Abstract
Fas and the tumor necrosis factor receptor (TNFR)1 regulate the programmed cell death of lymphocytes. The death domain kinase, receptor interacting protein (rip), is recruited to the TNFR1 upon receptor activation. In vitro, rip −/− fibroblasts are sensitive to TNF-induced cell death due to an impaired nuclear factor κB response. Because rip −/− mice die at birth, we were unable to examine the effects of a targeted rip mutation on lymphocyte survival. To address the contribution of RIP to immune homeostasis, we examined lethally irradiated mice reconstituted with rip −/− hematopoietic precursors. We observed a decrease in rip −/− thymocytes and T cells in both wild-type C57BL/6 and recombination activating gene 1−/− irradiated hosts. In contrast, the B cell and myeloid lineages are unaffected by the absence of rip. Thus, the death domain kinase rip is required for T cell development. Unlike Fas-associated death domain, rip does not regulate T cell proliferation, as rip −/− T cells respond to polyclonal activators. However, rip-deficient mice contain few viable CD4+ and CD8+ thymocytes, and rip −/− thymocytes are sensitive to TNF-induced cell death. Surprisingly, the rip-associated thymocyte apoptosis was not rescued by the absence of TNFR1, but appears to be rescued by an absence of TNFR2. Taken together, this study implicates RIP and TNFR2 in thymocyte survival.
Keywords: RIP, TNFR1, TNFR2, thymocyte survival, NF-κB
Introduction
Antigen-induced T cell apoptosis is the result of the expression of death cytokines FasL/APO-1L and TNF (for review see reference 1). The genes for FasL and TNF are induced in IL-2–stimulated T cells (2). These death cyto-kines engage the specific receptors Fas/APO-1 and the TNFR1 (p55) and TNFR2 (p75). Defects in T cell apoptosis, lymphoproliferation, and autoimmunity result in certain strains of mice homozygous for the gld and lpr alleles, which are genetic defects in FasL and Fas, respectively (3–7). In humans, mutation of the Fas/APO-1/CD95 receptor and other components of the death pathway also results in the development of autoimmune lymphoproliferative syndrome (for review see reference 8).
The deregulation of TNF has also been associated with autoimmune disease (9). TNF induces Fas-independent apoptosis of mouse and human lymphoblasts and has been implicated in thymocyte development (10–12). Furthermore, Fas-deficient T cells exhibit reduced but clearly evident TCR-induced cell death, and the residual apoptosis is blocked by anti-TNF antibodies (11, 13). TCR-induced apoptosis can also be inhibited in vivo using either anti-TNF antibodies or in tnf- and tnfr1-deficient mice (10, 14–16).
TNF and FasL mediate their biological effects through interaction with structurally related receptors. TNF binds two distinct TNFRs: TNFR1 (p55) and TNFR2 (p75; for review see reference 17). Most cell lines and tissues express both receptors, although T cells express more TNFR2 (18, 11). The signaling pathway(s) for TNFR1 is now well delineated. TNFR1, the major death-inducing receptor, recruits TNFR1-associated death domain (TRADD)*, Fas-associated death domain (FADD), receptor interacting protein (RIP), and TNFR-associated factor 2 (TRAF2) to the receptor complex after TNF binding. A death signal is induced by the recruitment of FADD and the subsequent recruitment, cleavage/activation of caspase 8. Protection from TNF-induced cell death in fibroblasts is mediated by the activation of the transcription factor, nuclear factor (NF)-κB (19–22). The death domain kinase rip plays a key role in TNFR1 signaling as Jurkat cells and mouse embryonic fibroblasts deficient for rip are highly sensitive to TNF-induced cell death due to a failure to activate the transcription factor NF-κB (23, 24).
TNFR2 lacks a cytoplasmic death domain and is thought to mediate proliferative signals to thymocytes (25, 26). TNFR2 also contributes to TNF-induced cell death (27), although the mechanism(s) by which R2 contributes to TNF-induced cell death is controversial. Some studies suggest that TNFR2-mediated death is mediated by TNFR1 (27, 28). However, studies in T cells suggest that TNFR2 contributes to cell death independently of TNFR1 (2, 29).
TNFR2 activates the transcription factor NF-κB presumably by recruiting TRAF1 and TRAF2 proteins to the receptor (30, 31). The regulation of TNFR2-induced cell death may involve the inhibitors of apoptosis (c-IAP–1 and c-IAP-2), which have also been found associated with the cytoplasmic domain of TNFR2 (32).
To determine whether the TRADD kinase rip participates in immune development and survival, we investigated the effect(s) of rip deletion in lymphocytes. Because targeted mutation of rip results in lethality, we examined the effects of rip mutation by reconstituting mice with rip −/− fetal liver precursors.
Materials and Methods
Adoptive Transfer of Fetal Liver Cells.
Timed matings were prepared from rip heterozygous mice. Fetal livers were harvested from embryonic day 14 embryos and single cell suspensions were prepared. DNA was prepared from the limbs and tails of embryos for genotyping. Approximately 2 × 106 fetal liver cells were injected into the tail vein of lethally irradiated recipient mice. Before injection, C57BL/6 recipients were exposed to a total of 1,200 rads of γ-irradiation in a split dose fashion. In other experiments, fetal liver cells were transplanted into recombination activating gene 1−/− (C57BL/6) recipient mice that had been exposed to 500 rads of γ-irradiation.
Generation of rip−/−/tnf−/−, rip−/−/tnfr1−/−, and rip−/−/tnfr2−/− Mice.
Double-mutant mice were generated by intercrossing rip +/− and tnf −/−, and tnfr1 −/− and tnfr2 −/− mice (provided by M. Marino, Memorial Sloan-Kettering Cancer Center, New York, NY). Tail biopsies were performed on 3-wk-old weanlings and DNA was isolated for genotyping. Rip genotyping was performed as previously described (23). Tnf, tnfr1, and tnfr2 genotyping was performed as previously described (33). Mice were weighed daily to identify double-mutant animals. Double-mutant mice were analyzed between 2 and 14 d after birth.
Flow Cytometry.
Thymus, spleen, and lymph nodes were removed from transplanted mice at various time points after reconstitution. Donor cells were detected by staining cell suspensions with FITC- or biotinylated-Ly9.1 followed by avidin-APC and PE-conjugated anti-CD3, -CD4, -CD8, -B220, –Mac-1, and –Gr-1 (BD PharMingen). Cells were stained with monoclonal antibodies for 30 min at 4°C in PBS containing 1% BSA and 0.1% sodium azide. Cells were analyzed on a FACScan™ (Becton Dickinson). To characterize the double negative (DN) thymocyte population, the thymus was stained with a cocktail of antibodies including biotinylated-IgM, –Ter 119, -Gr1, –Mac-1, -PanNK, -CD3, -CD4, and -CD8. Some samples were then stained with FITC-CD44, PE-CD25, and Streptavidin-CyChrome (BD PharMingen).
To quantitate apoptosis, thymocytes from wild-type, rip −/−, rip −/− /tnfr1 −/−, and rip −/− /tnfr2 −/− mice were stained directly with FITC–annexin V and propidium iodide or with 7-amino-actinomycin D and analyzed by flow cytometry.
