Abstract
The effector cytokine interferon γ (IFN-γ) may play a role in T cell homeostasis. We have examined the requirement for IFN-γ in one mechanism that regulates T cell expansion and survival, activation-induced cell death (AICD). CD4+ T cells lacking IFN-γ or the Stat1 transcription factor are resistant to AICD. IFN-γ is required for the production of caspases, and retrovirus-mediated expression of caspase-8 restores the sensitivity of Stat1-deficient T cells to AICD. In vitro, IFN-γ limits the expansion of T cells that are stimulated through their antigen receptors. Thus, IFN-γ may function to control the expansion and persistence of T cells by promoting caspase-8–dependent apoptosis.
Keywords: apoptosis, IFN-γ, Fas, caspases, Stat1
Introduction
IFN-γ is the principal effector cytokine of cell-mediated immunity. Recent studies suggest that IFN-γ also plays a previously unsuspected role in the homeostatic control of T cells. If mice lacking IFN-γ are infected with mycobacteria, there is a progressive expansion of T cells that is not attributable to increased numbers or persistence of bacteria (1). Similarly, after Listeria infection, the frequency of microbe-specific CD8+ T cells is greater in IFN-γ−/− than in wild-type mice, and the decline of T cells is delayed (2). This regulatory role of IFN-γ may also be relevant to immune responses to antigens other than microbial antigens. For instance, IFN-γ−/− mice and normal mice treated with anti–IFN-γ antibody show paradoxically more severe encephalitis after immunization with myelin proteins than do normal mice (3–5). Despite these studies suggesting an obligatory role of IFN-γ in T cell homeostasis, the mechanisms by which this cytokine regulates T cell expansion and survival are unknown.
Apoptotic death of lymphocytes is a major homeostatic mechanism in the immune system (6). One pathway of apoptosis, called activation-induced cell death (AICD), is induced by antigen stimulation under particular conditions. Repeated stimulation by antigen results in engagement of death receptors and activation of caspase-8 (7–9). In CD4+ T cells, the major death receptor responsible for triggering this pathway of AICD is Fas (CD95; reference 10). Fas-mediated AICD is known to play an essential role in the deletion of self-reactive lymphocytes, and may also be involved in the deletion of T cells chronically exposed to foreign antigens, such as persistent microbes. We and others have previously shown that in vitro, death of previously activated T cells induced by restimulation with anti-CD3 is largely dependent upon the ligation of Fas (10–12). A second pathway of AICD is induced by T cell stimulation in the absence of innate immunity or costimulation. This type of stimulation results in activation of pro-apoptotic members of the bcl-2 family, such as Bim, in the absence of antiapoptotic members, and T cell death without engagement of death receptors (13).
In previous studies of the regulation of AICD, we had noticed that differentiated Th2 cells were less sensitive to this death pathway than were Th1 cells. We hypothesized that the basis of this death resistance may be the absence of IFN-γ production in Th2 cells. Such a function of IFN-γ in potentiating AICD may also account for the role of this cytokine in T cell homeostasis. In this paper, we show that IFN-γ is indeed required for activation-induced death of T cells. Furthermore, IFN-γ functions by stimulating the expression of caspases downstream of the Fas death receptor, through the transcriptional activity of Stat1.
Materials and Methods
Mice.
3A9/+ and 3A9/IL-2−/− TCR transgenic mice have been described previously (14). IFN-γ−/− mice, on a C57BL/6 background, and normal C57BL/6 mice were obtained from the The Jackson Laboratory. Stat1−/− mice, on a 129sv background, were obtained from Dr. R.D. Schreiber (Washington University, St. Louis, MO) through Taconic Farms. All of the mice used in these experiments were 3–4-wk-old and were maintained in accordance with the guidelines of the Committee on Animals of the University of California San Francisco and those of the institute of Laboratory Animal Resources, National Research Council.
T Cell Purification and In Vitro Activation.
