Abstract
The recently described ligand–receptor pair, B7h–inducible costimulator (ICOS), is critical for germinal center formation and antibody responses. In contrast to the induced expression of the related costimulatory ligands B7.1 and B7.2, B7h is constitutively expressed on naive B cells and is surprisingly extinguished after antigen engagement and interleukin (IL)-4 cytokine signaling. Although signaling through both B cell receptor (BCR) and IL-4 receptor (R) converge on the extinction of B7h mRNA levels, BCR down-regulation occurs through Ca2+ mobilization, whereas IL-4R down-regulation occurs through a distinct Stat6-dependent pathway. During antigen-specific B cell activation, costimulation through CD40 signaling can reverse both BCR- and IL-4R–mediated B7h down-regulation. These data suggest that the CD40–CD40 ligand signaling pathway regulates B7h expression on activated B cells and may control whether antigen-activated B cells can express B7h and costimulate cognate antigen–activated T cells through ICOS.
Keywords: B7RP-1, ICOSL, GL-50, costimulation, CD40L
Introduction
Collaboration between cognate lymphocytes is essential for generating effective immune responses to T cell–dependent antigens. In addition to antigen–receptor engagement, both B and T cells must also receive costimulatory signals to proliferate, elicit specific downstream effector functions, and generate memory (1–3). Costimulatory ligand–receptor pairs expressed on B and T cells, respectively, include the well-known CD40-CD40 ligand (L;*CD154), and B7.1 (CD80)-CD28 and B7.2 (CD86)-CD28 ligand receptors (4, 5), as well as the more recently described B7h (also known as B7RP-1, GL50, inducible costimulator [ICOS]L)-ICOS ligand receptor (6–13). Mice deficient in CD40 (14), CD40L (CD154; references 15 and 16), B7.1 (CD80) and B7.2 (CD86; reference 17), CD28 (18, 19), and ICOS (20–23) all exhibit impaired germinal center formation and elicit defective humoral responses to antigen.
The CD40–CD40L signaling pathway is critical for B cell activation and subsequent differentiation including isotype switching and affinity maturation (5). Similarly, B7.1–CD28 and B7.2–CD28 signaling is crucial for the activation of naive T cells, particularly for IL-2 production, clonal expansion, and survival of antigen-specific T cells (4). However, although both CD28 and ICOS signaling can costimulate T cell proliferation, ICOS signaling does not costimulate IL-2 production, but instead appears more important for the secretion of several cytokines including IL-4, IL-5, GM-CSF, TNF-α, IFN-γ, and IL-10 (6, 9). Thus, these costimulatory receptor pairs have both overlapping and distinct functions in mediating the activation of antigen-specific B and T cells.
The expression of these three major costimulatory receptor pairs is highly regulated on B and T cells. B7.1 and B7.2 are induced on B cells after activation by innate and adaptive signals, whereas CD28 is constitutively expressed on naive and activated T cells (4). In contrast, CD40 is constitutively expressed on naive and activated B cells, whereas CD40L is only expressed on activated T cells (5). Analogous to CD40-CD40L expression, B7h is also constitutively expressed on B cells (7, 9, 10, 12), whereas ICOS requires T cell activation to induce its expression on naive T cells (6, 24).
The expression of these costimulatory molecules is not only highly regulated, but sequential cross talk between costimulatory receptor pairs during cognate interactions regulates the proper activation of antigen-specific B and T cells. Costimulation of T cells through CD28 helps stabilize CD40L expression on activated T cells (25, 26) and reciprocal costimulation of B cells through CD40 augments the expression of B7.1 and B7.2 molecules (27). Although B7.1–CD28 and B7.2–CD28 interactions are important in initiating immune responses, ICOS is induced on T cells after activation, which suggests that B7h–ICOS interactions may play a more important role in costimulating activated T cells. Indeed, costimulation through CD28 appears important for optimal expression of ICOS (24, 28). However, although multiple B cell stimulatory signals are able to induce B7.1 and B7.2 expression, little is known about the signals that regulate B7h expression on B cells.
Here we report that expression of B7h on activated B cells is highly regulated by these three critical B cell signaling pathways: B cell receptor (BCR), IL-4R, and CD40 receptor. After BCR engagement of antigen, constitutive B7h expression on naive B cells is progressively down-regulated on activated B cells. The hallmark Th2 cytokine IL-4 can also progressively down-regulate B7h on naive and activated B cells. Treatment with both antigen and IL-4 greatly enhance the kinetics and extent of B7h down-regulation, which leads to the extinction of cell surface B7h expression. Although BCR-mediated down-regulation occurs through Ca2+ mobilization, IL-4R–mediated down-regulation of B7h occurs through the Stat6 transcription factor, indicating that at least two distinct signaling pathways converge on the extinction of B7h mRNA and cell surface expression on B cells. Costimulation of activated B cells through the CD40 receptor, however, is capable of restoring the surface expression of B7h after both antigen- and IL-4–mediated down-regulation. Reversal of B7h down-regulation appears to be specific to CD40 signaling and was not observed with bacterial LPS or with a panel of innate and adaptive cytokines tested. These results suggest that the CD40–CD40L signaling pathway regulates B7h expression on activated B cells and may control whether antigen-activated B cells reexpress B7h and in turn costimulate cognate antigen–activated T cells through the ICOS receptor expressed on activated T cells.
Materials and Methods
Antibodies and Flow Cytometry.
Before staining, dead cells were removed by spinning through Histopaque 1119 (Sigma-Aldrich) and FcR was blocked by incubating cells with anti-FcR (2.4G2) hybridoma supernatant. Subsequent staining was performed in staining buffer (HBSS, 2% fetal bovine serum, 10 mM Hepes, and 0.05% NaN3). Levels of cell surface B7h expression were analyzed for all experiments (except that shown in Fig. 2 D) by staining with a biotinylated anti-B7h hamster mAb (clone 22D7) generated in Golden Syrian hamsters (29) followed by a second step phycoerythrin-conjugated streptavidin reagent (streptavidin-PE; BD PharMingen). For the anti-IgM time course (see Fig. 2 D), B7h staining was performed with anti-B7h mAb developed using a biotinylated goat anti–hamster antibody (Caltag) and streptavidin-PE. Levels of B7h staining on naive B cells were comparable between this 3-step method and the 2-step method that is used in other experiments for the detection of B7h (unpublished data). Expression of B7.1 and B7.2 was determined using a biotinylated anti-B7.1 mAb (16-10A1) or a biotinylated anti-B7.2 mAb (GL1; BD PharMingen). Background staining was determined using an appropriate isotype-specific hamster mAb as a control for anti-B7h and anti-B7.1 staining, or an isotype-specific rat mAb (BD PharMingen) for anti-B7.2 staining. Expression of I-Ad on Chinese hamster ovary (CHO) cells used in T cell activation assays was determined using an anti–mouse I-Adb/d mAb (25-9-17; BD PharMingen) followed by staining with biotinylated rat anti–mouse IgG2a (R19-15; BD PharMingen), and developed using streptavidin-PE. Cells were analyzed on a Coulter EPICS XL and data plotted using the WinMDI (The Scripps Institute Flow Cytometry Core Facility) program.
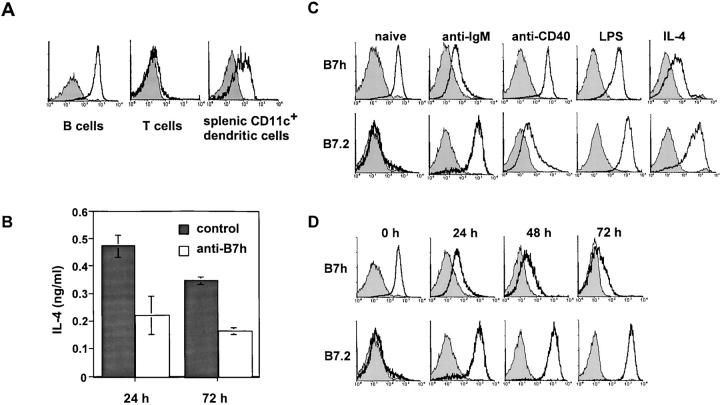
Figure 2.
