Abstract
Oral streptococci, such as Streptococcus gordonii, are the predominant early colonizers that initiate biofilm formation on tooth surfaces. Investigation of an S. gordonii::Tn917-lac biofilm-defective mutant isolated by using an in vitro biofilm formation assay showed that the transposon insertion is near the 3′ end of an open reading frame (ORF) encoding a protein homologous to Streptococcus mutans FruK. Three genes, fruR, fruK, and fruI, were predicted to encode polypeptides that are part of the fructose phosphotransferase system (PTS) in S. gordonii. These proteins, FruR, FruK, and FruI, are homologous to proteins encoded by the inducible fruRKI operon of S. mutans. In S. mutans, FruR is a transcriptional repressor, FruK is a fructose-1-phosphate kinase, and FruI is the fructose-specific enzyme II (fructose permease) of the phosphoenolpyruvate-dependent sugar PTS. Reverse transcription-PCR confirmed that fruR, fruK, and fruI are cotranscribed as an operon in S. gordonii, and the transposon insertion in S. gordonii fruK::Tn917-lac resulted in a nonpolar mutation. Nonpolar inactivation of either fruK or fruI generated by allelic replacement resulted in a biofilm-defective phenotype, whereas a nonpolar mutant with an inactivated fruR gene retained the ability to form a biofilm. Expression of fruK, as measured by the β-galactosidase activity of the fruK::Tn917-lac mutant, was observed to be growth phase dependent and was enhanced when the mutant was grown in media with high levels of fructose, sucrose, xylitol, and human serum, indicating that the fructose PTS operon was fructose and xylitol inducible, similar to the S. mutans fructose PTS. The induction by fructose was inhibited by the presence of glucose, indicating that glucose is able to catabolite repress fruK expression. Nonpolar inactivation of the fruR gene in the fruK::Tn917-lac mutant resulted in a greater increase in β-galactosidase activity when the organism was grown in media supplemented with fructose, confirming that fruR is a transcriptional repressor of the fructose PTS operon. These results suggest that the regulation of fructose transport and metabolism in S. gordonii is intricately tied to carbon catabolite control and the ability to form biofilms. Carbon catabolite control, which modulates carbon flux in response to environmental nutritional levels, appears to be important in the regulation of bacterial biofilms.
The process of bacterial accumulation and proliferation after initial bacterial adhesion leads to the formation of persistent, complex, organized sessile communities on oral surfaces. The multistep process of oral biofilm formation is a complex developmental process initiated by attachment to saliva-conditioned oral surfaces of primary colonizers, such as viridans streptococci (including Streptococcus gordonii), which constitute a majority of the cultivable bacteria found in dental plaque (21). Subsequent accumulation and growth of attached bacteria result in microcolonies that increase in size and eventually form biofilms. Fully developed oral biofilms (dental plaque) are similar to other complex sessile communities that have highly structured, distinct architecture and physiochemical properties (4, 37).
In nutritionally limited environments, such as oral surfaces, biofilm formation may represent a survival strategy (17). In the oral cavity, streptococci depend on sugars as an energy source; the main energy supply is carbohydrates. Thus, these bacteria are transiently exposed to a mixture of various sugars and live under feast-or-famine conditions. Oral streptococci constitute the dominant acidogenic population in supragingival plaque and are capable of transporting and fermenting a wide variety of sugars. The phosphotransferase system (PTS) is the major transport system for carbohydrates, which are phosphorylated during translocation through the membrane. In oral streptococci, the high-affinity phosphoenolpyruvate-dependent sugar PTS is the principal route for transport of most sugars and is primarily responsible for sugar transport at low sugar concentrations (43).
Comparative proteome and transcriptome analyses of biofilm and planktonic cells have revealed that various anabolic and catabolic operons are differentially expressed. These operons include operons involved in energy metabolism and in the biosynthesis, transport, and metabolism of carbon compounds, lipids, and amino acids in Pseudomonas aeruginosa (33, 49), Escherichia coli (34, 41), Bacillus subtilis (36), Bacillus cereus (22), Streptococcus mutans (39), and Listeria monocytogenes (42).
The transition from a planktonic existence to growth in a biofilm occurs primarily in response to environmental cues, including the availability of nutrients. Addition of carbohydrates to growth medium has been shown to affect biofilm formation by oral streptococci on abiotic surfaces (9, 18, 28, 51). In Streptococcus parasanguinis, addition of glucose enhanced biofilm formation in different types of media (9), whereas enriched media inhibited biofilm formation by S. gordonii (18) and S. mutans (51).
In contrast, glucose repressed biofilm formation by several species of the Enterobacteriaceae (13). In E. coli, this glucose effect or catabolite repression is partially mediated by cyclic AMP (cAMP) and cAMP receptor protein. Several other genes involved in global carbon regulation have been shown to be important in biofilm formation. A cAMP-independent catabolite repression control protein (Crc), which is a global carbon regulator, was found to be necessary for biofilm formation in P. aeruginosa (23). Another global regulatory factor that influences biofilm development in E. coli is the carbon storage regulator CsrA, which serves as both a repressor of biofilm formation and an activator of biofilm dispersal under a variety of culture conditions. The effects of CsrA on biofilm formation were found to be mediated largely through regulation of intracellular glycogen biosynthesis and catabolism (14). In gram-positive bacteria, the global carbon regulator CcpA (catabolite control protein) may regulate genes required for stable biofilm formation in S. mutans (48) and B. subtilis (36), as loss of CcpA resulted in an approximately 60% decrease in biofilm formation on an abiotic surface. In this report we describe isolation and characterization of an S. gordonii Tn917-lac mutant with a mutation in the fructose PTS operon and a biofilm-defective phenotype, and we provide additional evidence that regulation of carbon flux involving a sugar PTS also plays a significant role in the development of biofilms.
MATERIALS AND METHODS
Bacteria, media, and chemicals.
S. gordonii Challis 2, the rifamycin-resistant (500 μg/ml) strain of S. gordonii Challis (18), was used as the parent strain. Unless indicated otherwise, bacteria were subcultured and maintained routinely on brain heart infusion (BHI) agar (BBL, Becton Dickinson, Cockeysville, Md.) or Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.2% yeast extract (THBYE) at 37°C under anaerobic conditions (VWRbrand anaerobic chamber; VWR, Plainfield, N.J.).
All chemicals were purchased from Sigma (St. Louis, Mo.) or Fisher Scientific (Pittsburgh, Pa.). All enzymes used for DNA manipulations were purchased from Promega (Madison, Wis.) or Fisher Scientific unless indicated otherwise. Oligonucleotide primers were obtained from Invitrogen Life Technologies (Rockville, Md.).
Tn917-lac mutagenesis of S. gordonii Challis.
An in vitro microtiter plate biofilm formation assay was performed as previously described (18) by using a minimal defined medium as the biofilm medium (BM). This BM contained 58 mM K2HPO4, 15 mM KH2PO4, 10 mM (NH4)2SO4, 35 mM NaCl, 0.8% (wt/vol) glucose, 0.2% (wt/vol) Casamino Acids, 0.1 mM MnCl2 · 4H2O (pH 7.4), filter-sterilized vitamins (0.04 mM nicotinic acid, 0.1 mM pyridoxine HCl, 0.01 mM pantothenic acid, 1 μM riboflavin, 0.3 μM thiamine HCl, and 0.05 μM d-biotin), amino acids (4 mM l-glutamic acid, 1 mM l-arginine HCl, 1.3 mMl-cysteine HCl, and 0.1 mM l-tryptophan), and 2 mM MgSO2 · 7H2O.
S. gordonii Challis 2 was mutagenized with pTV32OK (6), and a lacZ fusion library was generated as described previously (19). Round-bottom polystyrene (Falcon 3918) microtiter plates (Becton-Dickinson Labware, Lincoln Park, N.J.) containing 100 μl of BM with erythromycin (10 μg/ml) in each well were inoculated with S. gordonii Challis::Tn917-lac mutants. After 24 to 48 h of incubation at 37°C under anaerobic conditions, bacterial growth and biofilm formation were quantified by measuring the absorbance at 575 nm of each bacterial culture and crystal violet-stained biofilm, respectively (19). Bacteria from a duplicate plate with corresponding wells having equivalent growth but poor crystal violet staining were used as a biofilm-defective mutant.
In addition to the microtiter plate assay, biofilm formation on borosilicate glass coverslips was visualized directly by phase-contrast microscopy (23). Cells from overnight colonies grown on BHI agar plates were inoculated into BM in 50-ml Falcon tubes to an A600 of ∼0.04. Borosilicate coverslips were coated with filter-sterilized, clarified whole saliva for 1 h at room temperature on a shaker. Then uncoated and saliva-coated coverslips were placed in the Falcon tubes containing BM inoculated with streptococci and incubated at 37°C under anaerobic conditions. At each time point, a coverslip was removed and rinsed with BM to remove nonadherent cells. Biofilm bacteria present on the coverslip were examined by phase-contrast microscopy by using a Micromaster phase-contrast microscope (Fisher Scientific), and images were captured with a Nikon Coolpix 950 digital camera (19).
Southern hybridization, localization of transposon insertion site, and sequence analyses.
Chromosomal DNA isolated from S. gordonii Challis 2 and the mutant strain were digested with HindIII and transferred onto a nitrocellulose membrane. Southern hybridization with a digoxigenin-labeled pTV32-OK probe was performed as described previously (19).
