independent of depolarization, whereas IP3R1 was highly sensitive to it. After 5 days in culture in the presence of low, nondepolarizing levels of K+ (5.3 mM), barely detectable amounts of the IP3R1 protein were expressed, whereas evident expression was observed in the presence of potassium concentrations higher than 15 mM (Fig. 1C). The expression of the SERCA2b pump, another protein involved in intracellular Ca2+ homeostasis, was also unaffected by the change in the extracellular K+ concentration (Fig. 1C). Next, factors that controlled IP3R1 expression were investigated. During depolarization, the cellular Ca2+ level increases, the increase being prevented by L type Ca2+-channel blockers, e.g., nifedipine (26). The treatment with nifedipine abolished IP3R1 expression but did not affect that of RyR2 (Fig. 1D). The nifedipine treatment reversed the K+-dependent increase of the resting intracellular Ca2+ concentration, which reached 150 ± 10 nM after 4–5 days in 25 mM KCl. In the presence of nifedipine, intracellular Ca2+ decreased to 60 ± 10 nM, as compared with 55 ± 10 nM for cells cultured under nondepolarizing conditions (6). The addition of nifedipine induced cell death after 6–7 days culture, as in the case of cells cultured in low potassium. Moreover, if neurons were treated with 10 or 15 mM K+ (concentrations that are at the border of effectiveness in up-regulating the IP3R1) in conjunction with an activator of L type Ca2+-channels, BayK8644, the level of IP3R1 increased, becoming similar to that observed in neurons cultured for 5 days in 25 mM KCl (Fig. 1D). Consistent with the nifedipine experiment, RyR2 expression was not affected by BayK8644 (Fig. 1D). The addition of antagonists of Q, N, and P type voltage-operated Ca2+-channels or of the NMDA receptor antagonist MK801 to the culture medium was ineffective in blocking the IP3R1 expression induced by 25 mM KCl (Fig. 1E), indicating that Ca2+ entering through the L type Ca2+-channel was preferentially responsible for the up-regulation of IP3R. However, Ca2+ entering through the NMDA receptor could also have a role, because the addition of the agonist NMDA (in the presence of 10 mM K+, necessary to relieve the physiological Mg2+ block of the channel) also induced IP3R1 expression (Fig. 1E). Again, RyR2 expression was not influenced by any of the above treatments (data not shown).
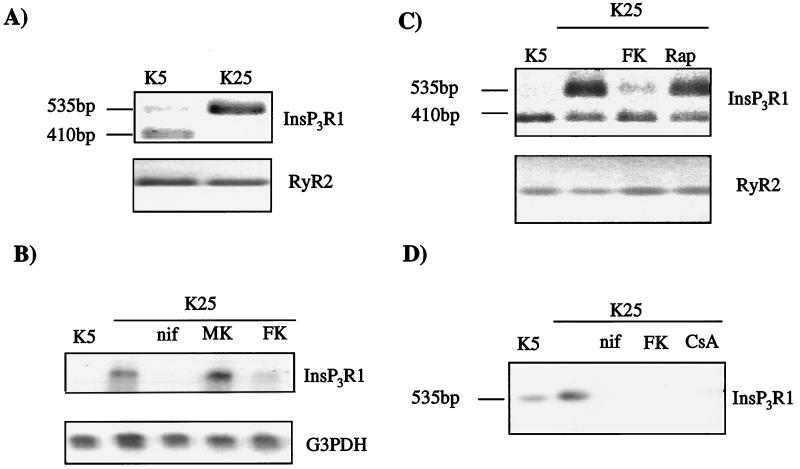
The up-regulation of the IP3R1 by Ca2+ influx through voltage-operated Ca2+ channels could be mediated by a number of mechanisms: (i) increased stability of the expressed protein; (ii) increased efficiency of its translation; (iii) positive regulation at the transcriptional level. The latter possibility was considered the most likely and was explored in RT-PCR experiments on cDNA prepared from cultures grown under different conditions. The analysis showed highly enhanced amplification of the transcript of the splicing variant IP3R1B− in cells grown under depolarizing conditions (Fig. 2A) whereas in control cells the amplified transcript was that of the variant IP3R1ABC− (27). The splice variant present in cells grown under depolarizing conditions corresponded to the dominant form present in neuronal tissues and its presence was, thus, not surprising, whereas the band amplified from control cells corresponded to the form usually present in nonneuronal tissue (28). Control experiments with G3PDH (glyceraldehyde-3-phosphate dehydrogenase) (data not shown) or RyR2 (Fig. 2A) specific primers failed to reveal differences among samples from cells grown in high or low KCl. Because RT-PCR is not quantitative, Northern blot analysis was also performed on RNAs obtained from cultures grown under different conditions. As shown in Fig. 2B, 25 mM K+ induced an increase in IP3R1 mRNA that was blocked by nifedipine but not by MK801, in agreement with the results of the Western blotting experiments.