Constitutive B7h expression on naive B cells is down-regulated after BCR signaling and by the cytokine, IL-4. (A) B7h is constitutively expressed on naive B cells and mature splenic dendritic cells. Naive splenic B cells, T cells, and CD11c+ splenic dendritic cells were stained for B7h expression (solid line) compared with staining with an isotype-matched control antibody (shaded histogram). (B) B7h expressed on B cells costimulates IL-4 production by T cells. Purified B cells were used as APCs to activate DO11.10 T cells in the presence of OVA peptide in cultures containing either a blocking anti-B7h mAb or control hamster IgG for 4 d. T cells were repurified and IL-4 production was assayed 24 and 72 h after rechallenge with splenic APCs presenting OVA peptide in the absence of blocking. (C) Rapid down-regulation of B7h is induced by BCR and IL-4R signaling. Naive B cells, or B cells activated for 24 h with anti-IgM F(ab′)2, LPS, anti-CD40 mAb, or with IL-4 were stained for B7h or B7.2 expression (solid line) compared with staining with an isotype-matched control antibody (shaded histogram). (D) B7h down-regulation induced by BCR signaling is progressive and occurs in concert with B7.2 up-regulation. Purified B cells, activated with anti-IgM F(ab′)2, were harvested at the indicated times and stained for B7h and B7.2 expression. Data shown in A and C are representative of at least three independent experiments.
Mice and Immunization.
5–8-wk-old C57BL/6 mice were obtained from The Jackson Laboratory and used within 1 mo of receipt. Hen egg lysozyme (HEL)-specific IgM and IgD BCR transgenic mice (IgHEL; MD4 line; provided by J.G. Cyster, University of California, San Francisco, CA) on a C57BL/6 background were used between the ages of 5 and 8 wk. DO11.10 TCR transgenic mice on either a BALB/c or B10.DR/recombination activating gene (RAG)1−/− background were used within 6 mo of age. Stat6−/− and littermate control mice were provided by K.M. Murphy (Washington University, St. Louis, MO). All animal procedures were performed in accordance with regulations set by the University of California Berkeley Animal Care and Use Committee.
C57BL/6 mice were immunized intraperitoneally with 100 μg (4-hydroxy-3-nitrophenyl)acetyl (NP) coupled to chicken-γ globulin (NP15-CG) in alum as previously described (30). For blocking experiments, mice were treated daily with 100 μg blocking anti-B7h mAb or control hamster IgG (Jackson ImmunoResearch Laboratories) for the course of the experiment, starting 1 d before immunization. On day 8, frozen splenic sections from cohorts of B7h-blocked and control mice were stained for Igλ+ cells using alkaline-phosphatase–labeled goat anti–λ antibody (Southern Biotechnology Associates, Inc.) or with biotinylated peanut agglutinin (Vector Laboratories) and streptavidin-AP (Boehringer), and developed with NBT/BCIP (Boehringer) according to the manufacturer's instructions.
RNase Protection Assay.
RNase protection assay was performed as previously described (10). Activated B cells were harvested and washed with 1× PBS. Total cellular RNAs were prepared using Tri-Reagent (Molecular Research Center) according to the manufacturer's protocol and 10–15 μg of total RNA was used to perform each RNase protection assay. Probes for B7h and β-actin were used as previously described (10). RNase protection assay was run simultaneously for the PMA/ionomycin, CD40L, and anti-IgM stimulated samples. A separate experiment compared IL-4– and anti-CD40–stimulated samples.
B Cell Activation.
B cells were purified from total splenocyte preparations as previously described (31). In brief, red blood cells were lysed with ammonium chloride and T cells were depleted by staining with anti-Thy1.2 (13H10) hybridoma supernatant and lysed using rabbit complement (CedarLane). Purified B cells (>90% B220+) were activated in 12-well plates at a density of 106 cells/ml in lymphocyte media (RPMI 1640, 10% fetal bovine serum, 50 μM 2-ME, 100 U penicillin, and 100 μg/ml streptomycin) in the presence of 10 μg/ml affinity-purified goat anti–IgM F(ab′)2 fragments (Jackson ImmunoResearch Laboratories), 10 μg/ml LPS (Sigma-Aldrich), 250 nM PMA, and 1 μM ionomycin or 200 ng/ml HEL (United States Biochemical) for the indicated times. For experiments examining the effects of CD40 signaling, 5 μg/ml purified activating anti-CD40 mAb (clone 3/23; BD PharMingen) was added with either HEL or anti-IgM F(ab′)2 during the initiation of stimulation, or added to cultures 24 h after activation with HEL. As a control for the possible effects of using whole anti-CD40 antibody molecules on B cell stimulation, a control-purified rat mAb to B7.2 was used with either HEL or anti-IgM F(ab′)2 to activate B cells. No effect of the control antibody on B cell activation or B7h expression was observed (unpublished data). For activation using membrane-bound CD40L (mCD40L), purified S19 membranes of baculovirus-expressed CD40L was used at a 1:500 dilution (32, 33). To determine the effects of cytokines on B7h expression after BCR activation, purified B cells were activated with HEL as described above in the presence of 5 ng/ml IL-4 (R&D Systems), IL-5 (1:500), 10 ng/ml IL-1α (R&D Systems), IL-2 (R&D Systems), 10 ng/ml TNF-α (R&D Systems), IFN-γ (provided by R. Schreiber, Washington University, St. Louis, MO), or 10 μg/ml LPS (Sigma-Aldrich). 10 μg/ml cyclosporin A (CsA) was added to unstimulated B cell cultures or to cultures activated with 10 μg/ml anti-IgM F(ab′)2 or PMA and ionomycin.
T Cell Activation.
To assess the function of B7h expressed on B cell APCs in priming T cell cytokine production, naive D011.10 TCR transgenic CD4+ T cells were isolated from the lymph nodes of DO11.10 transgenic mice in the B10.DR/RAG1−/− background by complement lysis with a cocktail of anti-MHC (28.16.8S and BP107), anti–heat-stable antigen (J11D), and anti-FcR (2.4G2) mAbs, and naive B cells (96% B220+) were purified from BALB/c mice as described above with an additional panning step to remove adherent cells bound to tissue culture plates at 37°C. 5 × 105 purified CD4+ T cells were cultured with 2.5 × 106 purified B cells in the presence of either 5 μg/ml blocking anti-B7h mAb (22D7) or control hamster IgG (Jackson ImmunoResearch Laboratories) and 0.3 μM OVA peptide (provided by K.M. Murphy) for 4 d. Activated T cells were purified as described above, rested for 24 h, and rechallenged with irradiated (1,000 rads) BALB/c splenocytes in the absence of blocking. Supernatants were harvested at the indicated times and IL-4 production was assayed by ELISA (BD PharMingen).
Naive DO11.10 TCR transgenic T cells were isolated from the lymph nodes of DO11.10 transgenic mice in the B10.DR/RAG1−/− background to examine the effects of costimulation on CD40L expression (>90% CD4+) as described above. For rechallenge experiments, DO11.10 transgenic T cells from DO11.10 transgenic mice in the BALB/c background were activated in bulk splenocyte cultures in the presence of 0.3 μM OVA peptide. Cells were expanded over 3 d, purified as described above, and rested overnight before restimulation. Purified T cells (>92% CD4+) were determined to express ICOS by staining with anti-ICOS mAb (provided by J.P. Allison, University of California, Berkeley, CA) and were CD40L− before rechallenge.
Purified T cells, either naive or previously activated, were activated using I-Ad–expressing CHO cells retrovirally transduced with green fluorescent protein, B7h, or B7.2 as previously described (29). 4 × 105 irradiated (2,000 rads) CHO cells were plated in 24-well dishes and prepulsed with varying OVA peptide concentrations (0–3 μM) for 2 h. For blocking experiments, anti-B7h, anti-B7.2, or control hamster IgG were added at a final concentration of 10 μg/ml. 5 × 105 T cells were added and CD40L expression was determined after stimulation for 24 h for naive T cells and 3 h for previously activated T cells by staining with biotinylated anti-CD40L (MR1; BD PharMingen) developed with streptavidin-PE and anti-CD4 FITC. The percentage of CD40L+CD4+ cells was determined by two-color flow cytometry.