The location of the transposon insertion was determined by sequence analysis of the region flanking the transposon. Initially, pBluescript vector and chromosomal DNA from the mutant were digested with HindIII, purified with a nucleotide removal kit (Qiagen, Valencia, Calif.), ligated to each other, and used as the PCR template. A PCR with primers IP917B and PBSSK3 (Table 1) was then performed under the following conditions: after an initial denaturation for 2 min at 95°C, 36 cycles of amplification (denaturation for 45 s at 94°C, annealing for 45 s at 53°C, and extension for 2 min at 72°C), followed by a final extension for 10 min at 72°C. The PCR products were analyzed by agarose gel electrophoresis, purified with a PCR purification kit (Qiagen), and sequenced at the Genetics Core Sequencing Facility of Boston University by using a model 377 automated sequencer (Applied Biosystems, Foster City, Calif.). The sequence obtained was compared with sequences in the GenBank database by using the BLASTX program (1) to identify homologous bacterial sequences. The putative protein sequences encoded by the genes identified were then compared to the sequences of FruR, FruK, and FruI homologs from different streptococci. Amino acid sequence alignment and phylogenetic analysis were performed by using the AlignX program in Vector NTI (Informax Inc., Bethesda, Md.), which utilizes the neighbor-joining algorithm (32).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequence (5′ to 3′)a | Location | Application | Amplicon |
|---|---|---|---|---|
| SSP-917B | AAC TGT ACC ACT AAT AAC TCA CAA T | Tn917-lac | PCR | |
| PBSSK3 | GTA AAA CGA CGG CCA GT | pBluescript | PCR | |
| Spec1 | CGA CGC GTC GAA ATC TAT AAA TAA ACT A | pSF152 | Mutagenesis | Spectinomycin cassette |
| Spec2 | CGG TCG ACC GAA ATA ATA AAA CAA AAA A | pSF152 | Mutagenesis | Spectinomycin cassette |
| Fru repressor5′ | CGA CGC GTC GGG TAC TAC AAA TGA GTT G | fruR | RT-PCR | fruR-fruI (3,330 bp) |
| FruPTS3′ | CGA CGC GTC GTT ATT YTG ACA ATG GTT T | fruI | RT-PCR | fruR-fruI (3,330 bp) |
| FruR1 | CGG GTA CCC GTC AAT ATC AAC TAC TGA AT | Hypothetical protein 1 | Mutagenesis of fruR, RT-PCR | fruR 5′ flanking region (907 bp) |
| FruR2 | CGA CGC GTC GTA TGA ACC AAA CTA TCT A | fruR | Mutagenesis of fruR, RT-PCR | fruR 5′ flanking region (907 bp) |
| FruR3 | CGG TCG ACC GTG TTA AAG TAG CTC CTC T | fruR | Mutagenesis of fruR | fruR 3′ flanking region (740 bp) |
| FruR4 | CGC TCG AGC GTA AGA TTT GAC GAG CAT A | fruK | Mutagenesis of fruR | fruR 3′ flanking region (740 bp) |
| FruK1 | CGG GTA CCC GAC AGG AGT AAA AAC AGA G | fruR | Mutagenesis of fruK | fruK 5′ flanking region (1,022 bp) |
| FruK2 | CGA CGC GTC GGG TTT AAC GTT ACA GTA T | fruK | Mutagenesis of fruK | fruK 5′ flanking region (1,022 bp) |
| FruK3 | CGG TCG ACC GTA TTA AAG AAA CAT ATG A | fruK | Mutagenesis of fruK | fruK 3′ flanking region (834 bp) |
| FruK4 | CGC TCG AGC GTC AGT TCT TCT GTC TTA C | fruI | Mutagenesis of fruK | fruK 3′ flanking region (834 bp) |
| PTS1 | CGG GTA CCC GTT GCT AAG GCA AAG ATA A | fruK | Mutagenesis of fruI | fruI 5′ flanking region (1,053 bp) |
| PTS2 | CGA CGC GTC GCT AGC AAC ATA ACG TCT T | fruI | Mutagenesis of fruI | fruI 5′ flanking region (1,053 bp) |
| PTS3 | CGG TCG ACC GTT TGG TTG GTG CAG TAG T | fruI | Mutagenesis of fruI | fruI 3′ flanking region (613 bp) |
| PTS4 | CGC TCG AGC GCA TTT AGA ACT CCA AAT TAA | Hypothetical protein 2 | Mutagenesis of fruI | frul 3′ flanking region (613 bp) |
| Hypo2rev | ATT CGA CCT TGA ATA TGG TTA ACT TGA G | Hypothetical protein 2 | RT-PCR | |
| Hypo3rev | CTC CAA GAT GTG TTC CAA TAA CAC TAC C | Hypothetical protein 3 | RT-PCR |
Engineered restriction sites are indicated by boldface type. The restriction endonuclease recognition sequences are as follows: KpnI, GGT/ACC; MluI, ACG/CGT; SalI, GTC/GAC; and XhoI, CTC/GAG.
RT-PCR of S. gordonii Challis 2 RNA.
In order to determine the genes that constitute the fructose PTS operon, reverse transcription-PCR (RT-PCR) was performed with total RNA extracted from S. gordonii Challis 2 grown to the mid-log phase (A600, 0.3 to 0.4) by using an RNeasy mini kit (Qiagen). The primer pairs used for RT-PCR analysis are listed in Table 1. Primers FruR1 and FruR2 are specific for an intergenic region extending from the open reading frame (ORF) encoding hypothetical protein 1 (upstream from fruR) to fruR; primers Fru repressor5′ and fruPTS3′ are specific for an intergenic region extending from fruR to fruI; primers PTS3 and Hypo2rev are specific for an intergenic region extending from fruI to the ORF encoding hypothetical protein 2 (downstream from fruI); and primers PTS3 and Hypo3rev are specific for an intergenic region extending from fruI to the ORF encoding hypothetical protein 3. The RT-PCR was performed by using the Access RT-PCR system (Promega) under the conditions that were described previously (19).
Expression of fruK in different environmental conditions.
The β-galactosidase activity of the biofilm-defective S. gordonii fruK::Tn917-lac mutant was determined by a fluorimetric assay (12, 19) by using 4-methylumbelliferyl-β-d-galactoside (MUG). Cells were grown in THBYE containing erythromycin, and the β-galactosidase activities of cells harvested at different growth phases over 24 h were determined. In order to determine the effects of various growth conditions on expression, bacteria were grown in 10 ml of THBYE or BM containing erythromycin at 37°C (unless indicated otherwise) in the anaerobic chamber for 18 h. The conditions tested were BHI agar, Todd-Hewitt broth, and THBYE without any supplement; THBYE without any supplement grown under aerobic conditions or at 30 or 42°C; THBYE without any supplement adjusted to various pHs (pH 5, 6, 7 8, or 9); and THBYE supplemented with zinc (1 mM), manganese (1 mM), 10% clarified whole human saliva, 10% human serum, NaCl (0.1, 0.2, 0.3, 0.4, or 0.5 M), a sugar (fructose, galactose, glucose, lactose, maltose, mannose, sucrose, mannitol, sorbitol, or xylitol) at a concentration of 0.8% (wt/vol), or an amino acid (alanine, arginine, aspartate, cysteine, glutamine, glutamate, glycine, histidine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine, or valine) at a concentration of 100 mM. In subsequent assays, the growth conditions tested were BM containing 0.8% glucose supplemented with various concentrations of fructose (0.1, 0.5, 0.8, 2, 5, or 10% [wt/vol]), xylitol (0.1, 0.5, 0.8, 2, 5, or 10% [wt/vol]), fructose 1-phosphate (50 μM), fructose 6-phosphate (50 μM), fructose 1,6-biphosphate (1 mM), 10% human serum, and 10% whole saliva. Growth in BM without glucose supplemented with various concentrations of glucose (0.1, 0.5, 0.8, 2, 5, or 10% [wt/vol]), fructose (0.1, 0.5, 0.8, 2, 5, or 10% [wt/vol]), lactose (0.1, 0.5, 0.8, 2, 5, or 10% [wt/vol]), or sucrose (0.1, 0.5, 0.8, 2, 5, or 10% [wt/vol]) or with 0.8% (wt/vol) galactose, 0.8% (wt/vol) maltose, or 0.8% (wt/vol) mannose was also tested. Cells were also grown in an alternative base medium, 3.5% (wt/vol) tryptone with vitamins (TV medium) (3) supplemented with 0.5% (wt/vol) glucose, 0.5% (wt/vol) fructose, or 0.5% (wt/vol) sucrose.