Figure 2.
IP3R1 is up-regulated at the transcriptional level. (A) RT-PCR using specific primers for IP3R1 and RyR2 with total RNA from cells cultured in the presence (K25) or absence (K5) of 25 mM KCl. RT-PCR samples were separated on agarose gels and stained with ethidium bromide. Negatives of the gels are shown. The two bands on the IP3R1 gel correspond to the neuronal (535 bp) and nonneuronal (410 bp) splice variants of the receptor. (B) Northern blot analysis of RNA extracted from cells cultured for 5 days with nifedipine (nif, 10 μM), MK801 (MK, 1 μM), or FK506 (FK, 300nM). Total RNA (15 μg) was separated on formaldehyde gels, transferred to Nytran, and probed with 32P-labeled RNA fragments. Equal loading was verified by hybridizing the membrane with a G3PDH-specific probe. (C) RT-PCR using specific primers for IP3R1 with RNA from cells cultured in the presence or absence of 25 mM KCl, 3 nM FK506 and rapamycin (1 μM). Bands as in Fig. 2A. (D) Southern blotting of a RT-PCR experiment. After separation on an agarose gel, the DNA was transferred to Nytran and probed with a 32P-labeled IP3R1B-specific probe.
The study was then extended to calcineurin, a Ca2+–calmodulin-regulated protein phosphatase (29) that is known to regulate gene transcription in T lymphocytes (30, 31), in heart (32) and in Saccharomices cerevisiae (33). Blockade of this phosphatase by FK506, an immunosuppressant that selectively inhibits calcineurin activation (34), abolished the increase in IP3R1 mRNA induced by depolarization (Fig. 2B). FK506 blocks calcineurin on binding to a specific family of proteins called FKBPs (34). In a control experiment, RT-PCR was performed using rapamycin, an immunosuppressant that binds to the same set of proteins as FK506, but that is not capable of inhibiting calcineurin (34). Treatment of granule cells with rapamycin failed to reverse the increase of IP3R1 transcript induced by depolarization (Fig. 2C). Southern blotting was performed by using a IP3R1 splice site specific probe to test the authenticity of the fragments amplified by PCR. As expected, the probe hybridized with a band of 535 bp, which was significantly higher in granule cells grown under depolarizing conditions (Fig. 2D). In cells treated with nifedipine or FK506, the 535-bp band was not visible, in agreement with the Northern blotting experiments. Cyclosporin A, an immunosuppressant that inhibits calcineurin by binding to an immunophilin distinct from FKBPs (34), also prevented the increase of the IP3R1-specific band (Fig. 2D).