Results
B7h Is Required for Germinal Center, but Not B Cell Foci, Formation.
To examine the function of B7h in regulating immune responses, we generated three independent IgG mAbs against B7h by immunizing hamsters with CHO cells expressing B7h. Two of these mAbs were effective in both blocking a B7h–Ig fusion protein from binding to ICOS-transfected 293 cells and in blocking B7h-mediated costimulation of T cell proliferation (unpublished data). We used one of these mAbs, clone 22D7, to block in vivo B7h–ICOS interactions and test the requirement for B7h in responses to T cell–dependent antigens.
Mice immunized with NP coupled to CG (NP15-CG) in alum were injected with daily doses of anti-B7h mAb. A control cohort of immunized mice was also injected in conjunction with nonspecific hamster IgG. Because the immune response to NP is characterized by a λ-specific response (34), formation of λ+ B cell foci early in the response could be examined. In cryosections of spleen at day 8, formation of λ+ foci was qualitatively and quantitatively similar in both control mice and mice treated with anti-B7h mAb (Fig. 1). Quantitation of λ+ foci/follicle was 1.65 ± 0.14 for cryosections from control mice and 1.73 ± 0.23 for cryosections from mice treated with anti-B7h mAb. Germinal center formation, which occurs later in an immune response, was also examined in the same splenic cryosections. In contrast to foci formation, germinal center formation was markedly impaired in mice treated with anti-B7h mAb. Quantitation of germinal centers/follicle was 0.27 ± 0.03 for cryosections from control mice and 0.03 ± 0.04 for cryosections from mice treated with anti-B7h mAb. These results correspond with the inability of ICOS−/− mice to form germinal centers (20, 21, 23) and suggest that B7h is the primary in vivo ligand for ICOS. In addition, these results indicate that although B7h–ICOS interactions are required later in an immune response for germinal center formation, they are not essential earlier during B cell foci formation.
Figure 1.
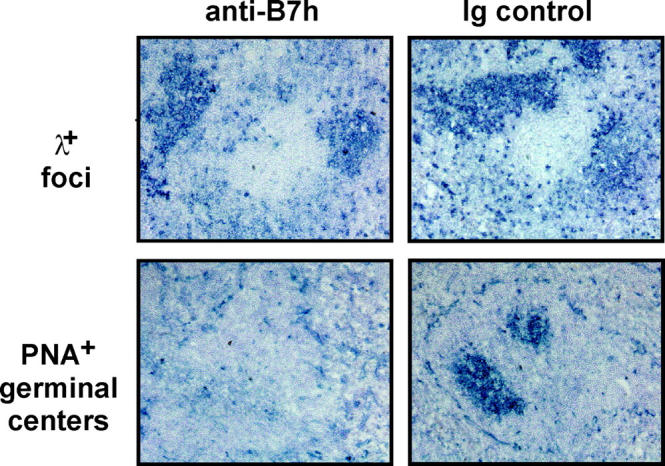
B7h is required for the formation of germinal centers, but not extrafollicular foci. Mice were immunized with the T cell–dependent antigen, NP-CG in alum, and were treated with daily intraperitoneal injections of 100 μg of either a blocking anti-B7h mAb or a hamster IgG control. Splenic cryosections at day 8 were analyzed by immunohistochemistry to detect λ+ NP-specific foci and peanut agglutinin+ germinal centers. ×200.
BCR and IL-4R Signaling Down-regulates Constitutive B7h on Naive B Cells.
A generally accepted paradigm in costimulation is that costimulatory molecules are appropriately up-regulated on APCs in response to proinflammatory innate and adaptive signals (4). For example, immature dendritic cells express low levels of costimulatory molecules. However, after activation, mature dendritic cells up-regulate the expression of B7.1 and B7.2 and are extremely effective APCs (35). B7h is also up-regulated on dendritic cells (12) and is highly expressed on splenic CD11c+ dendritic cells (Fig. 2 A). Similarly, B7.1 and B7.2 are not expressed on naive B cells, but are up-regulated after activation by numerous stimuli including engagement of BCR, IL-4R, CD40 receptor, and the B cell mitogen, LPS (36–40). B7h expression, however, differs from B7.1 and B7.2 in that it is highly expressed on naive B cells (7, 10, 12). In bone marrow, B7h is also expressed on IgM+B220+ cells (unpublished data), indicating that it is expressed before mature B cell entry into the periphery. Thus, in contrast to B7.1 and B7.2, which are both induced after B cell activation, B7h is highly expressed on naive B cells in the bone marrow and periphery.
The ICOS receptor expressed on activated T cells is essential for the generation of Th2 responses. In ICOS−/− mice (20–22) and in vitro cultures in which B7h–ICOS interactions were blocked (28), activated T cells were deficient for IL-4 and IL-10 production. To determine if B7h expressed on B cells was a functionally important source of costimulation for T cell cytokines, we used purified B cells as APCs to activate DO11.10 TCR transgenic T cells in the presence or absence of our blocking anti-B7h mAb. After this initial activation, T cells were rechallenged and IL-4 production was measured. We observed that IL-4 production was impaired when B7h–ICOS interactions were blocked compared with control hamster IgG–treated cultures (Fig. 2 B). These results demonstrate that B7h is not only highly expressed on B cells, but also has a nonredundant costimulatory function during cognate B cell–T cell interactions for costimulating IL-4 production (41).
The requirement for B7h–ICOS interactions in T cell–dependent B cell immune responses highlights the importance of this signaling interaction after the activation of antigen-specific B cells. Surprisingly, in examining the expression of B7h on activated B cells, we observed that both BCR engagement and treatment with IL-4 resulted in the rapid down-regulation of B7h expression (Fig. 2 C). This down-regulation of B7h did not appear to be a general consequence of B cell activation, as activation of naive B cells through CD40 receptor (42) or the B cell mitogen LPS did not result in the same rapid down-regulation of surface B7h levels observed with BCR or IL-4R signaling. In contrast, as has been previously reported (36–40), all of the activating stimuli examined rapidly induced B7.2 expression on naive B cells.
BCR engagement resulted in the down-regulation of B7h that was progressive and sustained (Fig. 2 D). By 72 h, the majority of B cells activated with anti-IgM antibodies no longer expressed B7h that was detectable by flow cytometry, and this occurred again in concert with the induction of B7.2 on activated B cells at all mitogenic concentrations of anti-IgM antibodies. At submitogenic concentrations of anti-IgM antibodies (<1 μg/ml), no changes in the surface expression of either B7h or B7.2 were detected (unpublished data). IL-4R–mediated signaling also resulted in the down-regulation of B7h that was progressive and sustained (unpublished data). Thus, B cell activation through either BCR or IL-4R engagement resulted in the down-regulation of B7h expression.
BCR and IL-4R Extinguish B7h mRNA Levels by Two Distinct Signaling Pathways.