For TNF-induced apoptosis, wild-type or rip −/− thymocytes were left untreated or treated with 50 ng/ml mTNF and 50 μg/ml cycloheximide for 18 h, stained with FITC–annexin V and propidium iodide (BD PharMingen), and analyzed by flow cytometry.
Fetal Thymic Organ Culture.
Thymic lobes were removed from fetuses on day 16, placed on a strip of nitrocellulose supported by a sterile metal grid, and incubated in RPMI supplemented with 10% fetal calf serum and 5 × 10−5 M β-mercaptoethanol, 1 mM glutamine, and 1% penicillin/streptomycin. At the indicated times, the thymic lobes were resuspended and stained with FITC-CD4 and PE-CD8 and analyzed on a FACScan™ instrument. To genotype fetuses, DNA was isolated from tail and limb biopsies.
Proliferation and NF-κB Assays.
Splenocytes from mice reconstituted with wild-type or rip −/− fetal liver precursors were stained with 2.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) and then left untreated or stimulated with 2 μg/ml Con A or 10 μg/ml plate-bound anti-CD3 (145-2C11; BD PharMingen) for 96 h. Cultures were then stained with antibodies to APC-Ly9.1, PE-CD4, or PE-CD8 and proliferation was assessed by CFSE fluorescence.
Splenocytes from rip +/+ /tnfr1 −/− and rip −/− /tnfr1 −/− neonatal mice were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin/streptomycin, and 2 mM glutamine in 96-well plates (105 cells/well). Splenocytes were stimulated with 2 μg/ml Con A and 10 μg/ml anti-CD3 (145-2C11; BD PharMingen) for 48 h. The cultures were pulsed with 50 μCi [3H]thymidine (NEN Life Science Products) for 18 h and harvested using a semiautomatic sample harvester. Incorporation of radioactivity was measured by scintillation counting.
To assay nuclear NF-κB, thymocytes were harvested from rip +/+ /tnfr1 −/−, rip +/− /tnfr1 −/−, or rip −/− /tnfr1 −/− neonatal mice at day 6 and incubated in media alone or in media containing 50 ng/ml mTNF for 1 h. Nuclear extracts were prepared and p65 was detected by immunoblotting with anti-p65 antisera (Santa Cruz Biotechnology Associates, Inc.). To confirm equivalent amounts of nuclear protein, immunoblots were reprobed with antibody to the nuclear corepressor protein msin3A.
Results
T Cell Development Is Affected in Mice Reconstituted with rip−/− Precursors.
To investigate the contribution of rip to lymphocyte development, lethally irradiated C57BL/6 mice were reconstituted with embryonic day 14 129/Sv wild-type and rip −/− fetal liver precursor cells. An antibody for the allelic Ly9.1 antigen distinguished donor (129/Sv mice are Ly9.1+) from host cells (B6 mice are Ly9.1−). 6 wk after reconstitution, three recipient mice were killed and the thymus, spleen, and lymph node were analyzed for the presence of Ly9.1+ cells. 10-fold decreases in the number of cells in the lymph nodes and thymus isolated from rip −/− reconstituted mice were observed. In contrast, similar numbers of splenocytes were observed in wild-type and rip −/− reconstituted mice.
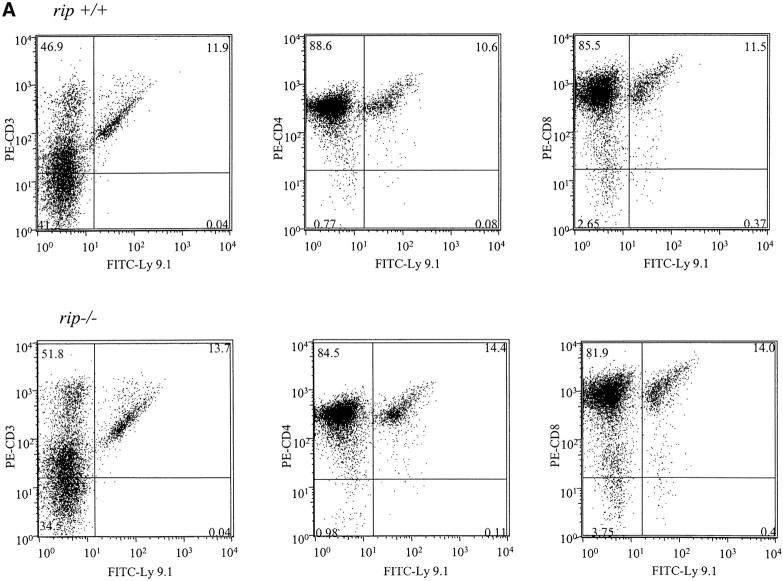
In addition to fewer cells in the rip −/− reconstituted thymus, the percentage of wild-type versus rip −/−-derived cells was also different. In rip +/+ reconstituted mice, 97% of the thymus contained Ly9.1+ cells, whereas only 3% of thymocytes in the rip −/− reconstituted mice were donor derived (Fig. 1 A). Although few rip −/− thymocytes were detected in the reconstituted mice, the CD4/CD8 profile did not reveal any developmental changes in rip −/− thymocytes.
Figure 1.

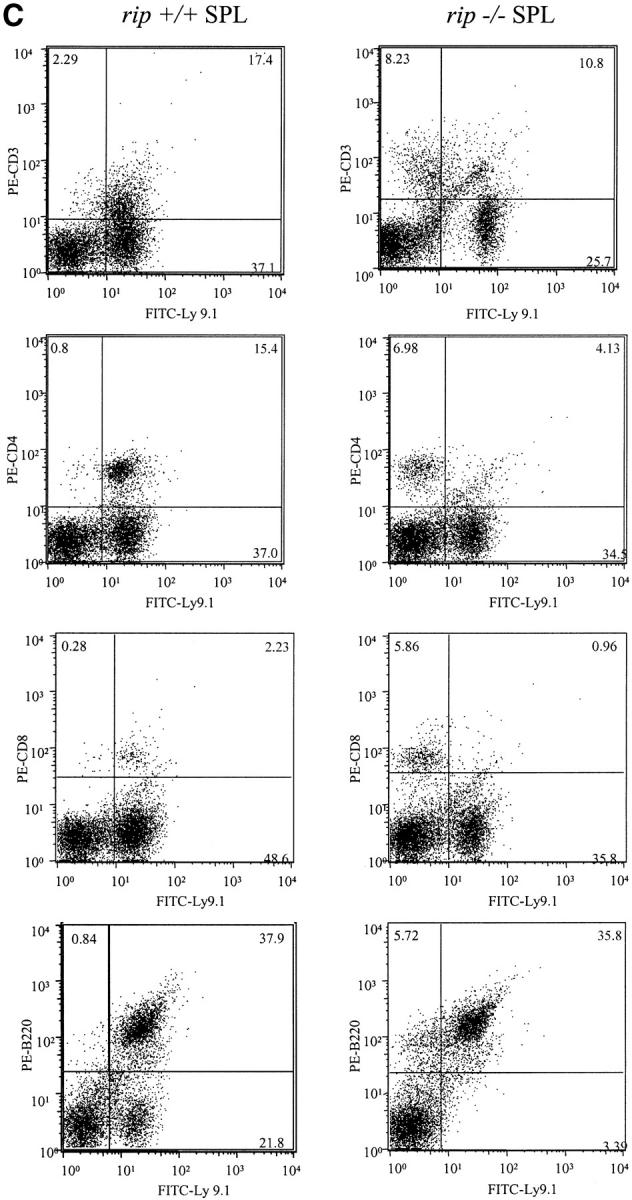
Flow cytometric analysis of mice reconstituted with rip +/+ or rip −/− fetal liver precursors. (A) Flow cytometric analysis of thymus. Single cell suspensions of thymocytes were stained for the donor-specific Ly9.1 marker and for CD4 or CD8 12 wk after reconstitution. (B) Flow cytometric analysis of peripheral lymphocytes. Single cell suspensions of cervical, inguinal, and mesenteric lymph nodes from rip +/+ and rip −/− reconstituted mice were stained with FITC-Ly9.1 and PE–anti-CD3, PE-CD4, or PE-CD8. (C) Splenocytes were also stained with FITC-Ly9.1 and CD3-PE, CD4-PE, CD8-PE, PE-B220, or PE–Mac-1. 10,000 events were collected. Plots are representative of three independent experiments.