Cells were centrifuged, resuspended in 10 ml of fresh, prewarmed liquid medium, and grown to the log phase (A600, 0.15 to 0.4) (∼3 h). The bacterial growth (A600) was recorded, and the cells were washed twice with 5 ml of TESC (10 mM Tris, 1 mM EDTA, 150 mM NaCl; pH 8.0), resuspended in 5 ml of TESC, and sonicated for 2 min. The cells were placed on ice for 5 min and centrifuged, and each clear sonicate was transferred to a new tube. For each sample, 100 μl of the sonicate was transferred to an opaque microtiter plate, and this was followed by addition of 100 μl of a solution containing 0.4 mg of MUG per ml in dimethyl sulfoxide. This preparation was mixed well and incubated for 30 min at room temperature. After addition of 200 μl of 4-methyl-umbelliferone standards (at concentrations ranging from 0.1 to 3.2 μM in dimethyl sulfoxide), the microtiter plate was read with a fluorescent plate reader (Spectrafluor plus; TECAN GmbH, Salzburg, Austria) at an excitation wavelength of 355 nm and an emission wavelength of 460 nm. A Bradford protein assay (Bio-Rad Laboratories, Richmond, Calif.) was performed according to the manufacturer's instructions to determine the protein concentration in each sonicate preparation. The activity was expressed in micromoles of 4-methyl-umbelliferone per milligram of protein per minute.
PCR ligation mutagenesis.
PCR ligation mutagenesis with vectorless intermediates was used to construct fruR, fruK, and fruI deletion mutants (15). A spectinomycin resistance gene, spec, amplified from plasmid pSF152, was used as the antibiotic marker insert. PCR amplification of the two flanking regions and the antibiotic marker insert was performed with the appropriate primers (Table 1). PCR products of the 5′ and 3′ flanking region and the spec cassette were ligated and used for transformation of S. gordonii Challis 2 as described previously (19).
Primers were designed for insertional inactivation so that allelic replacement resulted in a nonpolar mutation. PCR ligation mutagenesis (15) was used to replace most of the fruK gene (from position 9 to position 290 of the predicted 303-amino-acid sequence) on the chromosome of S. gordonii with spec. This method generated mutants with a fruK::spec allele. PCR ligation mutagenesis was also used to replace most of the fruR gene (encoding positions 28 to 214 of the predicted 247-amino-acid sequence) and the fruI gene (encoding positions 15 to 633 of the 653-amino-acid sequence) with spec in order to generate mutants with fruR::spec and fruI::spec alleles, respectively. The fruK::Tn917-lac biofilm-defective mutant was used for transformation instead of the Challis 2 strain, and this resulted in the double mutants fruK::Tn917-lac fruR::spec (fruK/fruR) and fruK::Tn917-lac fruI::spec (fruK/fruI). The transformants were then plated on BHI agar containing 1 mg of spectinomycin per ml and incubated at 37°C anaerobically for 3 to 5 days. RT-PCR was performed (19) by using RNA isolated from the fruK::Tn917-lac, fruR::spec, fruK::spec, fruI::spec, fruK/fruR, and fruK/fruI mutants to determine whether these strains had a polar or nonpolar mutation. S. gordonii Challis 2 RNA was use as a control. The primers used were FruK3 and FruK4, which are specific for an intergenic region extending from fruK to fruI.
Growth rate and xylitol toxicity assay.
The growth rates of S. gordonii strain Challis 2 and the fruK::Tn917-lac, fruK::spec, fruR::spec, and fruI::spec mutants were assessed by inoculating the strains from overnight THBYE cultures into 10 ml of fresh THBYE and growing them at 37°C under anaerobic conditions. Growth was quantified by recording the A600 at regular intervals for 24 h. The growth rates of the double mutants, fruK/fruR and fruK/fruI, were also assessed by the same method.
The sensitivity of these strains to xylitol was assessed by using the method of Wen et al. (47). Overnight cultures were grown in TV medium containing 0.5% (wt/vol) glucose and then diluted 1:10 in prewarmed TV medium containing 0.2% (wt/vol) glucose and incubated at 37°C anaerobically. TV medium was used because S. gordonii was not able to grow in TV medium without a carbohydrate supplement, so the experiment would not be confounded by the presence of significant quantities of uncharacterized sugars (3). Growth was monitored by determining the A575 of the cultures. At the early exponential phase (A575, 0.2 to 0.3), xylitol was added to a final concentration of 1% (wt/vol), and growth was monitored for an additional 22 h. Controls received an equivalent amount of sterile distilled water or 1% fructose instead of xylitol.
RESULTS
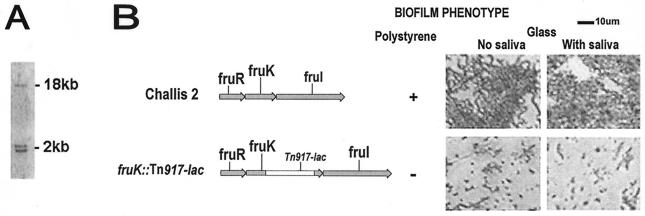
A biofilm-defective mutant was identified by screening an in-frame lacZ fusion library generated following Tn917-lac mutagenesis of S. gordonii Challis and was characterized further in subsequent experiments. The transposition was confirmed by Southern hybridization with Tn917-lac, which has two HindIII sites. Three hybridizing bands predicted to be present in HindIII-digested DNA from the biofilm-defective mutant were observed (Fig. 1A). This confirmed that a single transposon insertion had occurred in the biofilm-defective Tn917-lac mutant.
FIG. 1.
(A) Southern hybridization of HindIII-digested DNA from biofilm-defective S. gordonii fruK::Tn917-lac mutant. Digoxigenin-labeled pTV32-OK containing Tn917-lac (which has two HindIII sites) was used as the probe. Three hybridizing bands were produced with HindIII-digested DNA, demonstrating the presence of a single transposon insertion. (B) Gene organization of the fructose PTS operon. Genes of the fructose PTS operon are shown in grey. fruR encodes a putative transcriptional repressor, fruK encodes a fructose-1-phosphate kinase, and fruI encodes a fructose-specific enzyme II (EIIABC components). Maps of S. gordonii Challis 2 and fruK::Tn917-lac mutant are shown along with the corresponding biofilm phenotypes on polystyrene, uncoated glass, and saliva-coated glass surfaces as determined directly by phase-contrast microscopy. Cells were grown in BM for 24 h at 37°C under anaerobic conditions.
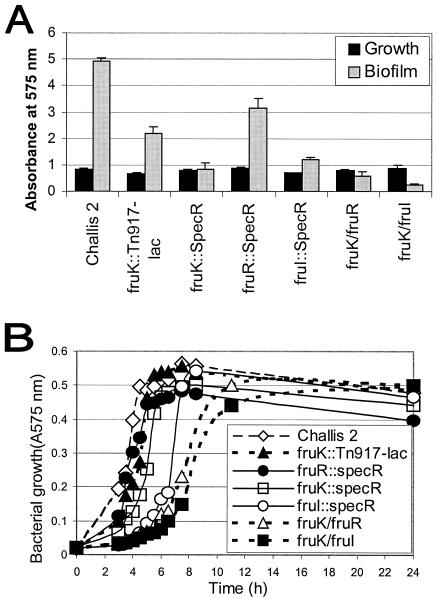
The ability of the biofilm-defective mutant to form biofilms on microtiter plates was measured. The biofilm assay showed that biofilm formation by this S. gordonii Tn917-lac mutant (A575, 2.19 ± 0.25 [mean ± standard deviation]) was reduced by 55.77% compared with the biofilm formation by the S. gordonii Challis 2 strain (A575, 4.94 ± 0.10).
In addition, biofilm formation on borosilicate glass coverslips was examined 24 h after inoculation. The abilities of the strains to form biofilms were confirmed by direct visualization of the biofilms formed on the glass surfaces by phase-contrast microscopy (Fig. 1B). After incubation for 1 h, small numbers of cells of both strains were observed, which probably represented reversible attachment of cells to the surfaces, and there was no obvious difference between the Challis 2 and mutant strains. After incubation for 24 h, the Challis 2 strain had formed large clusters of cells that were interspersed with sparsely covered areas. A number of dense microcolonies were also observed. In contrast, significantly fewer cells of the mutant strain had attached, and they formed a scattered pattern that was markedly different from the Challis 2 biofilm; large areas were devoid of cells. Biofilm formation on coverslips precoated with whole saliva was also visualized in order to examine the role of saliva in biofilm formation in this in vitro biofilm assay to mimic in vivo conditions. With both the Challis 2 strain and the Tn917-lac mutant, no difference was observed between biofilm formation on saliva-coated glass surfaces and biofilm formation on uncoated surfaces.
PCR performed with genomic DNA that was digested with HindIII and ligated with pBluescript resulted in a PCR product which was 3 kb long and contained the region 5′ to the transposon insertion. Sequence analysis of this PCR product revealed that transposition had occurred within an ORF that encoded a protein with a predicted amino acid sequence homologous to the sequence of FruK (Fig. 1B), a fructose-1-phosphate kinase encoded by the fruRKI operon of S. mutans (2, 47). This gene was not identified when Tn916 mutagenesis was used in a previous screening of biofilm-defective mutants of S. gordonii Challis 2 (18).
Genetic organization of the S. gordonii fructose PTS operon.
The nucleotide sequence of the region 5′ of the transposon insertion was used in sequence similarity BLASTN searches of the S. gordonii unfinished genome database (http://www.tigr.org). The S. gordonii nucleotide sequence obtained revealed that the closest homologs of the deduced amino acid sequences were proteins encoded by genes of the S. mutans fructose operon, which consists of fruR, fruK, and fruI (Fig. 1B). This operon is involved in fructose transport in S. mutans (2, 47).