Naive CD4+ T cells were isolated from pooled spleen and lymph nodes, as described previously (14). Briefly, cell suspensions were incubated with anti-CD4 coated magnetic beads (Dynal) for 45 min, at 4°C. The adherent cells were washed twice and incubated for 45 min with the Dynal Detach antibody. For in vitro activation, 2 × 105 naive CD4+ T cells were cultured with 2 × 106 mitomycin C (Sigma-Aldrich) treated H-2k spleen cells in the presence of 1 μg/ml of HEL(46–61) peptide. Some cultures were supplemented with IL-2 at 50 U/ml, and IFN-γ at 10 U/ml. The activation cultures were performed in RPMI 1640 with 10% heat inactivated fetal calf serum (GIBCO BRL), l-glutamine, penicillin, streptomycin, nonessential amino acids, sodium pyruvate, and 2-β mercaptoethanol (all from GIBCO BRL). To activate T cells without APCs, 106 CD4+ T cells were cultured with 1 μg/ml of soluble anti-CD3 antibody (2C11), and 10 μg/ml of anti-CD28 antibody (37N1; both from BD Biosciences), with or without added cytokines (R&D Systems). The in vitro primed T cells did not produce detectable IL-4 or IL-5 upon restimulation (unpublished data).
TCR-induced proliferation was assayed by incubating 106 CFSE-stained, naive CD4+ T cells with 1 μg/ml soluble anti-CD3 (clone 2C11; BD Biosciences) and 10 μg/ml soluble anti-CD28 (clone 37N1; BD Biosciences), for 3 d, and determining cell division number by flow cytometry. For the secondary proliferative responses to TCR stimulation, 5 × 103 previously activated cells were incubated with the indicated amounts of soluble anti-CD3 antibody (see Fig. 4) , and incubated in complete media for 48 h. The activated cells were pulsed with 1 μCi of [H3]thymidine for 12 h. The level of DNA replication was determined by quantifying the number of counts per minute using a PerkinElmer β-counter. All values were derived from the means of triplicate wells, and error bars represent the standard deviations.
Figure 4.
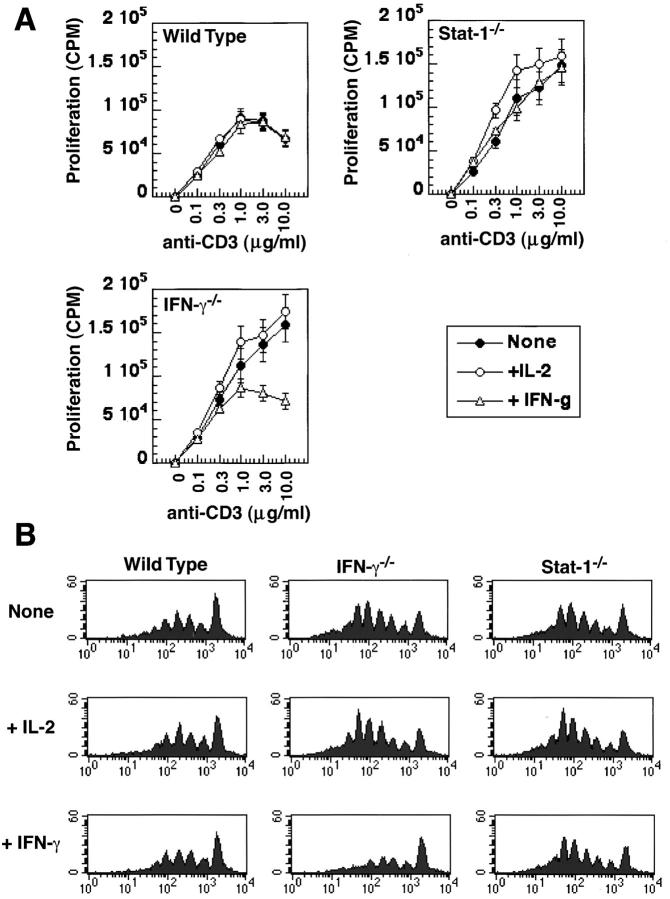
IFN-γ regulates the number of cells following antigenic stimulation by affecting apoptosis but not cell division and proliferation. CD4+ T cells were purified from pooled spleen and lymph node suspensions obtained from either C57BL/6 (wild type), IFN-γ−/− mice, or Stat-1−/− mice. (A) The cells were cultured with anti-CD3 and anti-CD28 antibodies for 4 d, rested in medium plus cytokines, as indicated, restimulated with soluble anti-CD3 antibodies at the indicated concentrations. These cultures were pulsed with [H3]thymidine for 12 h, 2 d after initial activation. (B) Freshly isolated cells were labeled with CFSE, activated with antibodies to CD3 and CD28, in the presence of exogenously added cytokines, as indicated. The activated T cells were analyzed by flow cytometry four days after activation.
