Abstract
The immunosurveillance of transformed cells by the immune system remains one of the most controversial and poorly understood areas of immunity. Gene-targeted mice have greatly aided our understanding of the key effector molecules in tumor immunity. Herein, we describe spontaneous tumor development in gene-targeted mice lacking interferon (IFN)-γ and/or perforin (pfp), or the immunoregulatory cytokines, interleukin (IL)-12, IL-18, and tumor necrosis factor (TNF). Both IFN-γ and pfp were critical for suppression of lymphomagenesis, however the level of protection afforded by IFN-γ was strain specific. Lymphomas arising in IFN-γ-deficient mice were very nonimmunogenic compared with those derived from pfp-deficient mice, suggesting a comparatively weaker immunoselection pressure by IFN-γ. Single loss of IL-12, IL-18, or TNF was not sufficient for spontaneous tumor development. A significant incidence of late onset adenocarcinoma observed in both IFN-γ– and pfp-deficient mice indicated that some epithelial tissues were also subject to immunosurveillance.
Keywords: immunosurveillance, effector, interferon, lymphoma, adenocarcinoma
Introduction
Although tumor immunosurveillance was first hypothesized more than four decades ago, the observed absence of spontaneous tumors in several immune-compromised mouse strains initially dampened enthusiasm for this idea. mAbs specific for immunoregulators have sometimes proven useful in establishing the importance of such molecules in immunity against experimental tumors (1–3). Production of gene-targeted mice for specific effector molecules has greatly aided this endeavor (4–14). Nevertheless, experimental tumor models examined to date have provided limited information on tumor initiation and development. IFN-γ (15) and perforin (pfp; references 5, 14, and 16), key molecules of both the innate and adaptive immune systems, contribute to tumor prevention in mice treated with the chemical carcinogen, methylcholanthrene (MCA), or in mice deficient for p53 expression. In MCA- (10) and other carcinogen-induced cutaneous malignancy models (17), the importance of antitumor immunity mediated by NK cells, NK T cells, and γδ+ T cells has also been illustrated. However, in most of these models tumor development involves the transformation of many cells at one time and only a defined spectrum of tumors (lymphomas and cutaneous malignancies) is observed. An alternative model of tumor induction is that spontaneous tumors can arise from a single cell of any tissue that has acquired the appropriate genetic mutations with age in any given environment. Herein, that environment has been controlled, and spontaneous tumor initiation compared in WT and gene-targeted mice of the same genetic background to illustrate the key role pfp and IFN-γ play in tumor immunosurveillance of lymphoma and lung adenocarcinoma.
Materials and Methods
Mice.
Inbred C57BL/6J and BALB/c WT mice were purchased from The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia. The following gene-targeted mice were bred and maintained at the Austin Research Institute Biological Research Laboratories, Heidelberg, Australia and the Peter MacCallum Cancer Institute, East Melbourne, Australia: C57BL/6J pfp-deficient (B6 pfp−/−) (targeted in C57BL/6J ES cells and provided by D. Kagi [Amgen Institute, Toronto, Canada]; reference 18); C57BL/6J TNF-deficient (B6 TNF−/−) (targeted in C57BL/6J ES cells [reference 19] and provided by Dr. J.D. Sedgwick and originally from the Centenary Institute of Cancer Medicine and Cell Biology, Sydney, Australia); C57BL/6 IFN-γ-deficient (B6 IFN-γ−/−)(backcrossed to C57BL/6J for 10 generations from Genentech, Inc.; reference 20); C57BL/6 IL-12p40-deficient (B6 IL-12−/−)(backcrossed to C57BL/6J for 10 generations from Hoffmann-La Roche; reference 21); C57BL/6 IL-18–deficient (B6 IL-18−/−) (backcrossed to C57BL/6J for eight generations and provided by Dr. S. Akira, Osaka University, Japan; reference 22); BALB/c pfp−/− (backcrossed to BALB/c for eight generations using microsatellite mapping), BALB/c IFN-γ−/− (backcrossed to BALB/c for 10 generations from Genentech, Inc., reference 20); and BALB/c pfp−/− IFN-γ−/−. F1 heterozygote progeny of some gene-targeted mice and their corresponding WT were bred as follows: (B6 × B6 IFN-γ−/−)F1; (BALB/c × BALB/c IFN-γ−/−)F1; and (BALB/c × BALB/c pfp−/−)F1. All aging mice were routinely screened for viruses, parasites, and other microbes and tested negative over the entire course of the experiment. Mice within the study were monitored for health and weighed twice weekly. Any mouse with an abnormality (palpable mass, abdominal distension, weight loss >10%, ruffled fur) was killed, its age recorded, and a postmortem performed. Mean lifespan ± SEM was calculated and probability of significance determined using a Mann-Whitney Rank Sum U-test. The significance of proportions of tumors and, in particular, disseminated lymphomas, was determined by a Fisher's exact test.