Interestingly, rip −/− T cells were detected in the periphery. The peripheral lymphoid organs of three wild-type or three rip −/− reconstituted mice were analyzed 12 wk after reconstitution. One representative experiment is shown in Fig. 1. In the cervical lymph nodes of rip −/− reconstituted mice, 17% of the CD3+ cells also stained positive for Ly9.1 and were derived from rip −/− cells (Fig. 1 B). In contrast, mice reconstituted with rip +/+ precursors contained 67% CD3+ Ly9.1+ cells in the cervical lymph nodes (Fig. 1 B). Wild-type reconstituted mice contained 53% Ly9.1+ CD4+ cells and 16% CD8+ T cells in the cervical lymph nodes. In contrast, 2.5% of Ly9.1+ CD4+ cells and 4% of the Ly9.1+ CD8+ cells were detected in the cervical lymph nodes of mice reconstituted with rip −/− precursors. Few peripheral rip −/− T cells were also observed in the spleen (Fig. 1 C) and the inguinal and mesenteric lymph nodes (unpublished data).
Previous studies have implicated Fas in CD4 T cell survival and TNF in CD8 T cell survival (11). Because rip mediates TNFR1 signaling, we expected the CD8 lineage to be affected by an absence of rip. However, both the CD4 and CD8 T cell lineages were affected by an absence of rip.
In contrast to the T cell lineage, rip −/− precursors contributed to the B lymphoid and myeloid cell lineages. Similar numbers of FITC-Ly9.1 and PE-B220+ cells were observed in the spleens of three rip +/+ (36, 38, and 35%) and three rip −/− (29, 35, and 36%) reconstituted mice (Fig. 1 C). Equal numbers of FITC-Ly9.1 and PE–Mac-1+ cells were also observed (unpublished data). Thus, the death domain kinase rip does not appear important in B cell or myeloid lineage development but is required for the normal development of the T lineage.
An Age-dependent Decrease in rip−/− Thymocytes.
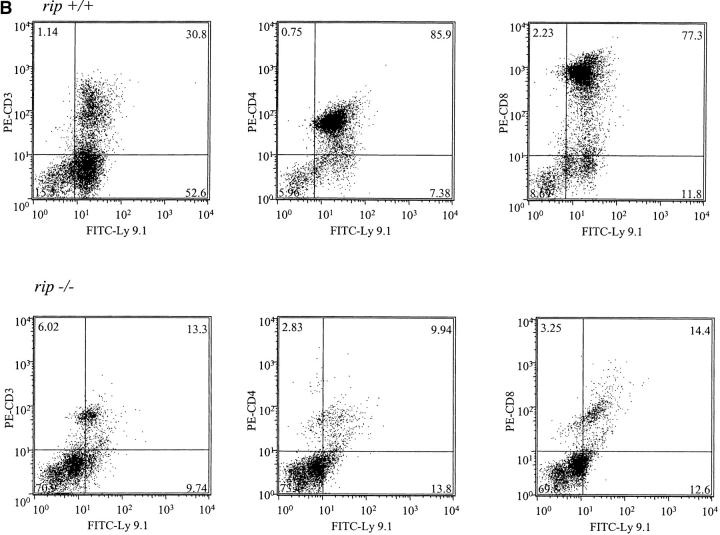
The decrease in rip −/− thymocytes and mature T cells in the reconstituted mice suggests that the lack of rip −/− T cells may reflect a deficiency in committed thymocyte precursors. To determine whether equal numbers of rip +/+ and rip −/− thymocytes could be detected at early time points, wild-type and rip −/− reconstituted recombination activating gene 1−/− mice were killed 2 wk after reconstitution and thymi were stained with FITC-Ly9.1 and PE-CD3, and PE-CD4 and PE-CD8. Similar numbers of Ly9.1+ donor-derived thymocytes were observed in mice reconstituted with wild-type (14%) or rip −/− (12%) precursors (Fig. 2 A). Additional reconstituted mice were then killed 6 wk after reconstitution. Although sufficient numbers of Ly9.1+ thymocytes were observed in three rip +/+ reconstituted mice (86, 83, and 87%), only 13% (11, 14, and 13%) of thymocytes were derived from rip −/− precursors (Fig. 2 B). These studies suggest that in the absence of rip, thymocytes either fail to proliferate or undergo cell death.
Figure 2.
Flow cytometric analysis of thymocytes from mice reconstituted with rip +/+ and rip −/− precursors 2 and 6 wk after reconstitution. (A) Thymocytes from three wild-type and three rip −/− reconstituted mice 2 and (B) 6 wk after reconstitution were analyzed with FITC–anti-Ly9.1 and PE–anti-CD3, PE–anti-CD4, or PE–anti-CD8.
Rip−/− T Cells Respond to Mitogens.