The three ORFs identified in S. gordonii were designated fruR, fruK, and fruI. The 741-bp fruR ORF encodes a 247-amino-acid sequence with a predicted molecular mass of 27.5 kDa. The deduced amino acid sequence showed a high degree of homology to the sequences of members of the deoxyribonucleoside repressor (DeoR) family of bacterial regulatory proteins (35).
Analysis of the region 3′ of fruR revealed the presence of two other ORFs, which were highly homologous to fruK and fruI of S. mutans. The fruK ORF was 909 bp long and started 1 bp upstream of the fruR stop codon. The fruK ORF was predicted to encode a 303-amino-acid protein with a predicted molecular mass of 32.8 kDa. The Tn917 insertion in S. gordonii fruK::Tn917-lac was at amino acid 210 of FruK.
The fruI ORF was 1,959 bp long and started 1 bp upstream of the fruK stop codon. This ORF encodes the fructose permease FruI, the EII enzyme of this PTS which allows uptake of extracellular fructose and concomitant phosphorylation of this fructose into fructose 1-phosphate (2, 47) and has a deduced 653-amino-acid sequence with a predicted molecular mass of 67.5 kDa. FruI consists of EII A, B, and C domains of phosphoenolpyruvate-dependent sugar PTSs. The deduced amino acid sequences of FruR, FruK, and FruI exhibited high levels of homology with the sequences of streptococcal and lactococcal homologs (Table 2).
TABLE 2.
Homology of the proteins encoded by the S. gordonii fructose PTS operon (FruR, FruK, and FruI), the proteins of other oral streptococci, and LacR, LacC, and FruA of Lactococcus lactis subsp. lactis
| Organism | % Identity (% similarity) with the following S. gordonii proteins:
|
||
|---|---|---|---|
| FruR | FruK | FruI | |
| Streptococcus pneumoniae | 69.6 (81.4) | 86.1 (90.4) | 81.6 (90.2) |
| Streptococcus mitis | 69.0 (81.0) | 87.2 (91.1) | 81.6 (90.2) |
| Streptococcus agalactiae | 53.2 (66.9) | 74.6 (82.5) | 77.7 (85.7) |
| Streptococcus mutans | 58.4 (72.4) | 74.3 (82.2) | 75.8 (83.7) |
| Streptococcus pyogenes | 54.3 (70.4) | 72.3 (82.8) | 77.2 (83.9) |
| Lactococcus lactis subsp. lactis | 42.0 (57.2) | 53.7 (66.8) | 67.0 (79.8) |
Located upstream of fruR is an ORF with a stop codon that is 139 bp upstream of the start codon of fruR, which encodes a putative 751-amino-acid protein designated hypothetical protein 1. The last 133 amino acids in the C-terminal region of hypothetical protein 1 exhibit homology to a chromosome segregation protein of Xanthomonas campestris pv. campestris, which is involved in cell division and chromosome partitioning. An ORF identified downstream of fruI and starting 48 bp from the stop codon of fruI encodes a 143-amino-acid protein designated hypothetical protein 2, which is homologous to a conserved hypothetical protein of unknown function in S. mutans. A second fructose PTS operon has been described in S. mutans (47). Likewise, a TBLASTN search of the S. gordonii unfinished genome database revealed a second homolog of FruK (data not shown).
Sequence and phylogenetic analyses of the fructose PTS operon.
The genetic organization, the phylogenetic relationship, and an amino acid alignment of the putative FruR, FruK, and FruI proteins were examined. Multiple alignments of all three proteins from S. gordonii, S. pneumoniae, S. mitis, S. mutans, S. equi, S. agalactaciae, and S. pyogenes suggested that they have extensive homologies.
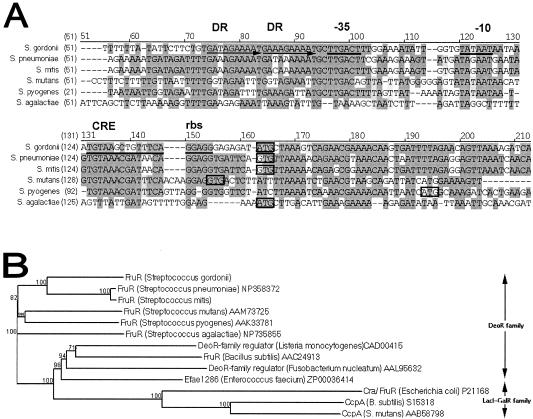
Catabolite repression occurs when enzymes involved in the metabolism of complex carbon and energy sources are repressed by glucose, as their functions are unnecessary when there are abundant, readily metabolized alternatives, such as glucose. Analysis of the nucleotide sequence 5′ of the fruR ORF resulted in identification of a catabolite responsive element (CRE) 7 bp downstream from the putative −10 promoter sequence (Fig. 2A). The CRE has a 14-bp palindromic sequence in concordance with the consensus sequence TGWAANCGNTNWCA (46), with one nucleotide mismatch. A 10-bp direct repeat with one mismatch was present 4 bp upstream from the putative −35 box of the promoter (Fig. 2A), and this repeat is probably also involved in regulation of the operon. A homologous direct repeat in the promoter region of the fruR homolog in S. mutans, fxpA, which is part of the fxpABC fructose and xylitol transport operon in S. mutans, has been proposed to be involved in binding of a trans-acting factor (2).
FIG. 2.
(A) Alignment of the promoter region of S. gordonii fruR with the fruR homologs in S. pneumoniae (accession no. NP345362), S. mitis, S. mutans (accession no. NC004350), S. pyogenes (accession no. 002737), and S. agalactiae (accession no. NC004116) determined by using the AlignX program in the Vector NTI software (Informax). Streptococcal sequences without accession numbers were obtained from the unfinished microbial genome database (www.ncbi.nlm.nih.gov). Nucleotides that are identical and conserved are indicated by dark grey and light grey backgrounds, respectively, and start codons are enclosed in boxes. In S. gordonii, direct repeat regions (DR) are indicated by horizontal arrows, while the CRE, putative ribosome binding site (rbs), putative −35, and putative −10 regions are underlined. (B) Phylogenetic tree of S. gordonii FruR and other bacterial sugar PTS regulatory proteins. The phylogenetic relatedness dendrogram was constructed on the basis of amino acid sequence similarities by using the AlignX program in the Vector NTI software (Informax Inc.), which utilizes the neighbor-joining algorithm (32). The reliability of the topology was estimated by performing 100 bootstrap trials, and the bootstrap values are expressed in percentages at branch points (www.genebee.msu.su). Accession numbers follow the species names, and sequences without accession numbers were obtained from the unfinished microbial genome sequence database (www.ncbi.nlm.nih.gov).
The first gene in the S. gordonii fructose PTS operon, fruR, encodes FruR, the putative regulator protein of the operon. FruR has significant similarity (Fig. 2B) with transcriptional regulator proteins of bacterial carbohydrate catabolic operons belonging to the DeoR family (45) and the same structural characteristics as other DeoR proteins, with a highly conserved N-terminal region that contains a helix-turn-helix motif at amino acid residues 38 to 56 which is probably involved in DNA binding.
The second gene, fruK, codes for a fructose-1-phosphate kinase (FruK), which belongs to the phosphofructokinase PfkB family of carbohydrate kinases (50). FruK uses ATP to phosphorylate fructose 1-phosphate in the cytoplasm to fructose 1,6-biphosphate. The third gene, fruI, encodes the fructose permease FruI, which allows uptake of extracellular fructose and concomitant phosphorylation of this fructose into fructose 1-phosphate. A palindromic region was observed upstream of fruK (TGATTATTCTTGG GCAGGTAAAACA; 34 bases upstream of the start codon) and also upstream of fruI (GGGAGTAGCTTGCG GAACAGCAACTACC; 68 bases upstream of the start codon). These sequences may be fru operator sequences recognized by the helix-turn-helix motif of FruR.
The third gene, fruI, codes for the fructose permease of the PTS. PTS sugar-specific permeases consist of three domains; two hydrophilic domains, IIA (formerly enzyme III) and IIB, possess the first and second phosphorylation sites, respectively, and a hydrophobic IIC domain is not phosphorylated but contains several membrane-spanning α-helices, forms the translocating transmembrane channel, and provides the sugar-binding site. IIB is phosphorylated by phospho-IIA before the phosphoryl group is transferred to the sugar substrate. The IIC domain catalyzes the transfer of a phosphoryl group from IIB to the sugar substrate (2, 16, 44).
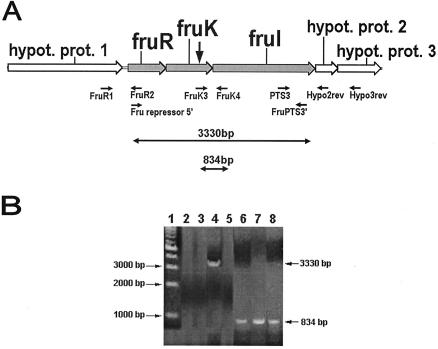
The sequence upstream of fruR was identified as ORF1, which codes for a hypothetical protein. The sequence downstream of fruI was identified as ORF2, which codes for another hypothetical protein. RT-PCR performed with RNA from the Challis 2 strain and primers Fru repressor5′ and FruPTS3′ (Table 1), which are specific for a 3.33-kb region that spans the fruR and fruI intergenic region, produced a product that was approximately 3,330 bp long (Fig. 3). This product spans the fruR and fruI ORFs, and its size coincides with the predicted amplicon size. RT-PCR performed with primers FruR1 and FruR2 (which are specific for a region extending from the ORF encoding hypothetical protein 1 to fruR), primers PTS3 and Hypo2rev (which are specific for a region extending from fruI to the ORF encoding hypothetical protein 2), and primers PTS3 and Hypo3rev (which are specific for a region extending from fruI to the ORF encoding hypothetical protein 3) produced no PCR products (Fig. 3). All the primer pairs (Table 1) used in the RT-PCR were also used in standard PCR amplifications with the S. gordonii Challis 2 DNA, which produced PCR products of the predicted sizes (data not shown). These results demonstrate that fruR, fruK, and fruI are cotranscribed as a single operon, while the genes encoding hypothetical proteins 1, 2, and 3 are probably not cotranscribed. Therefore, the fructose PTS operon in S. gordonii consists of only three ORFs, fruR, fruK, and fruI.