In vitro–activated T cells obtained from the various mutant mice used in the present studies were stained for surface expression of Fas and FasL as described previously (14). Activated T cells were reactivated by incubation for 12 h in anti-CD3 coated plates. These cells were collected and washed and incubated with PE-conjugated anti-Fas antibodies (Jo-2 clone; BD Biosciences), or with an anti-FasL monoclonal antibody (H11; a gift form Dr. Jürg Tschopp, University of Lausanne, Lausanne, Switzerland). These cells were incubated with a PE-conjugated secondary antibody (BD Biosciences).
Assays for Apoptosis.
T cells were activated as described above with the indicated cytokine added to the culture. After 3 d in culture, viable cells were isolated on a ficoll gradient. 106 activated T cells were then cultured in duplicate wells coated with 0–1 μg/ml anti-CD3 antibody, in the presence of IL-2 (50 U/ml). Controls in these experiments consisted of 106 cells cultured in IL-2 only. After 20 h, cells were collected, washed twice in PBS supplemented with 0.1% d-glucose, and fixed in 70% ethanol overnight at 4°C. The cells were spun and resuspended in 0.5 ml of a solution of 50 μg/ml Propidium Iodide containing 1 μg/ml RNaseA, diluted in PBS plus 0.1% d-glucose. Percentage apoptotic cells were determined by flow cytometry using a Becton Dickinson FACSCalibur™, by gating on the subdiploid population.
RNase Protection Assays.
RNA was obtained from 107 activated T cells using Trizol reagent (GIBCO BRL), following manufacturer's specifications. The RNase protection was performed using 5 μg of RNA with the Riboquant multi-probe RNase protection assay system (BD Biosciences), following the manufacturer's specifications. In brief, RNA was hybridized overnight with the in vitro translated 32P-labeled probe (mAPO-1 kit; BD Biosciences). After hybridization, samples were treated with RNase A and proteinase K, phenol/chloroform extracted, and ethanol precipitated. The protected fragments were resolved by electrophoresis on a 5% acrylamide/urea gel, and exposed on XAR film (Eastman Kodak Co.) as well as on a PhosphorImager cassette (Molecular Devices). The intensity of each band was determined using ImageQuant software, and normalized to an average of both control probes (GAPDH and L32).
Retroviral Infections.
The cDNA for human caspase-8 was cloned into a bicistronic IRES-GFP containing vector which is a variant of pMIG (15) that contains a posttranscriptional regulatory element from the woodchuck hepatitis virus (16) and is called pMIG-w. This virus shows 5 to 10-fold greater higher expression of genes in primary lymphocytes (unpublished data). Retroviral infection of primary murine CD4+ T cells were performed as described previously. Briefly, CD4+ T cells obtained from pooled spleen and lymph nodes from Stat1−/− mice were activated for 3 d with anti-CD3 and anti-CD28. Retrovirus infections were performed at 24 and 48 h of culture as described (15). Under these conditions, the transfection efficiency is routinely 40–60%. The cells were assayed for apoptosis, as described above, gating on GFP+ cells.
The levels of caspase-8 in retrovirus-infected cells were determined by Western blot analysis. The cells were washed in chilled Dulbecco's PBS (GIBCO BRL). Cells were lysed in 0.1% NP-40 containing lysis buffer, as described previously (14). Lysates were run on a 12% SDS-PAGE. Blots were probed with an anti-Caspase-8 monoclonal antibody (New England Biolabs, Inc./Cell Signaling Technologies), followed by a rabbit anti–mouse HRP-conjugated antibody (Santa Cruz Biotechnology, Inc.). Western blots were developed using an ECL kit (Amersham Biosciences) and exposed to XLS film (Eastman Kodak Co.).