Histopathology and Surface Phenotyping of Tumors.
A full autopsy was performed at sacrifice and tumor (macroscopically detected), spleen, liver, thymus, and lymph nodes were routinely examined by histology after fixing these tissues in formalin and on occasions also fresh frozen. The preparation and staining of sections for histology were performed by the Department of Anatomical Pathology, Austin and Repatriation Medical Centre, Heidelberg, Australia. Lymphomas from mice were also assessed for surface phenotype by multiparameter flow cytometric analysis. The following reagents were used: anti–αβ TCR-APC (H57–597; BD PharMingen); NK1.1-PE (PK136; BD PharMingen); CD4-FITC (CT4; Caltag Laboratories); CD8α-APC (53.6.7; BD PharMingen); CD8β-biotin (53.5.8; BD PharMingen); CD11c-FITC (HL3; BD PharMingen); B220-PE (RA3–6B2; Caltag Laboratories); Thy-1-PE (30-H12; BD PharMingen); CD45.2-FITC (104; BD PharMingen); γδ TCR-biotin (clone GL3; BD PharMingen); Mac-1-biotin (M170; Caltag Laboratories); goat anti–mouse Ig-FITC (Silenus Laboratories); and streptavidin-PerCP (BD PharMingen). Anti-Fc receptor (2.4G2) was used to prevent nonspecific binding by mAb. Staining was performed in PBS with 5% FCS and 0.02% sodium azide on ice. Fresh splenocytes from 6-wk-old B6 or BALB/c mice were always used as labeling controls. Analysis was performed on a FACScalibur™ using CELLQuest™ software (Becton Dickinson).
Tumor Transplantation Experiments.
A number of disseminated lymphomas were transplanted directly from B6.pfp−/−, BALB/c pfp−/−, or B6 IFN-γ−/−mice into WT mice or mice of the same genotype. Two representative experiments are shown using a B cell lymphoma from B6.pfp−/− mice, PNK7 (B220+Ig+TCR-αβ−), and a T cell lymphoma from B6 IFN-γ−/− mice, BG18 (CD8-αβ+CD4− TCR-αβ+Ig−). Groups of five WT or gene-targeted mice were injected intraperitoneally with increasing numbers of lymphoma cells and observed daily for tumor growth for >100 d.
Online Supplemental Materials.
The supplemental figures are a (Fig. S1) histology of spontaneous neoplasia in immunodeficient mice and a (Fig. S2) flow cytometric analysis of lymphomas arising in BALB pfp−/− and B6 pfp−/− mice. These can be found at http://www.jem.org/cgi/content/full/jem.20020063/DC1 or from the authors by request.
Results and Discussion
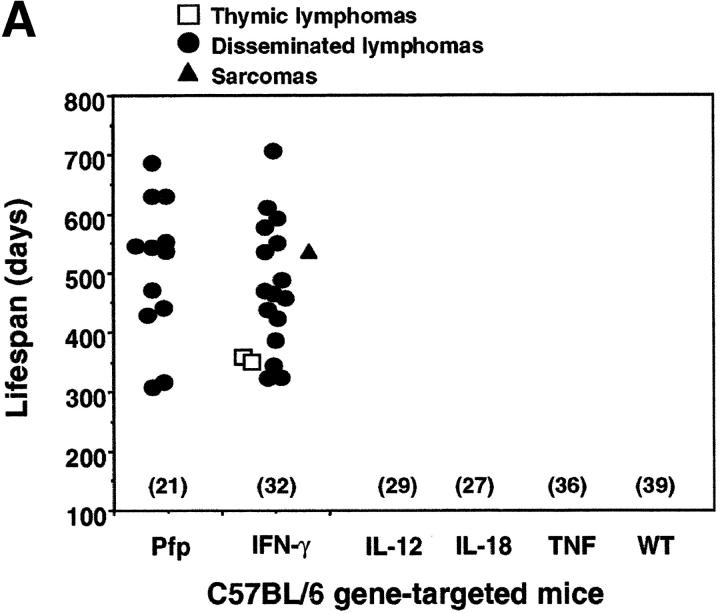
We undertook to monitor spontaneous tumor development in WT C57BL/6 (B6) and BALB/c mice or those that were deficient in IFN-γ and/or pfp. B6 mice deficient in IL-12, IL-18, or TNF were also examined, as all of these cytokines have been shown to regulate the expression of IFN-γ and other effector molecules in lymphocytes (23–27). B6 IFN-γ−/− mice developed disseminated lymphomas (16/32 (50%) and mean lifespan 491 ± 104 d) (Fig. 1 A). A few mice developed thymic lymphomas (n = 2) or sarcoma (n = 1). Like WT B6 mice (0/39 mice), B6 mice that were deficient in TNF (0/36), IL-12 (0/29), or IL-18 (0/27) did not develop any tumors over the same observation period (Fig. 1 A). These data illustrate spontaneous lymphoma formation in IFN-γ–deficient mice and suggest that more than one of the upstream mediators of IFN-γ or an alternative cytokine must be required to regulate the protective effects of IFN-γ. Consistent with our previous study (16), B6 pfp−/− mice died from aggressive disseminated lymphomas affecting the spleen, liver, and lymph nodes from 300 d onwards, with 57% (12/21) succumbing by the end of the experiment (mean lifespan = 510 ± 119 d; Fig. 1 A).