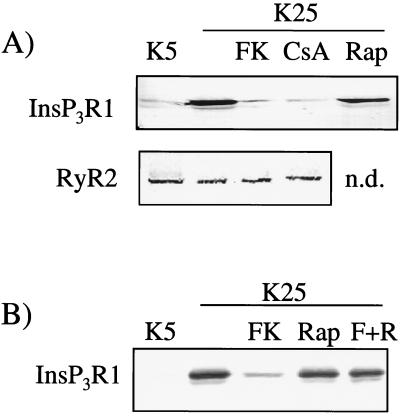
The immunosuppressant experiments were then extended to the receptor protein: both FK506 and cyclosporin A abolished the expression of the IP3R1 protein at concentrations as low as 3 nM and 100 nM, respectively (data not shown). Again, the effect was not mimicked by rapamycin (Fig. 3A). Because FK506 and rapamycin bind to the same FK-binding proteins, a competition experiment was performed by adding FK506 and a 1,000-fold higher concentration of rapamycin to cells cultured in the presence of 25 mM KCl. Under these conditions, FK506 failed to abolish IP3R1 expression, presumably reflecting its displacement from the immunophilin target protein.
Figure 3.
Characterization of the regulation by calcineurin of IP3R1 expression by Western immunoblotting. (A) Cells were treated on day 1 and harvested on day 5. FK, FK506, 3nM; CsA, cyclosporin, 300nM; Rap, rapamycin, 1 μM. (B) Same as A; FK, FK506, 3nM, Rap, rapamycin, 3 μM; F+R, FK506 and rapamycin.
FK506 has been shown to affect the activity of numerous plasma membrane channels (14), including L type Ca2+-channels. In principle, then, its effects on IP3R1 expression could be linked to a decreased Ca2+ influx. This, however, cannot be the case because both FK506 and cyclosporin A inhibit IP3R1 expression, whereas only FK506 binds to the FKBPs responsible for the change in L type Ca2+ channel activity. In addition, (i) the Ca2+-dependent up-regulation of c-fos (Fig. 4A) and of the plasma membrane Ca2+-ATPase isoform 1 (D.G. and E.C., unpublished data) were not affected by FK506 and (ii) the blockade of Ca2+ influx in these cells increased the proportion of cells that eventually died (35), whereas no significant differences in cell survival were observed between controls and FK506-treated cells.
Figure 4.
(A) RT-PCR using specific primers for c-fos. Cells were treated for 30 minutes with 50 mM KCl or treated with NaCl as control, in the presence or absence of FK (100 nM) and cyclosporin A (300nM). (B) Time course of c-fos and IP3R1 mRNA expression determined by RT-PCR. Cells were depolarized with 25 mM KCl on day 2 and harvested after different times.
We then studied the time course of the increase of the IP3R1 mRNA. RT-PCR experiments showed that an increase in messenger occurred after 6 h of exposure to the depolarizing stimulus (Fig. 4B). Parallel experiments showed that c-fos mRNA levels increased much faster, i.e., within 15 min (Fig. 4B). These experiments are consistent with an effect of calcineurin on the transcriptional rate of the IP3R1 mRNA but do not exclude an effect on the stability of the mRNA, which would also lead to its increase.
A major transcriptional pathway involving calcineurin in the central nervous system involves the activation of protein phosphatase 1 and the regulation of the phosphorylation state of cAMP response element-binding protein (CREB) (36). The CREB pathway is unlikely to be responsible for IP3R1 expression because the inhibition of calcineurin would increase the amount of phosphorylated CREB and the transcription efficiency (36). In the case of the IP3R1, the opposite occurred. Furthermore, a 5-day treatment of cerebellar granule cells with FK506 (1 mM) did not result in significant cell death (92 ± 6 of surviving cells assessed with the reduction of MTT), whereas higher levels were induced by inhibitors of other phosphatases (okadaic acid, 5 ± 2; calyculin, 8 ± 4 of surviving cells). This further differentiates the pathway involving calcineurin described here from the one involving the modulation of other phosphatases.