Results and Discussion
IFN-γ Signals Are Required for AICD.
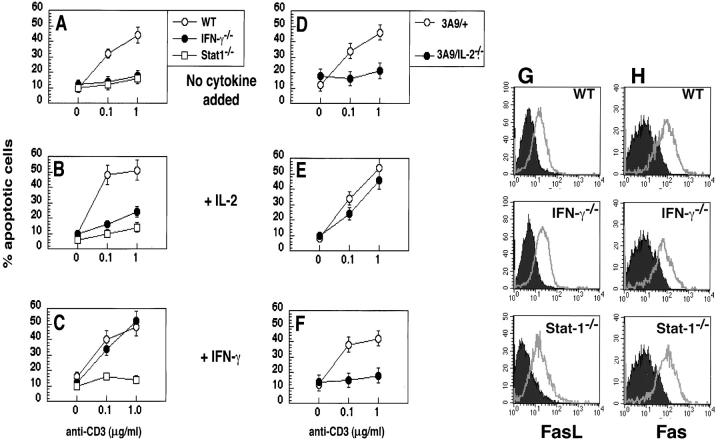
To assess the role of IFN-γ in AICD, we analyzed CD4+ T cells from knockout mice lacking IFN-γ or lacking the major transcription factor activated by the IFN receptor, Stat1. T cells activated with anti-CD3 plus anti-CD28 antibodies were exposed to anti-CD3 antibody, to mimic repeated antigen stimulation, and apoptosis was assayed. We have previously shown that under these conditions, death of CD4+ T cells is entirely dependent on Fas-Fas ligand (FasL) interactions (12, 14). Mice lacking IFN-γ or Stat1 did not undergo apoptosis whereas wild-type cells did (Fig. 1 A). The defect of the IFN-γ−/− mice was corrected by adding IFN-γ, showing that there is no intrinsic developmental defect in the T cells, and their resistance to AICD is entirely attributable to the lack of IFN-γ production. In addition, the activated IFN-γ−/− T cells did not produce IL-4 (data not shown), suggesting that these cells did not differentiate into Th2 type cells by default. As expected, adding IFN-γ to wild-type T cells (which produce IFN-γ and are not defective in AICD) had no detectable effect. The finding that Stat1−/− T cells show the same defect as IFN-γ−/− cells indicates that the role of this cytokine in AICD is dependent on the Stat1 transcription factor. As we and others have shown previously (14, 17), IL-2 is also required for AICD of CD4+ T cells (Fig. 1 B; note that to examine IL-2−/− T cells, we used mice expressing a TCR transgene, 3A9, crossed with IL-2 knockouts, because the transgenic antigen receptor retards the severe autoimmune disease of these knockouts, presumably by limiting the repertoire of autoreactive T cells). Addition of IL-2 restored the sensitivity of IL-2−/− T cells to AICD, and addition of IFN-γ made IFN-γ−/− T cells sensitive (Fig. 1). However, the effect of each cytokine was specific, suggesting that IFN-γ and IL-2 are independently required for AICD and may act by distinct mechanisms.
Figure 1.
IL-2 and IFN-γ independently regulate AICD. CD4+ T cells were purified from pooled spleen and lymph node suspensions obtained from C57BL/6 (WT), IFN-γ−/−, or Stat1−/− mice (A–C), or from 3A9/+ (wild-type) and 3A9/IL-2−/− mice (D–F). These cells were activated in vitro with anti-CD3 and anti-CD28, with the following cytokines added to the cultures: none (A and D), IL-2 (50 U/ml; B and E), or IFN-γ (10 U/ml; C and F). Activated cells were collected 3 d later, and incubated on anti-CD3 coated plates in the presence of IL-2. Apoptosis was determined by Propidium Iodide staining. The same cells were analyzed for the expression of FasL (G) and Fas (H) by staining and flow cytometry. The filled histograms in G represent resting cells and the empty histograms represent activated T cells. The filled histograms in H represent cells that were stained with an isotype-matched control antibody and the empty histograms represent cells that were stained with anti-Fas.