Figure 1.
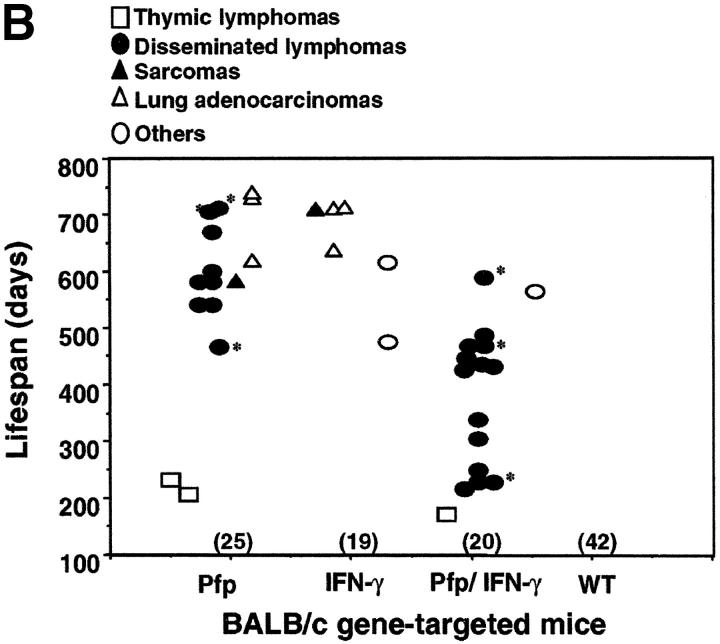
Pfp and IFN-γ protect mice from spontaneous lymphoma. The appearance of tumors was recorded in mice of (A) C57BL/6 and (B) BALB/c backgrounds as indicated. Groups of mice (number in parentheses) were evaluated on a weekly basis and, when moribund, tumor type (white squares, thymic lymphoma; black squares, disseminated lymphoma; black triangles, sarcoma; white triangles, lung adenocarcinoma; and white circles, other tumors) recorded against the age at the time of death/autopsy (in days). *, disseminated lymphomas of histiocyte morphology. No tumors were observed in heterozygote control groups of (B6 × B6 IFN-γ−/−)F1(n = 17), (BALB/c × BALB/c IFN-γ−/−)F1 (n = 12), and (BALB/c × BALB/c pfp−/−)F1 (n = 18) mice over a 750-d period. A small number of the oldest surviving BALB/c IFN-γ−/− mice (700–750 d) also developed nonmalignant mucosal hamartomas in the stomach.
Of great interest was a considerably distinct pattern of tumor development observed in similar gene-targeted mice on a BALB/c background (Fig. 1 B). In striking contrast to B6 IFN-γ−/− mice, BALB/c IFN-γ−/− mice did not develop disseminated lymphoma (0/19, P = 0.0001). The finding that IFN-γ was not essential for host protection from this type of tumor in BALB/c mice illustrated that strain-specific genetic factors can influence the nature of the immune response to these spontaneous lymphomas. By contrast, pfp was critical in host protection from disseminated lymphoma in both strains (Fig. 1 A versus Fig. 1 B). 9 of 25 BALB/c pfp−/− mice developed disseminated lymphoma (mean lifespan = 597 ± 82 d), and a few mice developed thymic lymphomas (n = 2) or sarcoma (n = 1; Fig. 1 B). The earlier onset (mean lifespan = 368 ± 120 d) (P < 0.0001, compared with pfp−/− mice) and greater frequency (14/20) of disseminated lymphoma in BALB/c mice deficient in both pfp and IFN-γ, revealed that in the absence of pfp, IFN-γ did play an important role in delaying the development of disseminated lymphoma (Fig. 1 B). These data supported a general role for IFN-γ in protection from spontaneous lymphoma, but suggested that the relative role of IFN-γ was strain specific.