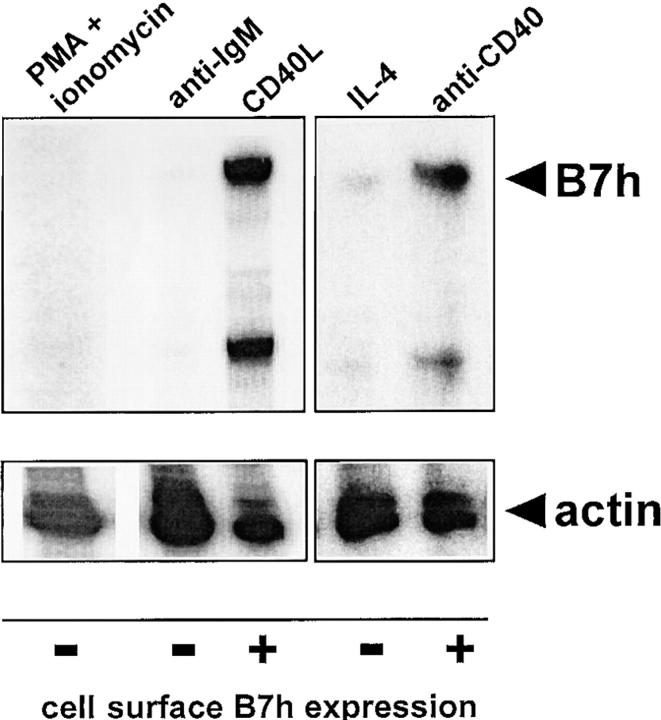
These results prompted us to examine whether BCR- and IL-4R–mediated down-regulation of surface B7h expression resulted from specific changes in the levels of B7h mRNA. B7h mRNA levels were examined in different activated populations of B cells that had either maintained or down-regulated surface B7h expression (Fig. 3). Levels of B7h and control β-actin mRNAs were assayed simultaneously using an RNase protection assay. B7h mRNA was readily detected in activated B cells treated with anti-CD40 mAb or membrane CD40 ligand (mCD40L), cell populations in which surface B7h expression was also observed by flow cytometry. In contrast, B cells activated with PMA and ionomycin or anti-IgM antibodies had severely reduced levels of B7h mRNA, and B cells treated with IL-4 also had decreased amounts of B7h mRNA. These decreases were specific to B7h mRNA, as β-actin mRNA was readily detected in all activated B cell populations. These results indicate that the down-regulation of surface B7h expression by both BCR and by IL-4R signaling lead to the extinction of B7h mRNA levels.
Figure 3.
BCR and IL-4 signaling induces the extinction of B7h mRNA in activated B cells. Total cellular RNA were prepared from B cells activated with PMA and ionomycin for 24 h, anti-IgM F(ab′)2 or membrane CD40L for 72 h, or IL-4 or anti-CD40 mAb for 48 h. The mRNA levels of B7h and β-actin were determined by RNase protection assay and sample lanes from autoradiographs exposed for the same amount of time are boxed. Cell surface B7h expression as determined by flow cytometry is indicated under each activation condition.
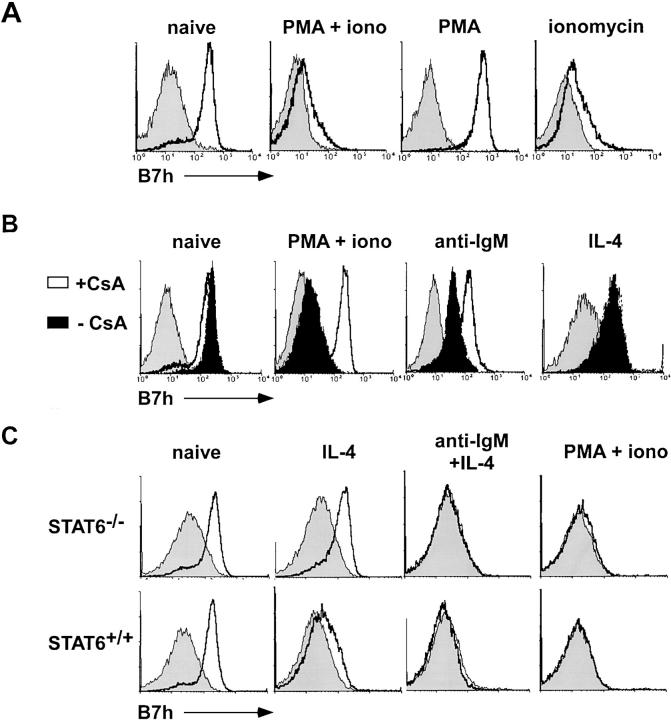
Next, we examined the proximal signaling pathways downstream of BCR engagement that lead to the extinction of B7h mRNA levels by activating naive B cells with the phorbol ester, PMA, and the Ca2+ ionophore, ionomycin (Fig. 4 A). Treatment with the combination of PMA and ionomycin was observed to down-regulate B7h surface expression, similar to the down-regulation observed with BCR signaling. In fact, ionomycin treatment alone was as effective as PMA and ionomycin, whereas treatment with PMA alone did not affect B7h surface expression. These data indicate that Ca2+ mobilization downstream of BCR signaling can mediate B7h down-regulation. Consistent with this observation, treatment with the inhibitor of calcineurin, CsA, blocked the down-regulation of B7h by both PMA and ionomycin treatment, and by anti-IgM antibodies (Fig. 4 B). In contrast, CsA treatment did not block IL-4–mediated B7h down-regulation. These data suggest that although Ca2+–dependent signaling is necessary for BCR-mediated down-regulation of B7h, IL-4–mediated down-regulation occurs through a different signaling mechanism that also converges on the extinction of B7h mRNA levels.
Figure 4.
BCR-mediated B7h down-regulation occurs through Ca2+ signaling, whereas IL-4–mediated down-regulation occurs through a distinct Stat6-dependent signaling pathway. (A) B7h down-regulation induced by BCR signaling is Ca2+ dependent. Purified B cells were treated for 24h with PMA alone, ionomycin alone, or a combination of both and stained for B7h expression. (B) B7h down-regulation by BCR signaling is CsA sensitive. Purified B cells were activated with PMA and ionomycin, anti-IgM F(ab′)2, IL-4, or untreated either in the presence (solid lines) or absence (solid filled) of CsA. After 24 h of stimulation, cells were stained for B7h expression compared with control staining (shaded histogram). (C) IL-4–mediated B7h down-regulation is Stat-6 dependent. Purified B cells from Stat6−/− and wild-type control mice were either untreated or activated with IL-4, anti-IgM F(ab′)2 and IL-4, or PMA and ionomycin. Cells were stained for B7h expression after 24 h of culture.
IL-4R signaling is mediated by several downstream events including the activation of the transcription factor, Stat6 (43). In Stat6−/− B cells (44), IL-4–mediated down-regulation of B7h was impaired (Fig. 4 C). In contrast, BCR-mediated down-regulation was not affected in Stat6−/− B cells. These results indicate that B7h down-regulation through BCR signaling and the cytokine IL-4 occur through distinct signaling mechanisms involving Ca2+ mobilization and the activation of Stat6, respectively.
CD40 Signaling Reverses Down-regulation of B7h on Activated B Cells.
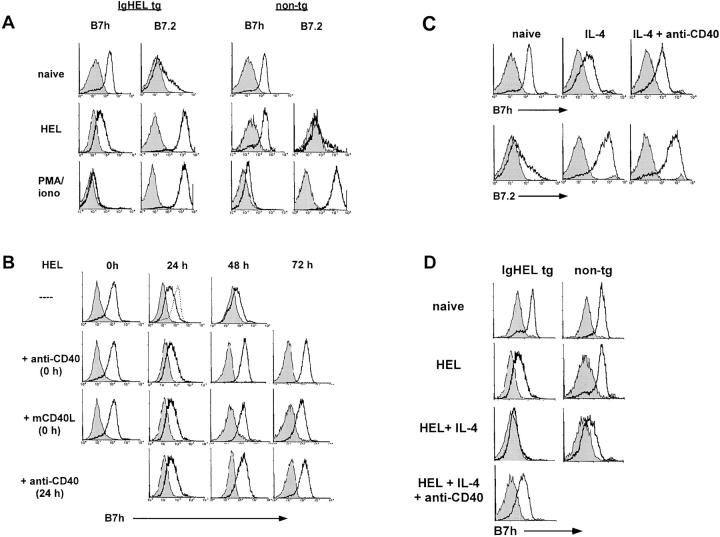
The requirement for B7h late in the immune response in germinal center formation suggested that for antigen-activated B cells to express sufficient B7h to engage ICOS on activated T cells, additional signaling events might be required to reverse antigen-mediated down-regulation of B7h. To explore what signaling pathways could lead to the up-regulation of B7h on antigen-activated B cells, we used IgHEL transgenic B cells, which express BCR that recognize the antigen, HEL (45). Transgenic B cells activated with soluble HEL antigen were observed to down-regulate B7h and induce B7.2 with similar kinetics to those seen with BCR stimulation by anti-IgM antibodies (Fig. 5 A).
Figure 5.