To ask if IFN-γ is required for the expression of Fas or FasL, activated T cells were stained with specific antibodies and analyzed by flow cytometry. As shown in Fig. 1, G and H, IFN- γ−/− and Stat-1−/− T cells expressed normal levels of FasL and Fas. Furthermore, these T cells were also resistant to apoptosis induced by antibody-mediated cross-linking of Fas (data not shown). Taken together, these results suggest that the defect in this pathway of AICD seen in IFN- γ−/− and Stat-1−/− T cells must be downstream of the expression of the death receptor and its ligand.
IFN-γ Is Required for Caspase Production.
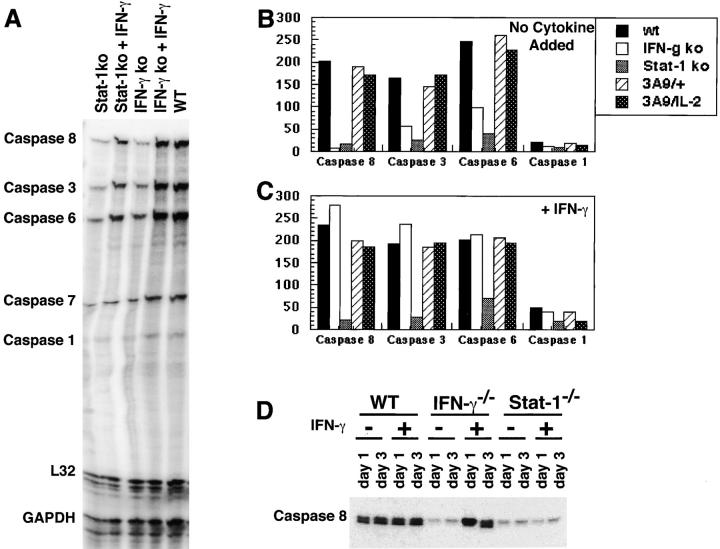
The sensitivity of cells to Fas-mediated apoptosis is controlled by the formation of the death-inducing signaling complex and the subsequent activation of caspases (7, 9). Fas is cross-linked by its ligand, resulting in the binding of an adaptor protein, which then recruits and activates pro-caspase-8. The endogenous inhibitor of apoptosis, c-FLIP, blocks the binding of pro-caspase-8 and thus prevents the propagation of the apoptotic signal. In this sequence, FasL is proapoptotic and FLIP is antiapoptotic. We have previously shown that IL-2 renders T cells sensitive to AICD by increasing FasL expression and inhibiting FLIP expression (14). Similar experiments with T cells lacking IFN-γ or Stat1 showed that IFN-γ is not required for enhancing the expression of FasL or inhibiting the transcription of FLIP (data not shown). We, therefore, postulated that the action of IFN-γ may be distal to formation of the death-inducing signaling complex, perhaps in the expression of caspases. To test this, we first assayed levels of RNA for several caspases in activated T cells from IFN-γ−/− and Stat1−/− mice. In the absence of IFN-γ signals, T cells contained virtually no RNA for the initiator caspase that is downstream of death receptors, caspase-8, and reduced RNA for effector caspases such as caspase-3 and caspase-6 (Fig. 2, A and B) . Furthermore, levels of caspase-8 RNA were restored by the addition of IFN-γ in IFN-γ−/− T cells but not, as expected, in Stat1−/− T cells (Fig. 2 C). The same results were seen when levels of caspase-8 protein were assayed by Western blotting (Fig. 2 D). Note that in wild-type T cells, the addition of IFN-γ does not increase the level of caspase-8, presumably because the cells make IFN-γ at optimal levels. As this caspase is the initiator caspase that is activated by death receptors, these results indicate that IFN-γ and Stat1 are uniquely involved in apoptosis mediated by death receptor triggering. Also, T cells express little or no caspase-1 mRNA, which is not surprising, as the function of this enzyme is to cleave the precursor form of IL-1β. In fibroblasts, caspase-1 production may also be stimulated by cytokines (18).
Figure 2.