Lymphocyte-mediated immunosurveillance of epithelial malignancy has never been demonstrated, except where viral infection may contribute to oncogenesis (e.g., EBV, HPV transplant recipients) or when carcinogens were also administered, and we therefore felt the development of spontaneous epithelial tumors in gene-targeted mice warranted particular attention. While IFN-γ and pfp were especially important for the control of disseminated lymphoma, a significant incidence of lung adenocarcinoma was detected in BALB/c IFN-γ−/− mice (3/19, P = 0.0269) and BALB/c pfp−/− mice (3/25, P = 0.048) (Fig. 1). These data directly demonstrated that IFN-γ and pfp suppressed some epithelial malignancies. Notably, it is relevant that the onset of lung adenocarcinomas was very late in BALB/c IFN-γ−/− (681 ± 43 d) and BALB/c pfp−/− (693 ± 67 d) mice. Indeed, the development of all tumors, including disseminated lymphomas was significantly delayed in BALB/c mice compared with B6 mice. Far fewer BALB/c pfp−/− IFN-γ−/−, B6 IFN-γ−/−, and B6 pfp−/− mice remained alive (free of lymphoma) over the first 600 d of life and therefore larger numbers of these will likely be required to observe adenocarcinomas. F1 progeny of gene-null mice and WT mice lacking either one allele of pfp or IFN-γ did not develop any spontaneous tumors (Fig. 1). Determining whether the immune system controls other epithelial malignancies is an important issue for future long-term studies and only a similar study of far larger groups of mice (≥100) will reveal whether significant numbers of other epithelial malignancies emerge in IFN-γ−/− and pfp−/− mice.
6 of the 16 lymphomas arising in B6 IFN-γ−/− mice were analyzed for phenotype and while five were TCR-αβ+, no unique lymphoma phenotype was apparent (unpublished data). Several previous studies have indicated that T cell death after antigen-driven expansion may be regulated by IFN-γ (28, 29), and therefore possibly disrupted homeostasis and failed immunosurveillance by IFN-γ loss, may be the cause of T cell lymphoma in B6 IFN-γ−/− mice. All of the disseminated lymphomas in B6 pfp−/− mice were of B cell origin (B220+ sIg+ CD4−CD8− TCR−), or plasmacytomas (B220+ CD4− CD8− TCR− sIglow; also defined histologically, unpublished data). Our previous study of pfp−/− mice with additional p53 loss indicated a majority of lymphomas of B cell origin, however some p53+/−pfp−/− mice also developed lymphomas of non-B cell origin (16). One explanation for the development of B cell lymphomas in pfp−/− mice is the recognized role for pfp in controlling the survival of some APCs, like B cells (30, 31). However, it remains unclear why only lymphomas of B cell origin developed in B6 pfp−/− mice when enhanced expansions of CD8+ T cells were observed in these mice, particularly after challenge with foreign antigens (e.g., viral or bacterial infection; references 28, and 32–34). Most of the lymphomas in B6 pfp−/− and B6 IFN-γ−/− mice were diffuse large cell lymphomas (see Online Supplemental Materials).
Flow cytometric analysis of disseminated lymphomas in BALB/c pfp−/− and BALB/c pfp−/−IFN-γ−/− mice revealed that all were also B220+ sIg+ CD4−CD8− TCR− (unpublished data). Importantly, these data supported a role for IFN-γ in also suppressing the earlier onset of B cell lymphomas. Interestingly, in the BALB/c strain, a small number of the lymphomas arising in pfp−/− and pfp−/− IFN-γ−/− mice showed an unusual histiocytic appearance (Fig. 1, asterisk) with a pale eosinophilic cytoplasm. These tumors additionally expressed CD11c and Mac-1 antigens (see Online Supplemental Materials), but did not all express other markers of B1 cells (e.g., CD5, unpublished data). Of note, this type of histopathology occurs only rarely in humans, and interestingly the accumulations of activated histiocytes (macrophages) observed in pfp-deficient humans displaying familial hemophagocytic lymphohistiocytosis (35) are one example. All the lung adenocarcinomas in both BALB/c pfp−/− and BALB/c IFN-γ−/− mice were well-differentiated papillary adenocarcinomas (see Online Supplemental Materials).