CD40 signaling rescues BCR-mediated B7h down-regulation. (A) Soluble HEL induces B7h down-regulation on naive transgenic B cells. Purified B cells from IgHEL transgenic or nontransgenic littermates were activated with HEL or PMA and ionomycin, or left untreated, and stained for B7h and B7.2 expression (solid lines) compared with staining by an isotype-matched control antibody (shaded histogram) after 48 h of stimulation. Data are representative of five similar experiments. (B) CD40 signaling rescues HEL-induced B7h down-regulation. B cells from transgenic or nontransgenic mice were activated with HEL and costimulated through CD40 using an activating anti-CD40 mAb or mCD40L. CD40 stimulation was provided either simultaneously with HEL 0 or 24 h after HEL activation. At the indicated times, cells were stained for B7h expression (solid line) compared with staining with an isotype-matched control antibody (shaded histogram). The level of B7h expression on unstimulated B cells is indicated by a dashed line. Data are representative of two similar experiments. (C) Costimulation through CD40 rescues IL-4–mediated B7h down-regulation. Purified B cells were untreated or stimulated with IL-4 or IL-4 and anti-CD40 mAb. After 48 h of activation, cells were stained for B7h (solid lines) compared with control staining (shaded histogram). (D) IL-4 signaling enhances HEL-mediated B7h down-regulation and costimulation through CD40 rescues B7h down-regulation by the combination of antigen and IL-4. Purified B cells from IgHEL transgenic and nontransgenic animals were activated with soluble HEL in the presence or absence of IL-4 and costimulation with anti-CD40 mAb. After 48 h of activation, cells were stained for B7h (solid lines) compared to control staining (shaded histogram).
An important signaling pathway in generating T cell–dependent B cell immune responses is the costimulation of B cells through CD40 (3, 46–48). To determine whether signals received through CD40 were capable of affecting antigen-mediated B7h down-regulation, we simultaneously treated HEL-activated B cells with an activating anti-CD40 mAb (Fig. 5 B). B cells treated with HEL and anti-CD40 mAb showed similar B7h down-regulation at 24 h compared with B cells activated with HEL alone. However, by 48 h B7h expression was restored to levels observed on naive B cells in cultures activated with HEL and anti-CD40 mAb. Treatment with membranes expressing CD40L (32), which has previously been shown to activate B cell proliferation and facilitate isotype switching (33), was also effective in rescuing antigen-mediated B7h down-regulation. The restoration of B7h expression was specific to CD40 signaling. Treatment with an isotype- and species-matched anti-B7.2 mAb did not alter antigen-mediated B7h down-regulation (unpublished data). CD40 signaling was also effective in reversing ongoing antigen-mediated B7h down-regulation. The addition of activating anti-CD40 mAb 24 h after HEL antigen activation could also reverse B7h down-regulation on antigen-activated B cells.
To examine whether costimulation through CD40 was also capable of reversing IL-4–mediated B7h down-regulation, IL-4–activated B cells were also stimulated with an anti-CD40 mAb (Fig. 5 C). CD40 signaling was also able to reverse IL-4–mediated down-regulation of B7h within 48 h. Thus, the costimulation of B cells through CD40 signaling was capable of restoring B7h expression on either antigen- or IL-4–activated B cells.
IL-4 treatment significantly enhanced the antigen-induced down-regulation of B7h on activated B cells (Fig. 5 D). This result may have physiological significance given the in vivo importance of B7h–ICOS signaling in regulating IL-4 production and Th2 responses. After the stimulation of B cells with both HEL antigen and IL-4, B7h was down-regulated more rapidly and completely in comparison to stimulation with either antigen or IL-4 alone. Again, CD40 signaling was able to restore B7h expression after activation with both HEL antigen and IL-4.
Specificity of CD40 Rescue of B7h Expression.
B7h expression in a variety of cell types is regulated by inflammatory signals. Fibroblasts (10) and bone marrow–derived macrophages treated with TNF-α (49) induce B7h expression and mice injected with LPS up-regulate B7h in a variety of peripheral organs including testes and kidney (10). These results suggested that in addition to T cell–specific CD40–CD40L interactions, inflammatory cytokines might also be important for regulating B7h induction on B cells. We tested the ability of a variety of inflammatory mediators, including bacterial LPS and the inflammatory cytokines TNF-α, IL-1, and IL-12 to up-regulate B7h on HEL-activated transgenic B cells (Fig. 6 A). In contrast to CD40 signaling, treatment with these stimuli was unable to rescue B7h expression on antigen-activated B cells. Thus, although inflammation, especially TNF-α treatment, is important for the induction of B7h in a variety of monocytic lineage cells, TNF-α signaling does not appear to regulate B7h expression on B cells.
Figure 6.
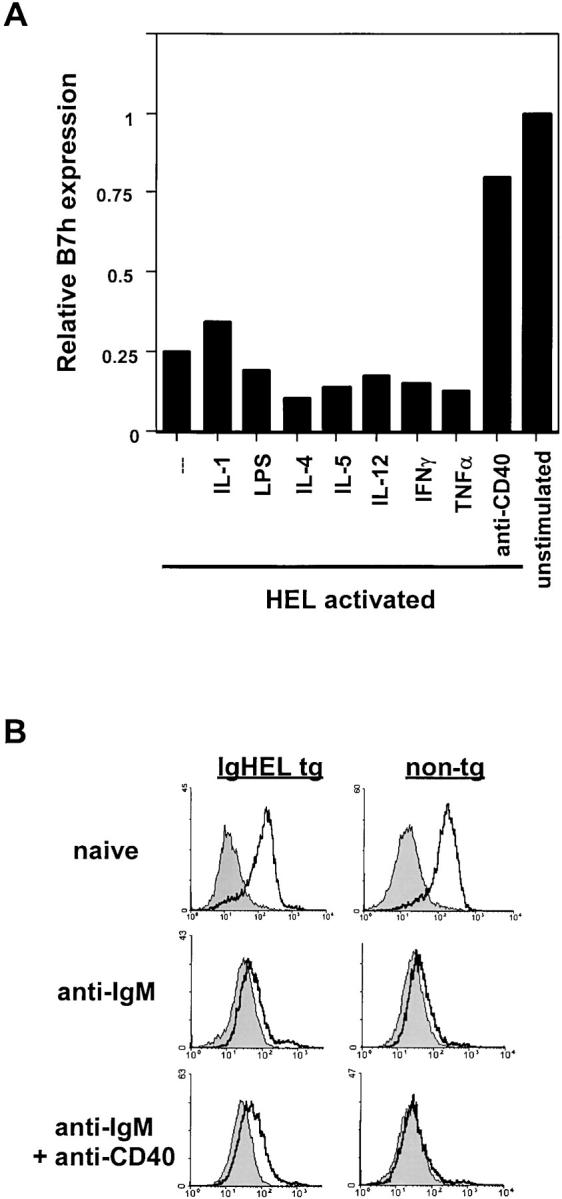
Reversal of antigen-induced B7h down-regulation is specific to CD40 signaling. (A) Cytokines and LPS do not prevent antigen-induced B7h down-regulation. Purified B cells were activated with soluble HEL antigen and the indicated treatments. After 48 h of stimulation, cells were stained for B7h expression. Relative B7h expression was determined as the ratio of mean B7h fluorescence intensity on experimental to unstimulated B cells. (B) CD40 signaling does not prevent B7h down-regulation by anti-IgM cross-linking. B cells from transgenic and nontransgenic mice were activated with anti-IgM F(ab′)2 in the presence or absence of anti-CD40 mAb. After 48 h, cells were stained for B7h expression.
The specificity of CD40 signaling suggested that T cell interactions were critical for the reexpression of B7h on B cells. In addition to CD40L expression, the production of a variety of cytokines by Th cells is also important for B cell activation. We tested a panel of T cell cytokines including IL-2, IL-4, IL-5, IL-13, and IFN-γ for their ability to rescue B7h expression (Fig. 6 A). Again, the rescue of B7h expression on B cells appeared to be specific to CD40 signaling. Thus, CD40 signaling appeared to be unique among the cytokine and signaling pathways we examined in its ability to up-regulate B7h expression.