FIG. 3.
RT-PCR analysis of RNA extracted from the S. gordonii Challis 2, fruK::Tn917-lac, and fruK/fruR strains. (A) Organization of the fructose PTS operon and adjacent genes, locations of primers, and predicted size of the successfully amplified RT-PCR product. See Table 1 for the primers used. The vertical arrow indicates the position of the Tn917-lac insertion in the biofilm-defective mutant. hypot. prot., hypothetical protein. (B) RT-PCR products obtained by using total RNA extracted from the Challis 2 strain, the fruK::Tn917-lac mutant, or the fruK/fruR double mutant as the template. Lane 1, 1-kb DNA marker; lane 2, Challis 2 RNA with primers FruR1 and FruR2; lane 3, Challis 2 RNA with primers PTS3 and Hypo2rev; lane 4, Challis 2 RNA with primers Fru repressor5′ and FruPTS3′; lane 5, Challis 2 RNA with primers PTS3 and Hypo3rev; lane 6, Challis 2 RNA with primers FruK3 and FruK4; lane 7, fruK::Tn917-lac RNA with primers FruK3 and FruK4; lane 8, fruK/fruR RNA with primers FruK3 and FruK4.
Regulation of fruK expression in response to changes in environmental conditions.
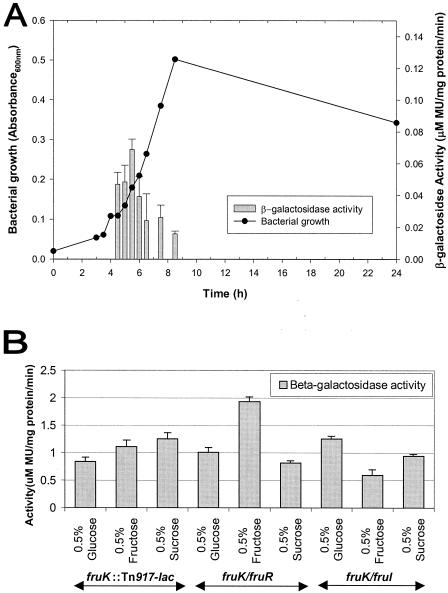
The effects of growth phase and various environmental conditions on the expression of fruK were examined by measuring the β-galactosidase activity of S. gordonii fruK::Tn917-lac grown in medium supplemented with a variety of nutrients and under various conditions. The initial β-galactosidase assays performed with cells grown in THBYE with various supplements and under various conditions did not reveal any significant differences in expression except for the differences observed after addition of 10% human serum or 10% whole saliva, which resulted in upregulation of fruK expression (data not shown). As THBYE contains various sugars, in all subsequent assays we used a base medium without any sugar so that the effects of various sugars on fruK expression could be evaluated. Two different base media, BM and TV medium, were used for comparison. The fruK::Tn917-lac mutant was not able to grow in BM without glucose supplemented with 0.1% sucrose, 0.1% glucose, or 0.1% lactose. Growth of this mutant was inhibited in BM containing 0.8% glucose supplemented with 5 or 10% xylitol.
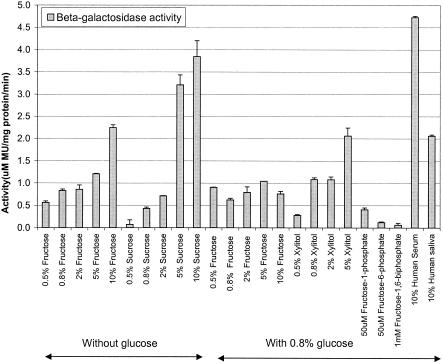
Assays in which cells grown in BM were used indicated that the expression of fruK was significantly increased in the presence of fructose, sucrose, and xylitol (Fig. 4). Expression of fruK was also upregulated in the presence of 10% human serum and 10% whole saliva, while none of the other conditions tested had a significant effect on expression (data not shown). The level of fruK induction by fructose increased with an increase in the concentration of fructose in the medium, which suggests that there is a fructose-titratable promoter system that results in different levels of gene expression. None of the other sugars tested (galactose, glucose, lactose, maltose, mannose) induced fruK activity, suggesting that fructose, sucrose, and xylitol are the only sugars capable of inducing fruK expression in S. gordonii. To investigate whether fruK is regulated by other mechanisms, such as catabolite repression by another sugar, the bacterium was grown in BM containing fructose supplemented with glucose at a final concentration of 0.8%. The resulting activities of the cells were lower the activities after induction with fructose in the absence of glucose, indicating that glucose is able to catabolite repress fruK expression (Fig. 4).
FIG. 4.
Effects of different concentrations of various sugars, human serum, and saliva on fruK expression. The biofilm-defective S. gordonii fruK::Tn917-lac mutant was grown in BM containing different concentrations of various sugars, human serum, and saliva at 37°C under anaerobic conditions as planktonic cells. The β-galactosidase activities of the fruK-lacZ transcriptional fusion were quantified by using MUG (12). All assays were performed in triplicate, and means and standard deviations are shown. MU, 4-methyl-umbelliferone.
DNA binding of Cra, the fructose repressor in E. coli, has been shown to be disrupted by a low concentration (50 μM) of fructose 1-phosphate and a high concentration (5 mM) of fructose 1,6-biphosphate (25). As growth was inhibited in the presence of 5 mM fructose 1,6-biphosphate, cells were grown in fructose 1,6-biphosphate (1 mM), fructose 1-phosphate (50 μM), or fructose 6-phosphate (50 μM). Expression assays indicated that only fructose 1-phosphate upregulated fruK expression (Fig. 4), suggesting that fructose 1-phosphate is also an inducer of the fructose operon in S. gordonii.
Expression of fruK was observed only between 4 and 8.5 h of growth in the assay, which corresponded to the early to mid-exponential growth phase of S. gordonii (Fig. 5A). The cells reached maximum growth by this time. When cells were grown in an alternative base medium (TV medium) supplemented with glucose, fructose, or sucrose, the expression of fruK was also observed to be higher in the presence of fructose and sucrose (Fig. 5B). These results were similar to those obtained when BM was used.
FIG. 5.
Effects of various growth conditions on fruK expression. Cells were grown at 37°C under anaerobic conditions in various liquid media. The β-galactosidase activities of the fruK-lacZ transcriptional fusion were quantified by using MUG (12). All assays were performed in triplicate, and means and standard deviations are shown. (A) Relationship between growth phase and fruK expression. The biofilm-defective fruK::Tn917-lac mutant was grown in THBYE. Growth and β-galactosidase activity were determined at 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8.5 and 24 h. (B) Effect of growth in TV medium (3) containing various sugars on fruK expression. The fruK::Tn917-lac, fruK/fruR, and fruK/fruI mutant strains were grown at 37°C under anaerobic conditions. MU, 4-methyl-umbelliferone.
Construction and characterization of fruR, fruK, fruI, fruK/fruR, and fruK/fruI mutants.
In order to confirm the biofilm-defective phenotype identified in S. gordonii fruK::Tn917-lac, an insertionally inactivated fruK gene was constructed by allelic exchange. The fruR gene was inactivated in the S. gordonii Challis 2 and fruK::Tn917-lac strains to confirm that fruR encodes a negative regulatory protein. The fruI gene was also inactivated in both the Challis 2 and fruK::Tn917-lac strains to investigate the role of FruI in biofilm formation and fructose transport. Mutants with either a fruR::spec or fruI::spec allele and two double mutants, fruK/fruR and fruK/fruI, were also generated by the same method. PCR confirmed that integration of the spec gene in fruR, fruK, or fruI in the chromosome had occurred as predicted. PCR was also used to confirm the integration of spec in fruR or fruI in the fruK::Tn917-lac mutant (data not shown).
RT-PCR with primers spanning the fruK and fruI intergenic region resulted in a PCR product when RNA isolated from either S. gordonii Challis 2 or fruK::Tn917-lac was used as the template (Fig. 3), indicating that the transposition resulted in a nonpolar fruK::Tn917-lac mutation. RT-PCR was also used to confirm that the fruK/fruR mutation constructed was nonpolar (Fig. 3). Similarly, RT-PCR was used to confirm that the fruR::spec and fruK::spec mutants were nonpolar (data not shown).