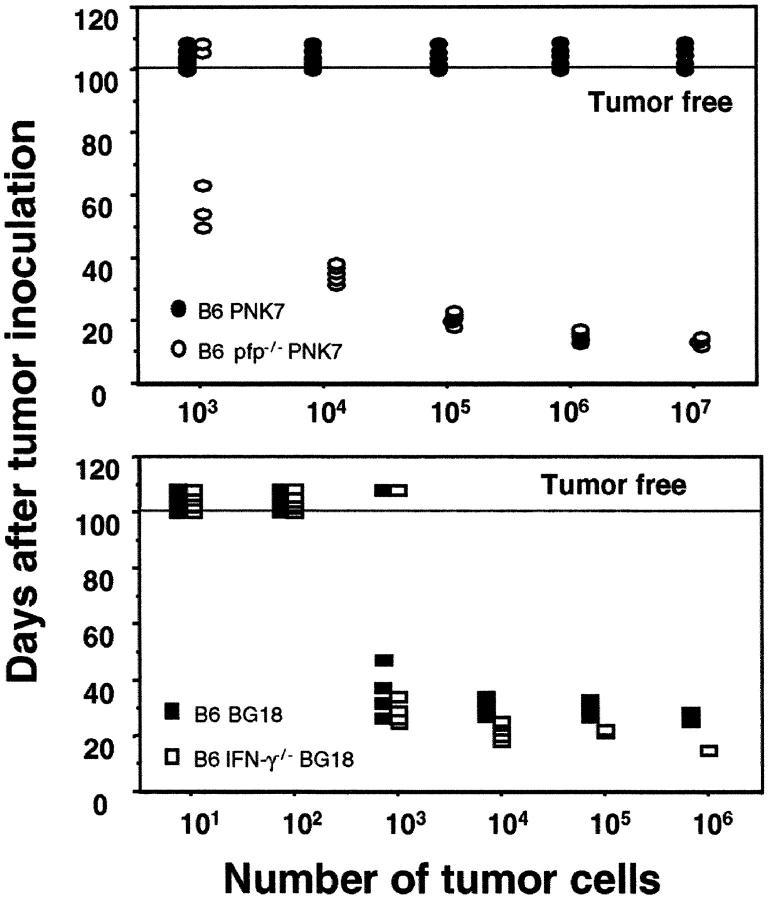
The malignancy of primary tumors arising was additionally confirmed by secondary transfer into mice of the same strain. Two representative experiments of >10 using different disseminated lymphomas transferred from either B6 pfp−/− or B6 IFN-γ−/− mice are depicted in Fig. 2. The transfer of three different T cell lymphomas from B6 IFN-γ−/− mice (one shown) demonstrated that these tumors grew at a similar rate in B6 IFN-γ−/− and B6 WT mice (Fig. 2). By contrast, and in concert with our previous data (16), all B cell lymphomas from B6 pfp−/− mice grew at low cell numbers in B6 pfp−/− mice, but were avidly rejected when transferred into B6 WT mice (Fig. 2). Thus far, all lymphomas of B cell origin arising in pfp−/− mice, including five described herein, were rejected in WT mice by CD8+ T cells (reference 16 and unpublished data) and in this study the donor tumor cells and recipient mice were genetically matched as closely as possible. Similar experiments with three different B cell lymphomas from BALB/c pfp−/− mice have also demonstrated rejection in BALB/c WT mice and (BALB/c × BALB/c. pfp−/−)F1 heterozygous mice, but not BALB/c pfp−/− mice (unpublished data). In concert with our previous lymphoma transfer studies (16), it is likely that the lymphomas of B cell origin are generally being detected by CD8+ T cells independently of any alloantigens potentially expressed by the tumor. It is probable that the lack of detectable immunogenicity of lymphomas from IFN-γ−/− mice indicates an important functional distinction between the activities of IFN-γ and pfp in eliminating potentially transformed cells. Consistent with the immune system functioning as a tumor-suppressor system, our results indicated that the immunoselection pressure of pfp on tumor cells was strong. Lymphomas from pfp-deficient mice have emerged in the absence of this significant immunoselection pressure, and they are avidly rejected in WT mice upon transfer. Immunoselection by IFN-γ appears far weaker from our spontaneous lymphoma transplant data, however it has been demonstrated in an experimental system using MCA fibrosarcomas arising in IFN-γ receptor-deficient mice (36).