We also examined whether the same stimuli could reverse B7h down-regulation induced through BCR cross-linking by anti-IgM antibodies (Fig. 6 B). Again, neither the cytokines tested nor LPS restored B7h expression on anti-IgM–treated B cells (unpublished data). Intriguingly, CD40 signaling was also not effective in restoring B7h expression on B cells in which BCR was highly cross-linked by anti-IgM treatment. These data suggest that the ability of B7h to be reexpressed may also depend on the type of antigen recognized by the B cell.
Stabilization of CD40L Expression on Activated T Cells by B7h and B7.2.
The CD40–CD40L signaling pathway plays an important regulatory role for B7h induction on antigen-activated B cells. Similarly, transient CD40L induction on activated T cells is also potentiated by other costimulatory pathways. Previous studies have demonstrated that costimulation by B7.1 and B7.2 through CD28 is critical in stabilizing CD40L expression on activated T cells (25, 26). A more recent study demonstrated that anti-ICOS antibodies enhanced the ability of anti-CD3 antibodies to up-regulate CD40L expression on bead-activated CD4+ T cells (21). Therefore, we directly compared the ability of B7.1–CD28, B7.2–CD28, and B7h–ICOS signaling pathways in regulating CD40L expression on T cells.
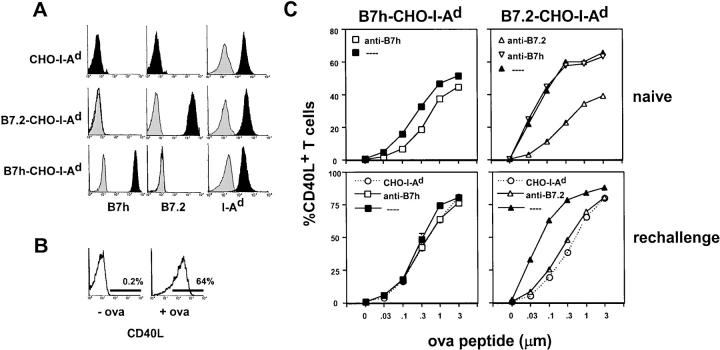
We compared the ability of B7h and B7.2 to costimulate the induction of CD40L expression on either naive or rechallenged transgenic DO11.10 T cells using I-Ad CHO cell lines expressing either B7h or B7.2 (Fig. 7 A). The percentage of T cells expressing CD40L was determined by flow cytometry over a broad range of OVA peptide concentrations that were effective in costimulating the proliferation of DO11.10 T cells (Fig. 7 B). For naive T cells, B7.2 was more effective than B7h in inducing CD40L expression after 24 h of contact with CHO cells (Fig. 7 C), and this could specifically be blocked using an anti-B7.2 mAb. Activation with B7h-CHO-IAd cells was less effective at inducing CD40L expression, however, this effect could still be inhibited by blocking B7h with an anti-B7h mAb. To test whether the differences in induction of CD40L by B7.2 compared with B7h-expressing CHO cells were due to ICOS expression by the T cells, we preactivated T cells to induce ICOS expression. CD40L expression was measured 3 h after rechallenge with the different CHO sublines. Again, B7.2 was more effective than B7h in stabilizing CD40L expression. These studies indicate that individually, B7.2 is more effective than B7h in stabilizing CD40L expression on T cells.
Figure 7.
Costimulation by B7.2 is more effective than B7h in stabilizing CD40L expression on activated T cells. (A) Expression of B7h, B7.2, and I-Ad (filled histograms) on CHO cell lines compared with control staining (shaded histograms). (B) Representative expression of CD40L on naive compared with activated T cells. (C) Induction of CD40L expression after the activation of naive or rechallenged DO11.10 transgenic T cells by B7h, B7.2, or control CHO cells prepulsed with different concentrations of OVA peptide in the presence or absence of blocking anti-B7h or anti-B7.2 mAb. CD40L expression was measured and plotted as the percentage of CD4+ T cells expressing CD40L. Error bars indicate the standard deviation of duplicate samples. Where not observed, the error is less than the symbol size.
Discussion
Costimulatory receptors convey important information about the pathogenic context in which antigens are recognized by B and T cells. The appropriate induction of costimulatory molecules by specific proinflammatory and adaptive signals is a well-established paradigm by which the transmission of costimulatory signals is controlled. The signals responsible for regulating the expression of the costimulatory ligands B7.1 and B7.2, as well as CD40L, have been well studied. Our results indicate that the expression of the B7h–ICOS costimulatory pair is also highly regulated not only at the level of ICOS receptor induction on activated T cells, but also at the level of B7h ligand expression on activated B cells.
Although B7h is present on naive B cells, B7h expression is regulated by the interplay between three important B cell signaling pathways after activation: BCR, IL-4R, and CD40 receptor. Engagement of antigen by naive B cells leads to the progressive and sustained down-regulation of B7h. This BCR-mediated B7h down-regulation is observed with both IgHEL transgenic B cells treated with soluble HEL antigen and with wild-type B cells treated with cross-linking anti-IgM antibodies. The down-regulation of B7h expression by BCR signaling occurs through Ca2+ mobilization and is CsA sensitive.
In addition to BCR signals, signaling by the Th2 cytokine IL-4 is also important for B cell activation and differentiation. Similar to BCR signaling, IL-4 signaling also down-regulates B7h expression. The combination of both BCR and IL-4R signaling results in a more rapid and complete extinction of B7h surface expression on activated B cells. Given the in vivo regulation of Th2 responses and IL-4 production by B7h–ICOS signaling, this observation is likely to be physiologically significant. However, although BCR-mediated B7h down-regulation occurs through a Ca2+–dependent mechanism, IL-4–mediated down-regulation occurs through a Stat6-dependent signaling pathway, which is not inhibited by CsA treatment.
Therefore, it appears that the convergence of the two signaling pathways, Ca2+ mobilization downstream of BCR engagement and Stat6-dependent signaling downstream of IL-4R engagement, results in the sustained extinction of cell surface B7h expression. Importantly, the loss of surface B7h expression is accompanied by the loss of B7h mRNA expression in both BCR- and IL-4–stimulated B cells. This suggests that the down-regulation of B7h may occur by signals that repress B7h transcription. Recent reports have shown that Ca2+ signaling in T cells is not only capable of activating the expression of a wide variety of genes, but can also repress the expression of a number of genes including cell surface molecules (50). In addition, Stat6-dependent events have also been shown to activate and repress a large number of different genes (51). Furthermore, the IL-4 effect on B7h expression on B cells can also be observed in other cell types. CD34+ bone marrow–derived monocytes induce B7h expression after TNF-α treatment, which is inhibited by IL-4 treatment (49). Additional studies, however, will be necessary to determine precisely what signaling molecules are directly responsible for B7h down-regulation.
Studies of ICOS−/− mice have revealed that in the absence of B7h–ICOS signaling, T cell proliferation and cytokine production are defective, especially the Th2 cytokines IL-4 and IL-13, and B cell responses to T cell–dependent antigens are impaired (20–23). The defects in ICOS−/− mice are similar to those observed in other costimulatory molecule–deficient mice, indicating that B7h–ICOS interactions are playing an important role for the generation of in vivo immune responses. Given this important in vivo function, the reestablishment of high levels of B7h expression on activated B cells is likely to be important for the appropriate progression of T cell–dependent B cell responses. Consistent with this notion, we observed that the costimulation of activated B cells through CD40 receptor was capable of restoring the surface expression of B7h after both antigen- and IL-4–mediated down-regulation. The reversal of B7h down-regulation was specific to CD40 signaling and was not observed with bacterial LPS or with a panel of innate and adaptive cytokines tested. These results indicate that the CD40–CD40L signaling pathway regulates B7h expression on activated B cells. Furthermore, because B7h induction is dependent on CD40–CD40L interactions, our results suggest that the reestablishment of B7h expression may serve as an important checkpoint to ensure proper B cell–T cell conjugate formation.