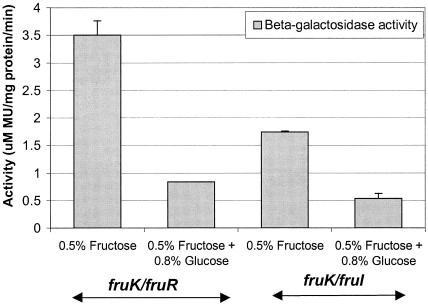
Expression of fruK in the two double deletion mutant strains, the fruK/fruR and fruK/fruI strains, was assessed by using cells grown in TV medium or BM. With the fruK/fruR mutant, expression of fruK was enhanced to a much larger degree when this organism was grown in TV medium in the presence of fructose (Fig. 5B) than when the fruK::Tn917-lac mutant was used. Similarly, growth of the fruK/fruR mutant in BM supplemented with fructose in the absence of glucose (Fig. 6) resulted in a greater response to fructose induction, probably due to the relief of repression by fruR. On the other hand, the fruK/fruI mutant grown in TV medium (Fig. 5B) or BM (Fig. 6) did not exhibit elevated fruK expression in the presence of fructose. This is probably because inactivation of fruI affected the transport of fructose into the cytoplasm, resulting in no induction of fruK.
FIG. 6.
fruK expression of the two biofilm-defective double mutants, fruK/fruR and fruK/fruI. Cultures were grown at 37°C under anaerobic conditions in BM containing different levels of fructose, with and without glucose. The β-galactosidase activities of the fruK-lacZ transcriptional fusion in the two double mutants were quantified by using MUG (12). All assays were performed in triplicate, and means and standard deviations are shown. MU, 4-methyl-umbelliferone.
To investigate whether glucose is a catabolite repressor of fruK in the fruK/fruR mutant, this strain was grown in BM containing fructose supplemented with glucose. The resulting activities of the cells were lower than the activities when the cells were grown in fructose in the absence of glucose (Fig. 6), indicating that fruK expression was affected by catabolite repression of glucose in the fruK/fruR mutant. None of the biofilm-defective mutant phenotypes could be rescued by addition of fructose 1-phosphate, fructose 6-phosphate, or fructose 1,6-biphosphate at various concentrations, indicating that alternative pathways are involved in biofilm formation.
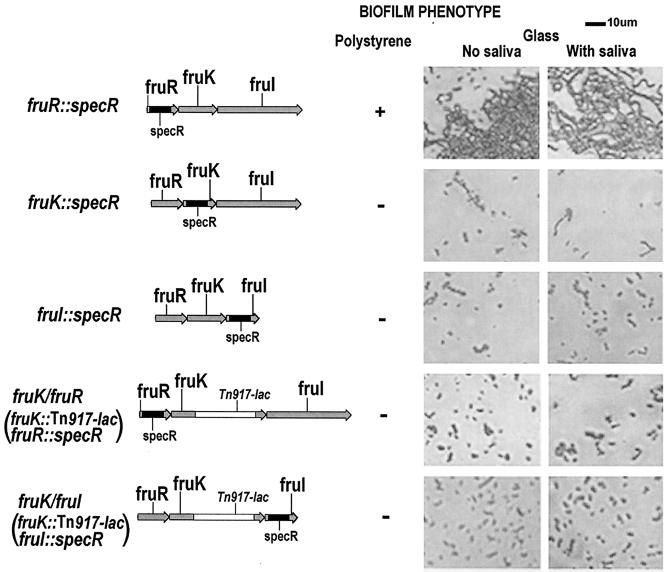
The effects of insertional inactivation on biofilm formation were assessed. Biofilm formation by the fruK::spec mutant (A575, 0.847 ± 0.211[mean ± standard deviation]) and biofilm formation by the fruI::spec mutant (A575, 1.196 ± 0.083) were similar to biofilm formation by the S. gordonii fruK::Tn917-lac mutant (A575, 2.185 ± 0.250), indicating that both fruK and fruI are involved in biofilm formation. The fruI mutant and both fruK mutants were defective in biofilm formation compared to the Challis 2 strain (A575, 4.94 ± 0.10) (Fig. 7A). On the other hand, the fruR::spec mutant was still able to form a biofilm (A575, 3.16 ± 0.36). The second biofilm formation assay performed on glass coverslips by using phase-contrast microscopy produced similar results (Fig. 8), with the Challis 2 and fruR::spec mutant strains able to form biofilms. The two double deletion mutant strains, the fruK/fruR and fruK/fruI strains, were also defective in biofilm formation on both polystyrene surfaces (Fig. 7A and 8) and uncoated and saliva-coated glass surfaces (Fig. 8). The biofilm phenotypes of the fruK and fruI mutants suggest that these two genes are important for biofilm formation.
FIG. 7.
(A) Bacterial growth and biofilm assay of the S. gordonii wild type and the fruK::Tn917-lac, fruK::spec, fruR::spec, fruI::spec, fruK/fruR, and fruK/fruI mutant strains in BM. Assays were performed by using BM and polystyrene plates under anaerobic conditions. All assays were performed in triplicate, and means and standard deviations are shown. (B) Growth curves for the S. gordonii wild type and the fruK::Tn917-lac, fruK::spec, fruR::spec, fruI::spec, fruK/fruR, and fruK/fruI mutant strains in 10 ml of THBYE over 24 h at 37°C under anaerobic conditions. Growth was measured by determining the A575.
FIG. 8.
Maps of the S. gordonii fruK::spec, fruR::spec, and fruI::spec mutants and fruK/fruR and fruK/fruI double mutants. The biofilm phenotypes of the strains on polystyrene, uncoated glass, and saliva-coated glass surfaces were observed directly by phase-contrast microscopy and are shown on the right. Cells were grown in BM for 24 h at 37°C under anaerobic conditions.
Growth rate and sensitivity to xylitol.
The growth rates of the S. gordonii Challis 2 strain and the fruK::Tn917-lac, fruR::spec, and fruK::spec mutants in THBYE were found to be similar, indicating that the growth rate was not affected by the mutations. On the other hand, S. gordonii fruI::spec and the double mutants fruK/fruR and fruK/fruI all grew at lower rates than the Challis 2 strain and the three other nonpolar mutants (Fig. 7B), indicating that fruI probably plays a role in the growth of S. gordonii, and the double mutations affected bacterial growth. However, the final growth yields of the fruI::spec, fruK/fruR, and fruK/fruI mutants after 24 h of growth appeared to be the same as those of Challis 2 and the fruK::Tn917-lac, fruK::spec, and fruI::spec mutants.
The fructose PTS of oral streptococci is thought to be responsible for the phosphorylation and uptake of xylitol (40). Xylitol is a naturally occurring five-carbon sugar alcohol and enters the bacterial cell as the nonmetabolizable xylitol 5-phosphate via the same PTS as fructose. As xylitol 5-phosphate is not catabolized, it accumulates and/or undergoes a futile phosphorylation-dephosphorylation cycle and becomes toxic to the cell (40, 47). The xylitol sensitivities of the Challis 2 strain and the fruK::Tn917-lac, fruR::spec, fruK::spec, fruI::spec, fruK/fruR, and fruK/fruI mutant strains were compared. After 4 h, the increases in the A575of the Challis 2 (0%) and fruR::spec (0.9%) cultures were minimal in the presence of 1% xylitol, whereas the increases in the A575 of the fruK::Tn917-lac (7.5%), fruK::spec (5.9%), fruI::spec (3.4%), fruK/fruR (30.9%), and fruK/fruI (31.5%) cultures were greater. After 24 h, the growth differences among the strains were minimal. Growth of the S. gordonii Challis 2, fruK::Tn917-lac, and fruR::spec strains was more sensitive to inhibition by 1% xylitol, whereas growth of the fruK::spec, fruI::spec, fruK/fruR, and fruK/fruI strains was less sensitive to xylitol inhibition, suggesting that a nonfunctional fruK or fruI gene increased S. gordonii resistance to xylitol.
DISCUSSION
Bacteria possess complex regulatory networks that are able to detect the frequent and abrupt changes that occur in the environment and to adapt their metabolism rapidly to such fluctuations; these networks include transport systems that transmit information about the nature, concentration, and diversity of the external energy sources (16, 24, 31). Fructose is a major constituent of the human diet and can be liberated from sucrose by glucosyltransferases and from fructans by fructanases of oral streptococci. A biofilm-defective mutant of S. gordonii::Tn917-lac revealed that a gene with a high degree of homology to fruK, which is part of the fructose PTS operon of S. mutans (2, 47), is required for biofilm formation. Although the S. gordonii fruK::Tn917-lac isolate exhibited a biofilm-defective phenotype on polystyrene, glass, and saliva-coated glass surfaces, these in vitro assay environments may not be representative of the in vivo environment.
The S. gordonii fructose PTS operon, consisting of the fruR, fruK, and fruI genes, was subsequently identified and was found to be homologous to the S. mutans fructose PTS operon, a member of the carbohydrate PTS family involved in fructose and xylitol transport in S. mutans (47). RT-PCR confirmed that fruR, fruK, and fruI are cotranscribed in S. gordonii.