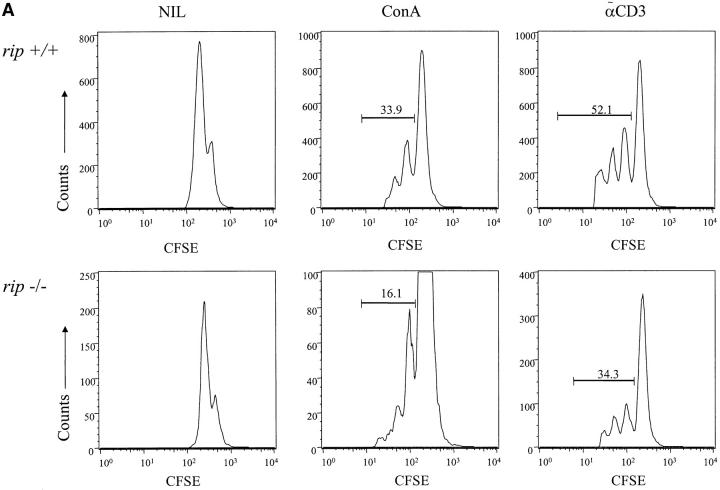
The age-dependent decrease in rip −/− thymocytes and the lack of mature rip −/− T cells in the reconstituted mice may reflect the fact that rip, like fadd, mediates proliferative pathways in lymphocytes (34). To examine this possibility, splenocytes from mice reconstituted with wild-type and rip −/− precursors were labeled with CFSE and then stimulated in vitro with anti-CD3 antibody or Con A. 4 d later, the cultures were stained with antibodies to Ly9.1, CD4, and CD8. To determine whether rip −/− T cells proliferate in response to mitogenic stimulation, we gated on the CFSE+ Ly9.1+ cells. Cells from mice reconstituted with wild-type or rip −/− cells proliferated in response to Con A or anti-CD3, as evidenced by the decrease in CFSE fluorescence relative to the unstimulated cells (Fig. 3 A). However, rip −/− T cells consistently exhibit a twofold decrease in the proliferative response when compared with wild-type T cells. This decreased response may reflect the limited numbers of rip −/− T cells in the splenocyte cultures (Fig. 3 B). In the experiment shown in Fig. 3 A, the rip −/− reconstituted spleen contains 2.8% Ly9.1+ CD4+ cells and 1% Ly9.1+ CD8+ cells, whereas wild-type reconstituted spleen contains 18% Ly9.1+ CD4+ cells and 5.8% Ly9.1+ CD8+ cells (Fig. 3 B).
Figure 3.
Rip −/− T cells respond to polyclonal activators. (A) Splenocytes from mice reconstituted with rip +/+ and rip −/− precursors were labeled with 2.5 μM CFSE and left untreated or stimulated with Con A or plate-bound anti-CD3 for 96 h. The proliferation of rip −/− T cells was determined by staining with APC-Ly9.1 and PE-CD3, PE-CD4, or PE-CD8. The results were reproduced in three independent experiments. (B) Few rip −/− T cells in the reconstituted spleen. Splenocytes from mice reconstituted with rip +/+ or rip −/− fetal liver precursors were stained with APC-Ly9.1 and PE-CD4 or PE-CD8. Three wild-type and three rip −/− reconstituted mice were analyzed. The data shown is representative of three independent experiments.
Although decreases in the rip −/− T cell proliferative responses were observed, we do not think this difference sufficiently explains the absence of rip −/− thymocytes and mature T cells in the reconstituted mice. Rip −/− T cells, like rip −/− murine embryonic fibroblasts, may fail to survive because they are sensitive to TNF-induced cell death (24). To test this hypothesis genetically, we examined thymocyte development in neonatal rip −/−, rip −/− /tnf −/−, rip −/− /tnfr1 −/−, and rip −/− /tnfr2 −/− mice.
An Absence of TNF or TNFR1, but Not TNFR2, Partially Rescues the RIP-associated Lethality.
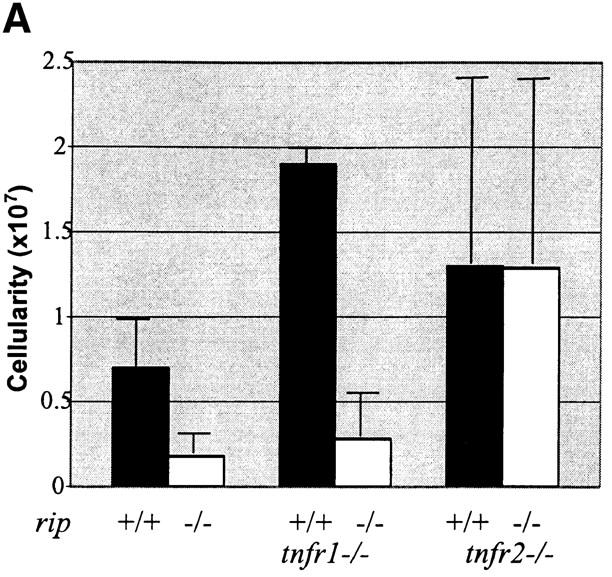
To determine whether the rip-associated T cell defect(s) were TNF-mediated, we generated rip −/− /tnf −/−, rip −/− /tnfr1 −/−, and rip −/− /tnfr2 −/− mice. The absence of TNF and TNFR1 improved the survival of rip −/− mice, with rip −/− /tnf −/− mice surviving an average of 5–6 d. Mice deficient for both rip and tnfr1 survived for the longest period, with double-mutant animals surviving an average of 12 d (Table I). The absence of the TNFR2 failed to rescue the rip-associated lethality, as both rip −/− and rip −/− /tnfr2 −/− mice died during the perinatal period. These data are consistent with the rip-associated lethality being TNFR1-mediated.
Table I.
Genetic Analysis of Offspring Obtained from rip+/−, rip+/−/tnf−/−, rip+/−/tnfr1−/−, and rip+/−/tnfr2−/− Heterozygous Matings
|
|
rip genotypes
|
|
+/+
|
+/−
|
−/−
|
Total
|
|---|---|---|---|---|---|---|
| Male rip +/− × Female rip +/− | 54 | 67 | <1 | 121 | ||
| Male rip +/−/tnf −/− × Female rip +/−/tnf−/− | tnf genotypes | −/− | 64 | 94 | 41 | 199 |
| Male rip +/−/tnfr1 −/− × Female rip +/−/tnfr1 −/− | tnfr1 genotypes | −/− | 49 | 118 | 106 | 273 |
| Male rip +/−/tnfr2 −/− × Female rip +/−/tnfr2 −/− | tnfr2 genotypes | −/− | 23 | 43 | <1 | 69 |
Breeding cages were examined daily and the number of pups born to each female was recorded. Tail biopsies were taken between days 2 and 6, and genotyping was performed as described in Materials and Methods.
The rip −/− /tnfr1 −/− mice appear normal at birth and are indistinguishable from littermates. However, by day 2, double-mutant animals are evident because they appear runt-like and cachectic. By day 7, rip −/− /tnfr1 −/− mice only weigh one third of control rip +/+ /tnfr1 −/− and rip +/− /tnfr1 −/− littermates (unpublished data). Histopathologic examination of the rip −/− and rip −/− /tnfr1 −/− mice failed to reveal the reason for the death of mutant animals. Studies on relA −/− /tnf −/−, relA −/− /tnfr1 −/−, and Ikk-β−/− /tnfr1 −/− mice have suggested that the mice are immunocompromised due to a failure to activate NF-κB and as a result die from opportunistic infections (35–37). Thus far, gram and silver staining of paraffin-embedded sections of rip −/− /tnfr1 −/− animals has failed to detect any evidence of infection.
Mitogenic Responsiveness in the Absence of RIP and TNFR1.