Previous studies (21) indicate that B7h–ICOS signaling stabilizes CD40L expression on activated T cells. However, our results, comparing the relative ability of B7.2 and B7h to costimulate CD40L expression on activated T cells, suggest that B7h is less effective than B7.2 in the induction/stabilization of CD40L expression. This greater effectiveness of B7.2 in costimulating CD40L expression on T cells is consistent with the established role of B7.1–CD28 and B7.2–CD28 interactions in the initial activation of naive T cells by mature dendritic cells (35). From this perspective, we note that CD11c+ splenic dendritic cells express lower levels of B7h than are observed on resting B cells. After activation, only the expression of B7.1 and B7.2, and not B7h, is highly induced (unpublished data). These observations are consistent with the notion that in initial interactions of T cells with dendritic T cells, costimulation by B7.1 and B7.2 play a more important role than costimulation by B7h. Additional studies, however, will be required to establish the precise sequence of cross-regulatory events involving B7.1–CD28, B7.2–CD28, CD40–CD40L, and B7h–ICOS costimulatory interactions in vivo.
The ability of BCR and IL-4R signaling to down-regulate B7h expression implies that similar to B7.1 and B7.2, B7h also likely needs to be up-regulated for activated B cells to maximize costimulatory ICOS signaling into antigen-activated T cells. We suggest that there may be several reasons why multiple B7 molecules are up-regulated during the immune response. First, the ability of B7h to be up-regulated on antigen-activated B cells appears to be more restricted than that of B7.1 and B7.2. Although multiple signals including CD40, BCR, and LPS are all capable of inducing B7.1 and B7.2 expression on activated B cells, only CD40 signaling was identified in our studies as being capable of up-regulating B7h. These results suggest that in the absence of appropriate CD40 signaling in B cells, ICOS signaling may be suboptimal. Therefore, B7h induction appears to be more critically dependent on the establishment of proper cognate interactions between activated T and B cells than B7.1 or B7.2 induction.
Second, different B7 molecules may play an important role at different phases during the immune response. Results from in vivo B7h blocking studies are consistent with a role for B7h–ICOS interactions in regulating later immune events after exposure to T cell–dependent antigens. Mice treated with the blocking anti-B7h antibody are still capable of forming antigen-specific extrafollicular foci, an event observed early during the immune response. However, later events such as germinal center formation are severely impaired. This observation is similar to data from ICOS−/− mice. Furthermore, the phenotype of ICOS−/− mice is similar to that observed in CD28−/− mice (19). Because of the role B7h-ICOS may play in later immune events, it is possible that B7h–ICOS interactions may play a more important role for germinal center formation as intact B7.1–CD28 and B7.2–CD28 signaling in ICOS-deficient mice is functionally unable to compensate for deficient signaling through B7h-ICOS. Data indicating that the costimulation of T cells through CD28 is necessary for optimal ICOS induction (24, 28) may further suggest that some of the defects in later immune events observed in CD28−/− mice may be due to the failure to induce ICOS. Although B7h–ICOS interactions may not be critical for initiating immune responses, this costimulatory pathway likely plays an important role later in the immune response for establishing correct cognate interactions between activated B and T cells, and subsequent differentiation.
Acknowledgments
We thank members of the Sha lab for helpful discussion, Jeff Wallin and Sia Bakardjiev for help with performing the experiments, Elizabeth Sweet for assistance with generating monoclonal antibodies, and Ken Murphy, Theresa Murphy, and members of Jim Allison's lab for advice and reagents for T cell costimulation studies.
This work was supported by grants from the National Institutes of Health (AI42252), a Pew Charitable Trusts Biomedical Scholar Award (W.C. Sha), a National Science Foundation Predoctoral Fellowship (L. Liang), and a Howard Hughes Medical Institute Undergraduate Fellows Award (E.M. Porter).
Footnotes
Abbreviations used in this paper: BCR, B cell receptor; CG, chicken-γ globulin; CHO, Chinese hamster ovary; CsA, cyclosporin A; HEL, hen egg lysozyme; ICOS, inducible costimulator; L, ligand; NP, (4-hydroxy-3-nitrophenyl)acetyl; RAG, recombination activating gene.
References
- 1.Chambers, C.A., and J.P. Allison. 1999. Costimulatory regulation of T cell function. Curr. Opin. Cell Biol. 11:203–210. [DOI] [PubMed] [Google Scholar]
- 2.McAdam, A.J., A.N. Schweitzer, and A.H. Sharpe. 1998. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 165:231–247. [DOI] [PubMed] [Google Scholar]
- 3.Noelle, R.J., J.A. Ledbetter, and A. Aruffo. 1992. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol. Today. 13:431–433. [DOI] [PubMed] [Google Scholar]
- 4.Lenschow, D.J., T.L. Walunas, and J.A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233–258. [DOI] [PubMed] [Google Scholar]
- 5.Foy, T.M., A. Aruffo, J. Bajorath, J.E. Buhlmann, and R.J. Noelle. 1996. Immune regulation by CD40 and its ligand GP39. Annu. Rev. Immunol. 14:591–617. [DOI] [PubMed] [Google Scholar]
- 6.Hutloff, A., A.M. Dittrich, K.C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R.A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 397:263–266. [DOI] [PubMed] [Google Scholar]
- 7.Ling, V., P.W. Wu, H.F. Finnerty, K.M. Bean, V. Spaulding, L.A. Fouser, J.P. Leonard, S.E. Hunter, R. Zollner, J.L. Thomas, et al. 2000. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J. Immunol. 164:1653–1657. [DOI] [PubMed] [Google Scholar]
- 8.Mages, H.W., A. Hutloff, C. Heuck, K. Buchner, H. Himmelbauer, F. Oliveri, and R.A. Kroczek. 2000. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur. J. Immunol. 30:1040–1047. [DOI] [PubMed] [Google Scholar]
- 9.Yoshinaga, S.K., J.S. Whoriskey, S.D. Khare, U. Sarmiento, J. Guo, T. Horan, G. Shih, M. Zhang, M.A. Coccia, T. Kohno, et al. 1999. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 402:827–832. [DOI] [PubMed] [Google Scholar]
- 10.Swallow, M.M., J.J. Wallin, and W.C. Sha. 1999. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFα. Immunity. 11:423–432. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga, S.K., M. Zhang, J. Pistillo, T. Horan, S.D. Khare, K. Miner, M. Sonnenberg, T. Boone, D. Brankow, T. Dai, et al. 2000. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int. Immunol. 12:1439–1447. [DOI] [PubMed] [Google Scholar]
- 12.Aicher, A., M. Hayden-Ledbetter, W.A. Brady, A. Pezzutto, G. Richter, D. Magaletti, S. Buckwalter, J.A. Ledbetter, and E.A. Clark. 2000. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 164:4689–4696. [DOI] [PubMed] [Google Scholar]
- 13.Coyle, A.J., S. Lehar, C. Lloyd, J. Tian, T. Delaney, S. Manning, T. Nguyen, T. Burwell, H. Schneider, J.A. Gonzalo, et al. 2000. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 13:95–105. [DOI] [PubMed] [Google Scholar]
- 14.Kawabe, T., T. Naka, K. Yoshida, T. Tanaka, H. Fujiwara, S. Suematsu, N. Yoshida, T. Kishimoto, and H. Kikutani. 1994. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1:167–178. [DOI] [PubMed] [Google Scholar]
- 15.Xu, J., T.M. Foy, J.D. Laman, E.A. Elliott, J.J. Dunn, T.J. Waldschmidt, J. Elsemore, R.J. Noelle, and R.A. Flavell. 1994. Mice deficient for the CD40 ligand. Immunity. 1:423–431. [DOI] [PubMed] [Google Scholar]