Figure 2.
Distinct behavior of lymphomas arising in B6 pfp−/− mice and B6 IFN-γ2/− mice. Primary lymphomas arising in B6 pfp−/− (top) and B6.IFN-γ2/− (bottom) were secondarily transplanted intraperitoneally (101–107 cells in 0.2 ml PBS, as indicated) into groups of five untreated B6 WT (black circles or squares), B6 pfp−/− (white circles) or B6 IFN-γ−/− (white squares) mice. Mice were monitored for 100 d and each symbol depicts an individual mouse. Tumor-free mice are indicated above the horizontal line. The results are representative of seven primary lymphomas and three primary lymphomas transplanted from B6 pfp−/− and B6 IFN-γ−/− mice, respectively. Top, PNK-7 (B cell lymphoma from B6 pfp−/− mouse); bottom, BG18 (T cell lymphoma from B6 IFN-γ−/− mouse).
Previous studies have demonstrated that T cells (2), NK T cells (37), and NK cells (37) can all produce IFN-γ with antitumor activity. It remains unclear which type of lymphocytes use IFN-γ to control spontaneous lymphomas or lung adenocarcinomas, and this issue is very difficult to address without mice conditionally deficient (e.g., NK cell, NK T cell, or T cell) in IFN-γ. Defining exactly how a pleiotropic cytokine like IFN-γ mediates host protection from spontaneous tumors will also be a challenging goal for future investigation given that the lymphomas arising in B6 IFN-γ−/− mice appear nonimmunogenic when transferred into WT mice. Many potential direct and indirect activities of IFN-γ could suppress spontaneous tumor formation. Although IFN-γ can suppress tumor angiogenesis, it is unlikely that this is the main mode of action in preventing the development of nonsolid tumors such as disseminated lymphomas. It also remains feasible that loss of IFN-γ may result in tumor promotion by another factor normally suppressed by IFN-γ.
Gene-targeted mice have been extremely valuable when used in models where tumors are induced by viruses or carcinogens or simply when the mice were aged for spontaneous tumor formation. The value of using several types of tumor induction models is no better illustrated by the lack of tumor development in TNF−/− mice, but the reduced predisposition of these mice to carcinogen-induced skin tumors (38). Although some other recent studies in aging mice have suggested that tumor immunosurveillance may exist (16, 36), our study directly demonstrated the specific importance of IFN-γ in the development of lymphomas and adenocarcinomas. Recently, a study of aging 129/Sv mice doubly deficient for recombination activating gene (RAG)-2 and signal transducer and activator of transcription (STAT)-1, the transcription factor mediating signaling by IFN-γ and IFN-α/β receptors (RAG-2−/−STAT-1−/−), suggested that lymphocytes and IFN-γ may play an important role in host protection from breast and colon carcinomas (36). Mice deficient in STAT-1 alone displayed a low incidence of mammary carcinoma, however STAT-1 regulates far more genes than IFNs alone, including the mammary tumor suppressor, BRCA-1 (39). The lack of lymphoma reported in 129/Sv STAT-1 mice (32) may be because 129/Sv behave like the BALB/c strain. Nevertheless, we did not observe colon or breast adenocarcinomas in either BALB/c or C57BL/6 strains of IFN-γ-deficient mice. The development of mammary and colon adenocarcinomas in 129/SvEv RAG-2−/−STAT-1−/− mice may be a consequence of intestinal hyperplasia peculiar to 129 RAG−/− mice. By contrast, the lung adenocarcinomas observed in BALB/c pfp−/− and BALB/c IFN-γ−/− mice occurred in strains of mice with no evidence of predisposing epithelial hyperplasia. The concept of whether lymphocytes play a key role in immunosurveillance beyond their own regulation (i.e., of nonlymphoid tissues) has been debated for years (40). The adenocarcinomas observed in mice in our study suggested that future studies, monitoring spontaneous tumor development in gene-targeted mice alone and bred with other strains genetically prone to epithelial malignancies, will be most informative.
Acknowledgments
We wish to thank the staff of the Biological Research Facility and Dr. Mauro Sandrin, Austin Research Institute and the animal staff at Peter MacCallum Cancer Institute for their assistance with the aging experiments. We also thank Drs. Andreas Strasser, Dale Godfrey, and Hideo Yagita for critically reviewing this manuscript.