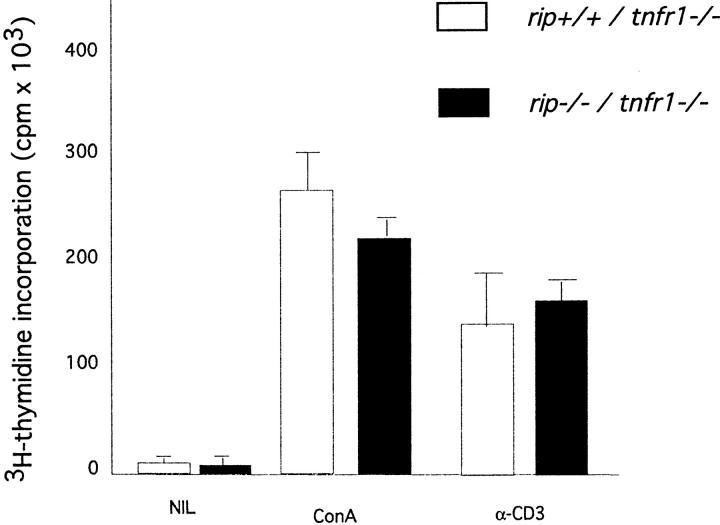
Rip-deficient T cells can be detected in the peripheral lymphoid organs of mice reconstituted with rip −/− precursors and in rip −/− /tnfr1 −/− neonatal mice. To further examine the contribution of rip to T cell proliferation, we compared the mitogenic response of rip +/+ /tnfr1 −/− and rip −/− /tnfr1 −/− splenic T cells. Consistent with our findings on the reconstituted mice, rip −/− /tnfr1 −/− T cells as well as control littermates proliferated when stimulated with Con A or anti-CD3 treatment (Fig. 4). These studies confirm our proliferative studies of rip −/− T cells in the reconstituted mice and fail to reveal a regulatory role for the death domain kinase rip in T cell proliferation.
Figure 4.
T cell proliferation in the absence of rip and tnfr1. Splenocytes from rip +/+ /tnfr1 −/−, rip +/− /tnfr1 −/−, and rip −/− /tnfr1 −/− neonatal mice were left untreated or stimulated with Con A and anti-CD3. Incorporation of [3H]thymidine was determined by standard procedures. Data shown is representative of three independent experiments.
Double Positive (DP) Thymocyte Apoptosis in the Absence of RIP.
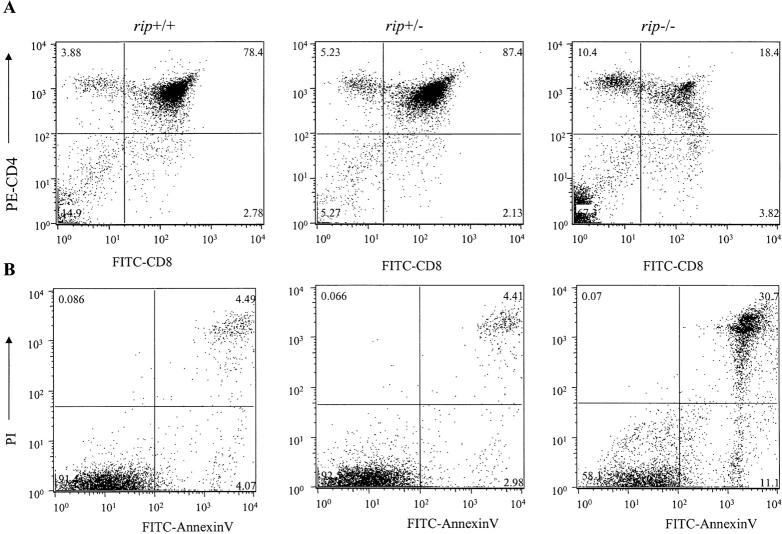
To determine whether targeted mutation of rip affects thymocyte survival, we isolated fetal thymus from a rip heterozygous mating and stained the thymocytes with anti-CD4 and -CD8 antibodies and analyzed them by flow cytometry. A decrease in rip −/− DP thymocytes was observed (Fig. 5 A). In contrast to thymus from control littermates, which on average contained 83% CD4 and CD8 DP cells, the rip −/− thymus only contained 18% viable DP thymocytes. Moreover, an average fivefold increase in the DN thymocyte population was also observed.
Figure 5.
Rip-deficient DP thymocytes are sensitive to TNF-induced cell death. (A) Thymocytes from rip +/− and rip −/− neonatal mice were stained with PE–anti-CD4, FITC–anti-CD8, and the fluorescent DNA binding dye, LDS-751. Viable cells were analyzed for the expression of CD4 and CD8 by flow cytometry. (B) Thymocytes from rip +/+, rip +/−, and rip −/− neonates were left untreated or treated with mTNF and cycloheximide for 18 h. Apoptotic cells were quantitated by FITC–annexin V/PI staining. Three rip heterozygous litters were examined. One representative experiment is shown. The percent of apoptotic cells is shown in the upper right quadrant.
Analysis of the fetal thymus from day 18 rip −/− embryos revealed variation in the penetrance of the DP thymocyte phenotype. For example, in four rip heterozygous matings analyzed at embryonic day 18, 2/3, 1/4, 2/2, and 1/3 of the rip −/− mutants were affected (i.e., exhibited >50% loss of DP thymocytes; unpublished data). Where rip −/− DP thymocyte loss is observed, we consistently observe an average of eightfold increase in the percent of apoptotic cells, detected by FITC–annexin V/propidium iodide or 7-AAD staining (unpublished data). Therefore, rip −/− embryos with normal CD4/CD8 profiles are observed, suggesting that thymocyte development/expansion occurs normally in the absence of rip, but that rip appears required for CD4 CD8 DP thymocyte survival.
To test whether the DP thymocyte loss may reflect sensitivity of rip −/− thymocytes to TNF-induced cell death, thymocytes were incubated with mTNF in the presence of cycloheximide for 18 h and the apoptotic cells were detected by FITC–annexin V/PI staining. Wild-type thymocytes treated with TNF were resistant to TNF-induced cell death with 4.5% of thymocyte staining with FITC–annexin V/PI (Fig. 5 B). A 7–10-fold increase in apoptotic thymocytes was observed when rip −/− thymocytes were treated with mTNF. Therefore, rip −/− thymocytes, like the rip −/− murine embryonic fibroblasts, appear sensitive to TNF-induced cell death. Consistent with the TNF sensitivity observed in vitro, DP thymocyte loss was not observed in neonatal rip −/− /tnf −/− mice (unpublished data).
The RIP-associated Thymocyte Apoptosis Is Not Mediated through TNFR1.