- 16.Renshaw, B.R., W.C. Fanslow III, R.J. Armitage, K.A. Campbell, D. Liggitt, B. Wright, B.L. Davison, and C.R. Maliszewski. 1994. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 180:1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borriello, F., M.P. Sethna, S.D. Boyd, A.N. Schweitzer, E.A. Tivol, D. Jacoby, T.B. Strom, E.M. Simpson, G.J. Freeman, and A.H. Sharpe. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 6:303–313. [DOI] [PubMed] [Google Scholar]
- 18.Shahinian, A., K. Pfeffer, K.P. Lee, T.M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P.S. Ohashi, C.B. Thompson, and T.W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 261:609–612. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson, S.E., S. Han, G. Kelsoe, and C.B. Thompson. 1996. CD28 is required for germinal center formation. J. Immunol. 156:4576–4581. [PubMed] [Google Scholar]
- 20.Tafuri, A., A. Shahinian, F. Bladt, S.K. Yoshinaga, M. Jordana, A. Wakeham, L.M. Boucher, D. Bouchard, V.S. Chan, G. Duncan, et al. 2001. ICOS is essential for effective T-helper-cell responses. Nature. 409:105–109. [DOI] [PubMed] [Google Scholar]
- 21.McAdam, A.J., R.J. Greenwald, M.A. Levin, T. Chernova, N. Malenkovich, V. Ling, G.J. Freeman, and A.H. Sharpe. 2001. ICOS is critical for CD40-mediated antibody class switching. Nature. 409:102–105. [DOI] [PubMed] [Google Scholar]
- 22.Dong, C., A.E. Juedes, U.A. Temann, S. Shresta, J.P. Allison, N.H. Ruddle, and R.A. Flavell. 2001. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 409:97–101. [DOI] [PubMed] [Google Scholar]
- 23.Dong, C., U.A. Temann, and R.A. Flavell. 2001. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166:3659–3662. [DOI] [PubMed] [Google Scholar]
- 24.Beier, K.C., A. Hutloff, A.M. Dittrich, C. Heuck, A. Rauch, K. Buchner, B. Ludewig, H.D. Ochs, H.W. Mages, and R.A. Kroczek. 2000. Induction, binding specificity and function of human ICOS. Eur. J. Immunol. 30:3707–3717. [DOI] [PubMed] [Google Scholar]
- 25.Johnson-Leger, C., J. Christensen, and G.G. Klaus. 1998. CD28 co-stimulation stabilizes the expression of the CD40 ligand on T cells. Int. Immunol. 10:1083–1091. [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal, A.I., and M. Croft. 1997. CD40 ligand induction on T cell subsets by peptide-presenting B cells: implications for development of the primary T and B cell response. J. Immunol. 159:2282–2291. [PubMed] [Google Scholar]
- 27.Azuma, M., D. Ito, H. Yagita, K. Okumura, J.H. Phillips, L.L. Lanier, and C. Somoza. 1993. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 366:76–79. [DOI] [PubMed] [Google Scholar]
- 28.McAdam, A.J., T.T. Chang, A.E. Lumelsky, E.A. Greenfield, V.A. Boussiotis, J.S. Duke-Cohan, T. Chernova, N. Malenkovich, C. Jabs, V.K. Kuchroo, et al. 2000. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 165:5035–5040. [DOI] [PubMed] [Google Scholar]
- 29.Wallin, J.J., L. Liang, A. Bakardjiev, and W.C. Sha. 2001. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J. Immunol. 167:132–139. [DOI] [PubMed] [Google Scholar]
- 30.Sha, W.C., H.C. Liou, E.I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 80:321–330. [DOI] [PubMed] [Google Scholar]
- 31.Liou, H.C., W.C. Sha, M.L. Scott, and D. Baltimore. 1994. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol. Cell. Biol. 14:5349–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehry, M.R., and B.E. Castle. 1994. Regulation of CD40 ligand expression and use of recombinant CD40 ligand for studying B cell growth and differentiation. Semin. Immunol. 6:287–294. [DOI] [PubMed] [Google Scholar]
- 33.Snapper, C.M., P. Zelazowski, F.R. Rosas, M.R. Kehry, M. Tian, D. Baltimore, and W.C. Sha. 1996. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 156:183–191. [PubMed] [Google Scholar]
- 34.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inaba, K., J.P. Metlay, M.T. Crowley, M. Witmer-Pack, and R.M. Steinman. 1990. Dendritic cells as antigen presenting cells in vivo. Int. Rev. Immunol. 6:197–206. [DOI] [PubMed] [Google Scholar]
- 36.Lenschow, D.J., A.I. Sperling, M.P. Cooke, G. Freeman, L. Rhee, D.C. Decker, G. Gray, L.M. Nadler, C.C. Goodnow, and J.A. Bluestone. 1994. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J. Immunol. 153:1990–1997. [PubMed] [Google Scholar]
- 37.Hathcock, K.S., G. Laszlo, C. Pucillo, P. Linsley, and R.J. Hodes. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenschow, D.J., G.H. Su, L.A. Zuckerman, N. Nabavi, C.L. Jellis, G.S. Gray, J. Miller, and J.A. Bluestone. 1993. Expression and functional significance of an additional ligand for CTLA-4. Proc. Natl. Acad. Sci. USA. 90:11054–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy, M.K., K.M. Mohler, K.D. Shanebeck, P.R. Baum, K.S. Picha, C.A. Otten-Evans, C.A. Janeway, Jr., and K.H. Grabstein. 1994. Induction of B cell costimulatory function by recombinant murine CD40 ligand. Eur. J. Immunol. 24:116–123. [DOI] [PubMed] [Google Scholar]
- 40.Yellin, M.J., J. Sinning, L.R. Covey, W. Sherman, J.J. Lee, E. Glickman-Nir, K.C. Sippel, J. Rogers, A.M. Cleary, M. Parker, et al. 1994. T lymphocyte T cell-B cell-activating molecule/CD40-L molecules induce normal B cells or chronic lymphocytic leukemia B cells to express CD80 (B7/BB-1) and enhance their costimulatory activity. J. Immunol. 153:666–674. [PubMed] [Google Scholar]
- 41.Mackay, C.R. 2000. Follicular homing T helper (Th) cells and the Th1/Th2 paradigm. J. Exp. Med. 192:F31–F34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasbold, J., C. Johnson-Leger, C.J. Atkins, E.A. Clark, and G.G. Klaus. 1994. Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur. J. Immunol. 24:1835–1842. [DOI] [PubMed] [Google Scholar]
- 43.Hou, J., U. Schindler, W.J. Henzel, T.C. Ho, M. Brasseur, and S.L. McKnight. 1994. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 265:1701–1706. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, K., T. Tanaka, W. Shi, M. Matsumoto, M. Minami, S. Kashiwamura, K. Nakanishi, N. Yoshida, T. Kishimoto, and S. Akira. 1996. Essential role of Stat6 in IL-4 signalling. Nature. 380:627–630. [DOI] [PubMed] [Google Scholar]
- 45.Goodnow, C.C., J. Crosbie, S. Adelstein, T.B. Lavoie, S.J. Smith-Gill, R.A. Brink, H. Pritchard-Briscoe, J.S. Wotherspoon, R.H. Loblay, K. Raphael, et al. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 334:676–682. [DOI] [PubMed] [Google Scholar]
- 46.Foy, T.M., J.D. Laman, J.A. Ledbetter, A. Aruffo, E. Claassen, and R.J. Noelle. 1994. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J. Exp. Med. 180:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foy, T.M., D.M. Shepherd, F.H. Durie, A. Aruffo, J.A. Ledbetter, and R.J. Noelle. 1993. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J. Exp. Med. 178:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Eertwegh, A.J., R.J. Noelle, M. Roy, D.M. Shepherd, A. Aruffo, J.A. Ledbetter, W.J. Boersma, and E. Claassen. 1993. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J. Exp. Med. 178:1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richter, G., M. Hayden-Ledbetter, M. Irgang, J.A. Ledbetter, J. Westermann, I. Korner, K. Daemen, E.A. Clark, A. Aicher, and A. Pezzutto. 2001. Tumor necrosis factor-α regulates the expression of inducible costimulator receptor ligand on CD34(+) progenitor cells during differentiation into antigen presenting cells. J. Biol. Chem. 276:45686–45693. [DOI] [PubMed] [Google Scholar]
- 50.Feske, S., J. Giltnane, R. Dolmetsch, L.M. Staudt, and A. Rao. 2001. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2:316–324. [DOI] [PubMed] [Google Scholar]
- 51.Schroder, A.J., P. Pavlidis, A. Arimura, D. Capece, and P.B. Rothman. 2002. Cutting edge: STAT6 serves as a positive and negative regulator of gene expression in IL-4-stimulated B lymphocytes. J. Immunol. 168:996–1000. [DOI] [PubMed] [Google Scholar]