Thus, the experiments described here have revealed a novel role for calcineurin in Ca2+ signaling in the central nervous system. The phosphatase had been previously reported to modulate the activity of IP3R and RyR (16), but no reports had indicated the involvement of the phosphatase in the control of the expression of IP3R. We have now shown that, in response to a rise in cytosolic Ca2+, calcineurin can increase IP3R levels through transcriptional regulation. The matter of transcriptional regulation by calcineurin has recently been explored in different cell types. In T lymphocytes, cytosolic calcineurin associates with the transcription factor NF-AT4 and dephosphorylates it while mediating its transport into the nucleus where the activated NF-AT4 acts (37). Calcineurin also regulates the expression of genes coding for Ca2+ transporters in the budding yeast S. cerevisiae; the target of the phosphatase is a Zn-finger transcription factor that contains a calcineurin transcriptional activation domain (33).
NF-AT has been detected in certain regions of the brain (38), but its role in transcriptional control and, particularly, its presence in the cerebellum have not been demonstrated. NF-AT seems unlikely to be the factor controlling the transcription of IP3R1, because no consensus binding site for NF-AT is present in the mouse or in the human promoter (39, 40). Although this does not rule out the presence of such a site in the rat promoter, the high conservation degree between the mouse and human promoters makes it unlikely. The role of other factors, suggested to act throughout a HRE (steroid responsive element) or the so-called E box located in the IP3R1 promoter (41, 42) is similarly unlikely. Finally, transforming growth factor (TGF)β1 has been shown to down-regulate IP3R1 in mesangial cells (43) but it is not clear whether the effect was mediated by calcineurin. Further investigations on the matter are clearly necessary.
Acknowledgments
We thank Dr. Joachim Krebs and Ms. Lei Li for fruitful and critical discussions and Dr. C. Taylor and Dr. V. Sorrentino for generous gifts of IP3R and RyR antibodies. The authors wish to acknowledge the support of European Molecular Biology Organization (EMBO) (long-term fellowship to A.A.G.). The support of the Italian Ministry of University and Scientific Research (P.R.I.N., Bioenergetics and Membrane Transport, to E.C.) is also gratefully acknowledged.
ABBREVIATIONS
- IP3R
inositol 1,4,5-trisphosphate receptor
- RyR
ryanodine receptor
- NMDA
N-methyl-d-aspartate
- RT-PCR
reverse-transcription–PCR
References
- 1.Berridge M J. Ann NY Acad Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- 2.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Galione A. Mol Cell Endocrinol. 1994;98:125–131. doi: 10.1016/0303-7207(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 4.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorrentino V, Volpe P. Trends Pharmacol Sci. 1993;14:98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- 6.Guerini D, Garcia-Martin E, Gerber A, Volbracht C, Leist M, Gutierrez Merino C, Carafoli E. J Biol Chem. 1999;274:1667–1676. doi: 10.1074/jbc.274.3.1667. [DOI] [PubMed] [Google Scholar]
- 7.Condorelli D, Dell’Albani P, Aronica E, Genazzani A, Casabona G, Corsaro M, Balazs R, Nicoletti F. J Neurochem. 1993;61:2133–2139. doi: 10.1111/j.1471-4159.1993.tb07451.x. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Hu L, Foulkes J. Curr Opin Biotechnol. 1996;7:608–615. doi: 10.1016/s0958-1669(96)80071-1. [DOI] [PubMed] [Google Scholar]
- 9.Halloran P. Clin Transplant. 1996;10:118–123. [PubMed] [Google Scholar]