In the biofilm-defective S. gordonii::Tn917-lac mutant, the transposon interrupted fruK, the second gene in the fructose PTS operon. fruK encodes a fructose-1-phosphate kinase, which phosphorylates fructose 1-phosphate to fructose 1,6-biphosphate. The first gene in the S. gordonii fructose PTS operon, fruR, encodes FruR, the putative regulator protein of the operon, which belongs to the DeoR family of regulators, which include transcriptional regulators of several carbohydrate catabolic operons. The third gene, fruI, encodes the fructose permease FruI. The EII enzymes encoded by fruI of S. mutans (47) and fruA of E. coli (26) appear to function as fructose and xylitol transporters as inactivation of these genes conferred resistance to growth inhibition by xylitol. Similarly, S. gordonii fruK::spec, fruI::spec, fruK/fruR, and fruK/fruI mutants were more resistant to growth in xylitol.
Expression studies showed that fructose, sucrose, and xylitol were the only sugars tested that were able to induce fruK expression. Expression of fruK was observed only during the exponential growth phase. The results also indicated that there was a fructose-titratable promoter system with different levels of gene expression. This is consistent with previous findings obtained with S. mutans, which showed that expression of fruI from the fructose operon was upregulated in response to growth on fructose or sucrose (47). In this study, the induction of fruK expression by fructose was inhibited by the presence of glucose, indicating that glucose is able to catabolite repress fruK expression in S. gordonii.
RT-PCR results showed that the Tn917-lac insertion into fruK was nonpolar. A previous study showed that Tn917 insertion can disrupt the expression of a particular gene and at the same time allow outwardly directed transcription initiating from within the transposon, resulting in complete expression of other individual genes of the Staphylococcus epidermidis ica operon (8). In the nonpolar fruK::Tn917-lac mutant, a second mutation created in fruR relieved the repression of the fructose PTS operon, as demonstrated by β-galactosidase assays of the fruK/fruR double mutant grown in the presence of fructose, confirming that FruR is indeed a transcriptional repressor of the fructose PTS operon in S. gordonii. In contrast, a second mutation in fruI in the nonpolar fruK::Tn917-lac mutant not only failed to relieve the repression of the operon but also resulted in a reduced response to induction by fructose, as inactivation of FruI probably affected fructose transport into the cell.
In the oral cavity, oral streptococci can use various carbohydrates as single sources of carbon and energy and must select the most appropriate energy source(s) from a constantly changing environment (43). The presence of a CRE in the promoter region of fruR suggests that catabolite repression is one of the regulatory mechanisms of the fructose PTS operon. The operator consensus sequence of the CREs, which govern carbon catabolite repression, was first identified in the B. subtilis α-amylase gene amyE (46). The S. gordonii fructose PTS operon may have regulatory mechanisms that are similar to those of the fructose PTS operon in S. mutans (2) and other oral streptococci that have homologous CRE sequences. This CRE sequence is homologous to an amyO palindromic sequence, the CcpA binding site of B. subtilis (11). CcpA is involved in catabolite repression in gram-positive bacteria (38) and in biofilm formation in S. mutans (48).
In addition to sugar transport and phosphorylation, PTSs are also involved in transcriptional regulation, catabolite repression, inducer exclusion, enzyme activity, and chemotaxis (43). Three mechanisms of bacterial catabolite repression have been described, the CcpA mechanism in gram-positive bacteria, a cAMP-independent mechanism, and a mechanism dependent on the cAMP receptor protein in E. coli (29).
Fructose is the only sugar found in nature that feeds directly into glycolysis, the central pathway of carbohydrate metabolism. Cra, the catabolite repressor activator formerly known as FruR in E. coli, is a member of the LacI-GalR family of transcriptional regulatory proteins and is the catabolite repressor-activator in the cAMP-independent system (30). Cra modulates the direction of carbon flow by transcriptional activation of the genes encoding enzymes concerned with oxidative and glyconeogenic carbon flow and by repression of the genes concerned with fermentative carbon flow (25). The cytoplasmic glycolytic intermediates fructose 1-phosphate and, to a lesser extent, fructose 1,6-biphosphate are inducers of the fructose operon and counteract both the repressive and the activating effects of Cra by displacing Cra from the DNA (25, 30).
Results of our expression assays showed that fructose 1-phosphate is an inducer of the S. gordonii fructose operon. Another DeoR family regulator, LacR from the lac operon in Lactococcus lactis, is induced by tagatose 6-phosphate (44). Fructose 1-phosphate has also been proposed to be a regulator of the DNA binding of FruR in Spiroplasma citri to a direct repeat in the promoter region of fruR (10).
Why would the fruK mutation affect biofilm formation in S. gordonii? Phosphorylation of the internalized fructose is a prerequisite for catabolism by glycolysis, and the fructose needs to be phosphorylated by a cytoplasmic sugar kinase. Since both fruK and fruI mutations in S. gordonii were biofilm defective, we speculate that the substrates of both fruK and fruI play a role in biofilm formation. The Cra protein in enteric bacteria is a global regulator of carbon and energy metabolism (25). Similar regulation in S. gordonii would require that a fruK mutation perturbs the cellular balance of fructose 1-phosphate and fructose 1-6 biphosphate so that the signal involved in the biofilm phenotype is altered. Inactivation of fruR, which relieved repression of the fructose PTS operon, did not affect the biofilm phenotype, suggesting that the fructose 1-phosphate and fructose1 6-biphosphate balance was not affected and hence the biofilm phenotype was retained. On the other hand, fructose 1-phosphate and fructose 1-6 biphosphate did not have significant effects on biofilm formation by the Challis 2 or mutant strains. The substrates may have been ineffective because they were not transported into the cell or because the concentrations examined were too low.
On the other hand, the disruption of biofilm formation by the fruK::Tn917-lac mutant may indicate that FruK has an additional function which is important in biofilm formation. Therefore, it is more likely that FruK could play a role in two-component signal transduction by acting as a kinase. This may occur via cross-regulation, which involves the phosphorylation of a response regulator of a two-component regulatory system by a different regulatory system (for example, the phosphorylation of a response regulator by a kinase other than a histidine protein kinase). In E. coli ANCC22, two histidine protein kinase-encoding genes, phoR and creC, are required for activation of their cognate response regulator, PhoB. In a recent study the workers found that a phosphofructokinase from Streptococcus thermophilus suppressed the phoR creC mutation in E. coli ANCC22, probably through a mechanism involving the production of acetyl phosphate (5), which may provide a regulatory link between glycolytic activity and two-component signal transduction regulation.
It has been demonstrated previously that cell-to-cell signaling is involved in biofilm formation by S. gordonii (18). Insertional inactivation of luxS, which is responsible for AI-2 synthesis, disrupted the ability of S. gordonii to form a mixed-species biofilm with a LuxS-null strain of Porphyromonas gingivalis, suggesting that LuxS-dependent intercellular communication is essential for biofilm formation by nongrowing cells of P. gingivalis and S. gordonii (20). S. gordonii AI-2 was also found to regulate aspects of carbohydrate metabolism, as fruA (which codes for the fructan β-frutosidase precursor), gtfG (the glucosyltransferase gene), and rgg (which positively regulates gtfG) were downregulated in the S. gordonii luxS mutant (20). The AI-2 signaling molecule of E. coli significantly upregulated frwC, which encodes a fructose-like EII component of a PTS in E. coli (7) with a high level of homology to the EIIC domain (32% identity, 50% similarity) of FruI of the fructose PTS in S. gordonii. FrwC is one of the three silent gene clusters that encode silent backup fructose PTSs with unknown physiological functions in E. coli (27). Alternatively, a mutation in fruK may have affected the expression of other surface proteins that could influence initial adhesion and biofilm formation.
A common theme in microbial development involves an input of environmental cues that results in an output of an altered physiological state or behavior (23). The transition from a planktonic existence to a sessile existence occurs in response to environmental factors, including the availability of nutrients. Regulatory proteins and signaling pathways play a role in the transduction of environmental signals and precipitation of developmental changes that allow a bacterium to adapt to its environment. In P. aeruginosa, nutritional cues are integrated by the catabolite repression control (Crc) protein as part of a signal transduction pathway, which regulates biofilm formation (23). The P. aeruginosa Crc represses the levels of enzymes involved in mannitol and glucose catabolism in the presence of succinate and is involved in the expression of pilA, the pilin structural gene. Crc may be part of a signal transduction pathway that can sense and respond to nutritional signals, such as carbon availability, and thereby may play a role in the bacterium's transition from planktonic growth to biofilm growth.
Transcriptome analysis of B. subtilis biofilms suggests that nonoptimal growth conditions, such as catabolite repression, starvation, and possibly high cell density, stimulate biofilm formation (36). In addition, biofilm formation by B. subtilis (36) and E. coli (13) is inhibited in the presence of a rapidly metabolized carbon source, such as a higher concentration of glucose or fructose. The correlation between the induction of glucose-repressed genes and biofilm formation also suggests that biofilm formation is subject to catabolite repression (36).
The fructose PTS of oral streptococci, as well as a number of other bacteria, is thought to be responsible for the phosphorylation and uptake of fructose (47). When S. gordonii encounters high levels of extracellular fructose, the fructose PTS operon could function as a sensory mechanism to enable the switch from a sessile phenotype to a planktonic phenotype, thus facilitating the dispersal and spread of the bacterium. Alternatively, FruK may play a role in cross-regulation of a two-component signal transduction system by phosphorylation of a response regulator.