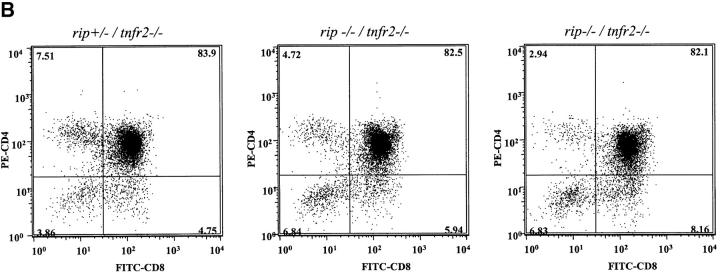
To determine whether the rip-associated thymocyte apoptosis was TNFR1-mediated, we examined the CD4/CD8 profiles of the rip +/− /tnfr1 −/− and rip −/− /tnfr1 −/− neonatal thymus. Dramatic differences in the overall cellularity were observed, suggesting that survival is not mediated through the TNFR1. The rip-deficient thymus consists of 10× fewer thymocytes than rip +/+ /tnfr1 −/− littermates (Fig. 6 B). Similar to what was observed in the rip −/− thymus, four- to sevenfold increases in the percent of apoptotic cells were also observed in the rip −/− /tnfr1 −/− thymocytes by staining with FITC–annexin V/PI or 7-AAD (unpublished data). In addition to the decreased cellularity and increase in apoptotic cells, there were concomitant decreases in the DP thymocyte population (31, 66, 29, and 56% in four age-matched rip −/− /tnfr1 −/− mice compared with 84, 83, 86, and 88% in littermate controls). Thus, the absence of RIP resulted in an average 22-fold decrease in the absolute numbers of DP thymocytes.
Figure 6.
DP thymocyte apoptosis in the absence of rip and tnfr1. Flow cytometric analysis of thymocytes from rip +/− /tnfr1 −/− and rip −/− /tnfr1 −/− mice. (A) Thymocytes from day-6 rip +/− /tnfr1 −/− and rip −/− /tnfr1 −/− mice were stained with PE–anti-CD4, FITC–anti-CD8, and LDS-751. The percent of viable cells are indicated in each quadrant. (B) Thymocyte and spleen cell counts of rip +/+ /tnfr1 −/− and rip −/− /tnfr1 −/− mice. Cell counts were performed in triplicate. Results are expressed ± SEM of at least six animals between 6 to 8 d old. (C) Thymus from control littermates and rip −/− /tnfr1 −/− mice was stained with a lineage-specific cocktail containing biotinylated-IgM, -Ter 119, -Gr1, –Mac-1, -PanNK, -CD3, -CD4, and -CD8. Some samples were then stained with FITC-CD44, PE-CD25, and Streptavidin-CyChrome. The Cy− or DN cells were further analyzed according to their expression of CD44 and CD25. The percent of positive cells are indicated in each quadrant. Seven rip −/− /tnfr1 −/− mice and seven littermate controls were analyzed. One representative experiment is shown.
There were concomitant increases in the relative percentage of CD4− CD8− DN thymocytes (35, 23, 12, and 17% in rip −/− /tnfr1 −/− mice compared with 7, 4, 3, and 8% in control littermates; Fig. 6 A). Additional analysis of the DN thymocytes isolated from the rip −/− /tnfr1 −/− mice revealed an increase in the CD44− CD25+ DN precursor thymocytes, suggesting that an absence of rip induces a partial thymocyte arrest at the DN3 stage.
Thymocyte Survival in rip− /−/tnfr2− /− Mice.
In the absence of TNFR1, rip −/− DP thymocytes undergo apoptosis and fail to survive, which suggests that rip −/− and rip −/− /tnfr1 −/− thymocytes may undergo TNFR2-induced cell death. The TNFR2 (p75) has also been implicated in immune homeostasis (for review see reference 8). To test whether the thymocyte apoptosis observed in the rip −/− /tnfr1 −/− mice is TNFR2-mediated, we examined the neonatal rip +/− /tnfr2 −/− and rip −/− /tnfr2 −/− thymus. In contrast to the decreased cellularity observed in the rip −/− and rip −/− /tnfr1 −/− thymus, the neonatal rip −/− /tnfr2 −/− thymus contained similar numbers of total thymocytes as control littermates (Fig. 7 A). Surprisingly, in the absence of rip and TNFR2, no decreases in the relative percentage of DP thymocytes were observed (Fig. 7 B). Similar numbers of DP thymocytes were detected in rip −/− /tnfr2 −/− mice (72, 84, 82, 95, and 92%) as seen in littermate controls (86, 83, 85, 93, 92, and 90%). Consistent with these studies, no significant increase in the percent of apoptotic cells was observed (unpublished data). These studies implicate TNFR2 in DP thymocyte survival and suggest that rip participates in TNFR2 signaling.
Figure 7.
DP thymocyte survival in the absence of rip and tnfr2. To determine the contribution of TNFR2 to rip −/− thymocyte apoptosis, thymus was harvested at day 2 from rip −/− /tnfr2 −/− mice and control littermates. Thymocytes were stained with PE–anti-CD4 and FITC–anti-CD8. Viable cells expressing CD4 and CD8 are shown. Six rip −/− /tnfr2 −/− and 36 control littermates were analyzed. Two representative plots are shown for rip −/− /tnfr2 −/− mice.
Thymocyte Apoptosis in the Presence of Nuclear p65.
The nature of the rip-mediated survival signal in rip +/+/tnfr1 −/− thymocytes does not appear to involve the activation of p65 subunit of NF-κB. We observed nuclear NF-κB (p65) in untreated rip +/+ /tnfr1 −/− and rip +/− /tnfr1 −/− thymocytes (Fig. 8 A, lanes 2 and 3, respectively) and rip −/− /tnfr1 −/− thymocytes (Fig. 8 A, lane 1). Furthermore, an increase in nuclear p65 was not observed when either wild-type or rip −/− /tnfr1 −/− thymocytes were treated with mTNF, suggesting that TNFR2 activates other survival pathways (Fig. 8 A, lane 4, rip −/− /tnfr1 −/− , 5 rip +/− /tnfr1 −/−, and 6 rip +/+ /tnfr1 −/−). As expected, nuclear translocation of p65 was observed when murine embryonic fibroblasts were treated with mTNF (Fig. 8 A, lane 8). To control for equivalent amounts of nuclear protein, the immunoblots were probed with an antibody to the nuclear corepressor protein, msin3A.
Figure 8.
Nuclear NF-κB in rip +/+ /tnfr1 −/− and rip −/− /tnfr1 −/− thymocytes. (A) Nuclear p65 in rip +/+ /tnfr1 −/−, rip +/− /tnfr1 −/−, and rip −/− /tnfr1 −/− thymocytes. Thymocytes from rip +/+ /tnfr1 −/− (lanes 3 and 6), rip +/− /tnfr1 −/− (lanes 2 and 5), and rip −/− /tfnr1 −/− (lanes 1 and 4) mice were left untreated or treated with 50 ng/ml mTNF for 60 min. Wild-type murine embryonic fibroblasts were also left untreated (lane 7) or treated with 50 ng/ml mTNF for 60 min (lane 8). Nuclear extracts were prepared and p65 detected by immunoblotting with anti-p65 antisera (Santa Cruz Biotechnology, Inc.). The immunoblots were then reprobed with an anti-mSin3A antibody to ensure that equal amounts of nuclear protein were analyzed.
Discussion
Previous work has revealed that the death domain kinase rip is an important mediator in TNFR1 signaling. rip expression results in the induction of cell death and NF-κB activation, and dominant negative forms of rip inhibit NF-κB activation by TNFR1 (38, 23). Consistent with these studies, mouse fibroblasts deficient in rip are TNF sensitive due to an impaired antiapoptotic NF-κB response (23, 24).