- 10.de la Pompa J, Timmerman L, Takimoto H, Yoshida H, Elia A, Samper E, Potter J, Wakeham A, Marengere L, Langille B, et al. Nature (London) 1998;392:182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 11.Ranger A, Grusby M, Hodge M, Gravallese E, de la Brousse F, Hoey T, Mickanin C, Baldwin H, Glimcher L. Nature (London) 1998;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 12.Sussman M, Lim H, Gude N, Taigen T, Olson E, Robbins J, Colbert M, Gualberto A, Wieczorek D, Molkentin J. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 13.Molkentin J, Lu J, Antos C, Markham B, Richardson J, Robbins J, Grant S, Olson E. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder S H, Lai M M, Burnett P E. Neuron. 1998;21:283–294. doi: 10.1016/s0896-6273(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 15.Gallo V, Ciotti M T, Aloisi F, Levi G. J Neurosci Res. 1986;15:289–301. doi: 10.1002/jnr.490150302. [DOI] [PubMed] [Google Scholar]
- 16.Cameron A, Steiner J, Roskams A, Ali S, Ronnett G, Snyder S. Cell. 1995;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 17.D’Mello S, Galli C, Ciotti T, Calissano P. Proc Natl Acad Sci USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmichael J, DeGraff W, Gazdar A, Minna J, Mitchell J. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 19.Laemmli U. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardy T, Traynor D, Taylor C. Biochem J. 1997;328:785–793. doi: 10.1042/bj3280785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 24.Mignery G, Newton C, Archer B, Sudhof T. J Biol Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- 25.Oberdorf J, Vallano M, Wojcikiewicz R. J Neurochem. 1997;69:1897–1903. doi: 10.1046/j.1471-4159.1997.69051897.x. [DOI] [PubMed] [Google Scholar]
- 26.Balazs R, Gallo V, Kingsbury A. Brain Res. 1988;468:269–276. doi: 10.1016/0165-3806(88)90139-3. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T, Okano H, Furuichi T, Aruga J, Mikoshiba K. Proc Natl Acad Sci USA. 1991;88:6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danoff S K, Ferris C D, Donath C, Fischer G A, Munemitsu S, Ullrich A, Snyder S H, Ross C A. Proc Natl Acad Sci USA. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 30.Loh C, Shaw K T, Carew J, Viola J P, Luo C, Perrino B A, Rao A. J Biol Chem. 1996;271:10884–10991. doi: 10.1074/jbc.271.18.10884. [DOI] [PubMed] [Google Scholar]
- 31.Guerini D. Biochem Biophys Res Comm. 1997;235:271–275. doi: 10.1006/bbrc.1997.6802. [DOI] [PubMed] [Google Scholar]
- 32.Izumo S, Aoki H. Nat Med. 1998;4:661–662. doi: 10.1038/nm0698-661. [DOI] [PubMed] [Google Scholar]
- 33.Matheos D, Kingsbury T, Ahsan U, Cunningham K. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder S H, Sabatini D M, Lai M M, Steiner J P, Hamilton G S, Suzdak P D. Trends Pharmacol Sci. 1998;19:21–26. doi: 10.1016/s0165-6147(97)01146-2. [DOI] [PubMed] [Google Scholar]
- 35.Franklin J L, Johnson E M., Jr Trends Neurosci. 1992;15:501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- 36.Bito H, Deisseroth K, Tsien R W. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 37.Shibasaki F, Price E, Milan D, McKeon F. Nature (London) 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 38.Ho A M, Jain J, Rao A, Hogan P G. J Biol Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 39.Deelman L E, Jonk L J, Henning R H. Gene. 1998;207:219–225. doi: 10.1016/s0378-1119(97)00630-6. [DOI] [PubMed] [Google Scholar]
- 40.Furutama D, Shimoda K, Yoshikawa S, Miyawaki A, Furuichi T, Mikoshiba K. J Neurochem. 1996;66:1793–1801. doi: 10.1046/j.1471-4159.1996.66051793.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirkwood K L, Homick K, Dragon M B, Bradford P G. J Biol Chem. 1997;272:22425–22431. doi: 10.1074/jbc.272.36.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konishi Y, Kobayashi Y, Kishimoto T, Makino Y, Miyawaki A, Furuichi T, Okano H, Mikoshiba K, Tamura T. J Neurochem. 1997;69:476–484. doi: 10.1046/j.1471-4159.1997.69020476.x. [DOI] [PubMed] [Google Scholar]
- 43.Sharma K, Wang L, Zhu Y, Bokkala S, Joseph S K. J Biol Chem. 1997;272:14617–14623. doi: 10.1074/jbc.272.23.14617. [DOI] [PubMed] [Google Scholar]