The ability of the PTS proteins to modulate biofilm formation points to the possibility that specific applications of basic PTS research could be used in combating infections by pathogenic bacteria. Rational drug design in which PTS proteins are used may be possible as PTSs are present only in prokaryotes. Further characterization of the fructose PTS operon should provide insight into the molecular mechanisms of biofilm formation by oral streptococci and a possible role in the pathogenicity of these organisms. Interference with the function(s) of the fructose transport system described here is potentially a novel approach to develop methods for the prevention of streptococcal biofilm formation.
Acknowledgments
DNA and protein sequences used in this study were retrieved from the National Center for Biotechnology Information genomic BLAST pages that contain sequences of completed and unfinished microbial genomes (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi).
This work was supported by PHS grant RO1-DE13328 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Benchabane, H., L.-A. Lortie, N. D. Buckley, L. Trahan, and M. Frenette. 2002. Inactivation of the Streptococcus mutans fxpC gene confers resistance to xylitol, a caries-preventive natural carbohydrate sweetener. J. Dent. Res. 81:380-386. [DOI] [PubMed] [Google Scholar]
- 3.Burne, R. A., Z. T. Wen, Y.-Y. M. Chen, and J. E. C. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 181:2863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 5.Crispie, F., J. Anba, P. Renault, D. Ehrlich, G. Fitzgerald, and D. van Sinderen. 2002. Identification of a phosphofructokinase-encoding gene from Streptococcus thermophilus CNR1205—a novel link between carbon metabolism and gene regulation? Mol. Genet. Genomics 268:500-509. [DOI] [PubMed] [Google Scholar]
- 6.Cvitkovitch, D. G., J. A. Gutierrez, J. Behari, P. J. Youngman, J. E. Wetz, P. J. Crowley, J. D. Hillman, l. J. Brady, and A. S. Bleiweis. 2000. Tn917-lac mutagenesis of Streptococcus mutans to identify environmentally regulated genes. FEMS Microbiol. Lett. 182:149-154. [DOI] [PubMed] [Google Scholar]
- 7.DeLisa, M. P., C.-F. Wu, L. Wang, J. J. Valdes, and W. E. Bentley. 2001. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J. Bacteriol. 183:5239-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobinsky, S., K. Bartscht, and D. Mack. 2002. Influence of Tn917 insertion on transcription of the icaADBC operon in six biofilm-negative transposon mutants of Staphylococus epidermidis. Plasmid 47:10-17. [DOI] [PubMed] [Google Scholar]
- 9.Froeliger, E. H., and P. Fives-Taylor. 2001. Streptococcus parasanguis fimbria-associated adhesin Fap1 is required for biofilm formation. Infect. Immun. 69:2512-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaurivaud, P., F. Laigret, M. Garnier, and J. M. Bove. 2001. Characterization of FruR as a putative activator of the fructose operon of Spiroplasma citri. FEMS Microbiol. Lett. 198:73-78. [DOI] [PubMed] [Google Scholar]
- 11.Henkin, T. M. 1996. The role of the CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 135:9-15. [DOI] [PubMed] [Google Scholar]
- 12.Honeyman, A. L., C. K. Cote, and R. Curtiss III. 2002. Construction of transcriptional and translational lacZ gene reporter plasmids for use in Streptococcus mutans. J. Microbiol. Methods 49:163-171. [DOI] [PubMed] [Google Scholar]
- 13.Jackson, D. W., J. W. Simecka, and T. Romeo. 2002. Catabolite repression of Escherichia coli biofilm formation. J. Bacteriol. 184:3406-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 16.Lengeler, J. W., F. Titgemeyer, A. P. Vogler, and B. M. Wohrl. 1990. Structures and homologies of carbohydrate:phosphotransferase system (PTS) proteins. Philos. Trans. R. Soc. Lond. B 326:489-504. [DOI] [PubMed] [Google Scholar]
- 17.Liljemark, W. F., C. G. Bloomquist, B. E. Reilly, C. J. Bernards, D. W. Townsend, A. T. Pennock, and J. L. LeMoine. 1997. Growth dynamics in a natural biofilm and its impact on oral disease management. Adv. Dent. Res. 11:14-23. [DOI] [PubMed] [Google Scholar]
- 18.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation. J. Bacteriol. 185:2887-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNab, R., S. K. Ford, A. El-Sabaeny, B. Barbieri, G. S. Cook, and R. J. Lamont. 2003. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 185:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyvad, B., and M. Kilian. 1990. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 24:267-272. [DOI] [PubMed] [Google Scholar]
- 22.Oosthuizen, M. C., B. Steyn, J. Theron, P. Cosette, D. Lindsay, A. Von Holy, and V. S. Brozel. 2002. Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Appl. Environ. Microbiol. 68:2770-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postma, P. W., and J. W. Lengeler. 1985. Phosphoenolpyruvate:carbohydrate phosphotransferase system in bacteria. Microbiol. Rev. 49:232-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramseier, T. M., S. Bledig, V. Michotey, R. Feghali, and M. H. Saier, Jr. 1995. The global regulatory protein FruR modulates the direction of carbon flow in Escherichia coli. Mol. Microbiol. 16:1157-1169. [DOI] [PubMed] [Google Scholar]
- 26.Reiner, A. M. 1977. Xylitol and d-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J. Bacteriol. 132:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reizer, J., and A. Reizer. 1996. A voyage along the bases: novel phosphotransferase genes revealed by in silico analyses of the Escherichia coli genome. Res. Microbiol. 147:458-471. [DOI] [PubMed] [Google Scholar]
- 28.Rogers, J. D., R. J. Palmer, Jr., P. E. Kolenbrander, and F. A. Scannapieco. 2001. Role of Streptococcus gordonii amylase-binding protein A in adhesion to hydroxyapatite, starch metabolism, and biofilm formation. Infect. Immun. 69:7046-7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saier, M. H., Jr. 1996. Cyclic AMP-independent catabolite repression in bacteria. FEMS Microbiol. Lett. 138:97-103. [DOI] [PubMed] [Google Scholar]
- 30.Saier, M. H., Jr., and T. M. Ramseier. 1996. The catabolite repressor/activator (Cra) protein of enteric bacteria. J. Bacteriol. 178:3411-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saier, M. H., Jr., and J. Reizer. 1994. The bacterial phosphotransferase system: new frontiers 30 years later. Mol. Microbiol 13:755-764. [DOI] [PubMed] [Google Scholar]
- 32.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 33.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schembri, M. A., K. Kjaergaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-267. [DOI] [PubMed] [Google Scholar]
- 35.Schweizer, H. P., and C. Po. 1996. Regulation of glycerol metabolism of Pseudomonas aeruginosa: characterization of the glpR repressor gene. J. Bacteriol. 178:5215-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley, N. R., R. A. Britton, A. D. Grossman, and B. A. Lazazzera. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 38.Stulke, J., and W. Hillen. 1999. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 39.Svensater, G., J. Welin, J. C. Wilkins, D. Beighton, and I. R. Hamilton. 2001. Protein expression by planktonic and biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 205:139-146. [DOI] [PubMed] [Google Scholar]
- 40.Trahan, L., S. Neron, and M. Bareil. 1991. Intracellular xylitol-phosphate hydrolysis and efflux of xylitol in Streptococcus sobrinus. Oral Microbiol. Immunol. 6:41-50. [DOI] [PubMed] [Google Scholar]
- 41.Tremoulet, F., O. Duche, A. Namane, B. Martinie, and J. C. Labadie. 2002. A proteomic study of Escherichia coli O157:H7 NCTC 12900 cultivated in biofilm or in planktonic growth mode. FEMS Microbiol. Lett. 215:7-14. [DOI] [PubMed] [Google Scholar]
- 42.Tremoulet, F., O. Duche, A. Namane, B. Martinie, and J. C. Labadie. 2002. Comparison of protein patterns of Listeria monocytogenes grown in biofilm or in planktonic mode by proteomic analysis. FEMS Microbiol. Lett. 210:25-31. [DOI] [PubMed] [Google Scholar]
- 43.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotranserase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 44.van Rooijen, R. J., K. J. Dechering, C. Niek, J. Wilmink, and W. M. de Vos. 1993. Lysine 72, 80 and 213 and aspartic acid 210 of the Lactococcus lactis LacR repressor are involved in the response to the inducer tagatose-6-phosphate leading to induction of lac operon expression. Protein Eng. 6:201-206. [DOI] [PubMed] [Google Scholar]
- 45.van Rooijen, R. N., and W. M. de Vos. 1990. Molecular cloning, transcriptional analysis, and nucleotide sequence of lacR, a gene encoding the repressor of the lactose phosphotransferase system of Lactococcus lactis. J. Biol. Chem. 265:18499-18503. [PubMed] [Google Scholar]
- 46.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:6238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen, Z. T., C. Browngardt, and R. A. Burne. 2001. Characterization of two operons that encode components of fructose-specific enzyme II of the sugar:phosphotransferase system of Streptococcus mutans. FEMS Microbiol. Lett. 205:337-342. [DOI] [PubMed] [Google Scholar]
- 48.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 68:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitely, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 50.Wu, L. F., A. Reizer, J. Reizer, B. Cai, J. M. Tomich, and M. H. Saier, Jr. 1991. Nucleotide sequence of the Rhodobacter capsulatus fruK gene, which encodes fructose-1-phosphate kinase: evidence for a kinase superfamily including both phosphofructokinases of Escherichia coli. J. Bacteriol. 173:3117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida, A., and H. K. Kuramitsu. 2002. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 68:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]