To address the role of the death domain kinase rip in immune regulation, we reconstituted lethally irradiated mice with rip −/− fetal liver precursors. Mice reconstituted with rip −/− precursors contained few thymocytes and mature T cells, whereas the B lymphocyte and myeloid lineages were unaffected. In contrast to mice reconstituted with wild-type cells, few rip −/− T cells were detected in the thymus, spleen, or lymph node. Although TNF has been implicated in the regulation of the CD8 lineage (11), both the CD4 and CD8 single positive T cells were affected by an absence of rip. These studies reveal a specific T cell survival function for the TRADD kinase, rip.
The lack of rip −/− T cells in the reconstituted mice does not reflect proliferative defects as has been described for FADD-deficient T cells (34). Surviving rip −/− T cells in the reconstituted mice were capable of responding to Con A or anti-CD3 stimulation. Furthermore, no significant difference in the mitogenic response was observed when rip +/+ /tnfr1 −/− and rip −/− /tnfr1 −/− T cells were compared. Taken together, these studies fail to demonstrate a direct role for rip in T cell proliferation.
DP thymocytes that lack rip exhibit increased apoptosis and fail to survive. The rip-deficient thymocytes are sensitive to TNF-induced cell death, suggesting that the lack of rip −/− thymocytes and T cells is due to TNFR1-induced cell death. Although the absence of the TNFR1 partially rescues the RIP-associated lethality, it fails to rescue the RIP-associated DP thymocyte apoptosis. In the absence of RIP and TNFR1, mouse DP thymocytes fail to survive and DN thymocytes that accumulate are arrested at the DN3 stage (CD44− CD25+). The increase in DN3-stage thymocytes suggests that rip participates in thymocyte development. Analysis of transgenic mice expressing a dominant negative FADD has implicated death receptors in the regulation of the preTCR checkpoint (39). Thus, it remains possible that rip and a TNFR work in concert with the preTCR to regulate DN3 survival and expansion. Consistent with this notion is the fact that TNFR2 is expressed in DN3 precursors and preTCR signal has been suggested to involve NF-κB activation (39, 40). Yet, in the absence of rip, we do not observe significant increases in the absolute numbers of DN precursors, but instead observe on average a 22-fold decrease in the absolute numbers of DP thymocytes. These data are consistent with a primary function for rip in DP thymocyte survival.
The absence of TNFR2 appears to rescue rip −/− DP thymocytes from cell death as thymocyte cell number is normal in rip −/− /tnfr2 −/− neonates. Thus, rip −/− and rip −/− /tnfr1 −/− thymocytes undergo TNFR2-induced cell death, which suggests that rip mediates TNFR2 survival signals in thymocytes. Rip has been implicated in TNFR2 signaling and shown to associate with TNFR2 in a TRAF2-dependent manner (29). In activated T cells, RIP has been proposed to recruit FADD to the TNFR2 and function as a molecular switch, stimulating T cell death/survival. In activated T cells, TNFR2-mediated death appears to require RIP and in contrast to TNFR1, occurs in the presence of nuclear NF-κB.
Although our data also implicates rip in TNFR2 signaling, we find that in developing mouse thymocytes, rip retains its antiapoptotic activity. However, the nature of the rip-mediated TNFR2 survival signal is unclear. Thymocytes from wild-type and rip −/− /tnfr1 −/− mice exhibit nuclear NF-κB. Thus, rip −/− /tnfr1 −/− thymocytes undergo cell death in spite of nuclear p65, suggesting that the TNFR2 survival signal involves mechanism(s) other than activation of NF-κB. However, it remains possible that NF-κB activation participates in TNFR2-mediated survival, as decreases in overall thymocyte cellularity have been reported for IKK-β −/− /tnfr1 −/− mice (35).
Thymocyte apoptosis is observed in rip −/− and rip −/− /tnfr1 −/− thymocytes, suggesting that an absence of rip sensitizes thymocytes to TNFR2-induced cell death. Sensitivity to TNFR2-induced cell death has recently been shown to reflect cellular inhibitor of apoptosis 1 (c-IAP-1)–induced ubiquitination and degradation of TRAF2 (41). Here we show that the absence of rip sensitizes tnfr1 −/− thymocytes to TNFR2-induced cell death, indicating that rip, like TRAF2, has antiapoptotic functions in TNFR2 signaling. Rip and TRAF2 interact at the TNFR1 and RIP recruitment to TNFR2 appears TRAF2 dependent (29). Thus, it remains possible c-IAP-1–mediated degradation of TRAF2 may interfere with the stable recruitment of RIP to TNFR2. Alternatively, RIP may also serve as a c-IAP-1 substrate and TNFR2-induced thymocyte cell death might involve the degradation of both RIP and TRAF2.
Acknowledgments
We thank Dr. Leslie Berg for critical reading of the manuscript and members of the Berg laboratory, specifically Luana Atherly for help with the FTOC and Julie Lucas with the characterization of the DN thymocytes.
This work was supported by grant RPG 00-120 from the American Cancer Society and R01-GM61298 grant from the National Institutes of Health. M. Kelliher is a recipient of a Sidney Kimmel Cancer Scholar Award.
N. Cusson and S. Oikemus contributed equally to this work.
Footnotes
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate succinimidyl ester; DN, double negative; DP, double positive; FADD, Fas-associated death domain; NF, nuclear factor; rip, receptor interacting protein; TRADD, TNFR1-associated death domain; TRAF2, TNFR-associated factor 2.