This work was supported by fellowship and project grants from the National Health & Medical Research Council of Australia to M.J. Smyth and J.A. Trapani and by a Dora Lush Postgraduate Scholarship (NH&MRC) to S.E.A. Street.
The online version of this article contains supplemental material.
References
- 1.Gresser, I., F. Belardelli, C. Maury, M.T. Maunoury, and M.G. Tovey. 1983. Injection of mice with antibody to interferon enhances the growth of transplantable murine tumors. J. Exp. Med. 158:2095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuttle, T.M., C.W. McCrady, T.H. Inge, M. Salour, and H.D. Bear. 1993. γ-interferon plays a key role in T-cell-induced tumor regression. Cancer Res. 53:833–839. [PubMed] [Google Scholar]
- 3.Takeda, K., Y. Hayakawa, M.J. Smyth, N. Kayagaki, N. Yamaguchi, S. Kakuta, Y. Iwakura, H. Yagita, and K. Okumura. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7:94–100. [DOI] [PubMed] [Google Scholar]
- 4.van den Broek, M.F., D. Kagi, R.M. Zinkernagel, and H. Hengartner. 1995. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur. J. Immunol. 25:3514–3516. [DOI] [PubMed] [Google Scholar]
- 5.van den Broek, M.E., D. Kagi, F. Ossendorp, R. Toes, S. Vamvakas, W.K. Lutz, C.J. Melief, R.M. Zinkernagel, and H. Hengartner. 1996. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 184:1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth, M.J., J.M. Kelly, A.G. Baxter, H. Korner, and J.D. Sedgwick. 1998. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J. Exp. Med. 188:1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodama, T., K. Takeda, O. Shimozato, Y. Hayakawa, M. Atsuta, K. Kobayashi, M. Ito, H. Yagita, and K. Okumura. 1999. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur. J. Immunol. 29:1390–1396. [DOI] [PubMed] [Google Scholar]
- 8.Smyth, M.J., K.Y. Thia, E. Cretney, J.M. Kelly, M.B. Snook, C.A. Forbes, and A.A. Scalzo. 1999. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 162:6658–6662. [PubMed] [Google Scholar]
- 9.Qin, Z., and T. Blankenstein. 2000. CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN γ receptor expression by nonhematopoietic cells. Immunity. 12:677–686. [DOI] [PubMed] [Google Scholar]
- 10.Smyth, M.J., K.Y. Thia, S.E. Street, E. Cretney, J.A. Trapani, M. Taniguchi, T. Kawano, S.B. Pelikan, N.Y. Crowe, and D.I. Godfrey. 2000. Differential tumor surveillance by natural killer (NK) and NK T cells. J. Exp. Med. 191:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyth, M.J., M. Taniguchi, and S.E. Street. 2000. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J. Immunol. 165:2665–2670. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima, C., Y. Uekusa, M. Iwasaki, N. Yamaguchi, T. Mukai, P. Gao, M. Tomura, S. Ono, T. Tsujimura, H. Fujiwara, and T. Hamaoka. 2001. A role of interferon-γ (IFN-γ) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN-γ-deficient mice. Cancer Res. 61:3399–3405. [PubMed] [Google Scholar]
- 13.Dobrzanski, M.J., J.B. Reome, and R.W. Dutton. 2001. Role of effector cell-derived IL-4, IL-5, and perforin in early and late stages of type 2 CD8 effector cell-mediated tumor rejection. J. Immunol. 167:424–434. [DOI] [PubMed] [Google Scholar]
- 14.Street, S.E., E. Cretney, and M.J. Smyth. 2001. Perforin and interferon-γ activities independently control tumor initiation, growth, and metastasis. Blood. 97:192–197. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan, D.H., V. Shankaran, A.S. Dighe, E. Stockert, M. Aguet, L.J. Old, and R.D. Schreiber. 1998. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 95:7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth, M.J., K.Y. Thia, S.E. Street, D. MacGregor, D.I. Godfrey, and J.A. Trapani. 2000. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardi, M., D.E. Oppenheim, C.R. Steele, J.M. Lewis, E. Glusac, R. Filler, P. Hobby, B. Sutton, R.E. Tigelaar, and A.C. Hayday. 2001. Regulation of cutaneous malignancy by γδT Cells. Science. 294:604–609. [DOI] [PubMed] [Google Scholar]
- 18.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K.J. Olsen, E.R. Podack, R.M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 369:31–37. [DOI] [PubMed] [Google Scholar]