IFN-γ and Stat1 increase the levels of caspase transcripts. CD4+ T cells were purified from pooled lymph node and spleen suspensions obtained from C57BL6 (WT), IFN-γ−/−, Stat1−/−, 3A9/+, or 3A9/IL-2−/− mice. These cells were activated with antibodies to CD3 and CD28, with no added cytokine, or with added IFN-γ. Activated cells were collected after 3 d, and mRNA for caspases was assayed by RNase protection. The protected species were resolved on a TBE/Urea gel, and the bands quantified using a PhosphorImager, a representative example is shown in A. Levels of mRNA (B and C) were calculated by averaging the two bands for the housekeeping genes (L32 and GAPDH), and determining the percentage of the value for each of the caspase probes. The data were pooled from two independent experiments. (D) The levels of caspase-8 protein in CD4+ T cells from C57BL-6 (WT), IFN-γ−/−, and Stat1−/− mice activated for 1 or 3 d with no added cytokine or with IFN-γ (10 U/ml) were determined by Western blot analysis.
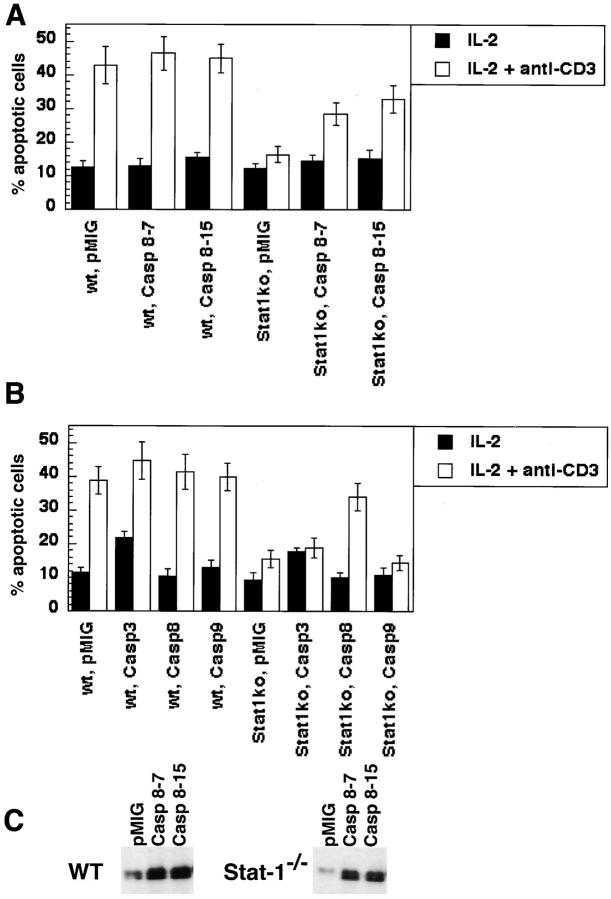
If the principal reason why IFN-γ−/− or Stat1−/− T cells are insensitive to AICD is a deficiency of caspase-8, enforced expression of this enzyme should restore the sensitivity of the T cells to Fas-mediated apoptosis. To test this possibility, we introduced three different caspases into wild-type and Stat1−/− T cells using a bicistronic retroviral vector in which GFP serves as a marker for viral infection, and assayed AICD in GFP+ cells. As shown in Fig. 3 , expression of caspase-8, but not caspase- 3 or caspase-9, made Stat1−/− T cells sensitive to AICD. Furthermore, upon repeated activation, we detected two bands for caspase-8 by Western blotting, consistent with cleavage of the enzyme as a result of Fas-signaling (data not shown).
Figure 3.
Retrovirally transduced caspase-8 is able to correct the AICD defect observed in Stat1−/− T cells. (A) CD4+ T cells were purified from pooled spleen and lymph node suspensions obtained from either C57BL-6 (wt), or Stat1−/− mice. These cells were activated and infected in vitro with bicistronic retroviral vectors encoding caspase-8 (two different clones, 8–7 and 8–15) or empty vector (pMIG). The infected cells were then incubated on anti-CD3 coated plates for 20 h to induce AICD, and apoptosis was assayed in GFP+ cells by flow cytometry. The data presented here were obtained from averaging the results of triplicate wells, from one experiment representative of three. (B) The specificity of the requirement for caspase-8 was determined by retrovirally transducing CD4+ T cells from either C57BL/6 (wt), or Stat1−/− mice with viruses encoding caspase-3 (Casp3), caspase-8 (Casp8), or caspase-9 (Casp9), and processed as in A. (C) The levels of caspase-8 expression in retrovirus-infected activated C57BL/6 (WT) and Stat1−/− cells were verified by Western blot analysis.