References
- 1.Lenardo, M., F.K.M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis-immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221–253. [DOI] [PubMed] [Google Scholar]
- 2.Zheng, L., C.L. Trageser, D.M. Willerford, and M. Lenardo. 1998. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J. Immunol. 160:763–769. [PubMed] [Google Scholar]
- 3.Itoh, N., S. Yonehara, A. Ishii, M. Yonehara, S. Mizushima, M. Sameshima, A. Hase, Y. Seto, and S. Nagata. 1991. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 66:233–243. [DOI] [PubMed] [Google Scholar]
- 4.Suda, T., T. Takahashi, P. Golstein, and S. Nagata. 1993. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 75:1169–1178. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi, T., M. Tanaka, C.I. Brannan, N.A. Jenkins, N.G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice caused by a point mutation in the Fas ligand. Cell. 76:969–976. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe-Fukunuga, R., C.I. Brannan, N.G. Copeland, N.A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 356:314–317. [DOI] [PubMed] [Google Scholar]
- 7.Ramsdell, F., M.S. Seaman, R.E. Miller, T.W. Tough, M.R. Alderson, and D.H. Lynch. 1994. Gld/gld mice are unable to express a functional ligand for Fas. Eur. J. Immunol. 24:928–933. [DOI] [PubMed] [Google Scholar]
- 8.Chan, F.K.M., R.M. Siegel, and M. Lenardo. 2001. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 13:419–422. [DOI] [PubMed] [Google Scholar]
- 9.Jacob, C.O., G.D. Lewis, and H.O. McDevitt. 1991. MHC class II-associated variation in the production of tumor necrosis factor in mice and humans: relevance to the pathogenesis of autoimmune diseases. Immunol. Res. 10:156–168. [DOI] [PubMed] [Google Scholar]
- 10.Sarin, A., M. Conan-Cibotti, and P.A. Henkart. 1995. Cytotoxic effect of TNF and lymphotoxin on T lymphoblasts. J. Immunol. 155:3716–3718. [PubMed] [Google Scholar]
- 11.Zheng, L., G. Fisher, R.E. Miller, J. Peschon, D.H. Lynch, and M.J. Lenardo. 1995. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 377:348–351. [DOI] [PubMed] [Google Scholar]
- 12.Zuniga-Pflucker, J.C., D. Jiang, and M. Lenardo. 1995. Requirement for TNF-α and IL-α in fetal thymocyte commitment and differentiation. Science. 268:1906–1909. [DOI] [PubMed] [Google Scholar]
- 13.Tucek-Szabo, C.L., S. Andjelic, E. Lacy, K.B. Elkon, and J. Nikoloci-Zugic. 1996. Surface T cell Fas receptor/CD95 regulation, in vivo activation and apoptosis. Activation-induced death can occur without Fas receptor. J. Immunol. 156:192–200. [PubMed] [Google Scholar]
- 14.Speiser, D.E., E. Sebzda, T. Ohteki, M.F. Bachman, K. Pfeffer, T.W. Mak, and P.S. Ohashi. 1996. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur. J. Immunol. 26:3055–3060. [DOI] [PubMed] [Google Scholar]
- 15.Sytwu, H.K., R.S. Liblau, and H.O. McDevitt. 1996. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 5:17–30. [DOI] [PubMed] [Google Scholar]
- 16.Marino, M.W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, et al. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA. 94:8093–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, C.A., T. Farrah, and R.G. Goodwin. 1994. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell. 76:959–962. [DOI] [PubMed] [Google Scholar]
- 18.Ware, C.F., P.D. Crowe, T.L. Vanarsdale, J.L. Andrew, M.H. Grayson, R. Jerzy, C.A. Smith, and R.G. Goodwin. 1991. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type 1 TNF receptor during activation of resting and effector T cells. J. Immunol. 147:4229–4238. [PubMed] [Google Scholar]
- 19.Beg, A.A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNFα-induced cell death. Science. 274:782–784. [DOI] [PubMed] [Google Scholar]
- 20.Van Antwerp, D.J., S.J. Martin, T. Kafri, D.R. Green, and I.M. Verma. 1996. Suppression of TNFα-induced apoptosis by NF-κB. Science. 274:787–789. [DOI] [PubMed] [Google Scholar]
- 21.Li, Z.W., W.M. Chu, Y.L. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor-kB activation and prevention of apoptosis. J. Exp. Med. 189:1839–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu, M., H.Y. Lee, R.E. Bellas, S.L. Schauer, M. Arsura, D. Katz, M.J. Fitzgerald, T.L. Rothstein, D.H. Sherr, and G.E. Sonenshein. 1996. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 23.Ting, A.T., F.X. Pimentel-Muinos, and B. Seed. 1996. RIP mediates tumor necrosis factor receptor type 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 24.Kelliher, M.A., S. Grimm, Y. Ishida, F. Kuo, B.Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 8:297–303. [DOI] [PubMed] [Google Scholar]
- 25.Tartaglia, L., R.F. Weber, I.S. Figari, C. Reynolds, M.A. Palladino, and D.V. Goeddel. 1991. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc. Natl. Acad. Sci. USA. 88:9292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grell, M., F.M. Becke, H. Wajant, D.N. Mannel, and P. Scheurich. 1998. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur. J. Immunol. 28:257–263. [DOI] [PubMed] [Google Scholar]
- 27.Grell, M., G. Zimmerman, E. Gottfried, C.M. Chen, U. Grunwald, D.C.S. Huang, Y.H.W. Lee, H. Durkop, H. Engelmann, P. Scheurich, et al. 1999. Induction of cell death by tumour necrosis factor (TNF) receptor type 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 18:3034–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tartaglia, L.A., D.V. Goeddel, C. Reynolds, I.S. Figari, R.F. Weber, B.M. Fendly, and M.A. Palladino. 1993. Stimulation of human T cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J. Immunol. 151:4637–4641. [PubMed] [Google Scholar]
- 29.Pimentel-Muinos, F.X., and B. Seed. 1999. Regulated commitment of TNF receptor signaling: a molecular switch of death or activation. Immunity. 11:783–793. [DOI] [PubMed] [Google Scholar]
- 30.Rothe, M., S.C. Wong, W.J. Henzel, and D.V. Goeddel. 1994. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 78:681–692. [DOI] [PubMed] [Google Scholar]
- 31.Rothe, M., V. Sarma, V.M. Dixit, and D.V. Goeddel. 1995. TRAF2-mediated activation of NF-kappa-B by TNF receptor 2 and CD40. Science. 269:1424–1427. [DOI] [PubMed] [Google Scholar]
- 32.Rothe, M., M.G. Pan, W.J. Henzel, T.M. Ayres, and D.V. Goeddel. 1995. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 83:1243–1252. [DOI] [PubMed] [Google Scholar]
- 33.Peshcon, J.J., D.S. Torrance, K.L. Stocking, M.B. Glaccum, C. Otten, C.R.Willis, K. Charrier, P.J. Morrissey, C.B. Ware, and K.M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several modes of inflammation. J. Immunol. 160:943–952. [PubMed] [Google Scholar]
- 34.Zhang, J., D. Cado, A. Chen, N.H. Kabra, and A. Winoto. 1998. Fas-mediated apoptosis and activation-induced T cell proliferation are defective in mice lacking FADD/Mort 1. Nature. 392:296–299. [DOI] [PubMed] [Google Scholar]
- 35.Doi, T.S., M.W. Marino, T. Takahashi, T. Yoshida, T. Sakakura, L.J. Old, and Y. Obata. 1999. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl. Acad. Sci. USA. 96:2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenfeld, M.E., L. Prichard, N. Shiojiri, and N. Fausto. 2000. Prevention of hepatic apoptosis and embryonic lethality in relA/TNFR-1 double knockout mice. Am. J. Pathol. 156:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sentftleben, U., Z.W. Li, V. Baud, and M. Karin. 2001. IKK-β is essential for protecting T cells from TNF-α-induced apoptosis. Immunity. 14:217–230. [DOI] [PubMed] [Google Scholar]
- 38.Hsu, H., J. Huang, H.B. Shu, V. Baichwal, and D.V. Goeddel. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 4:387–396. [DOI] [PubMed] [Google Scholar]
- 39.Newton, K., A.W. Harris, and A. Strasser. 2000. Fadd/Mort 1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. EMBO J. 19:931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voll, R.E., E. Jimi, R.J. Phillips, D.F. Barber, M. Rincon, A. Hayday, R.A. Flavell, and S. Ghosh. 2000. NF-κB activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. 2000. Immunity. 13:677–689. [DOI] [PubMed] [Google Scholar]
- 41.Li, X., Y. Yang, and J.D. Ashwell. 2002. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 416:345–349. [DOI] [PubMed] [Google Scholar]