- 19.Korner, H., D.S. Riminton, D.H. Strickland, F.A. Lemckert, J.D. Pollard, and J.D. Sedgwick. 1997. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J. Exp. Med. 186:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalton, D.K., S. Pitts-Meek, S. Keshav, I.S. Figari, A. Bradley, and T.A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 259:1739–1742. [DOI] [PubMed] [Google Scholar]
- 21.Magram, J., S.E. Connaughton, R.R. Warrier, D.M. Carvajal, C.Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D.A. Faherty, and M.K. Gately. 1996. IL-12-deficient mice are defective in IFN γ production and type 1 cytokine responses. Immunity. 4:471–481. [DOI] [PubMed] [Google Scholar]
- 22.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 8:383–390. [DOI] [PubMed] [Google Scholar]
- 23.Sinigaglia, F., D. D'Ambrosio, P. Panina-Bordignon, and L. Rogge. 1999. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol. Rev. 170:65–72. [DOI] [PubMed] [Google Scholar]
- 24.Wiltrout, R.H. 2000. Regulation and antimetastatic functions of liver-associated natural killer cells. Immunol. Rev. 174:63–76. [DOI] [PubMed] [Google Scholar]
- 25.Boehm, U., T. Klamp, M. Groot, and J.C. Howard. 1997. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15:749–795. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello, C.A. 2000. Interleukin-18, a proinflammatory cytokine. Eur. Cytokine Netw. 11:483–486. [PubMed] [Google Scholar]
- 27.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251–276. [DOI] [PubMed] [Google Scholar]
- 28.Badovinac, V.P., A.R. Tvinnereim, and J.T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science. 290:1354–1358. [DOI] [PubMed] [Google Scholar]
- 29.Chu, C.Q., S. Wittmer, and D.K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shustov, A., I. Luzina, P. Nguyen, J.C. Papadimitriou, B. Handwerger, K.B. Elkon, and C.S. Via. 2000. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J. Clin. Invest. 106:R39–R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepp, S.E., P.A. Mathew, M. Bennett, G. de Saint Basile, and V. Kumar. 2000. Perforin: more than just an effector molecule. Immunol. Today. 21:254–256. [DOI] [PubMed] [Google Scholar]
- 32.Matloubian, M., M. Suresh, A. Glass, M. Galvan, K. Chow, J.K. Whitmire, C.M. Walsh, W.R. Clark, and R. Ahmed. 1999. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 73:2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sad, S., D. Kagi, and T.R. Mosmann. 1996. Perforin and Fas killing by CD8+ T cells limits their cytokine synthesis and proliferation. J. Exp. Med. 184:1543–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klenerman, P., and R.M. Zinkernagel. 1998. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 394:482–485. [DOI] [PubMed] [Google Scholar]
- 35.Stepp, S.E., R. Dufourcq-Lagelouse, F. Le Deist, S. Bhawan, S. Certain, P.A. Mathew, J.I. Henter, M. Bennett, A. Fischer, G. de Saint Basile, et al. 1999. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 286:1957–1959. [DOI] [PubMed] [Google Scholar]
- 36.Shankaran, V., H. Ikeda, A.T. Bruce, J.M. White, P.E. Swanson, L.J. Old, and R.D. Schreiber. 2001. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 410:1107–1111. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa, Y., K. Takeda, H. Yagita, S. Kakuta, Y. Iwakura, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Critical contribution of IFN-γ and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of α-galactosylceramide. Eur. J. Immunol. 31:1720–1727. [PubMed] [Google Scholar]
- 38.Moore, R.J., D.M. Owens, G. Stamp, C. Arnott, F. Burke, N. East, H. Holdsworth, L. Turner, B. Rollins, M. Pasparakis, et al. 1999. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat. Med. 5:828–831. [DOI] [PubMed] [Google Scholar]
- 39.Ouchi, T., S.W. Lee, M. Ouchi, S.A. Aaronson, and C.M. Horvath. 2000. Collaboration of signal transducer and activator of transcription 1 (STAT1) and BRCA1 in differential regulation of IFN-γ target genes. Proc. Natl. Acad. Sci. USA. 97:5208–5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smyth, M.J., and J.A. Trapani. 2001. Lymphocyte-mediated immunosurveillance of epithelial cancers? Trends Immunol. 22:409–411. [DOI] [PubMed] [Google Scholar]