Role of IFN-γ in T Cell Expansion.
If IFN-γ enhances AICD in T cells, then it should reduce the accumulation of T cells that are repeatedly stimulated by their antigen receptors, which is the situation most likely to trigger AICD. To test this possibility, we stimulated wild-type, IFN-γ−/− or Stat1−/− T cells with anti-CD3 plus anti-CD28 antibodies, and assayed the incorporation of [3H]thymidine as a measure of cell survival and proliferation. T cells lacking IFN-γ or Stat1 showed significantly greater [3H]thymidine incorporation than wild-type cells, and the response of the IFN-γ−/− cells was reduced to wild-type levels by culture in the presence of the cytokine (Fig. 4 A). To more directly determine if these differences were attributable to cycling, we labeled cells with CFSE and measured dilution of the dye as an assay for cycling. As shown in Fig. 4 B, there was no difference in the cycling of normal, IFN-γ−/− or Stat1−/− cells, and cycling was not inhibited by the cytokine. Therefore, IFN-γ reduces T cell expansion, presumably by reducing survival and not by inhibiting cell cycling. This is the likely explanation for the finding that IFN-γ inhibits T cell expansion (19), which was previously attributed to an antiproliferative effect.
The proapoptotic activity of IFN-γ that we describe supports an emerging idea that this cytokine may function not only as an effector of cell-mediated immunity but also as a regulator of T cell survival (1–5). It is possible that IFN-γ production by differentiated Th1 cells contributes to the elimination of these cells, especially in situations of prolonged antigen stimulation, such as chronic infections and responses to self-antigens. This mechanism of T cell elimination will be important for preventing damage to host tissues during infections, because Th1 cells are pro-inflammatory and potentially injurious. The finding that two cytokines, IL-2 and IFN-γ, are both required for Fas-mediated apoptosis raises the possibility that these cytokines may function in homeostasis at different stages of immune responses. IL-2 may act early, even on T cells that have not differentiated into Th1 effectors, whereas IFN-γ may act only on differentiated Th1 cells. T cells that differentiate along the Th1 pathway may transiently reduce their expression of IFN-γ receptors (20, 21) and become resistant to the cytokine. It is possible that IFN-γ receptors are reexpressed at different stages of Th1 responses, allowing responses to be terminated by IFN-γ. It is also interesting that mutations in IL-2 or the α or β chain of the IL-2 receptor result in autoimmunity (22), but IFN-γ−/− mice do not develop autoimmune disease. We do not know if this difference is because of the stage or type of T cell response that is regulated by these cytokines, or because IL-2 has functions important for maintaining self-tolerance in addition to sensitizing T cells for AICD.
Acknowledgments
We thank Dr. Robert D. Schreiber (Washington University, St. Louis, MO) for providing us with Stat1−/− mice, Dr. Andrew Lichtman (Brigham and Women's Hospital, Boston, MA) for helpful discussions and advice, as well as members of the Bishop laboratory for helpful discussions.
Y. Refaeli was supported by a Howard Hughes Medical Institute predoctoral fellowship and is currently a Merck Fellow of the Life Sciences Research Foundation. L. Van Parijs was supported by an Arthritis Foundation Fellowship, S.I. Alexander was supported by a fellowship from the American Society of Transplantation. This work was supported by National Institutes of Health grants R01 AI42100 and P01 AI35297 (A.K. Abbas).
Y. Refaeli's present address is G.W. Hooper Foundation and Department of Microbiology and Immunology, University of California San Francisco, San Francisco, CA 94143.
S.I. Alexander's present address is Center for Kidney Research, Children's Hospital at Westmead, Westmead NSW 2145, Australia.
References
- 1.Dalton, D.K., L. Haynes, C.Q. Chu, S.L. Swain, and S. Wittmer. 2000. Interferon gamma eliminates responding CD4+ T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badovinac, V.P., A.R. Tvinnereim, and J.T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 290:1354–1358. [DOI] [PubMed] [Google Scholar]
- 3.Krakowski, M., and T. Owens. 1996. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26:1641–1646. [DOI] [PubMed] [Google Scholar]
- 4.Ferber, I.A., S. Brocke, C. Taylor-Edwards, W. Ridgway, C. Dinisco, L. Steinman, D. Dalton, and C.G. Fathman. 1996. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156:5–7. [PubMed] [Google Scholar]
- 5.Chu, C.Q., S. Wittmer, and D.K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Parijs, L., and A.K. Abbas. 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 280:243–248. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi, A., and V.M. Dixit. 1998. Death receptors: signaling and modulation. Science. 281:1305–1308. [DOI] [PubMed] [Google Scholar]
- 8.Siegel, R.M., and M.J. Lenardo. 2001. To B or not to B: TNF family signaling in lymphocytes. Nat. Immunol. 2:577–578. [DOI] [PubMed] [Google Scholar]
- 9.Rathmell, J.C., and C.B. Thompson. 2002. Pathways of apoptosis in lymphocyte development, homeostasis and disease. Cell. 109:S97–S107. [DOI] [PubMed] [Google Scholar]
- 10.Ju, S.T., D.J. Panka, H. Cui, R. Ettinger, M. el-Khatib, D. Sherr, B.Z. Stanger, and A. Marshak-Rothstein. 1995. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 373:444–448. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, M.R., T.W. Tough, T. Davis-Smith, S. Braddy, B. Falk, K.A. Schooley, R.G. Goodwin, C.A. Smith, F. Ramsdell, and D.H. Lynch. 1995. Fas ligand mediates activation-induced cell death in human T-lymphocytes. J. Exp. Med. 181:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Parijs, L., A. Buikians, and A.K. Abbas. 1998. Functional roles of Fas and Bcl-2 regulated apoptosis of T lymphocytes. J. Immunol. 160:2065–2071. [PubMed] [Google Scholar]
- 13.Hildeman, D.A., Y. Zhu, T.C. Mitchell, P. Bouillet, A. Strasser, J. Kappler, and P. Marrack. 2002. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 16:759–760. [DOI] [PubMed] [Google Scholar]
- 14.Refaeli, Y., L. Van Parijs, C.A. London, J. Tschopp, and A.K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 8:615–623. [DOI] [PubMed] [Google Scholar]
- 15.Van Parijs, L., Y. Refaeli, J.D. Lord, B.H. Nelson, A.K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 11:281–288. [DOI] [PubMed] [Google Scholar]
- 16.Zufferey, R.D.J., D. Trono, and T.J. Hope. 1999. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 73:2886–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenardo, M.J. 1991. Interleukin-2 programs mouse alpha-beta T lymphocytes for apoptosis. Nature. 353:858–861. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, A., M. Commane, T.W. Flickinger, C.M. Horvath, and G.R. Stark. 1997. Defective TNF-alpha-induced apoptosis in Stat1-null cells due to low constitutive levels of caspases. Science. 278:1630–1632. [DOI] [PubMed] [Google Scholar]
- 19.Gajewski, T.F., J. Joyce, and F.W. Fitch. 1989. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J. Immunol. 143:15–22. [PubMed] [Google Scholar]
- 20.Bach, E.A., S.J. Szabo, A.S. Dighe, A. Ashkenazi, M. Aguet, K.M. Murphy, and R.D. Schreiber. 1995. Ligand-induced autoregulation of IFN-gamma receptor beta chain expression in T helper cell subsets. Science. 270:1215–1218. [DOI] [PubMed] [Google Scholar]
- 21.Skrenta, H., Y. Yang, S. Pestka, and C.G. Fathman. 2000. Ligand-independent down-regulation of IFN-gamma receptor 1 following TCR engagement. J. Immunol. 164:3506–3511. [DOI] [PubMed] [Google Scholar]
- 22.Refaeli, Y., L. Van Parijs, and A.K. Abbas. 1999. Genetic models of abnormal apoptosis in lymphocytes. Immunol. Rev. 169:273–282. [DOI] [PubMed] [Google Scholar]